Effect of Organomontmorillonite-Cloisite® 20A Incorporation on the Structural and Drug Release Properties of Ureasil–PEO Hybrid
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
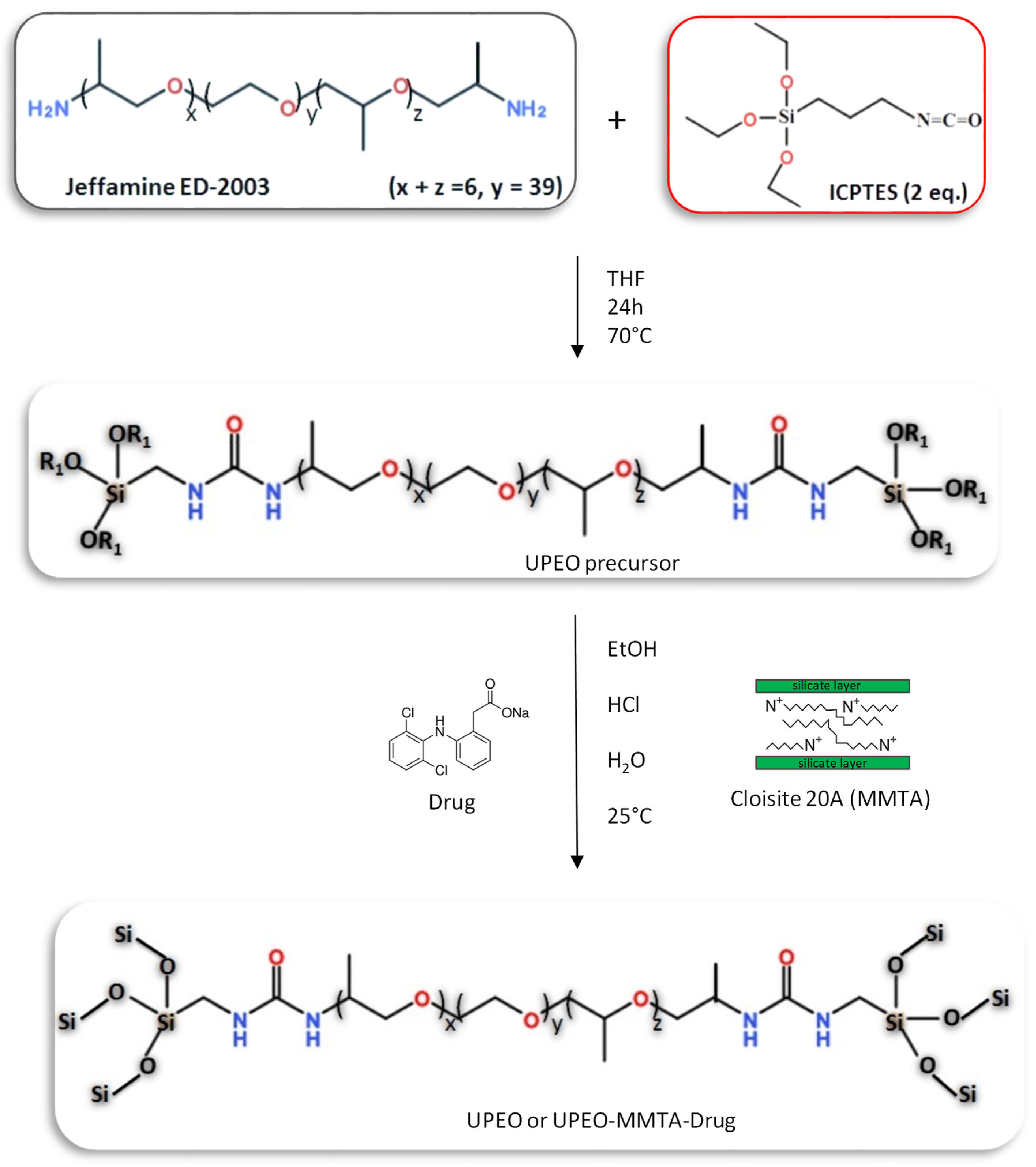
2.2. Synthesis of the Ureasil–PEO Hybrid Precursor
2.3. Synthesis of the Ureasil–PEO Nanocomposites Containing Cloisite®20A and Sodium Diclofenac (DCF)
2.4. Characterization
3. Results and Discussion
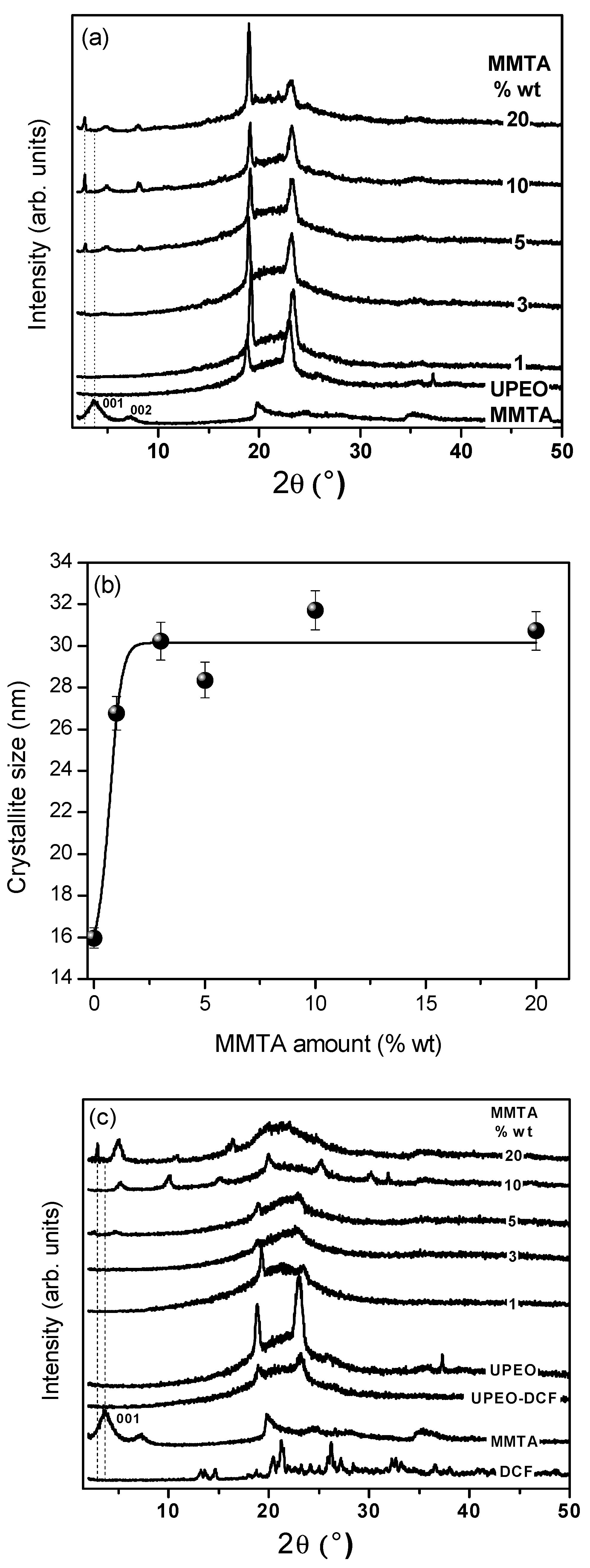
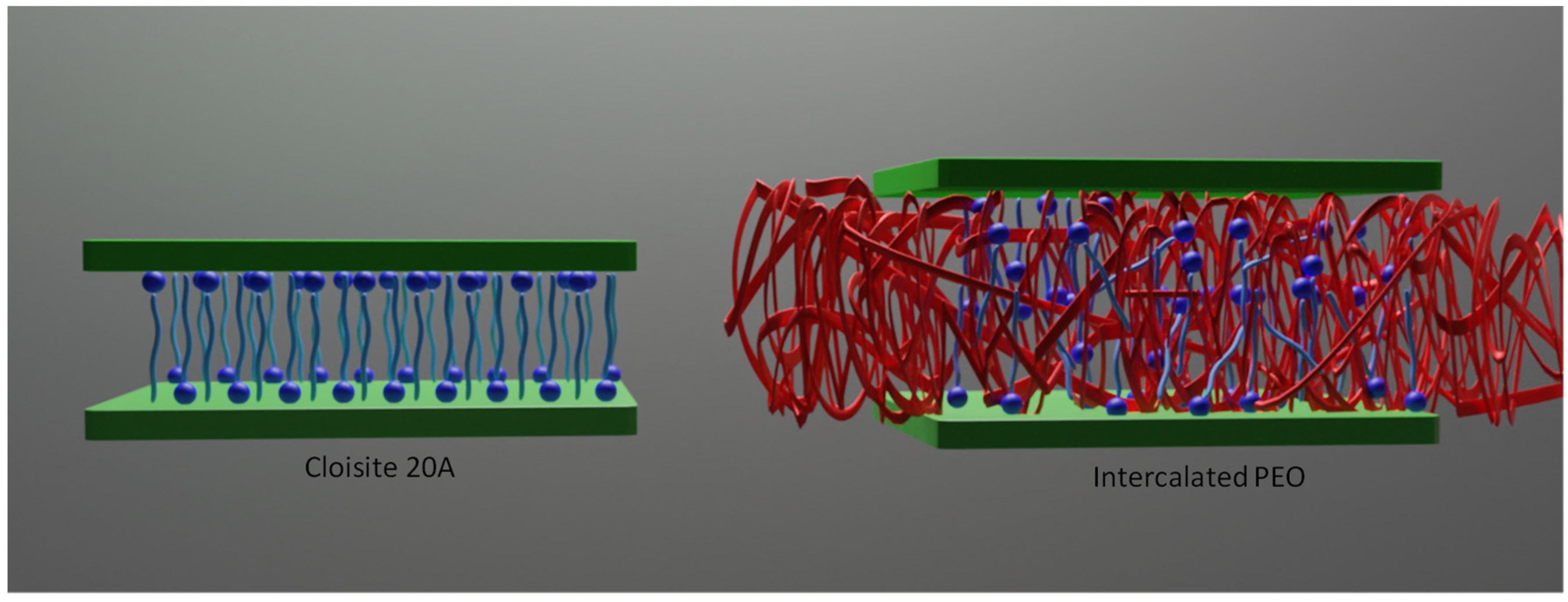
3.1. Structural Evaluation
3.2. Thermal Features
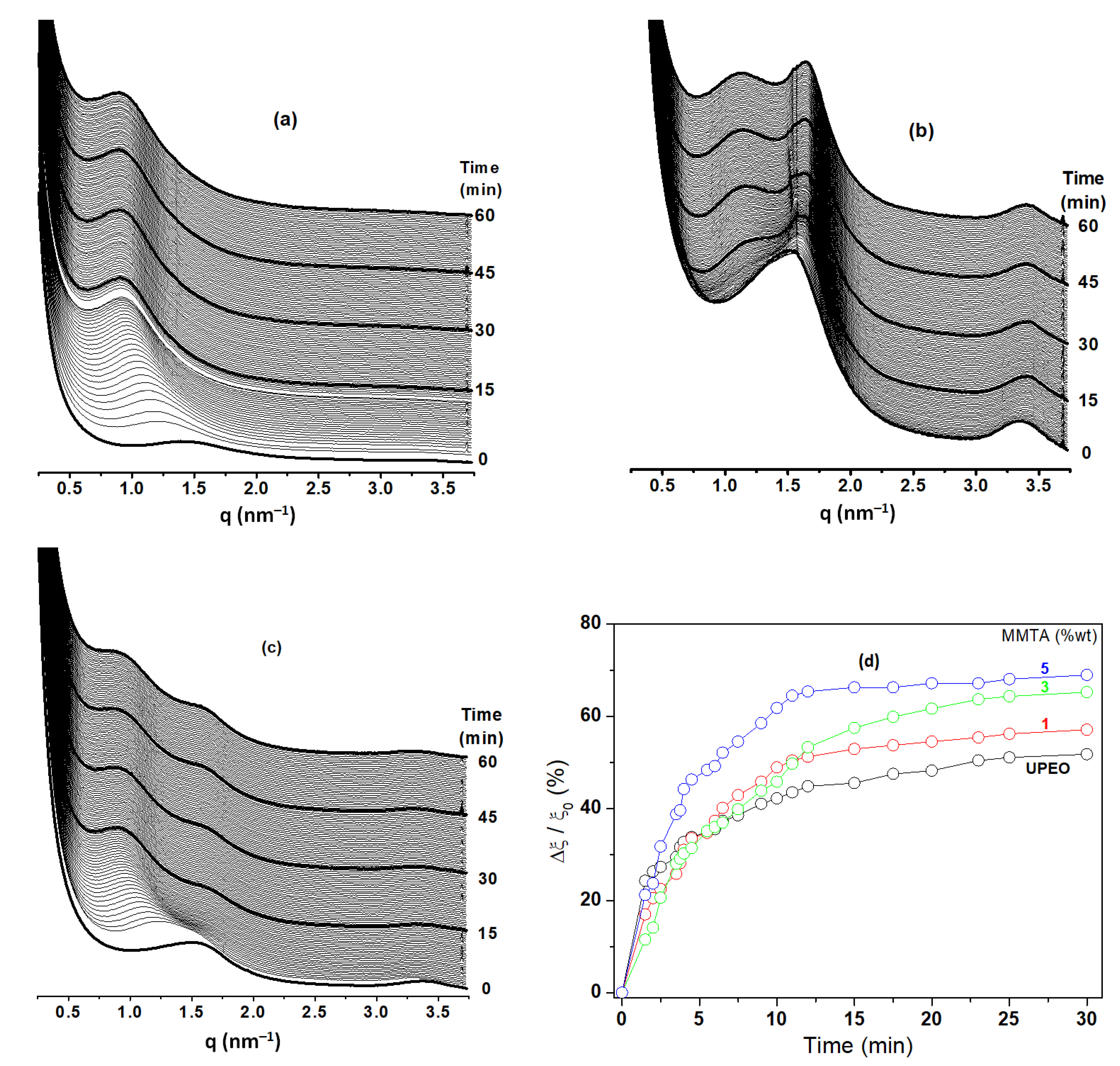
3.3. Swelling Behavior from SAXS Studies
3.4. Release Assays
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doménech, A.; Doménech-Carbó, M.T.; Agredos-Pascual, M.L.V. From Maya Blue to “Maya Yellow”: A Connection between Ancient Nanostructured Materials from the Voltammetry of Microparticles. Angew. Chem. Int. Ed. 2011, 50, 5741–5744. [Google Scholar] [CrossRef]
- Viguerie, L.; Jaber, M.; Pasco, H.; Lalevèe, J.; Morlet-Savary, F.; Ducouret, G.; Rigaud, B.; Pouget, T.; Sanchez, C.; Walter, P. A 19th Century “Ideal” Oil Paint Medium: A Complex Hybrid Organic–Inorganic Gel. Angew. Chem. Int. Ed. 2017, 56, 1619–1623. [Google Scholar] [CrossRef] [PubMed]
- Faustini, M.; Nicole, L.; Ruiz-Hitzky, E.; Sanchez, C. History of Organic–Inorganic Hybrid Materials: Prehistory, Art, Science, and Advanced Applications. Adv. Funct. Mater. 2018, 28, 1704158. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Olphen, H.V. Maya Blue: A Clay-Organic Pigment? Science 1966, 154, 645–646. [Google Scholar] [CrossRef]
- José-Yacamán, M.; Rendón, L.; Arenasand, J.; Puche, M.C.S. Maya Blue Paint: An Ancient Nanostructured Material. Science 1996, 273, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Humayun, A.; Murray, T.A.; Kemp, B.S.; McFarland, A.; Liu, X.; Mills, D.K. Cellular Analysis and Chemotherapeutic Potential of a Bi-Functionalized Halloysite Nanotube. Pharmaceutics 2020, 12, 962. [Google Scholar] [CrossRef]
- Pagano, C.; Latterini, L.; Matlou, G.G.; Abrahamse, H. Hybrid Inorganic-Organic Core-Shell Nanodrug Systems in Targeted Photodynamic Therapy of Cancer. Pharmaceutics 2021, 13, 1773. [Google Scholar]
- Sanchez, C.; Belleville, P.; Popalld, M.; Nicole, L. Applications of advanced hybrid organic–inorganic nanomaterials: From laboratory to Market. Chem. Soc. Rev. 2011, 40, 696–753. [Google Scholar] [CrossRef]
- Faccendini, A.; Ruggeri, M.; Miele, D.; Rossi, S.; Bonferoni, M.C.; Aguzzi, C.; Grisoli, P.; Viseras, C.; Vigani, B.; Sandri, G.; et al. Norfloxacin-Loaded Electrospun Scaffolds: Montmorillonite Nanocomposite vs. Free Drug. Pharmaceutics 2020, 12, 325. [Google Scholar] [CrossRef]
- Kojima, Y.; Usuki, A.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Mechanical properties of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1185–1189. [Google Scholar] [CrossRef]
- Sharma, B.; Malik, P.; Jain, P. Biopolymer Reinforced Nanocom-posites: A Comprehensive Review. Mater. Today Commun. 2018, 16, 353–363. [Google Scholar] [CrossRef]
- Pagano, C.; Latterini, L.; Michele, A.; Luzi, F.; Puglia, D.; Ricci, M.; Iborra, C.A.V.; Perioli, L. Polymeric Bioadhesive Patch Based on Ketoprofen-Hydrotalcite Hybrid for Local Treatments. Pharmaceutics 2020, 12, 733. [Google Scholar] [CrossRef]
- Borrego-Sánchez, A.; Sánchez-Espejo, R.; García-Villén, F.; Viseras, C.; Sainz-Díaz, C.I. Praziquantel–Clays as Accelerated Release Systems to Enhance the Low Solubility of the Drug. Pharmaceutics 2020, 12, 914. [Google Scholar] [CrossRef] [PubMed]
- Cirri, M.; Mura, P.; Valleri, M.; Brunetti, L. Devel-opment and Characterization of Liquisolid Tablets Based on Mesoporous Clays or Silicas for Improving Glyburide Dissolution. Pharmaceutics 2020, 12, 503. [Google Scholar] [CrossRef]
- Meazzini, I.; Blayo, C.; Arlt, J.; Marques, A.; Scherf, U.; Burrows, H.D.; Evans, R.C. Ureasil organic–inorganic hybrids as photoactive waveguides for conjugated polyelectrolyte luminescent solar concentrators. Mater. Chem. Front. 2017, 1, 2271–2282. [Google Scholar] [CrossRef]
- Fang, M.; Neto, A.N.C.; Fu, L.; Ferreira, R.A.S.; Bermudez, V.Z.; Carlos, L.D. A Hybrid Materials Approach for Fabricating Efficient WLEDs Based on Di-Ureasils Doped with Carbon Dots and a Europium Complex. Adv. Mater. Technol. 2021, 7, 2100727. [Google Scholar] [CrossRef]
- Molina, E.F.; Pulcinelli, S.H.; Santilli, C.V.; Briois, V. Ligand exchange inducing efficient incorporation of CisPt derivatives into ureasil-PPO hybrid and their interactions with the multifunctional hybrid Network. J. Phys. Chem. B 2012, 116, 7931–7939. [Google Scholar] [CrossRef]
- Molina, E.F.; Parreira, R.L.T.; De Faria, E.H.; Carvalho, H.W.P.; Caramori, G.F.; Coimbra, D.F.; Nassar, E.J.; Ciuffi, K.J. Ureasil-poly(ethylene oxide) hybrid matrix for selective adsorption and separation of dyes from water. Langmuir 2014, 30, 3857–3868. [Google Scholar] [CrossRef]
- Caravieri, B.B.; de Oliveira, P.F.; Furtado, R.A.; Tavares, D.C.; Nassar, E.J.; Ciuffi, K.J.; Molina, E.F. Solubility enhancement of ibuprofen using tri-ureasil-PPO hybrid: Structural, cytotoxic, and drug release investigation. J. Solgel Sci. Technol. 2014, 72, 627–636. [Google Scholar] [CrossRef]
- de Jesus, N.A.M.; de Oliveira, A.H.P.; Tavares, D.C.; Furtado, R.A.; Silva, M.A.L.; Cunha, W.R.; Molina, E.F. Biofilm formed from a tri-ureasil organic−inorganic hybrid gel for use as a cubebin release system. J. Solgel Sci. Technol. 2018, 88, 192–201. [Google Scholar] [CrossRef]
- Paredes, M.; Pulcinelli, S.H.; Peniche, C.; Gonçalves, V.; Santilli, C.V. Chitosan/(ureasil–PEO hybrid) blend for drug delivery. J. Solgel Sci. Technol. 2014, 72, 233–238. [Google Scholar] [CrossRef]
- Molina, E.F.; Jesus, C.R.N.; Chiavacci, L.A.; Pulcinelli, S.H.; Briois, V.; Santilli, C.V. Ureasil–polyether hybrid blend with tuneable hydrophilic/hydrophobic features based on U-PEO1900 and U-PPO400 mixtures. J. Solgel Sci. Technol. 2014, 70, 317–328. [Google Scholar] [CrossRef]
- Chaker, J.A.; Santilli, C.V.; Pulcinelli, S.H.; Dahmouche, K.; Briois, V.; Judeinstein, P. Multi-scale structural description of siloxane–PPO hybrid ionic conductors doped by sodium salts. J. Mater. Chem. 2007, 17, 744–757. [Google Scholar] [CrossRef]
- Santana, W.M.O.S.; Abramson, S.; Fini, R.; Caetano, B.L.; Ménager, C.; Pulcinelli, S.H.; Santilli, C.V. Ureasil−Polyether−CoFe2O4 Nanocomposites: Coupling a Drug Delivery System and Magnetic Hyperthermia. ACS Appl. Polym. Mater. 2021, 3, 4837–4848. [Google Scholar] [CrossRef]
- Molina, E.F.; Marçal, L.; Carvalho, H.W.P.; Nassar, E.J.; Ciuffi, K.C. Tri-ureasil gel as a multifunctional organic–inorganic hybrid matrix. Polym. Chem. 2013, 4, 1575–1582. [Google Scholar] [CrossRef]
- Li, H.; Zhao, J.; Huang, L.; Xia, P.; Zhou, Y.; Wang, J.; Jiang, L. A Constrained Assembly Strategy for High-Strength Natural Nanoclay Film. ACS Nano 2022, 16, 6224–6232. [Google Scholar] [CrossRef]
- Chiu, Y.; Liao, Y. Nanocomposite Coatings to Induce Pigment Precipitation for Durable Paper-Based Foldable Electronics. ACS Appl. Electron. Mater. 2022, 4, 3938–3946. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z.; Li, J.; Wu, X.; Lin, J.; He, S. Mechanical performance design via regulating the interactions in acrylonitrile-butadiene rubber/clay nanocomposites by small molecule compounds. Polym. Test. 2022, 110, 107565. [Google Scholar] [CrossRef]
- He, S.; He, T.; Wang, J.; Wu, X.; Xue, Y.; Zhang, L.; Lin, L. A novel method to prepare acrylonitrile-butadiene rubber/clay nanocomposites by compounding with clay gel. Compos. Part B 2019, 167, 356–361. [Google Scholar] [CrossRef]
- Papadopoulos, L.; Terzopoulou, Z.; Vlachopoulos, A.; Klonos, P.A.; Kyritsis, A.; Tzetzis, D.; Papageorgiou, G.Z.; Bikiaris, D. Synthesis and characterization of novel polymer/clay nanocomposites based on poly (butylene 2,5-furan dicarboxylate). Appl. Clay Sci. 2020, 190, 105588. [Google Scholar] [CrossRef]
- Hojiyev, R.; Ulcay, Y.; Çelik, M.S. Development of a clay-polymer compatibility approach for nanocomposite applications. Appl. Clay Sci. 2017, 146, 548–556. [Google Scholar] [CrossRef]
- Jesus, C.R.N.; Molina, E.F.; Pulcinelli, S.H.; Santilli, C.V. Highly Controlled Diffusion Drug Release from Ureasil−Poly(ethylene oxide)−Na+−Montmorillonite Hybrid Hydrogel Nanocomposites. ACS Appl. Mater. Interfaces 2018, 10, 19059–19068. [Google Scholar] [CrossRef] [PubMed]
- Santilli, C.V.; Chiavacci, L.A.; Lopes, L.; Pulcinelli, S.H.; Oliveira, A.G. Controlled Drug Release from Ureasil#Polyether Hybrid Materials. Chem. Mater. 2009, 21, 463–467. [Google Scholar]
- Oshiro, J.A.; Lusuardi, A.; Beamud, E.M.; Chiavacci, L.A.; Cuberes, M.T. Nanostructural Arrangements and Surface Morphology on Ureasil-Polyether Films Loaded with Dexamethasone Acetate. Nanomaterials 2021, 11, 1362. [Google Scholar]
- Lyu, G.; Southern, T.J.F.; Charles, B.L.; Roger, M.; Gerbier, P.; Clément, S.; Evans, R.C. Aggregation-induced emission from silole-based lumophores embedded in organic–inorganic hybrid hosts. J. Mater. Chem. C 2021, 9, 13914. [Google Scholar] [CrossRef]
- Scherrer, P. Göttinger Nachrichten. Math. Phys. 1918, 2, 98–100. [Google Scholar]
- Klug, H.P.; Alexander, L.E. X-Ray Diffraction Procedures, 2nd ed.; Wiley: Hoboken, NJ, USA, 1974; Chapter 9. [Google Scholar]
- Torin, R.F.S.; Camani, P.H.; Silva, L.N.; Sato, J.A.P.; Ferreira, F.F.; Rosa, D.S. Effect of clay and clay/essential oil in packaging films. Polym. Compos. 2018, 39, 4034–4040. [Google Scholar] [CrossRef]
- Lopes, L.; Molina, E.F.; Chiavacci, L.A.; Santilli, C.V.; Briois, V.; Pulcinelli, S.H. Drug-matrix interaction of sodium diclofenac incorporated into ureasil-poly(ethylene oxide) hybrid materials. RSC Advances 2012, 2, 5629–5636. [Google Scholar] [CrossRef]
- Mya, K.Y.; Pramoda, K.P.; He, C.B. Crystallization behavior of star-shaped poly(ethylene oxide) with cubic silsesquioxane (CSSQ) core. Polymer 2006, 47, 5035–5043. [Google Scholar] [CrossRef]
- Giordano, F.; Rossi, A.; Pasquali, I.; Bettini, R.; Frigo, E.; Gazzaniga, A.; Sangalli, M.E.; Mileo, V.; Catinella, S. Thermal degradation and melting point determination of diclofenac. J. Therm. Anal. Calorim. 2003, 73, 509–518. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Liu, X.; Lu, X.; Su, Y.; Kun, E.; Zhang, F. Clay-Polymer Nanocomposites Prepared by Reactive Melt Extrusion for Sustained Drug Release. Pharmaceutics 2020, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Adrover, A.; Paolicelli, P.; Petralito, S.; Muzio, L.; Trilli, J.; Cesa, S.; Tho, I.; Casadei, M.A. Gellan Gum/Laponite Beads for the Modified Release of Drugs: Experimental and Modeling Study of Gastrointestinal Release. Pharmaceutics 2019, 11, 187. [Google Scholar] [CrossRef]


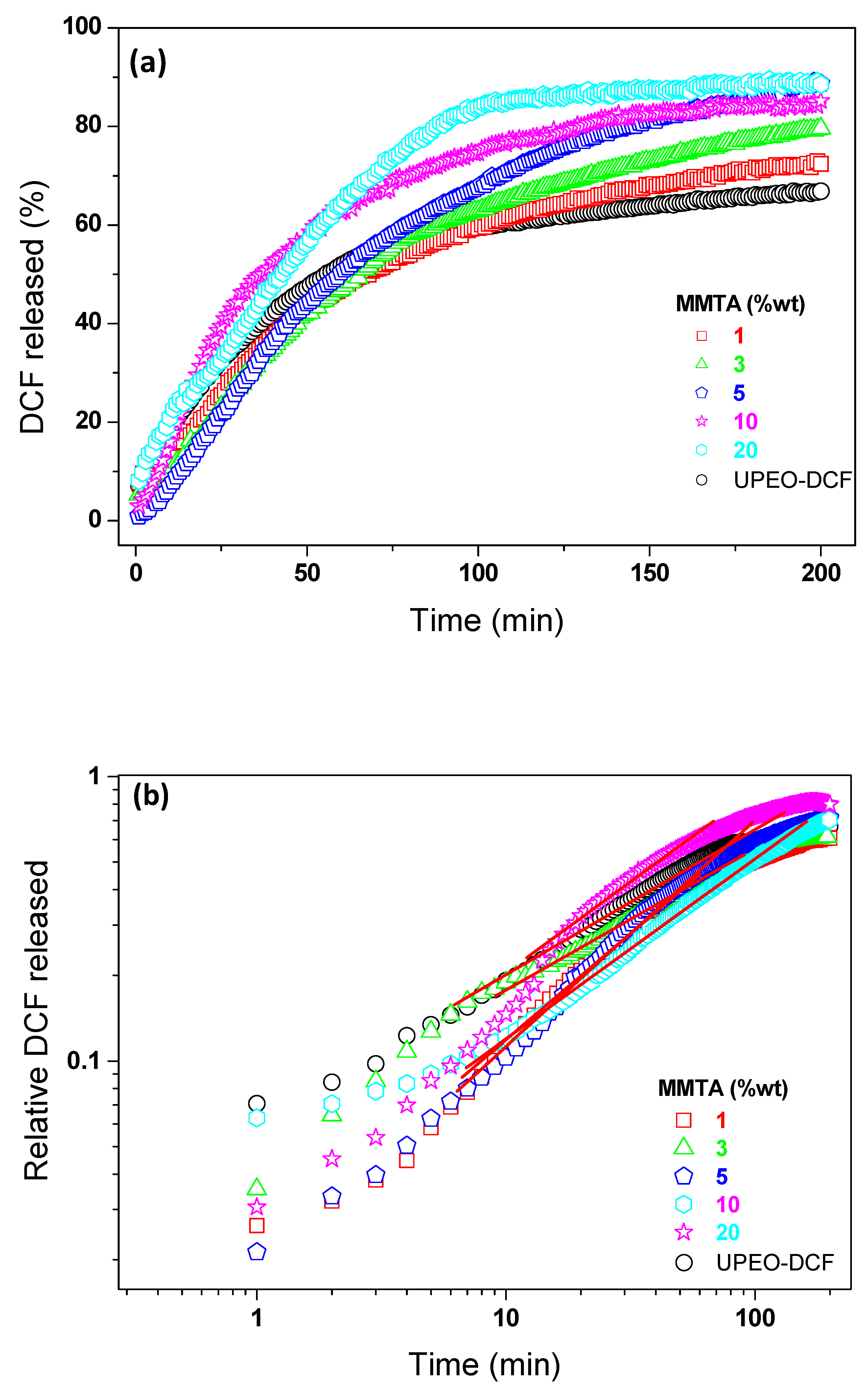
| Amount Released (%) | Korsmeyer–Peppas Parameters | Release Mechanism | ||
|---|---|---|---|---|
| Sample | n | R2 | ||
| UPEO | 67 | 0.55 | 0.99 | Fickian diffusion |
| UPEO–MMTA1% | 72 | 0.68 | 0.98 | anomalous transport |
| UPEO–MMTA3% | 80 | 0.82 | 0.99 | anomalous transport |
| UPEO–MMTA5% | 86 | 1.09 | 0.99 | Case II |
| UPEO–MMTA10% | 88 | 0.99 | 0.99 | anomalous transport |
| UPEO–MMTA20% | 0.75 | 0.99 | anomalous transport | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jesus, C.R.N.; Molina, E.F.; de Oliveira, R.; Pulcinelli, S.H.; Santilli, C.V. Effect of Organomontmorillonite-Cloisite® 20A Incorporation on the Structural and Drug Release Properties of Ureasil–PEO Hybrid. Pharmaceutics 2023, 15, 33. https://doi.org/10.3390/pharmaceutics15010033
Jesus CRN, Molina EF, de Oliveira R, Pulcinelli SH, Santilli CV. Effect of Organomontmorillonite-Cloisite® 20A Incorporation on the Structural and Drug Release Properties of Ureasil–PEO Hybrid. Pharmaceutics. 2023; 15(1):33. https://doi.org/10.3390/pharmaceutics15010033
Chicago/Turabian StyleJesus, Celso R. N., Eduardo F. Molina, Ricardo de Oliveira, Sandra H. Pulcinelli, and Celso V. Santilli. 2023. "Effect of Organomontmorillonite-Cloisite® 20A Incorporation on the Structural and Drug Release Properties of Ureasil–PEO Hybrid" Pharmaceutics 15, no. 1: 33. https://doi.org/10.3390/pharmaceutics15010033
APA StyleJesus, C. R. N., Molina, E. F., de Oliveira, R., Pulcinelli, S. H., & Santilli, C. V. (2023). Effect of Organomontmorillonite-Cloisite® 20A Incorporation on the Structural and Drug Release Properties of Ureasil–PEO Hybrid. Pharmaceutics, 15(1), 33. https://doi.org/10.3390/pharmaceutics15010033