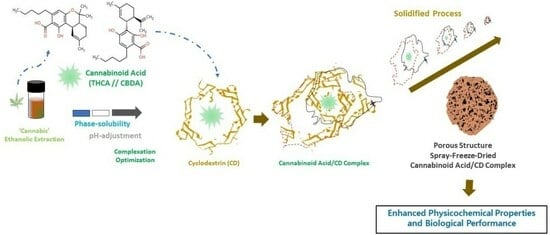
Investigation of Cannabinoid Acid/Cyclodextrin Inclusion Complex for Improving Physicochemical and Biological Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HPLC Analysis of Cannabinoids
2.3. Phase Solubility Studies of Cannabinoids According to the Types of Cyclodextrin
2.4. Preparation of Cannabinoids/M-β-CD Inclusion Complex
2.5. Solid-State Characterization of Cannabinoids and Cyclodextrin Inclusion Complex
2.5.1. Determination of Cannabinoid Solubility
2.5.2. Field-Emission Scanning Electron Microscopy (FE-SEM)
2.5.3. Differential Scanning Calorimetry (DSC)
2.5.4. X-ray Diffraction (XRD)
2.6. Proton Nuclear Magnetic Resonance (1H NMR) Spectroscopy
2.7. Stability Studies of the Inclusion Complex in Simulated Physiological Conditions
2.8. In Vitro Permeation Studies
2.9. In Vitro Anti-Cancer Activity Assay Using Human Breast (MCF-7) Cancer Cell Lines
3. Results and Discussion
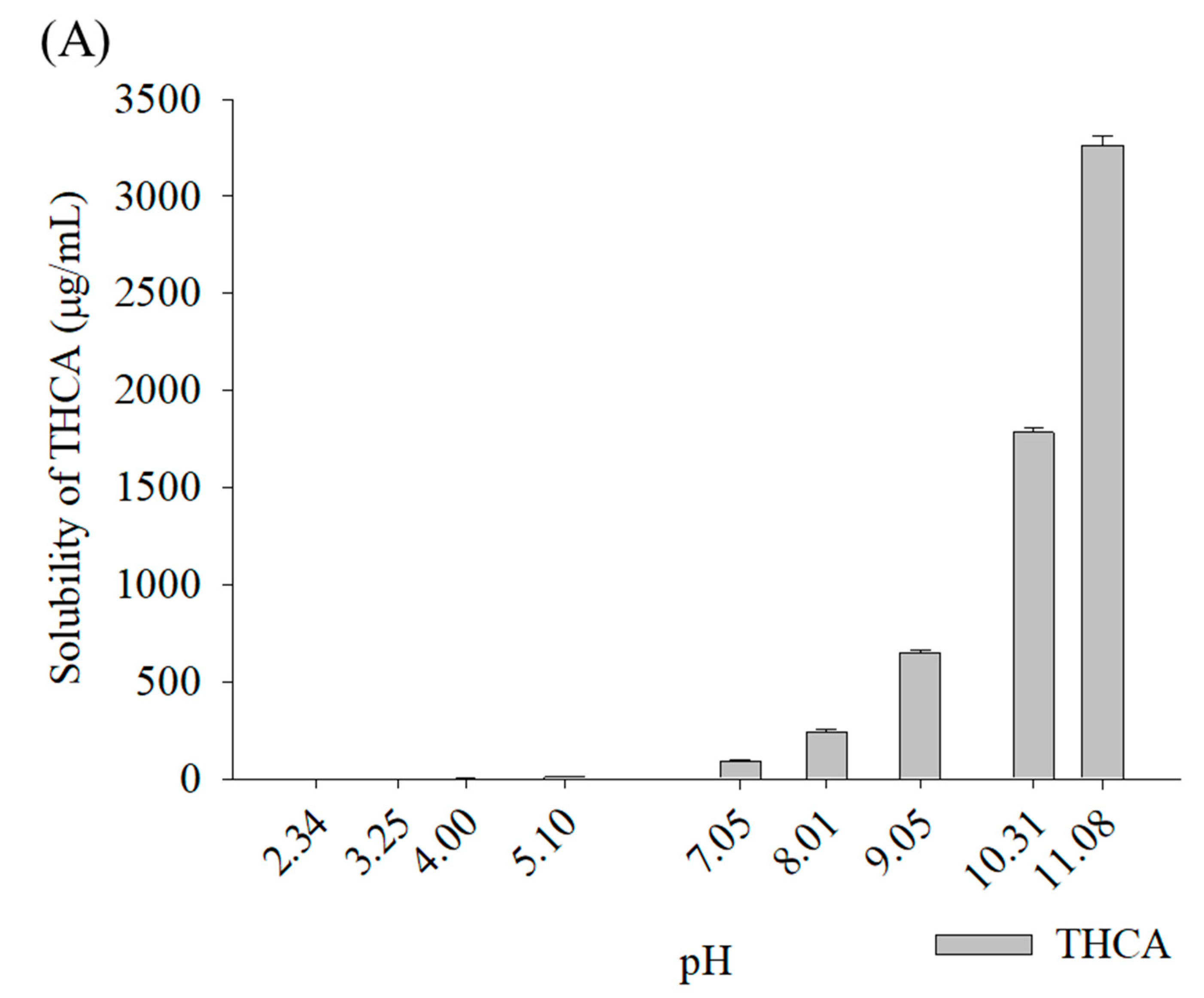
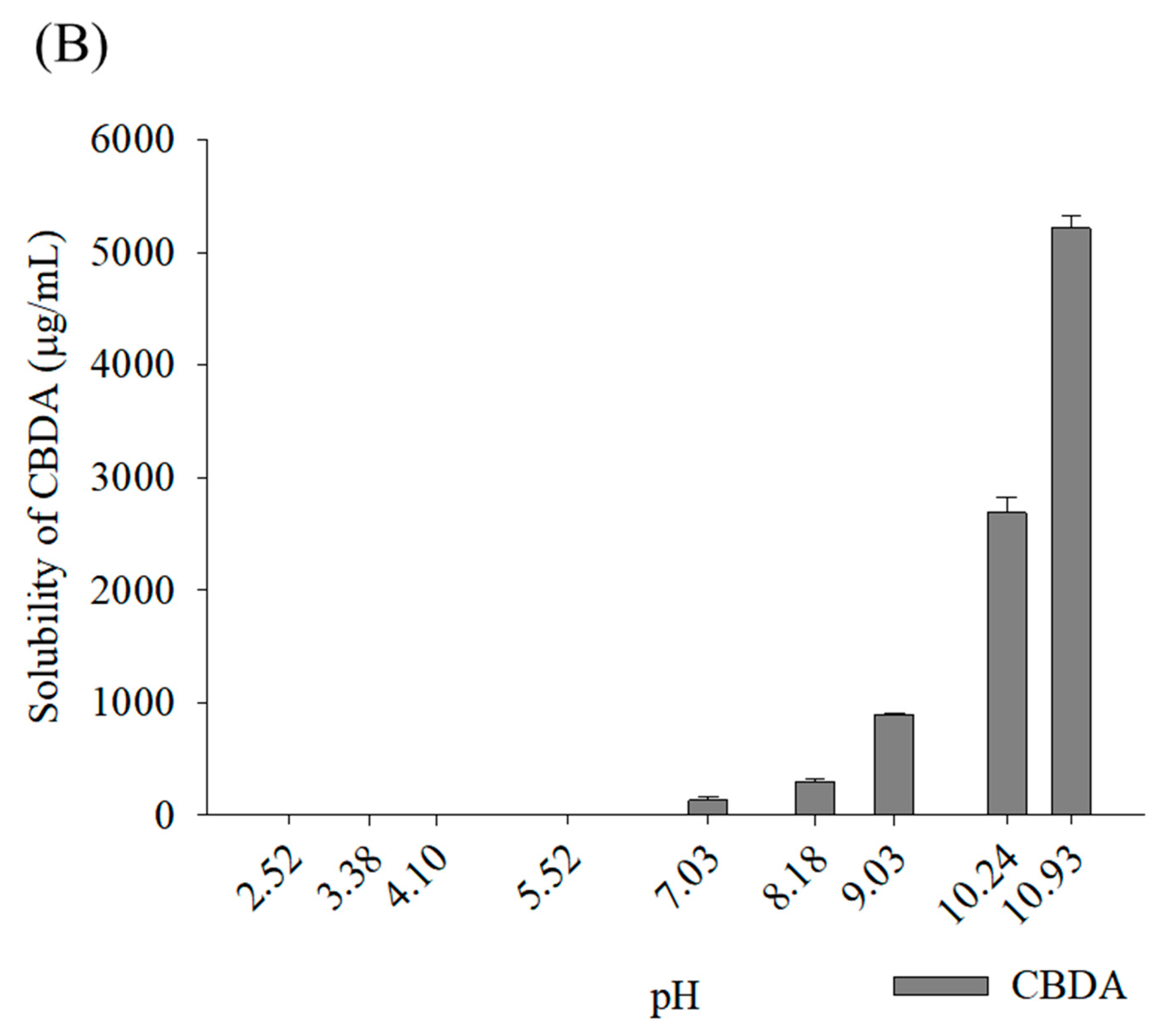
3.1. Phase Solubility Studies of Cannabinoids According to Types of Cyclodextrin
3.2. Solid-State Characterization of Cannabinoids and CD Inclusion Complex
3.2.1. Solubility Determination
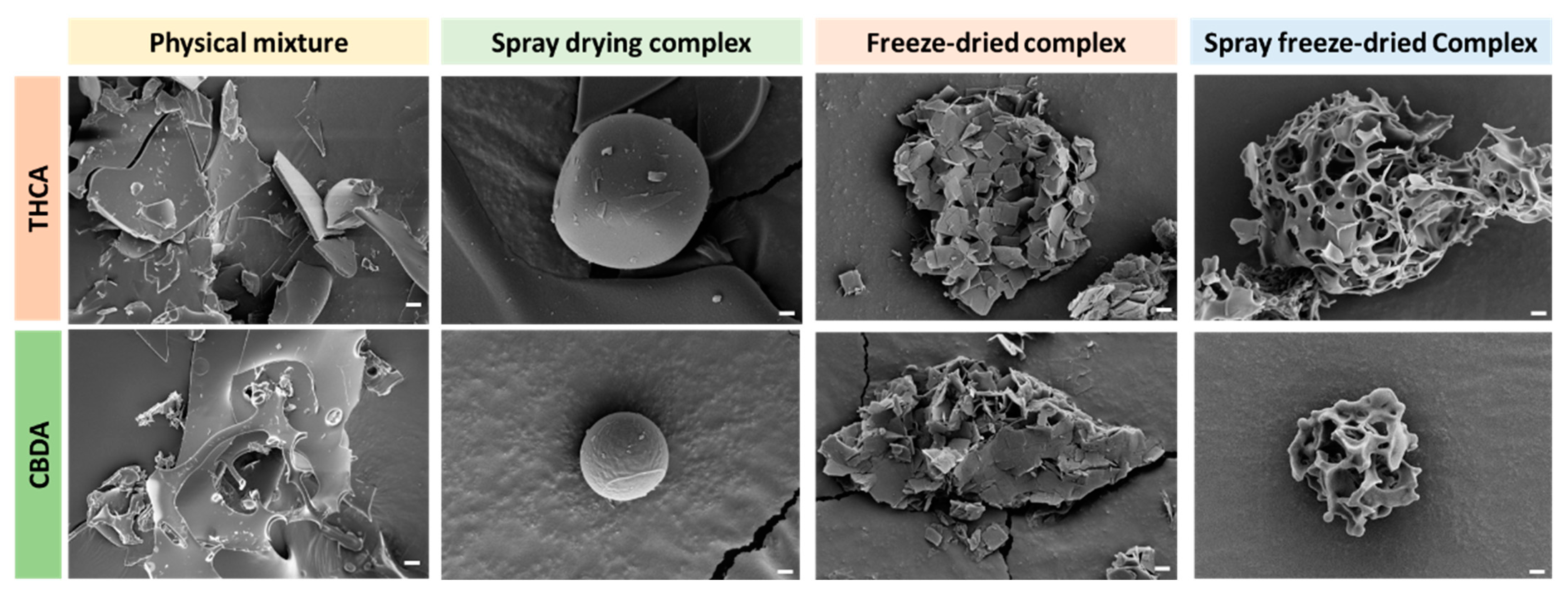
3.2.2. FE-SEM Analysis
3.2.3. DSC Analysis
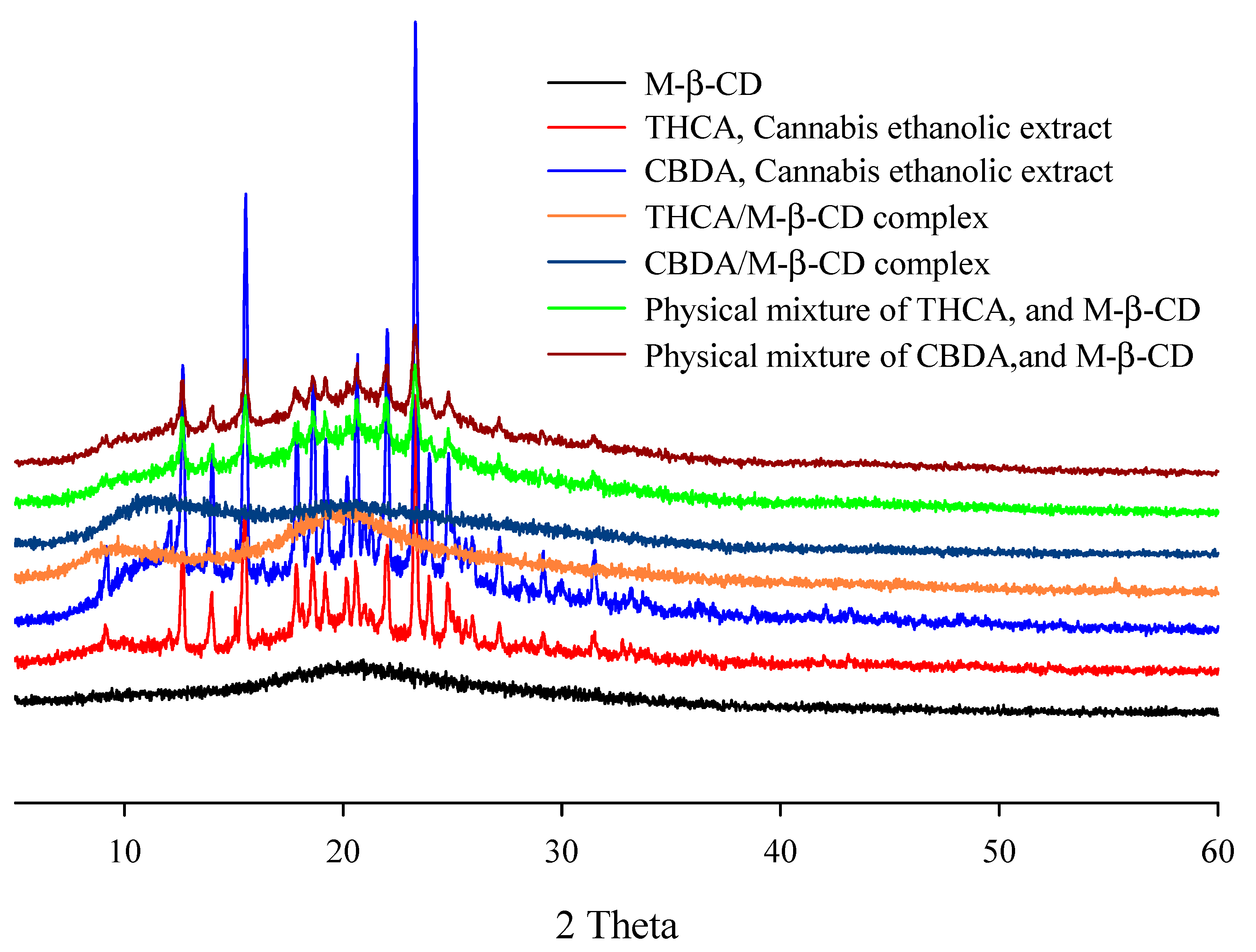
3.2.4. XRD Analysis
3.3. 1H NMR Studies of Cannabinoids/M-β-CD Inclusion Complex
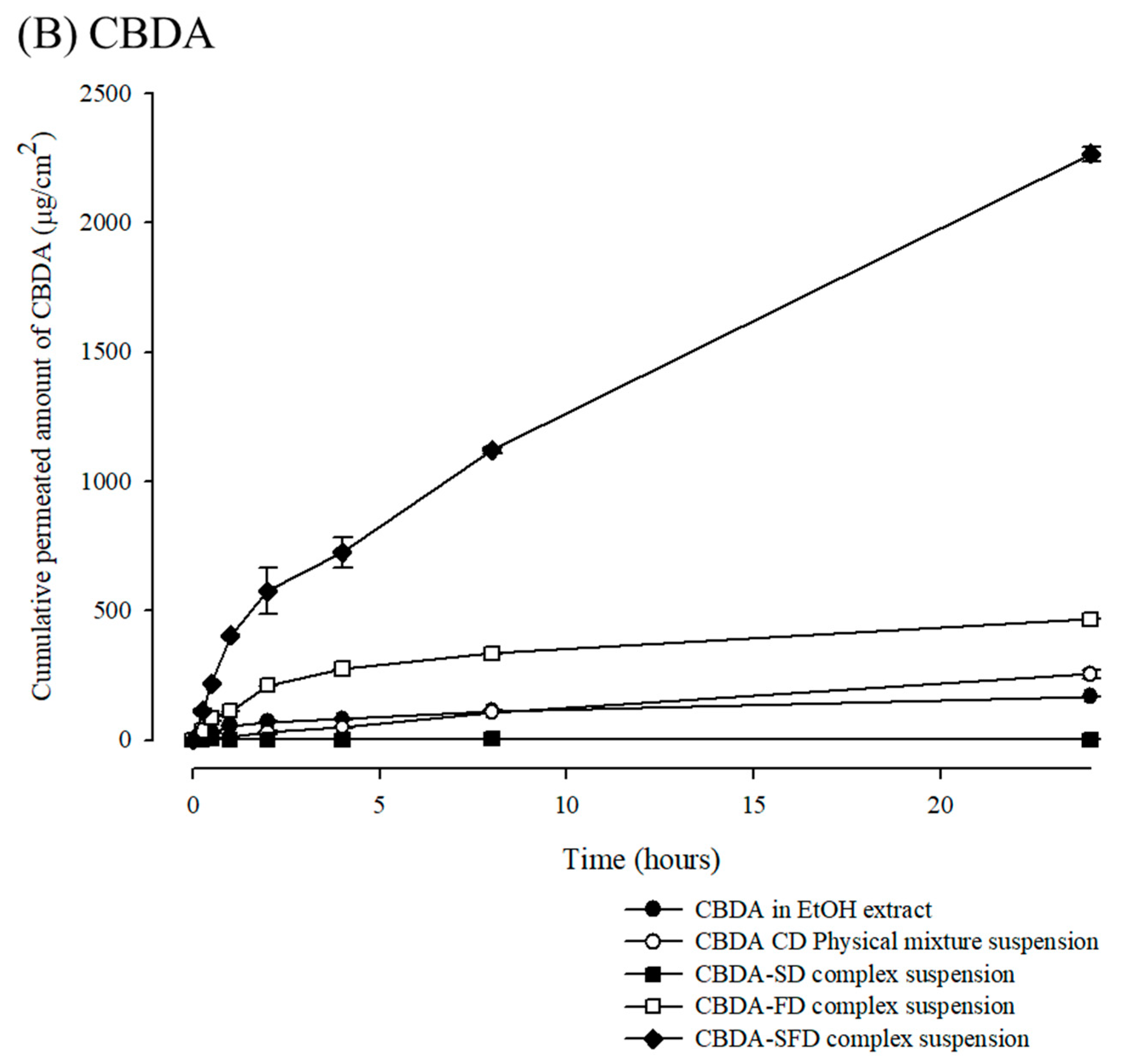
3.4. In Vitro Permeation Profiles of Cannabinoids/M-β-CD Inclusion Complex
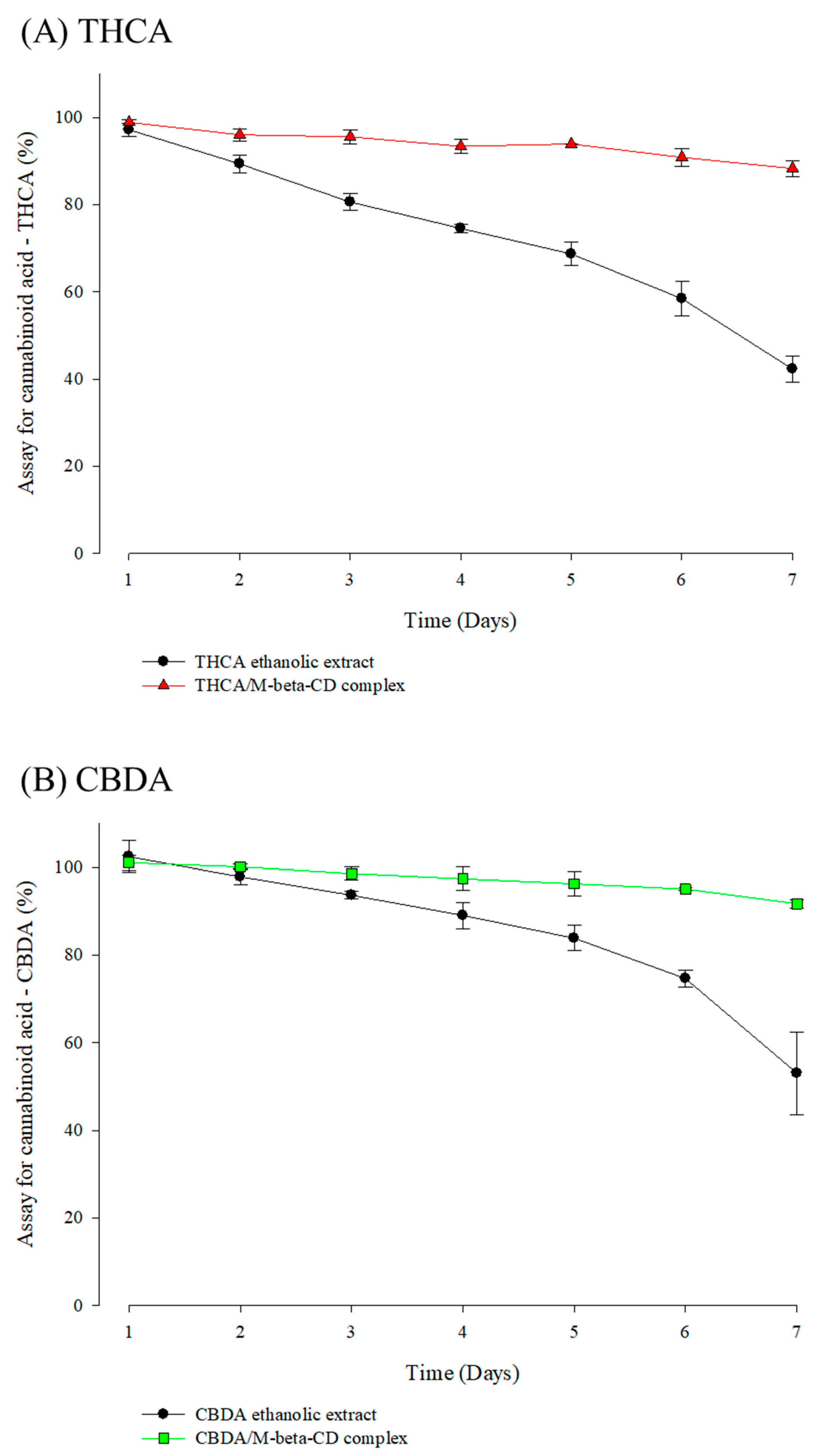
3.5. Stability Profiles of Cannabinoid Acids and CD Inclusion Complex in Simulated Physiological Condition
3.6. In Vitro Anti-Cancer Activities of Cannabinoids/M-β-CD Inclusion Complex
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CD | cyclodextrin |
| α-CD | α-cyclodextrin |
| β-CD | β-cyclodextrin |
| γ-CD | γ-cyclodextrin |
| HP-α-CD | hydroxypropyl-α-cyclodextrin |
| M-β-CD | methylated-β-cyclodextrin; |
| DSC | differential scanning calorimetry |
| FE-SEM | field-emission scanning electron microscopy |
| 1H NMR | proton nuclear magnetic resonance spectroscopy |
| THC | tetrahydrocannabinol |
| CBD | cannabidiol |
| THCA | Tetrahydro-cannabinolic acid |
| CBDA | cannabidiolic acid |
| COX | cyclooxygenase |
| RP | reversed-phase |
| HPLC | high-performance liquid chromatographic system |
| PVDF | Polyvinylidene Fluoride |
References
- Baswan, S.M.; Klosner, A.E.; Glynn, K.; Rajgopal, A.; Malik, K.; Yim, S.; Stern, N. Therapeutic potential of cannabidiol (CBD) for skin health and disorders. Clin. Cosmet. Investig. Dermatol. 2020, 13, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Pagano, C.; Navarra, G.; Coppola, L.; Avilia, G.; Bifulco, M.; Laezza, C. Cannabinoids: Therapeutic Use in Clinical Practice. Int. J. Mol. Sci. 2022, 23, 3344. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Nassar, N.; Bachari, A.; Schanknecht, E.; Telukutla, S.; Zomer, R.; Piva, T.J.; Mantri, N. The pathophysiology and the therapeutic potential of cannabinoids in prostate cancer. Cancers 2021, 13, 4107. [Google Scholar] [CrossRef]
- Weigelt, M.A.; Sivamani, R.; Lev-Tov, H. The therapeutic potential of cannabinoids for integumentary wound management. Exp. Dermatol. 2021, 30, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Freeman, T.P.; Hindocha, C.; Green, S.F.; Bloomfield, M.A.P. Medicinal use of cannabis based products and cannabinoids. BMJ 2019, 365, l1141. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A. Human cannabinoid pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef]
- Hazekamp, A. The trouble with CBD oil. Med. Cannabis Cannabinoids 2018, 1, 65–72. [Google Scholar] [CrossRef]
- Jehangir, A.; Parkman, H.P. Cannabinoid use in patients with gastroparesis and related disorders: Prevalence and benefit. Off. J. Am. Coll. Gastroenterol. 2019, 114, 945–953. [Google Scholar] [CrossRef]
- Millar, S.A.; Maguire, R.F.; Yates, A.S.; O’Sullivan, S.E. Towards Better Delivery of Cannabidiol (CBD). Pharmaceuticals 2020, 13, 219. [Google Scholar] [CrossRef]
- Child, R.B.; Tallon, M.J. Cannabidiol (CBD) Dosing: Plasma Pharmacokinetics and Effects on Accumulation in Skeletal Muscle, Liver and Adipose Tissue. Nutrients 2022, 14, 2101. [Google Scholar] [CrossRef]
- Knaub, K.; Sartorius, T.; Dharsono, T.; Wacker, R.; Wilhelm, M.; Schön, C. A Novel Self-Emulsifying Drug Delivery System (SEDDS) Based on VESIsorb(®) Formulation Technology Improving the Oral Bioavailability of Cannabidiol in Healthy Subjects. Molecules 2019, 24, 2967. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Sanz, G. Can you pass the acid test? critical review and novel therapeutic perspectives of Δ9-tetrahydrocannabinolic acid A. Cannabis Cannabinoid Res. 2016, 1, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Nadal, X.; Del Río, C.; Casano, S.; Palomares, B.; Ferreiro-Vera, C.; Navarrete, C.; Sánchez-Carnerero, C.; Cantarero, I.; Bellido, M.L.; Meyer, S. Tetrahydrocannabinolic acid is a potent PPARγ agonist with neuroprotective activity. Br. J. Pharmacol. 2017, 174, 4263–4276. [Google Scholar] [CrossRef] [PubMed]
- Palomares, B.; Ruiz-Pino, F.; Garrido-Rodriguez, M.; Prados, M.E.; Sánchez-Garrido, M.A.; Velasco, I.; Vazquez, M.J.; Nadal, X.; Ferreiro-Vera, C.; Morrugares, R. Tetrahydrocannabinolic acid A (THCA-A) reduces adiposity and prevents metabolic disease caused by diet-induced obesity. Biochem. Pharmacol. 2020, 171, 113693. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Misawa, K.; Yamamoto, I.; Watanabe, K. Cannabidiolic acid as a selective cyclooxygenase-2 inhibitory component in cannabis. Drug Metab. 2008, 36, 1917–1921. [Google Scholar] [CrossRef]
- Takeda, S.; Okajima, S.; Miyoshi, H.; Yoshida, K.; Okamoto, Y.; Okada, T.; Amamoto, T.; Watanabe, K.; Omiecinski, C.J.; Aramaki, H. Cannabidiolic acid, a major cannabinoid in fiber-type cannabis, is an inhibitor of MDA-MB-231 breast cancer cell migration. Toxicol. Lett. 2012, 214, 314–319. [Google Scholar] [CrossRef]
- Pereira, S.R.; Hackett, B.; O’Driscoll, D.N.; Sun, M.C.; Downer, E.J. Cannabidiol modulation of oxidative stress and signalling. Neuronal Signal. 2021, 5, NS20200080. [Google Scholar] [CrossRef]
- Sunda, F.; Arowolo, A. A molecular basis for the anti-inflammatory and anti-fibrosis properties of cannabidiol. FASEB J. 2020, 34, 14083–14092. [Google Scholar] [CrossRef]
- Martinez, A.S.; Lanaridi, O.; Stagel, K.; Halbwirth, H.; Schnürch, M.; Bica-Schröder, K. Extraction techniques for bioactive compounds of cannabis. Nat. Prod. Rep. 2023, 40, 676–717. [Google Scholar] [CrossRef]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations I: Structure and physicochemical properties, formation of complexes, and types of complex. Drug Discov. Today 2016, 21, 356–362. [Google Scholar] [CrossRef]
- Tian, B.; Liu, Y.; Liu, J. Cyclodextrin as a magic switch in covalent and non-covalent anticancer drug release systems. Carbohydr. Polym. 2020, 242, 116401. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Rai, A.K. Cyclodextrins in delivery systems: Applications. J. Pharm. Bioallied Sci. 2010, 2, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.; Mascarenhas-Melo, F.; Rabaça, S.; Mathur, A.; Sharma, A.; Giram, P.S.; Pawar, K.D.; Rahdar, A.; Raza, F.; Veiga, F.; et al. Cyclodextrin-based dermatological formulations: Dermopharmaceutical and cosmetic applications. Colloids Surf. B 2023, 221, 113012. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chang, S.-L.; Chang, T.-R.; You, Y.; Wang, X.-D.; Wang, L.-W.; Yuan, X.-F.; Tan, M.-H.; Wang, P.-D.; Xu, P.-W. Inclusion complexes of cannabidiol with β-cyclodextrin and its derivative: Physicochemical properties, water solubility, and antioxidant activity. J. Mol. Liq. 2021, 334, 116070. [Google Scholar] [CrossRef]
- Mannila, J.; Järvinen, T.; Järvinen, K.; Jarho, P. Precipitation complexation method produces cannabidiol/β-cyclodextrin inclusion complex suitable for sublingual administration of cannabidiol. J. Pharm. Sci. 2007, 96, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Mannila, J.; Järvinen, T.; Järvinen, K.; Tervonen, J.; Jarho, P. Sublingual administration of Δ9-tetrahydrocannabinol/β-cyclodextrin complex increases the bioavailability of Δ9-tetrahydrocannabinol in rabbits. Life Sci. 2006, 78, 1911–1914. [Google Scholar] [CrossRef] [PubMed]
- Triamchaisri, N.; Toochinda, P.; Lawtrakul, L. Structural Investigation of Beta-Cyclodextrin Complexes with Cannabidiol and Delta-9-Tetrahydrocannabinol in 1: 1 and 2: 1 Host-Guest Stoichiometry: Molecular Docking and Density Functional Calculations. Int. J. Mol. Sci. 2023, 24, 1525. [Google Scholar] [CrossRef]
- Hazekamp, A.; Verpoorte, R. Structure elucidation of the tetrahydrocannabinol complex with randomly methylated β-cyclodextrin. Eur. J. Pharm. Sci. 2006, 29, 340–347. [Google Scholar] [CrossRef]
- Paudel, K.S.; Hammell, D.C.; Agu, R.U.; Valiveti, S.; Stinchcomb, A.L. Cannabidiol bioavailability after nasal and transdermal application: Effect of permeation enhancers. Drug Dev. Ind. Pharm. 2010, 36, 1088–1097. [Google Scholar] [CrossRef]
- Lv, P.; Zhang, D.; Guo, M.; Liu, J.; Chen, X.; Guo, R.; Xu, Y.; Zhang, Q.; Liu, Y.; Guo, H. Structural analysis and cytotoxicity of host-guest inclusion complexes of cannabidiol with three native cyclodextrins. J. Drug Deliv. Sci. Technol. 2019, 51, 337–344. [Google Scholar] [CrossRef]
- Stella, B.; Baratta, F.; Della Pepa, C.; Arpicco, S.; Gastaldi, D.; Dosio, F. Cannabinoid formulations and delivery systems: Current and future options to treat pain. Drugs 2021, 81, 1513–1557. [Google Scholar] [CrossRef] [PubMed]
- Greish, K.; Mathur, A.; Al Zahrani, R.; Elkaissi, S.; Al Jishi, M.; Nazzal, O.; Taha, S.; Pittalà, V.; Taurin, S. Synthetic cannabinoids nano-micelles for the management of triple negative breast cancer. J. Control. Release 2018, 291, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Izgelov, D.; Shmoeli, E.; Domb, A.J.; Hoffman, A. The effect of medium chain and long chain triglycerides incorporated in self-nano emulsifying drug delivery systems on oral absorption of cannabinoids in rats. Int. J. Pharm. 2020, 580, 119201. [Google Scholar] [CrossRef] [PubMed]
- Tamba, B.I.; Stanciu, G.D.; Urîtu, C.M.; Rezus, E.; Stefanescu, R.; Mihai, C.T.; Luca, A.; Rusu-Zota, G.; Leon-Constantin, M.-M.; Cojocaru, E. Challenges and opportunities in preclinical research of synthetic cannabinoids for pain therapy. Medicina 2020, 56, 24. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Zuo, J.; Somayaji, V.; Lee, B.-J.; Löbenberg, R. Development of a novel cannabinoid-loaded microemulsion towards an improved stability and transdermal delivery. Int. J. Pharm. 2021, 604, 120766. [Google Scholar] [CrossRef]
- Higuchi, T. Phase-solubility techniques. Adv. Anal. Chem. Instr. 1965, 4, 117–212. [Google Scholar]
- Zachari, M.A.; Chondrou, P.S.; Pouliliou, S.E.; Mitrakas, A.G.; Abatzoglou, I.; Zois, C.E.; Koukourakis, M.I. Evaluation of the alamarblue assay for adherent cell irradiation experiments. Dose Response 2014, 12, 246–258. [Google Scholar] [CrossRef]
- Kwakye, A.K.; Kampo, S.; Lv, J.; Ramzan, M.N.; Richard, S.A.; Falagán, A.A.; Agudogo, J.; Atito-Narh, E.; Yan, Q.; Wen, Q.P. Levobupivacaine inhibits proliferation and promotes apoptosis of breast cancer cells by suppressing the PI3K/Akt/mTOR signalling pathway. BMC Res. Notes 2020, 13, 386. [Google Scholar] [CrossRef]
- Qiu, N.; Zhao, X.; Liu, Q.; Shen, B.; Liu, J.; Li, X.; An, L. Inclusion complex of emodin with hydroxypropyl-β-cyclodextrin: Preparation, physicochemical and biological properties. J. Mol. Liq. 2019, 289, 111151. [Google Scholar] [CrossRef]
- Figueiras, A.; Carvalho, R.A.; Ribeiro, L.; Torres-Labandeira, J.J.; Veiga, F.J.B. Solid-state characterization and dissolution profiles of the inclusion complexes of omeprazole with native and chemically modified β-cyclodextrin. Eur. J. Pharm. Biopharm. 2007, 67, 531–539. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Vieira, M.T.; Veiga, F.J.B. Physicochemical characterization and in vitro dissolution behavior of nicardipine–cyclodextrins inclusion compounds. Eur. J. Pharm. Sci. 2002, 15, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, P.; Jin, M.; Kim, D.; Yang, J.; Maharjan, A.; Shin, M.C.; Cho, K.H.; Kim, M.S.; Min, K.A. Evaluation of epithelial transport and oxidative stress protection of nanoengineered curcumin derivative-cyclodextrin formulation for ocular delivery. Arch. Pharm. Res. 2019, 42, 909–925. [Google Scholar] [CrossRef] [PubMed]
- Andriotis, E.G.; Chachlioutaki, K.; Monou, P.K.; Bouropoulos, N.; Tzetzis, D.; Barmpalexis, P.; Chang, M.-W.; Ahmad, Z.; Fatouros, D.G. Development of Water-Soluble Electrospun fibers for the oral delivery of cannabinoids. AAPS PharmSciTech 2021, 22, 23. [Google Scholar] [CrossRef]
- Belica-Pacha, S.; Daśko, M.; Buko, V.; Zavodnik, I.; Miłowska, K.; Bryszewska, M. Thermodynamic studies of interactions between sertraline hydrochloride and randomly methylated β-cyclodextrin molecules supported by circular dichroism spectroscopy and molecular docking results. Int. J. Mol. Sci. 2021, 22, 12357. [Google Scholar] [CrossRef]
- Bruni, N.; Della Pepa, C.; Oliaro-Bosso, S.; Pessione, E.; Gastaldi, D.; Dosio, F. Cannabinoid delivery systems for pain and inflammation treatment. Molecules 2018, 23, 2478. [Google Scholar] [CrossRef] [PubMed]
- Kirk, R.D.; Akanji, T.; Li, H.; Shen, J.; Allababidi, S.; Seeram, N.P.; Bertin, M.J.; Ma, H. Evaluations of Skin Permeability of Cannabidiol and Its Topical Formulations by Skin Membrane-Based Parallel Artificial Membrane Permeability Assay and Franz Cell Diffusion Assay. Med. Cannabis Cannabinoids 2022, 5, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Tabboon, P.; Pongjanyakul, T.; Limpongsa, E.; Jaipakdee, N. In vitro release, mucosal permeation and deposition of cannabidiol from liquisolid systems: The influence of liquid vehicles. Pharmaceutics 2022, 14, 1787. [Google Scholar] [CrossRef]
- Kim, D.; Park, C.; Meghani, N.M.; Tran, T.T.D.; Tran, P.H.L.; Park, J.-B.; Lee, B.-J. Utilization of a fattigation platform gelatin-oleic acid sodium salt conjugate as a novel solubilizing adjuvant for poorly water-soluble drugs via self-assembly and nanonization. Int. J. Pharm. 2020, 575, 118892. [Google Scholar] [CrossRef]
- Welzel, J.; Wilhelm, K.P.; Wolff, H.H. Skin permeability barrier and occlusion: No delay of repair in irritated human skin. Contact Dermat. 1996, 35, 163–168. [Google Scholar] [CrossRef]
- Belenguer-Sapiña, C.; Saez-Hernandez, R.; Pellicer-Castell, E.; Armenta, S.; Mauri-Aucejo, A.R. Simultaneous determination of third-generation synthetic cannabinoids in oral fluids using cyclodextrin-silica porous sorbents. Microchem. J. 2022, 172, 106915. [Google Scholar] [CrossRef]
- Lou, J.; Teng, Z.; Zhang, L.; Yang, J.; Ma, L.; Wang, F.; Tian, X.; An, R.; Yang, M.; Zhang, Q. β-Caryophyllene/hydroxypropyl-β-cyclodextrin inclusion complex improves cognitive deficits in rats with vascular dementia through the cannabinoid receptor type 2-mediated pathway. Front. Pharmacol. 2017, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Quintans-Júnior, L.J.; Araújo, A.A.S.; Brito, R.G.; Santos, P.L.; Quintans, J.S.S.; Menezes, P.P.; Serafini, M.R.; Silva, G.F.; Carvalho, F.M.S.; Brogden, N.K. β-caryophyllene, a dietary cannabinoid, complexed with β-cyclodextrin produced anti-hyperalgesic effect involving the inhibition of Fos expression in superficial dorsal horn. Life Sci. 2016, 149, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Sripetch, S.; Prajapati, M.; Loftsson, T. Cyclodextrins and Drug Membrane Permeation: Thermodynamic Considerations. J. Pharm. Sci. 2022, 111, 2571–2580. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Bakker, M.M.; Yang, Y.; Vyawahare, R.; Kotra, L.P. Extractions of Medical Cannabis Cultivars and the Role of Decarboxylation in Optimal Receptor Responses. Cannabis Cannabinoid Res. 2019, 4, 183–194. [Google Scholar] [CrossRef]





| Types of Cannabinoid Acid | Types of Cyclodextrin | Slope | Y-Intercept (So, mM) | Stability Constant (KS, M−1) | Regression Coefficient (R2) | Complexation Efficiency (%) | Molar Ratio (Cannabinoids:CD) |
|---|---|---|---|---|---|---|---|
| THCA | α-CD | 0.086 | 0.052 | 642 | 0.998 | 9.43 | 1.00:11.39 |
| β-CD | 0.262 | 0.359 | 2140 | 0.997 | 35.5 | 1.00:3.80 | |
| γ-CD | 0.096 | 0.109 | 1280 | 0.998 | 10.6 | 1.00:10.38 | |
| M-β-CD | 0.344 | 0.448 | 2360 | 0.999 | 52.5 | 1.00:2.88 | |
| HP-β-CD | 0.227 | 0.241 | 1400 | 0.999 | 29.4 | 1.00:4.35 | |
| CBDA | α-CD | 0.098 | 0.018 | 191 | 0.998 | 10.8 | 1.00:10.27 |
| β-CD | 0.115 | 0.018 | 159 | 0.997 | 13.0 | 1.00:8.64 | |
| γ-CD | 0.185 | 0.035 | 199 | 0.998 | 22.6 | 1.00:5.45 | |
| M-β-CD | 0.390 | 0.141 | 420 | 0.999 | 63.9 | 1.00:2.55 | |
| HP-β-CD | 0.138 | 0.023 | 172 | 1.000 | 15.9 | 1.00:7.25 |
| Types of Cannabinoids | Ratio of EtOH/Water | Inclusion Complex | |
|---|---|---|---|
| Drug Loading Content (%) | Molar Ratio | ||
| THCA | 20% EtOH (v/v) | 11.05 ± 0.02 | 1:1.93 |
| 15% EtOH (v/v) | 11.18 ± 0.03 | 1:1.91 | |
| 10% EtOH (v/v) | 8.37 ± 0.01 | 1:2.62 | |
| 5% EtOH (v/v) | 4.98 ± 0.02 | 1:4.58 | |
| 2.5% EtOH (v/v) | 2.38 ±0.04 | 1:9.82 | |
| CBDA | 20% EtOH (v/v) | 10.51 ± 0.05 | 1:2.27 |
| 15% EtOH (v/v) | 12.25 ± 0.09 | 1:1.72 | |
| 10% EtOH (v/v) | 5.34 ± 0.01 | 1:4.26 | |
| 5% EtOH (v/v) | 2.83 ± 0.00 | 1:8.25 | |
| 2.5% EtOH (v/v) | 1.02 ± 0.01 | 1:23.28 | |
| Preparation Method | Types of Cannabinoids | Aqueous Solubility of Cannabinoid Acids (µg/mL) | ||
| Molar Ratio (Cannabinoids: M-β-CD) | ||||
| 1:1 | 1:2 | 1:5 | ||
| Spray-drying | THCA | 220 ± 9 | 480 ± 20 | 338 ± 3 |
| CBDA | 300 ± 70 | 554 ± 4 | 440 ± 10 | |
| Freeze-drying | THCA | 78 ± 5 | 190 ± 10 | 110 ± 30 |
| CBDA | 93 ± 8 | 200 ± 20 | 90 ± 10 | |
| Spray-freeze-drying | THCA | 310 ± 20 | 930 ± 20 | 470 ± 60 |
| CBDA | 450 ± 40 | 1090 ± 40 | 540 ± 60 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.; Zuo, J.; Gil, M.-C.; Löbenberg, R.; Lee, B.-J. Investigation of Cannabinoid Acid/Cyclodextrin Inclusion Complex for Improving Physicochemical and Biological Performance. Pharmaceutics 2023, 15, 2533. https://doi.org/10.3390/pharmaceutics15112533
Park C, Zuo J, Gil M-C, Löbenberg R, Lee B-J. Investigation of Cannabinoid Acid/Cyclodextrin Inclusion Complex for Improving Physicochemical and Biological Performance. Pharmaceutics. 2023; 15(11):2533. https://doi.org/10.3390/pharmaceutics15112533
Chicago/Turabian StylePark, Chulhun, Jieyu Zuo, Myung-Chul Gil, Raimar Löbenberg, and Beom-Jin Lee. 2023. "Investigation of Cannabinoid Acid/Cyclodextrin Inclusion Complex for Improving Physicochemical and Biological Performance" Pharmaceutics 15, no. 11: 2533. https://doi.org/10.3390/pharmaceutics15112533
APA StylePark, C., Zuo, J., Gil, M.-C., Löbenberg, R., & Lee, B.-J. (2023). Investigation of Cannabinoid Acid/Cyclodextrin Inclusion Complex for Improving Physicochemical and Biological Performance. Pharmaceutics, 15(11), 2533. https://doi.org/10.3390/pharmaceutics15112533