Microfluidic Manipulation for Biomedical Applications in the Central and Peripheral Nervous Systems
Abstract
1. Introduction
2. Neurological Injury and Disorders of the CNS and PNS
2.1. Basic Anatomical Structure and Functionality of the Nervous System
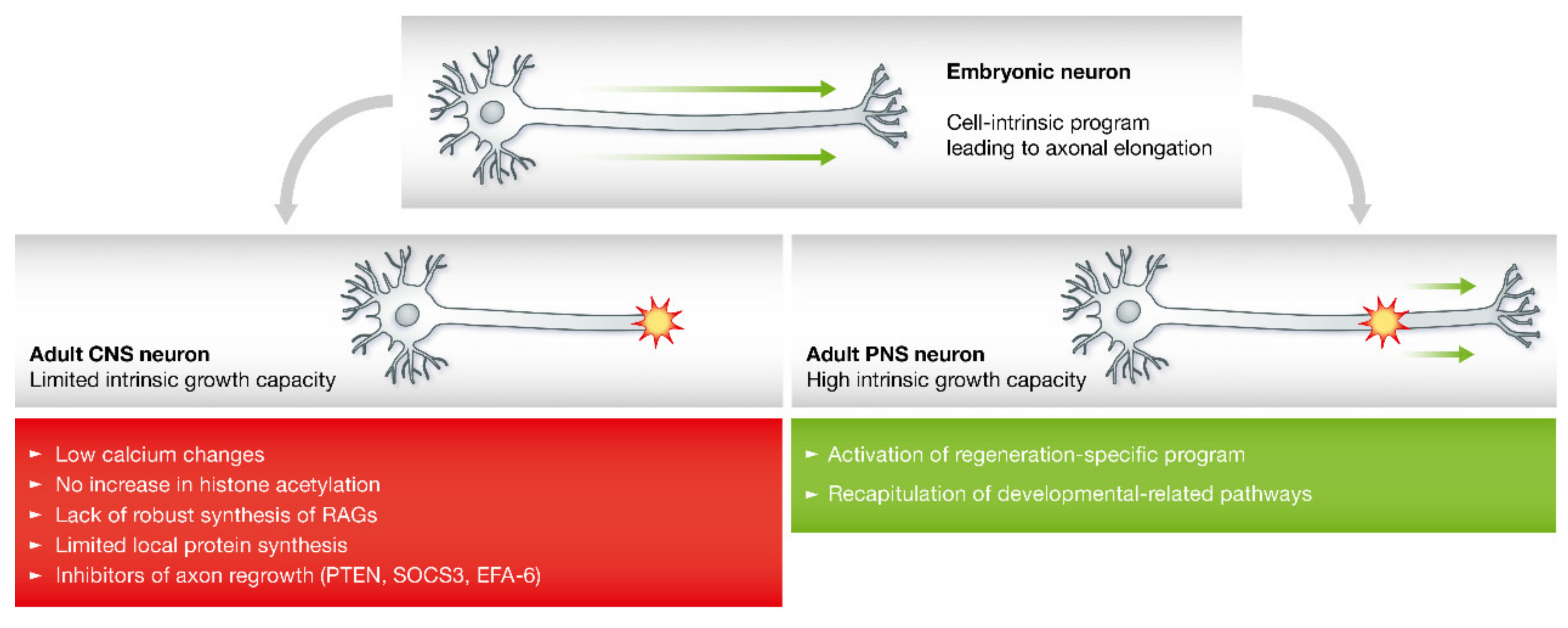
2.2. Axon Regeneration in the Peripheral Nervous System
2.3. Axon Regeneration in the CNS
3. Microfluidic Platforms for Exploring Nerve Injury and Regeneration
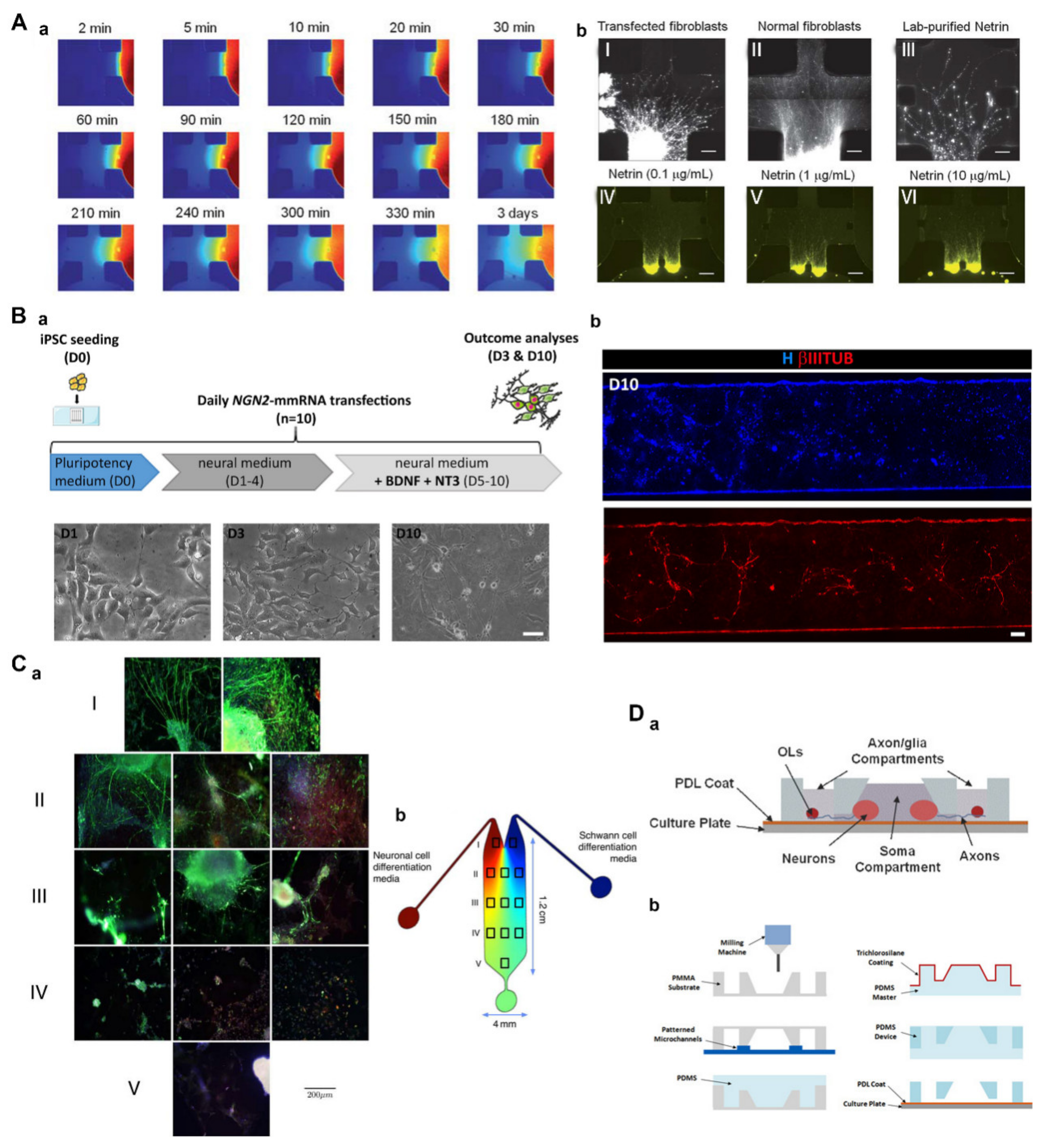
3.1. Engineering Neural Damage Using Microfluidics
3.1.1. Traumatic Neural Injury Models
3.1.2. Neurodegenerative Disease Models
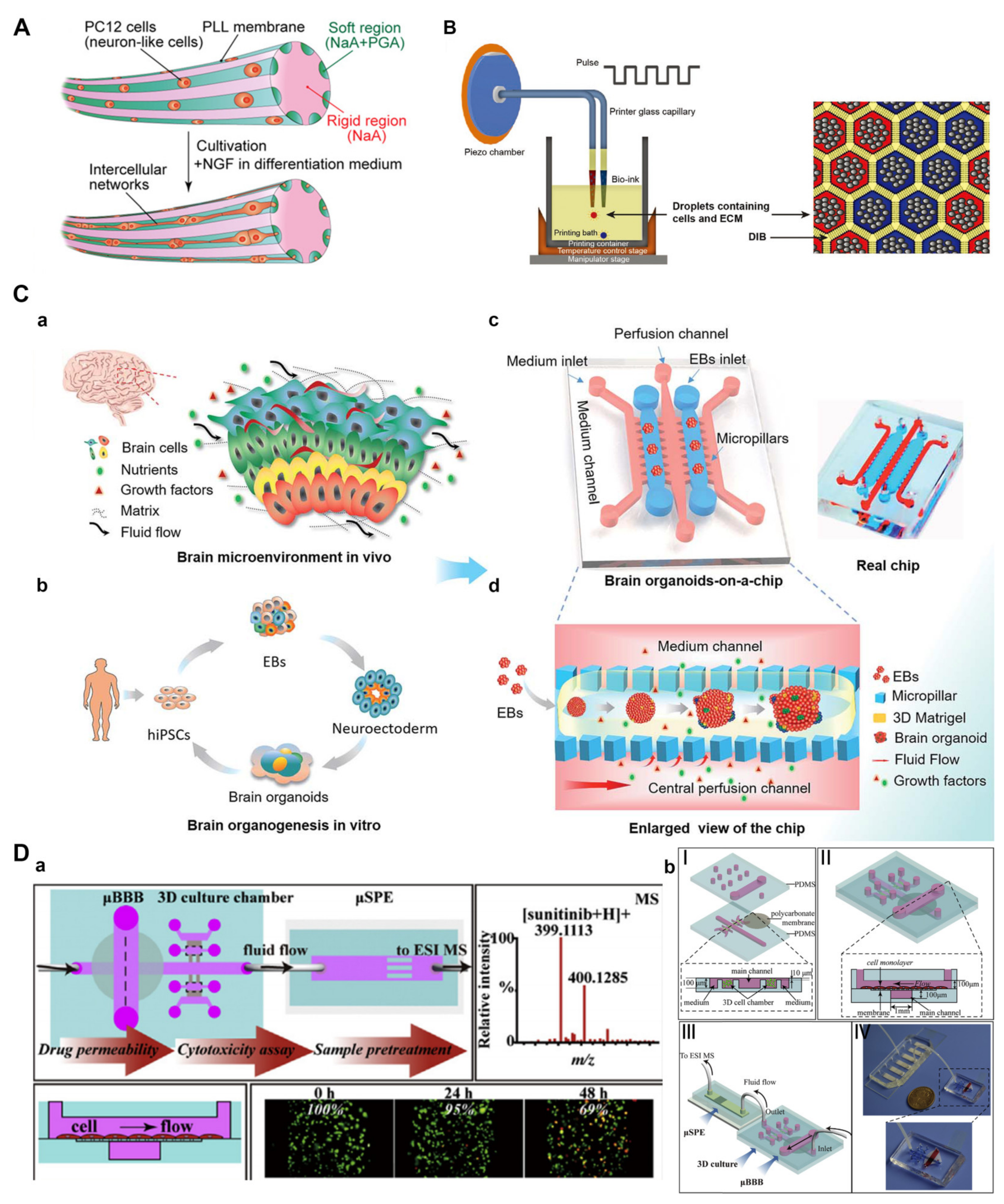
3.2. Engineering Neural Microenvironment Using Microfluidics
3.2.1. Engineering the Neural Microenvironment to Differentiate and Grow
3.2.2. Engineering Neuronal Networks and Organoids
3.2.3. Engineering Drug Delivery for Neural Tissue
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carvalho, C.R.; Reis, R.L.; Oliveira, J.M. Fundamentals and Current Strategies for Peripheral Nerve Repair and Regeneration. Adv. Exp. Med. Biol. 2020, 1249, 173–201. [Google Scholar]
- Beris, A.; Gkiatas, I.; Gelalis, I.; Papadopoulos, D.; Kostas-Agnantis, I. Current concepts in peripheral nerve surgery. Eur. J. Orthop. Surg. Traumatol. 2019, 29, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Trehan, S.K.; Model, Z.; Lee, S.K. Nerve Repair and Nerve Grafting. Hand Clin. 2016, 32, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Varadarajan, S.G.; Hunyara, J.L.; Hamilton, N.R.; Kolodkin, A.L.; Huberman, A.D. Central nervous system regeneration. Cell 2022, 185, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Curcio, M.; Bradke, F. Axon Regeneration in the Central Nervous System: Facing the Challenges from the Inside. Annu. Rev. Cell Dev. Biol. 2018, 34, 495–521. [Google Scholar] [CrossRef]
- Palmisano, I.; Di Giovanni, S. Advances and Limitations of Current Epigenetic Studies Investigating Mammalian Axonal Regeneration. Neurotherapeutics 2018, 15, 529–540. [Google Scholar] [CrossRef]
- Abu-Rub, M.; McMahon, S.; Zeugolis, D.I.; Windebank, A.; Pandit, A. Spinal cord injury in vitro: Modelling axon growth inhibition. Drug Discov. Today 2010, 15, 436–443. [Google Scholar] [CrossRef]
- Ndyabawe, K.; Kisaalita, W.S. Engineering Microsystems to Recapitulate Brain Physiology on a Chip. Drug Discov. Today 2019, 24, 1725–1730. [Google Scholar] [CrossRef]
- Karimi, M.; Bahrami, S.; Mirshekari, H.; Basri, S.M.M.; Nik, A.B.; Aref, A.R.; Akbari, M.; Hamblin, M.R. Microfluidic systems for stem cell-based neural tissue engineering. Lab Chip 2016, 16, 2551–2571. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, L.; Zhang, H.; Shang, L.; Zhao, Y. Microfluidics for Drug Development: From Synthesis to Evaluation. Chem. Rev. 2021, 121, 7468–7529. [Google Scholar] [CrossRef]
- Nikolakopoulou, P.; Rauti, R.; Voulgaris, D.; Shlomy, I.; Maoz, B.M.; Herland, A. Recent progress in translational engineered in vitro models of the central nervous system. Brain 2020, 143, 3181–3213. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhou, S.; Mea, H.J.; Guo, Y.; Ku, H.; Urbina, B.M. Emerging Roles of Microfluidics in Brain Research: From Cerebral Fluids Manipulation to Brain-on-A-Chip and Neuroelectronic Devices Engineering. Chem. Rev. 2022, 122, 7142–7181. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Micholt, L.; Friedrich, S.; Rand, D.R.; Bartic, C.; Braeken, D.; Levchenko, A. Superimposed topographic and chemical cues synergistically guide neurite outgrowth. Lab Chip 2013, 13, 3070–3081. [Google Scholar] [CrossRef] [PubMed]
- Siddique, R.; Thakor, N. Investigation of nerve injury through microfluidic devices. J. R. Soc. Interface 2013, 11, 20130676. [Google Scholar] [CrossRef] [PubMed]
- Jortner, B.S. Preparation and Analysis of the Peripheral Nervous System. Toxicol. Pathol. 2011, 39, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Echternacht, S.R.; Chacon, M.A.; Leckenby, J.I. Central versus peripheral nervous system regeneration: Is there an exception for cranial nerves? Regen. Med. 2021, 16, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Petrova, V.; Nieuwenhuis, B.; Fawcett, J.W.; Eva, R. Axonal organelles as molecular platforms for axon growth and regeneration after injury. Int. J. Mol. Sci. 2021, 22, 1798. [Google Scholar] [CrossRef] [PubMed]
- De Vries, G.H.; Boullerne, A.I. Glial cell lines: An overview. Neurochem. Res. 2010, 35, 1978–2000. [Google Scholar] [CrossRef]
- Wang, H.; Xu, C. A Novel Progress: Glial Cells and Inflammatory Pain. ACS Chem. Neurosci. 2022, 13, 288–295. [Google Scholar] [CrossRef]
- Hughes, A.N. Glial Cells Promote Myelin Formation and Elimination. Front. Cell Dev. Biol. 2021, 9, 661486. [Google Scholar] [CrossRef]
- Allen, N.J.; Lyons, D.A. Glia as architects of central nervous system formation and function. Science 2018, 362, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Höke, A. Mechanisms of Disease: What factors limit the success of peripheral nerve regeneration in humans? Nat. Clin. Pract. Neurol. 2006, 2, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Nave, K.A.; Werner, H.B. Myelination of the nervous system: Mechanisms and functions. Annu. Rev. Cell Dev. Biol. 2014, 30, 503–533. [Google Scholar] [CrossRef] [PubMed]
- Topp, K.S.; Boyd, B.S. Peripheral nerve: From the microscopic functional unit of the axon to the biomechanically loaded macroscopic structure. J. Hand Ther. 2012, 25, 142–152. [Google Scholar] [CrossRef] [PubMed]
- López-Cebral, R.; Silva-Correia, J.; Reis, R.L.; Silva, T.H.; Oliveira, J.M. Peripheral Nerve Injury: Current Challenges, Conventional Treatment Approaches, and New Trends in Biomaterials-Based Regenerative Strategies. ACS Biomater. Sci. Eng. 2017, 3, 3098–3122. [Google Scholar] [CrossRef] [PubMed]
- Grinsell, D.; Keating, C.P. Peripheral Nerve Reconstruction after Injury: A Review of Clinical and Experimental Therapies. BioMed Res. Int. 2014, 2014, 698256. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Yi, S. Pathophysiological changes of physical barriers of peripheral nerves after injury. Front. Neurosci. 2018, 12, 597. [Google Scholar] [CrossRef]
- Kanda, T. Biology of the blood-nerve barrier and its alteration in immune mediated neuropathies. J. Neurol. Neurosurg. Psychiatry 2013, 84, 208–212. [Google Scholar] [CrossRef]
- Menorca, R.M.G.; Fussell, T.S.; Elfar, J.C. Peripheral Nerve Trauma: Mechanisms of Injury and Recovery. Hand Clin. 2013, 29, 317–330. [Google Scholar] [CrossRef]
- Mahar, M.; Cavalli, V. Intrinsic mechanisms of neuronal axon regeneration. Nat. Rev. Neurosci. 2018, 19, 323–337. [Google Scholar] [CrossRef]
- Saheb-Al-Zamani, M.; Yan, Y.; Farber, S.J.; Hunter, D.A.; Newton, P.; Wood, M.D.; Stewart, S.A.; Johnson, P.J.; Mackinnon, S.E. Limited regeneration in long acellular nerve allografts is associated with increased Schwann cell senescence. Exp. Neurol. 2013, 247, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Mar, F.M.; Bonni, A.; Sousa, M.M. Cell intrinsic control of axon regeneration. EMBO Rep. 2014, 15, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Berdan, R.C.; Easaw, J.C.; Wang, R. Alterations in membrane potential after axotomy at different distances from the soma of an identified neuron and the effect of depolarization on neurite outgrowth and calcium channel expression. J. Neurophysiol. 1993, 69, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Rishal, I.; Fainzilber, M. Axon-soma communication in neuronal injury. Nat. Rev. Neurosci. 2014, 15, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Sloutsky, R.; Naegle, K.M.; Cavalli, V. Injury-induced HDAC5 nuclear export is essential for axon regeneration. Cell 2013, 155, 894–908. [Google Scholar] [CrossRef]
- Ghosh-Roy, A.; Wu, Z.; Goncharov, A.; Jin, Y.; Chisholm, A.D. Calcium and cyclic AMP promote axonal regeneration in Caenorhabditis elegans and require DLK-1 kinase. J. Neurosci. 2010, 30, 3175–3183. [Google Scholar] [CrossRef]
- Verma, P.; Chierzi, S.; Codd, A.M.; Campbell, D.S.; Meyer, R.L.; Holt, C.E.; Fawcett, J.W. Axonal protein synthesis and degradation are necessary for efficient growth cone regeneration. J. Neurosci. 2005, 25, 331–342. [Google Scholar] [CrossRef]
- Song, W.; Cho, Y.; Watt, D.; Cavalli, V. Tubulin-tyrosine ligase (TTL)-mediated increase in tyrosinated α-tubulin in injured axons is required for retrograde injury signaling and axon regeneration. J. Biol. Chem. 2015, 290, 14765–14775. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Baig, H.S.; Hammarlund, M. Mitochondria Localize to Injured Axons to Support Regeneration. Neuron 2016, 92, 1308–1323. [Google Scholar] [CrossRef]
- Perlson, E.; Hanz, S.; Ben-Yaakov, K.; Segal-Ruder, Y.; Seger, R.; Fainzilber, M. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron 2005, 45, 715–726. [Google Scholar] [CrossRef]
- Barnat, M.; Enslen, H.; Propst, F.; Davis, R.J.; Soares, S.; Nothias, F. Distinct roles of c-Jun N-terminal kinase isoforms in neurite initiation and elongation during axonal regeneration. J. Neurosci. 2010, 30, 7804–7816. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yaakov, K.; Dagan, S.Y.; Segal-Ruder, Y.; Shalem, O.; Vuppalanchi, D.; Willis, D.E.; Yudin, D.; Rishal, I.; Rother, F.; Bader, M.; et al. Axonal transcription factors signal retrogradely in lesioned peripheral nerve. EMBO J. 2012, 31, 1350–1363. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; He, H.; Qiu, J.; Lorber, B.; Bryson, J.B.; Filbin, M.T. Increased synthesis of spermidine as a result of upregulation of arginase I promotes axonal regeneration in culture and in vivo. J. Neurosci. 2009, 29, 9545–9552. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.J.; Park, M.; Spillane, M.; Yoo, S.; Pacheco, A.; Gomes, C.; Vuppalanchi, D.; Mcdonald, M.; Kim, H.K.; Merianda, T.T.; et al. Axonally synthesized β-actin and GAP-43 proteins support distinct modes of axonal growth. J. Neurosci. 2013, 33, 3311–3322. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Gao, Y.; Bryson, J.B.; Hou, J.; Chaudhry, N.; Siddiq, M.; Martinez, J.; Spencer, T.; Carmel, J.; Hart, R.B.; et al. The cytokine interleukin-6 is sufficient but not necessary to mimic the peripheral conditioning lesion effect on axonal growth. J. Neurosci. 2006, 26, 5565–5573. [Google Scholar] [CrossRef]
- Scheib, J.; Höke, A. Advances in peripheral nerve regeneration. Nat. Rev. Neurol. 2013, 9, 668–676. [Google Scholar] [CrossRef]
- Namikawa, K.; Okamoto, T.; Suzuki, A.; Konishi, H.; Kiyama, H. Pancreatitis-associated protein-III is a novel macrophage chemoattractant implicated in nerve regeneration. J. Neurosci. 2006, 26, 7460–7467. [Google Scholar] [CrossRef]
- Napoli, I.; Noon, L.A.; Ribeiro, S.; Kerai, A.P.; Parrinello, S.; Rosenberg, L.H.; Collins, M.J.; Harrisingh, M.C.; White, I.J.; Woodhoo, A.; et al. A Central Role for the ERK-Signaling Pathway in Controlling Schwann Cell Plasticity and Peripheral Nerve Regeneration In vivo. Neuron 2012, 73, 729–742. [Google Scholar] [CrossRef]
- Gomez-Sanchez, J.A.; Carty, L.; Iruarrizaga-Lejarreta, M.; Palomo-Irigoyen, M.; Varela-Rey, M.; Griffith, M.; Hantke, J.; Macias-Camara, N.; Azkargorta, M.; Aurrekoetxea, I.; et al. Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J. Cell Biol. 2015, 210, 153–168. [Google Scholar] [CrossRef]
- Terenghi, G. Peripheral nerve regeneration and neurotrophic factors. J. Anat. 1999, 194, 1–14. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The success and failure of the schwann cell response to nerve injury. Front. Cell. Neurosci. 2019, 13, 33. [Google Scholar] [CrossRef]
- Zigmond, R.E.; Echevarria, F.D. Macrophage biology in the peripheral nervous system after injury. Prog. Neurobiol. 2019, 173, 102–121. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, J.A.; Pilch, K.S.; Van Der Lans, M.; Fazal, S.V.; Benito, C.; Wagstaff, L.J.; Mirsky, R.; Jessen, K.R. After nerve injury, lineage tracing shows that myelin and Remak Schwann cells elongate extensively and branch to form repair Schwann cells, which shorten radically on remyelination. J. Neurosci. 2017, 37, 9086–9099. [Google Scholar] [CrossRef] [PubMed]
- Burnett, M.G.; Zager, E.L. Pathophysiology of peripheral nerve injury: A brief review. Neurosurg. Focus 2004, 16, 1–7. [Google Scholar] [CrossRef]
- Klimovich, P.; Rubina, K.; Sysoeva, V.; Semina, E. New frontiers in peripheral nerve regeneration: Concerns and remedies. Int. J. Mol. Sci. 2021, 22, 13380. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Piao, X.; Bonaldo, P. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol. 2015, 130, 605–618. [Google Scholar] [CrossRef]
- Cattin, A.L.; Burden, J.J.; Van Emmenis, L.; MacKenzie, F.E.; Hoving, J.J.A.; Garcia Calavia, N.; Guo, Y.; McLaughlin, M.; Rosenberg, L.H.; Quereda, V.; et al. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Allodi, I.; Udina, E.; Navarro, X. Specificity of peripheral nerve regeneration: Interactions at the axon level. Prog. Neurobiol. 2012, 98, 16–37. [Google Scholar] [CrossRef] [PubMed]
- Stassart, R.M.; Fledrich, R.; Velanac, V.; Brinkmann, B.G.; Schwab, M.H.; Meijer, D.; Sereda, M.W.; Nave, K.A. A role for Schwann cell-derived neuregulin-1 in remyelination. Nat. Neurosci. 2013, 16, 48–54. [Google Scholar] [CrossRef]
- Liu, K.; Tedeschi, A.; Park, K.K.; He, Z. Neuronal intrinsic mechanisms of axon regeneration. Annu. Rev. Neurosci. 2011, 34, 131–152. [Google Scholar] [CrossRef]
- Tedeschi, A.; Bradke, F. Spatial and temporal arrangement of neuronal intrinsic and extrinsic mechanisms controlling axon regeneration. Curr. Opin. Neurobiol. 2017, 42, 118–127. [Google Scholar] [CrossRef]
- Case, L.C.; Tessier-Lavigne, M. Regeneration of the adult central nervous system. Curr. Biol. 2005, 15, R749–R753. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, A. Tuning the orchestra: Transcriptional pathways controlling axon regeneration. Front. Mol. Neurosci. 2012, 4, 60. [Google Scholar] [CrossRef] [PubMed]
- Mar, F.M.; Simões, A.R.; Leite, S.; Morgado, M.M.; Santos, T.E.; Rodrigo, I.S.; Teixeira, C.A.; Misgeld, T.; Sousa, M.M. CNS axons globally increase axonal transport after peripheral conditioning. J. Neurosci. 2014, 34, 5965–5970. [Google Scholar] [CrossRef] [PubMed]
- Ertürk, A.; Hellal, F.; Enes, J.; Bradke, F. Disorganized microtubules underlie the formation of retraction bulbs and the failure of axonal regeneration. J. Neurosci. 2007, 27, 9169–9180. [Google Scholar] [CrossRef] [PubMed]
- Ruschel, J.; Bradke, F. Systemic administration of epothilone D improves functional recovery of walking after rat spinal cord contusion injury. Exp. Neurol. 2018, 306, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Spira, M.E.; Benbassat, D.; Dormann, A. Resealing of the proximal and distal cut ends of transected axons: Electrophysiological and ultrastructural analysis. J. Neurobiol. 1993, 24, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Lindner, R.; Puttagunta, R.; Di Giovanni, S. Epigenetic Regulation of Axon Outgrowth and Regeneration in CNS Injury: The First Steps Forward. Neurotherapeutics 2013, 10, 771–781. [Google Scholar] [CrossRef]
- Gumy, L.F.; Tan, C.L.; Fawcett, J.W. The role of local protein synthesis and degradation in axon regeneration. Exp. Neurol. 2010, 223, 28–37. [Google Scholar] [CrossRef]
- Park, K.K.; Liu, K.; Hu, Y.; Smith, P.D.; Wang, C.; Cai, B.; Xu, B.; Connolly, L.; Kramvis, I.; Sahin, M.; et al. Promoting Axon Regeneration in the Adult CNS by Modulation of the PTEN/mTOR Pathway. Science 2008, 322, 963–966. [Google Scholar] [CrossRef]
- Rawlings, J.S.; Rosler, K.M.; Harrison, D.A. The JAK/STAT signaling pathway. J. Cell Sci. 2004, 117, 1281–1283. [Google Scholar] [CrossRef] [PubMed]
- Eva, R.; Koseki, H.; Kanamarlapudi, V.; Fawcett, J.W. EFA6 regulates selective polarised transport and axon regeneration from the axon initial segment. J. Cell Sci. 2017, 130, 3663–3675. [Google Scholar] [CrossRef] [PubMed]
- Filbin, M.T. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat. Rev. Neurosci. 2003, 4, 703–713. [Google Scholar] [CrossRef]
- Niederöst, B.P.; Zimmermann, D.R.; Schwab, M.E.; Bandtlow, C.E. Bovine CNS myelin contains neurite growth-inhibitory activity associated with chondroitin sulfate proteoglycans. J. Neurosci. 1999, 19, 8979–8989. [Google Scholar] [CrossRef] [PubMed]
- Pasterkamp, R.J.; Anderson, P.N.; Verhaagen, J. Peripheral nerve injury fails to induce growth of lesioned ascending dorsal column axons into spinal cord scar tissue expressing the axon repellent Semaphorin3A. Eur. J. Neurosci. 2001, 13, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Bundesen, L.Q.; Scheel, T.A.; Bregman, B.S.; Kromer, L.F. Ephrin-B2 and EphB2 regulation of astrocyte-meningeal fibroblast interactions in response to spinal cord lesions in adult rats. J. Neurosci. 2003, 23, 7789–7800. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Sun, Z.L.; Yang, X.T.; Zhu, L.; Feng, D.F. Exploring Optic Nerve Axon Regeneration. Curr. Neuropharmacol. 2017, 15, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, G.; Khakh, B.S.; Deming, T.J.; Sofroniew, M.V. Astrocyte scar formation AIDS central nervous system axon regeneration. Nature 2016, 532, 195–200. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. New insights into glial scar formation after spinal cord injury. Cell Tissue Res. 2022, 387, 319–336. [Google Scholar] [CrossRef]
- Li, H.Y.; Ruan, Y.W.; Ren, C.R.; Cui, Q.; So, K.F. Mechanisms of secondary degeneration after partial optic nerve transection. Neural Regen. Res. 2014, 9, 565–574. [Google Scholar]
- Amor, S.; Peferoen, L.A.N.; Vogel, D.Y.S.; Breur, M.; van der Valk, P.; Baker, D.; Van Noort, J.M. Inflammation in neurodegenerative diseases—An update. Immunology 2014, 142, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.H.; Siddique, R.; Hosmane, S.; Thakor, N.; Höke, A. Compartmentalized microfluidic culture platform to study mechanism of paclitaxel-induced axonal degeneration. Exp. Neurol. 2009, 218, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ren, L.; Liu, W.; Wang, J.C.; Wang, Y.; Tu, Q.; Xu, J.; Liu, R.; Zhang, Y.; Yuan, M.S.; et al. Spatiotemporally controlled and multifactor involved assay of neuronal compartment regeneration after chemical injury in an integrated microfluidics. Anal. Chem. 2012, 84, 6444–6453. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Vahidi, B.; Taylor, A.M.; Rhee, S.W.; Jeon, N.L. Microfluidic culture platform for neuroscience research. Nat. Protoc. 2006, 1, 2128–2136. [Google Scholar] [CrossRef]
- Dollé, J.P.; Morrison, B., III; Schloss, R.S.; Yarmush, M.L. Brain-on-a-chip microsystem for investigating traumatic brain injury: Axon diameter and mitochondrial membrane changes play a significant role in axonal response to strain injuries. Technology 2014, 2, 106. [Google Scholar] [CrossRef]
- Kim, Y.T.; Karthikeyan, K.; Chirvi, S.; Davé, D.P. Neuro-optical microfluidic platform to study injury and regeneration of single axons. Lab Chip 2009, 9, 2576–2581. [Google Scholar] [CrossRef]
- Shin, Y.; Choi, S.H.; Kim, E.; Bylykbashi, E.; Kim, J.A.; Chung, S.; Kim, D.Y.; Kamm, R.D.; Tanzi, R.E. Blood–Brain Barrier Dysfunction in a 3D In Vitro Model of Alzheimer’s Disease. Adv. Sci. 2019, 6, 1900962. [Google Scholar] [CrossRef]
- Park, J.; Wetzel, I.; Marriott, I.; Dréau, D.; D’Avanzo, C.; Kim, D.Y.; Tanzi, R.E.; Cho, H. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat. Neurosci. 2018, 21, 941–951. [Google Scholar] [CrossRef]
- Moreno, E.L.; Hachi, S.; Hemmer, K.; Trietsch, S.J.; Baumuratov, A.S.; Hankemeier, T.; Vulto, P.; Schwamborn, J.C.; Fleming, R.M.T. Differentiation of neuroepithelial stem cells into functional dopaminergic neurons in 3D microfluidic cell culture. Lab Chip 2015, 15, 2419–2428. [Google Scholar] [CrossRef]
- Osaki, T.; Uzel, S.G.M.; Kamm, R.D. Microphysiological 3D model of amyotrophic lateral sclerosis (ALS) from human iPS-derived muscle cells and optogenetic motor neurons. Sci. Adv. 2018, 4, eaat5847. [Google Scholar] [CrossRef]
- Kothapalli, C.R.; van Veen, E.; de Valence, S.; Chung, S.; Zervantonakis, I.K.; Gertler, F.B.; Kamm, R.D. A high-throughput microfluidic assay to study neurite response to growth factor gradients. Lab Chip 2011, 11, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Tolomeo, A.M.; Laterza, C.; Grespan, E.; Michielin, F.; Canals, I.; Kokaia, Z.; Muraca, M.; Gagliano, O.; Elvassore, N. NGN2 mmRNA-Based Transcriptional Programming in Microfluidic Guides hiPSCs Toward Neural Fate with Multiple Identities. Front. Cell. Neurosci. 2021, 15, 602888. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.G.; Flanagan, L.A.; Rhee, S.W.; Schwartz, P.H.; Lee, A.P.; Monuki, E.S.; Jeon, N.L. Human neural stem cell growth and differentiation in a gradient-generating microfluidic device. Lab Chip 2005, 5, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, P.; White, J.B.; Yull Park, J.; Hume, R.I.; Ebisu, F.; Mendez, F.; Takayama, S.; Barald, K.F. Concomitant differentiation of a population of mouse embryonic stem cells into neuron-like cells and schwann cell–like cells in a slow-flow microfluidic device. Dev. Dyn. 2017, 246, 7–27. [Google Scholar] [CrossRef]
- Park, J.; Koito, H.; Li, J.; Han, A. A multi-compartment CNS neuron-glia co-culture microfluidic platform. J. Vis. Exp. 2009, e1399. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Guo, Y.; Zhu, Y.; Qin, J. Engineering stem cell-derived 3D brain organoids in a perfusable organ-on-a-chip system. RSC Adv. 2018, 8, 1677–1685. [Google Scholar] [CrossRef]
- Noorani, B.; Bhalerao, A.; Raut, S.; Nozohouri, E.; Bickel, U.; Cucullo, L. A quasi-physiological microfluidic blood-brain barrier model for brain permeability studies. Pharmaceutics 2021, 13, 1474. [Google Scholar] [CrossRef]
- Shao, X.; Gao, D.; Chen, Y.; Jin, F.; Hu, G.; Jiang, Y.; Liu, H. Development of a blood-brain barrier model in a membrane-based microchip for characterization of drug permeability and cytotoxicity for drug screening. Anal. Chim. Acta 2016, 934, 186–193. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Naganuma, Y.; Yajima, Y.; Yamada, M.; Seki, M. Patterned hydrogel microfibers prepared using multilayered microfluidic devices for guiding network formation of neural cells. Biofabrication 2014, 6, 035011. [Google Scholar] [CrossRef]
- Wei, G.; Jiang, D.; Hu, S.; Yang, Z.; Zhang, Z.; Li, W.; Cai, W.; Liu, D. Polydopamine-Decorated Microcomposites Promote Functional Recovery of an Injured Spinal Cord by Inhibiting Neuroinflammation. ACS Appl. Mater. Interfaces 2021, 13, 47341–47353. [Google Scholar] [CrossRef]
- Wang, K.; Kumar, U.S.; Sadeghipour, N.; Massoud, T.F.; Paulmurugan, R. A Microfluidics-Based Scalable Approach to Generate Extracellular Vesicles with Enhanced Therapeutic MicroRNA Loading for Intranasal Delivery to Mouse Glioblastomas. ACS Nano 2021, 15, 18327–18346. [Google Scholar] [CrossRef] [PubMed]
- Hsu, R.S.; Chen, P.Y.; Fang, J.H.; Chen, Y.Y.; Chang, C.W.; Lu, Y.J.; Hu, S.H. Adaptable Microporous Hydrogels of Propagating NGF-Gradient by Injectable Building Blocks for Accelerated Axonal Outgrowth. Adv. Sci. 2019, 6, 1900520. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Tang, X.; Wang, T.; Wei, X.; Zhang, J.; Lu, L.; Liu, Y.; Yang, B. A Dual-Modal Magnetic Resonance/Photoacoustic Imaging Tracer for Long-Term High-Precision Tracking and Facilitating Repair of Peripheral Nerve Injuries. Adv. Healthc. Mater. 2022, 11, e2200183. [Google Scholar] [CrossRef]
- Zhou, L.; Wolfes, A.C.; Li, Y.; Chan, D.C.W.; Ko, H.; Szele, F.G.; Bayley, H. Lipid-Bilayer-Supported 3D Printing of Human Cerebral Cortex Cells Reveals Developmental Interactions. Adv. Mater. 2020, 32, e2002183. [Google Scholar] [CrossRef]
- Terrell-Hall, T.B.; Ammer, A.G.; Griffith, J.I.G.; Lockman, P.R. Permeability across a novel microfluidic blood-tumor barrier model. Fluids Barriers CNS 2017, 14, 3. [Google Scholar] [CrossRef]
- Brown, J.A.; Codreanu, S.G.; Shi, M.; Sherrod, S.D.; Markov, D.A.; Neely, M.D.; Britt, C.M.; Hoilett, O.S.; Reiserer, R.S.; Samson, P.C.; et al. Metabolic consequences of inflammatory disruption of the blood-brain barrier in an organ-on-chip model of the human neurovascular unit. J. Neuroinflammation 2016, 13, 306. [Google Scholar] [CrossRef]
- Chen, X.; Liu, C.; Muok, L.; Zeng, C.; Li, Y. Dynamic 3D On-Chip BBB Model Design, Development, and Applications in Neurological Diseases. Cells 2021, 10, 3183. [Google Scholar] [CrossRef] [PubMed]
- Samiei, E.; Seyfoori, A.; Toyota, B.; Ghavami, S.; Akbari, M. Investigating programmed cell death and tumor invasion in a three-dimensional (3d) microfluidic model of glioblastoma. Int. J. Mol. Sci. 2020, 21, 3162. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Sun, A.X.; An, J.; Chua, C.K.; Chew, S.Y. 3D neural tissue models: From spheroids to bioprinting. Biomaterials 2018, 154, 113–133. [Google Scholar] [CrossRef] [PubMed]
- Hur, E.M.; Yang, I.H.; Kim, D.H.; Byun, J.; Saijilafu; Xu, W.L.; Nicovich, P.R.; Cheong, R.; Levchenko, A.; Thakor, N.; et al. Engineering neuronal growth cones to promote axon regeneration over inhibitory molecules. Proc. Natl. Acad. Sci. USA 2011, 108, 5057–5062. [Google Scholar] [CrossRef]
- Taylor, A.M.; Blurton-Jones, M.; Rhee, S.W.; Cribbs, D.H.; Cotman, C.W.; Jeon, N.L. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat. Methods 2005, 2, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Santos-Ferreira, T.; Herbig, M.; Otto, O.; Carido, M.; Karl, M.O.; Michalakis, S.; Guck, J.; Ader, M. Morpho-Rheological Fingerprinting of Rod Photoreceptors Using Real-Time Deformability Cytometry. Cytom. Part A 2019, 95, 1145–1157. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.D.; Hung, W.C.; Wirtz, D.; Konstantopoulos, K. Engineered Models of Confined Cell Migration. Annu. Rev. Biomed. Eng. 2016, 18, 159–180. [Google Scholar] [CrossRef] [PubMed]
- Mahto, S.K.; Song, H.; Rhee, S.W. Functional synapse formation between compartmentalized cortical neurons cultured inside microfluidic devices. BioChip J. 2011, 5, 289–298. [Google Scholar] [CrossRef]
- Peyrin, J.M.; Deleglise, B.; Saias, L.; Vignes, M.; Gougis, P.; Magnifico, S.; Betuing, S.; Pietri, M.; Caboche, J.; Vanhoutte, P.; et al. Axon diodes for the reconstruction of oriented neuronal networks in microfluidic chambers. Lab Chip 2011, 11, 3663–3673. [Google Scholar] [CrossRef]
- Kunze, A.; Giugliano, M.; Valero, A.; Renaud, P. Micropatterning neural cell cultures in 3D with a multi-layered scaffold. Biomaterials 2011, 32, 2088–2098. [Google Scholar] [CrossRef]
- LaPlaca, M.C.; Cullen, D.K.; McLoughlin, J.J.; Cargill, R.S. High rate shear strain of three-dimensional neural cell cultures: A new in vitro traumatic brain injury model. J. Biomech. 2005, 38, 1093–1105. [Google Scholar] [CrossRef]
- Zhang, X.X.; Tian, Y.; Wang, Z.T.; Ma, Y.H.; Tan, L.; Yu, J.T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimer’s Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef]
- Varma, P.; Narayan, L.; Alty, J.; Painter, V.; Padmakumar, C. An innovative personalised management program for older adults with parkinson’s disease: New concepts and future directions. J. Pers. Med. 2021, 11, 43. [Google Scholar] [CrossRef]
- Krishna, S.; Prasad, S.; Goel, R.; Prasad, B.K. Parkinson’s disease—A review. J. Evol. Med. Dent. Sci. 2018, 7, 1294–1297. [Google Scholar] [CrossRef]
- Feldman, E.L.; Goutman, S.A.; Petri, S.; Mazzini, L.; Savelieff, M.G.; Shaw, P.J.; Sobue, G. Amyotrophic lateral sclerosis. Lancet 2022, 400, 1363–1380. [Google Scholar] [CrossRef] [PubMed]
- Antony, P.M.A.; Diederich, N.J.; Krüger, R.; Balling, R. The hallmarks of Parkinson’s disease. FEBS J. 2013, 280, 5981–5993. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Yildirimer, L.; Zhang, Q.; Kuang, S.; Cheung, C.W.J.; Chu, K.A.; He, Y.; Yang, M.; Zhao, X. Engineering three-dimensional microenvironments towards in vitro disease models of the central nervous system. Biofabrication 2019, 11, 032003. [Google Scholar] [CrossRef]
- Alsmadi, N.Z.; Bendale, G.S.; Kanneganti, A.; Shihabeddin, T.; Nguyen, A.H.; Hor, E.; Dash, S.; Johnston, B.; Granja-Vazquez, R.; Romero-Ortega, M.I. Glial-Derived Growth Factor and Pleiotrophin Synergistically Promote Axonal Regeneration in Critical Nerve Injuries. Acta Biomater. 2018, 78, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xia, B.; Shi, X.; Gao, J.; Yang, Y.; Xu, F.; Qi, F.; Liang, C.; Huang, J.; Luo, Z. Time-restricted release of multiple neurotrophic factors promotes axonal regeneration and functional recovery after peripheral nerve injury. FASEB J. 2019, 33, 8600–8613. [Google Scholar] [CrossRef]
- Markus, A.; Patel, T.D.; Snider, W.D. Neurotrophic factors and axonal growth. Curr. Opin. Neurobiol. 2002, 12, 523–531. [Google Scholar] [CrossRef]
- Giobbe, G.G.; Michielin, F.; Luni, C.; Giulitti, S.; Martewicz, S.; Dupont, S.; Floreani, A.; Elvassore, N. Functional differentiation of human pluripotent stem cells on a chip. Nat. Methods 2015, 12, 637–640. [Google Scholar] [CrossRef]
- Luo, C.; Lancaster, M.A.; Castanon, R.; Nery, J.R.; Knoblich, J.A.; Ecker, J.R. Cerebral Organoids Recapitulate Epigenomic Signatures of the Human Fetal Brain. Cell Rep. 2016, 17, 3369–3384. [Google Scholar] [CrossRef]
- Camp, J.G.; Badsha, F.; Florio, M.; Kanton, S.; Gerber, T.; Wilsch-Bräuninger, M.; Lewitus, E.; Sykes, A.; Hevers, W.; Lancaster, M.; et al. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc. Natl. Acad. Sci. USA 2015, 112, 15672–15677. [Google Scholar] [CrossRef]
- Rolland, J.P.; Maynor, B.W.; Euliss, L.E.; Exner, A.E.; Denison, G.M.; DeSimone, J.M. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J. Am. Chem. Soc. 2005, 127, 10096–10100. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.Y.; Utada, A.S.; Shah, R.K.; Kim, J.W.; Weitz, D.A. Controllable Monodisperse Multiple Emulsions. Angew. Chem., Int. Ed. 2007, 46, 8970–8974. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Cheng, Y.; Zhao, Y. Emerging Droplet Microfluidics. Chem. Rev. 2017, 117, 7964–8040. [Google Scholar] [CrossRef] [PubMed]
- Gonidec, M.; Puigmartí-Luis, J. Continuous-versus segmented-flow microfluidic synthesis in materials science. Crystals 2019, 9, 12. [Google Scholar] [CrossRef]
- Casadevall i Solvas, X.; de Mello, A. Droplet Microfluidics: Recent Developments and Future Applications. Chem. Commun. 2011, 47, 1936–1942. [Google Scholar] [CrossRef]
- Tahara, Y.; Mukai, S.A.; Sawada, S.I.; Sasaki, Y.; Akiyoshi, K. Nanocarrier-Integrated Microspheres: Nanogel Tectonic Engineering for Advanced Drug-Delivery Systems. Adv. Mater. 2015, 27, 5080–5088. [Google Scholar] [CrossRef]
- Liu, H.W.; Wen, W.S.; Hu, M.; Bi, W.T.; Chen, L.J.; Liu, S.X.; Chen, P.; Tan, X.Y. Chitosan conduits combined with nerve growth factor microspheres repair facial nerve defects. Neural Regen. Res. 2013, 8, 3139–3147. [Google Scholar]
- Kittel, Y.; Kuehne, A.J.C.; De Laporte, L. Translating Therapeutic Microgels into Clinical Applications. Adv. Healthc. Mater. 2022, 11, e2101989. [Google Scholar] [CrossRef]
- Velasco, D.; Tumarkin, E.; Kumacheva, E. Microfluidic encapsulation of cells in polymer microgels. Small 2012, 8, 1633–1642. [Google Scholar] [CrossRef]



| Type of Device | Application to the Nervous System | References |
|---|---|---|
| Polymer-based microfluidic devices | Chemical injury models of axons | [82,83] |
| Mechanical injury models of axons | [84,85,86] | |
| Models of Alzheimer’s disease | [87,88] | |
| Models of Parkinson’s disease | [89] | |
| Models of amyotrophic lateral sclerosis | [90] | |
| Study of neurite guidance | [91] | |
| Inducing pluripotent stem cells toward neural fate | [92] | |
| Inducing the proliferation and differentiation of neural stem cells | [93] | |
| Induced stem cell-derived neuron-like cells and Schwann cell-like cells | [94] | |
| Study of the signaling networks of CNS | [95] | |
| Engineering stem cell-derived 3D brain organoids | [96] | |
| Brain permeability studies | [97,98] | |
| Capillary-based microfluidic devices | Guiding network formation of neural cells | [99] |
| Promoting the functional recovery of an injured spinal cord | [100] | |
| Enhanced therapeutic microRNA loading for CNS diseases | [101] | |
| Accelerating axonal regeneration | [102] | |
| Long-term tracking and repair of PNI | [103] | |
| 3D-printed devices | Constructing 3D tissue models and guiding self-organization | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Jiang, Z.; Lu, L.; Liu, Y. Microfluidic Manipulation for Biomedical Applications in the Central and Peripheral Nervous Systems. Pharmaceutics 2023, 15, 210. https://doi.org/10.3390/pharmaceutics15010210
Li Z, Jiang Z, Lu L, Liu Y. Microfluidic Manipulation for Biomedical Applications in the Central and Peripheral Nervous Systems. Pharmaceutics. 2023; 15(1):210. https://doi.org/10.3390/pharmaceutics15010210
Chicago/Turabian StyleLi, Zhenghang, Zhenmin Jiang, Laijin Lu, and Yang Liu. 2023. "Microfluidic Manipulation for Biomedical Applications in the Central and Peripheral Nervous Systems" Pharmaceutics 15, no. 1: 210. https://doi.org/10.3390/pharmaceutics15010210
APA StyleLi, Z., Jiang, Z., Lu, L., & Liu, Y. (2023). Microfluidic Manipulation for Biomedical Applications in the Central and Peripheral Nervous Systems. Pharmaceutics, 15(1), 210. https://doi.org/10.3390/pharmaceutics15010210





