Synergistic Pro-Apoptotic Effect of a Cyclic RGD Peptide-Conjugated Magnetic Mesoporous Therapeutic Nanosystem on Hepatocellular Carcinoma HepG2 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of RGDSPIO@MSN NPs
2.2.1. Preparation of SPIO NPs
2.2.2. Preparation of SPIO@MSN NPs
2.2.3. Amination of SPIO@MSN NPs by APTES
2.2.4. NH2-SPIO@MSN NPs and Cyclic RGD Peptides Were Activated by the Bifunctional Cross-Linker Sulfo-SMCC and Traut’s Reagent, Respectively
2.2.5. Cyclic RGD Peptide-Modified SPIO@MSN NPs
2.3. Stability Analysis of SPIO@MSN NPs and RGDSPIO@MSN NPs in Different Media
2.4. Hemolytic Analysis of RGDSPIO@MSN NPs
2.5. Drug Loading and Drug Loading Content Analysis
2.6. Cell Culture
2.7. RBITC-Labeled SPIO@MSN NPs and RGDSPIO@MSN NPs
2.8. Cellular Uptake Analysis
2.9. Cytotoxicity Analysis
2.10. Reactive Oxygen Species Assay
2.11. Apoptosis Analysis of the Treated HepG2 Cells
3. Results and Discussion
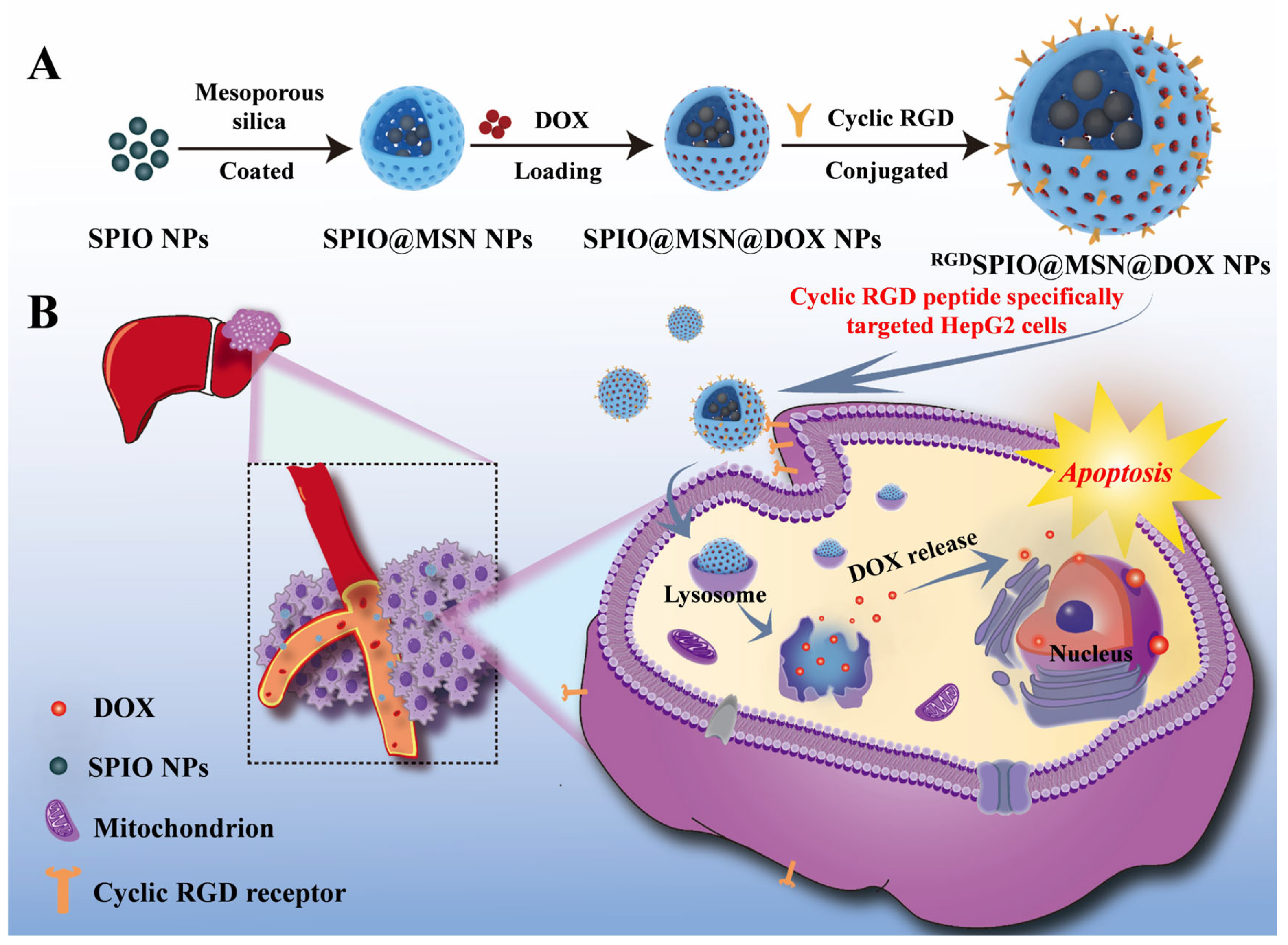
3.1. Synthesis and Characterization of RGDSPIO@MSN@DOX NPs
3.2. Analysis of Stability and Drug Loading Performance

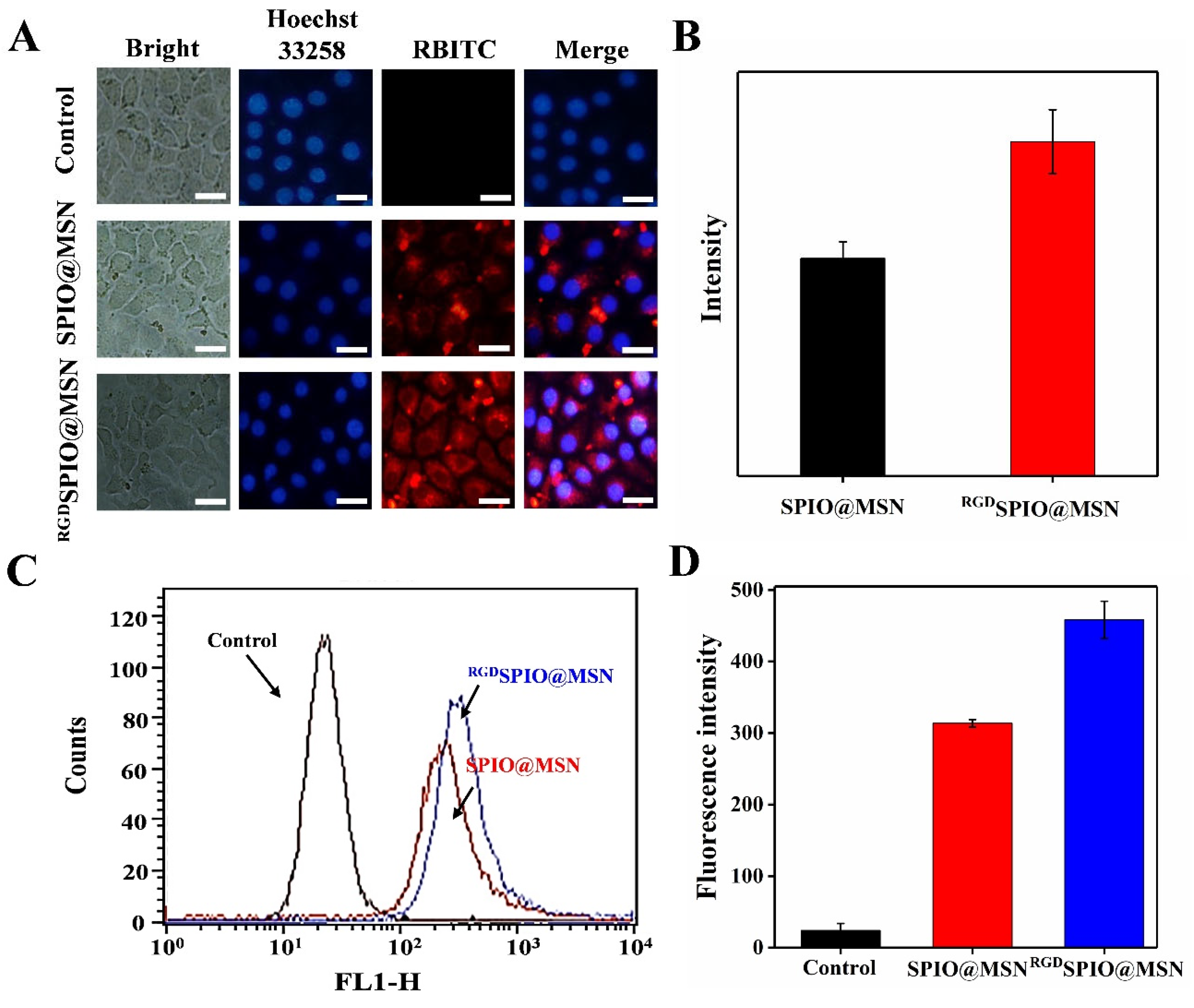
3.3. RBITC-Labeled SPIO@MSN NPs and RGDSPIO@MSN NPs
3.4. Cellular Uptake Evaluation
3.5. Cytotoxicity and Anti-Proliferative Activity Analysis
3.6. The Effect on the Level of Reactive Oxygen Species

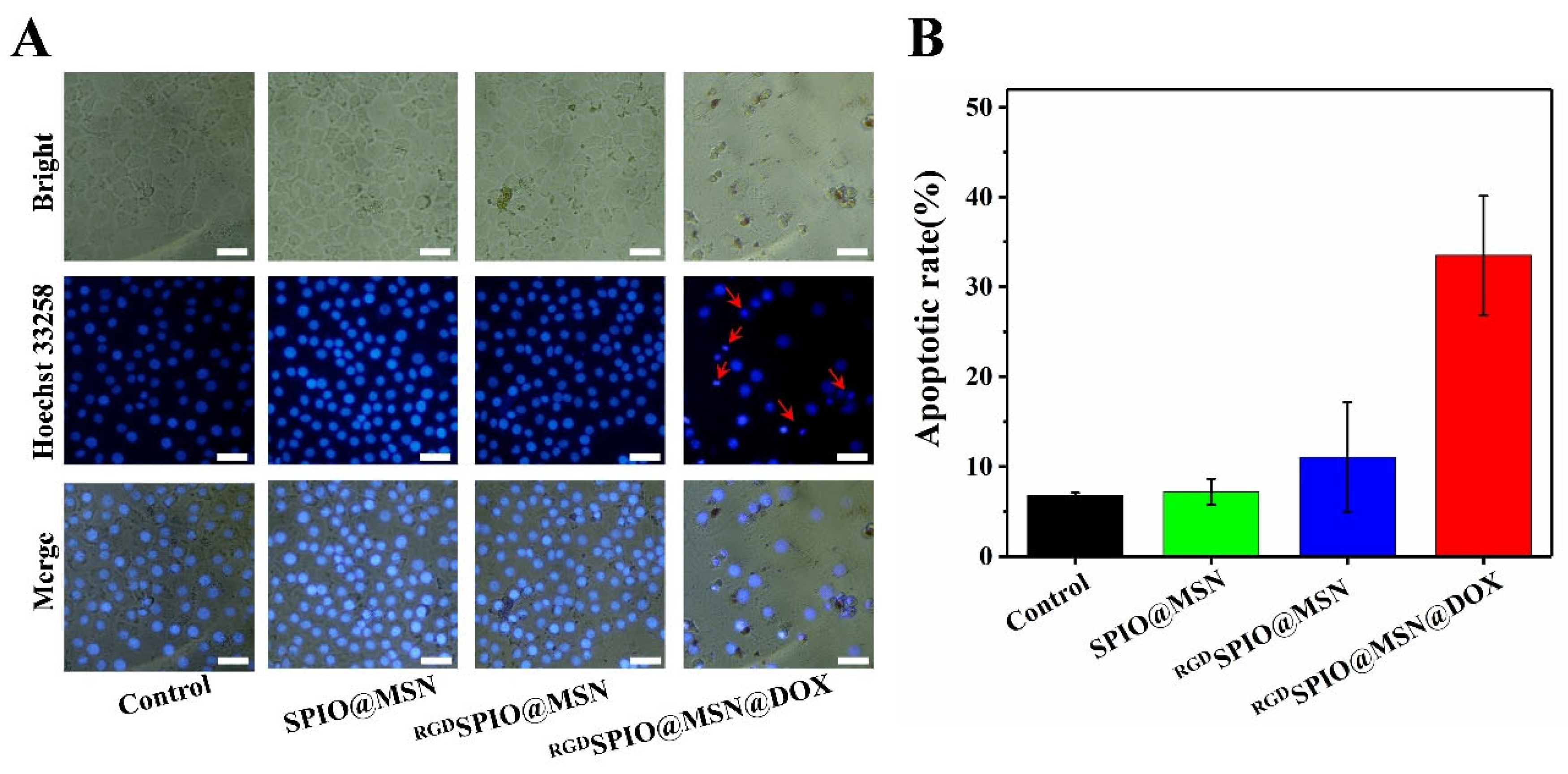
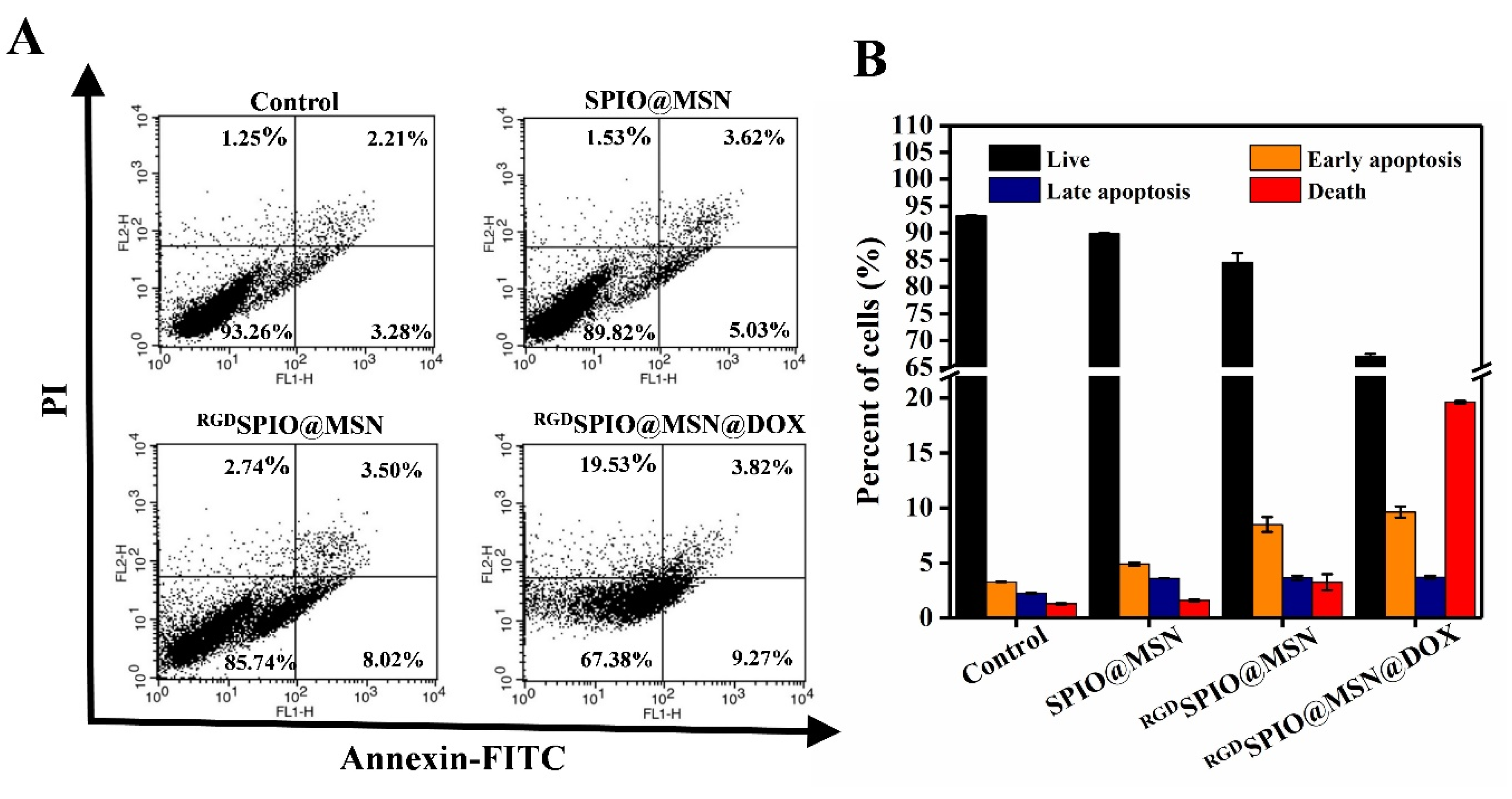
3.7. Analysis of Cell Apoptosis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA-Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A. Hepatocellular carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. Biophys. Acta-Rev. Cancer 2020, 1873, 188314. [Google Scholar] [CrossRef]
- Mak, L.-Y.; Cruz-Ramón, V.; Chinchilla-López, P.; Torres, H.A.; LoConte, N.K.; Rice, J.P.; Foxhall, L.E.; Sturgis, E.M.; Merrill, J.K.; Bailey, H.H.; et al. Global epidemiology, prevention, and management of hepatocellular carcinoma. Am. Soc. Clin. Oncol. Educ. Book. 2018, 38, 262–279. [Google Scholar] [CrossRef]
- Sugawara, Y.; Hibi, T. Surgical treatment of hepatocellular carcinoma. BioSci. Trends 2021, 15, 138–141. [Google Scholar] [CrossRef]
- Vogel, A.; Martinelli, E. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the esmo clinical practice guidelines. Ann. Oncol. 2021, 32, 801–805. [Google Scholar] [CrossRef]
- Kim, E.; Viatour, P. Hepatocellular carcinoma: Old friends and new tricks. Exp. Mol. Med. 2020, 52, 1898–1907. [Google Scholar] [CrossRef]
- Usmani, A.; Mishra, A.; Ahmad, M. Nanomedicines: A theranostic approach for hepatocellular carcinoma. Artif. Cell Nanomed. Biotechnol. 2018, 46, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, M.-D.; Liang, L.; Xing, H.; Zhang, C.-W.; Shen, F.; Huang, D.-S.; Yang, T. Nanotechnology for hepatocellular carcinoma: From surveillance, diagnosis to management. Small 2021, 17, 2005236. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Singh, S.; Lillard, J.W.; Singh, R. Drug delivery approaches for breast cancer. Int. J. Nanomed. 2017, 12, 6205–6218. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef]
- Hanušová, V.; Boušová, I.; Skálová, L. Possibilities to increase the effectiveness of doxorubicin in cancer cells killing. Drug Metab. Rev. 2011, 43, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Da Won Bae, S.; Nguyen, R.; Huo, X.; Han, S.; Zhang, Z.; Hebbard, L.; Duan, W.; Eslam, M.; Liddle, C.; et al. An aptamer-based drug delivery agent (CD133-APT-DOX) selectively and effectively kills liver cancer stem-like cells. Cancer Lett. 2021, 501, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, S.; Salehi, D.; Mahdipoor, P.; Beuttler, R.; Tiwari, R.; Aliabadi, H.M.; Parang, K. Design and application of hybrid cyclic-linear peptide-doxorubicin conjugates as a strategy to overcome doxorubicin resistance and toxicity. Eur. J. Med. Chem. 2021, 226, 113836. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Xie, X.; Cao, P. Magnoflorine improves sensitivity to doxorubicin (dox) of breast cancer cells via inducing apoptosis and autophagy through akt/mtor and p38 signaling pathways. Biomed. Pharm. 2020, 121, 109139. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Shim, M.K.; Kim, W.J.; Choi, J.; Nam, G.-H.; Kim, J.; Kim, J.; Moon, Y.; Kim, H.Y.; Park, J.; et al. Cancer-activated doxorubicin prodrug nanoparticles induce preferential immune response with minimal doxorubicin-related toxicity. Biomaterials 2021, 272, 120791. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; An, Y.; Li, X.; Li, Z.; Duan, J.; Yang, X.-D. Targeted therapy of colon cancer by aptamer-guided holliday junctions loaded with doxorubicin. Int. J. Nanomed. 2020, 15, 2119–2129. [Google Scholar] [CrossRef]
- Nitica, S.; Fizesan, I.; Dudric, R.; Loghin, F.; Lucaciu, C.M.; Iacovita, C. Doxorubicin loaded thermosensitive magneto-liposomes obtained by a gel hydration technique: Characterization and in vitro magneto-chemotherapeutic effect assessment. Pharmaceutics 2022, 14, 2501. [Google Scholar] [CrossRef]
- Tadokoro, T.; Ikeda, M.; Ide, T.; Deguchi, H.; Ikeda, S.; Okabe, K.; Ishikita, A.; Matsushima, S.; Koumura, T.; Yamada, K.-I.; et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight 2020, 5, 132747. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.; Zhang, H.; Tian, B.; Li, R.; Hou, X.; Wei, F. Fluorescent magnetic PEI-PLGA nanoparticles loaded with paclitaxel for concurrent cell imaging, enhanced apoptosis and autophagy in human brain cancer. Colloid Surf. B Biointerfaces 2018, 172, 708–717. [Google Scholar] [CrossRef]
- Chung, S.; Revia, R.A.; Zhang, M. Iron Oxide Nanoparticles for immune cell labeling and cancer immunotherapy. Nanoscale Horiz. 2021, 6, 696–717. [Google Scholar] [CrossRef]
- Alphandéry, E. Bio-synthesized iron oxide nanoparticles for cancer treatment. Int. J. Pharm. 2020, 586, 119472. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Guo, R.; Huang, S.; Zhang, F.; Liu, J.; Wang, Z.; Yang, X.; Shuai, X.; Cao, Z. Mesoporous polydopamine carrying sorafenib and spio nanoparticles for mri-guided ferroptosis cancer therapy. J. Control. Release 2020, 320, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, B.; Li, R.; Yang, Y.; Zhang, H.; Tian, B.; Cui, L.; Weng, H.; Wei, F. Anti-CD133 monoclonal antibody conjugated immunomagnetic nanosensor for molecular imaging of targeted cancer stem cells. Sens. Actuator B-Chem. 2018, 255, 3447–3457. [Google Scholar] [CrossRef]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B. 2018, 8, 165–177. [Google Scholar] [CrossRef]
- Wen, J.; Yang, K.; Liu, F.; Li, H.; Xu, Y.; Sun, S. Diverse Gatekeepers for Mesoporous silica nanoparticle based drug delivery systems. Chem. Soc. Rev. 2017, 46, 6024–6045. [Google Scholar] [CrossRef] [PubMed]
- van der Kooij, R.S.; Steendam, R.; Frijlink, H.W.; Hinrichs, W.L.J. An overview of the production methods for core–shell microspheres for parenteral controlled drug delivery. Eur. J. Pharm. Biopharm. 2022, 170, 24–42. [Google Scholar] [CrossRef]
- Timon-David, E.; Perez, C.; Rodallec, A. Nanotherapeutics plus immunotherapy in oncology: Who brings what to the table? Pharmaceutics 2022, 14, 2326. [Google Scholar] [CrossRef]
- Yan, H.; You, Y.; Li, X.; Liu, L.; Guo, F.; Zhang, Q.; Liu, D.; Tong, Y.; Ding, S.; Wang, J. Preparation of RGD peptide/folate acid double-targeted mesoporous silica nanoparticles and its application in human breast cancer MCF-7 cells. Front. Pharmacol. 2020, 11, 898. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Z.-C.; Liu, Y.; Chen, Y.-R.; Deng, R.-H.; Zhang, Z.-N.; Yu, J.-K.; Yuan, F.-Z. Function and mechanism of rgd in bone and cartilage tissue engineering. Front. Bioeng. Biotechnol. 2021, 9, 773636. [Google Scholar] [CrossRef]
- Garanti, T.; Alhnan, M.A.; Wan, K.-W. RGD-decorated solid lipid nanoparticles enhance tumor targeting, penetration and anticancer effect of asiatic acid. Nanomedicine 2020, 15, 1567–1583. [Google Scholar] [CrossRef]
- Danhier, F.; Le Breton, A.; Préat, V. RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis. Mol. Pharm. 2012, 9, 2961–2973. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; Alhakamy, N.A.; Md, S.; Kesharwani, P. Recent progress of RGD modified liposomes as multistage rocket against cancer. Front. Pharmacol. 2021, 12, 803304. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Qiu, S.; Fang, Y.; Wu, J.; Li, Q. Comparison of linear vs cyclic RGD pentapeptide interactions with integrin αvβ3 by molecular dynamics simulations. Biology 2021, 10, 688. [Google Scholar] [CrossRef]
- Liu, S. Radiolabeled cyclic RGD peptide bioconjugates as radiotracers targeting multiple integrins. Bioconjug. Chem. 2015, 26, 1413–1438. [Google Scholar] [CrossRef]
- Zou, Y.; Wei, Y.; Sun, Y.; Bao, J.; Yao, F.; Li, Z.; Meng, F.; Hu, C.; Storm, G.; Zhong, Z. Cyclic RGD-functionalized and disulfide-crosslinked iodine-rich polymersomes as a robust and smart theranostic agent for targeted ct imaging and chemotherapy of tumor. Theranostics 2019, 9, 8061–8072. [Google Scholar] [CrossRef]
- Qu, S.; Yang, H.; Ren, D.; Kan, S.; Zou, G.; Li, D.; Li, M. Magnetite nanoparticles prepared by precipitation from partially reduced ferric chloride aqueous solutions. J. Colloid Interface Sci. 1999, 215, 190–192. [Google Scholar] [CrossRef]
- Wang, X.; Xiong, T.; Cui, M.; Guan, X.; Yuan, J.; Wang, Z.; Li, R.; Zhang, H.; Duan, S.; Wei, F. Targeted self-activating Au-Fe3O4 composite nanocatalyst for enhanced precise hepatocellular carcinoma therapy via dual nanozyme-catalyzed cascade reactions. Appl. Mater. Today 2020, 21, 100827. [Google Scholar] [CrossRef]
- Luo, W.; Zhu, C.; Su, S.; Li, D.; He, Y.; Huang, Q.; Fan, C. Self-Catalyzed, Self-limiting growth of glucose oxidase-mimicking gold nanoparticles. ACS Nano 2010, 4, 7451–7458. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, P.; Zhu, L.; Qin, L.; Zhu, Y.; Yan, G.; Duan, S.; Guo, Y. Anti-CD133 antibody-targeted therapeutic immunomagnetic albumin microbeads loaded with vincristine-assisted to enhance anti-glioblastoma treatment. Mol. Pharm. 2019, 16, 4582–4593. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the MTT assay. Cold Spring Harb Protoc. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kılınç, E. Fullerene C60 functionalized γ-Fe2O3 magnetic nanoparticle: Synthesis, characterization, and biomedical applications. Artif. Cell Nanomed. Biotechnol. 2016, 44, 298–304. [Google Scholar] [CrossRef]
- Liochev, S.I. Reactive oxygen species and the free radical theory of aging. Free Radic. Biol. Med. 2013, 60, 1–4. [Google Scholar] [CrossRef]
- Zhou, Z.; Ni, K.; Deng, H.; Chen, X. Dancing with reactive oxygen species generation and elimination in nanotheranostics for disease treatment. Adv. Drug Deliv. Rev. 2020, 158, 73–90. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Reactive oxygen species (ROS)-based nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Ferrell, J.E. Apoptosis propagates through the cytoplasm as trigger waves. Science 2018, 361, 607–612. [Google Scholar] [CrossRef]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-X.; Brantley, S.L.; Corcelli, S.A.; Tokmakoff, A. DNA minor-groove binder Hoechst 33258 destabilizes base-pairing adjacent to its binding site. Commun. Biol. 2020, 3, 525. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Chen, X. Apoptosis imaging: Beyond Annexin V. J. Nucl. Med. 2010, 51, 1659–1662. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Liu, C.; Wang, Z.; Zhao, Y.; Chen, X.; Tao, H.; Chen, H.; Wang, X.; Duan, S. Synergistic Pro-Apoptotic Effect of a Cyclic RGD Peptide-Conjugated Magnetic Mesoporous Therapeutic Nanosystem on Hepatocellular Carcinoma HepG2 Cells. Pharmaceutics 2023, 15, 276. https://doi.org/10.3390/pharmaceutics15010276
Zhao X, Liu C, Wang Z, Zhao Y, Chen X, Tao H, Chen H, Wang X, Duan S. Synergistic Pro-Apoptotic Effect of a Cyclic RGD Peptide-Conjugated Magnetic Mesoporous Therapeutic Nanosystem on Hepatocellular Carcinoma HepG2 Cells. Pharmaceutics. 2023; 15(1):276. https://doi.org/10.3390/pharmaceutics15010276
Chicago/Turabian StyleZhao, Xuanping, Chuan Liu, Zichao Wang, Yingyuan Zhao, Xuyang Chen, Haizhen Tao, Hong Chen, Xueqin Wang, and Shaofeng Duan. 2023. "Synergistic Pro-Apoptotic Effect of a Cyclic RGD Peptide-Conjugated Magnetic Mesoporous Therapeutic Nanosystem on Hepatocellular Carcinoma HepG2 Cells" Pharmaceutics 15, no. 1: 276. https://doi.org/10.3390/pharmaceutics15010276
APA StyleZhao, X., Liu, C., Wang, Z., Zhao, Y., Chen, X., Tao, H., Chen, H., Wang, X., & Duan, S. (2023). Synergistic Pro-Apoptotic Effect of a Cyclic RGD Peptide-Conjugated Magnetic Mesoporous Therapeutic Nanosystem on Hepatocellular Carcinoma HepG2 Cells. Pharmaceutics, 15(1), 276. https://doi.org/10.3390/pharmaceutics15010276






