Copaiba Oil-Loaded Polymeric Nanocapsules: Production and In Vitro Biosafety Evaluation on Lung Cells as a Pre-Formulation Step to Produce Phytotherapeutic Medicine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Copaiba Oil Chemical Characterization
2.2.1. Gas Chromatography—Mass Spectroscopy (GC-MS)
2.2.2. Fourier-Transform Infrared Spectroscopy (FTIR)
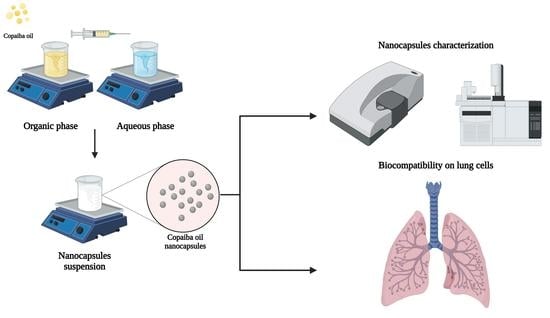
2.3. Copaiba Oil Nanocapsules Production
2.4. Physicochemical Characterization and Stability Evaluation of the Nanocapsules
2.5. Biocompatibility Assessment of Cop and CopNc
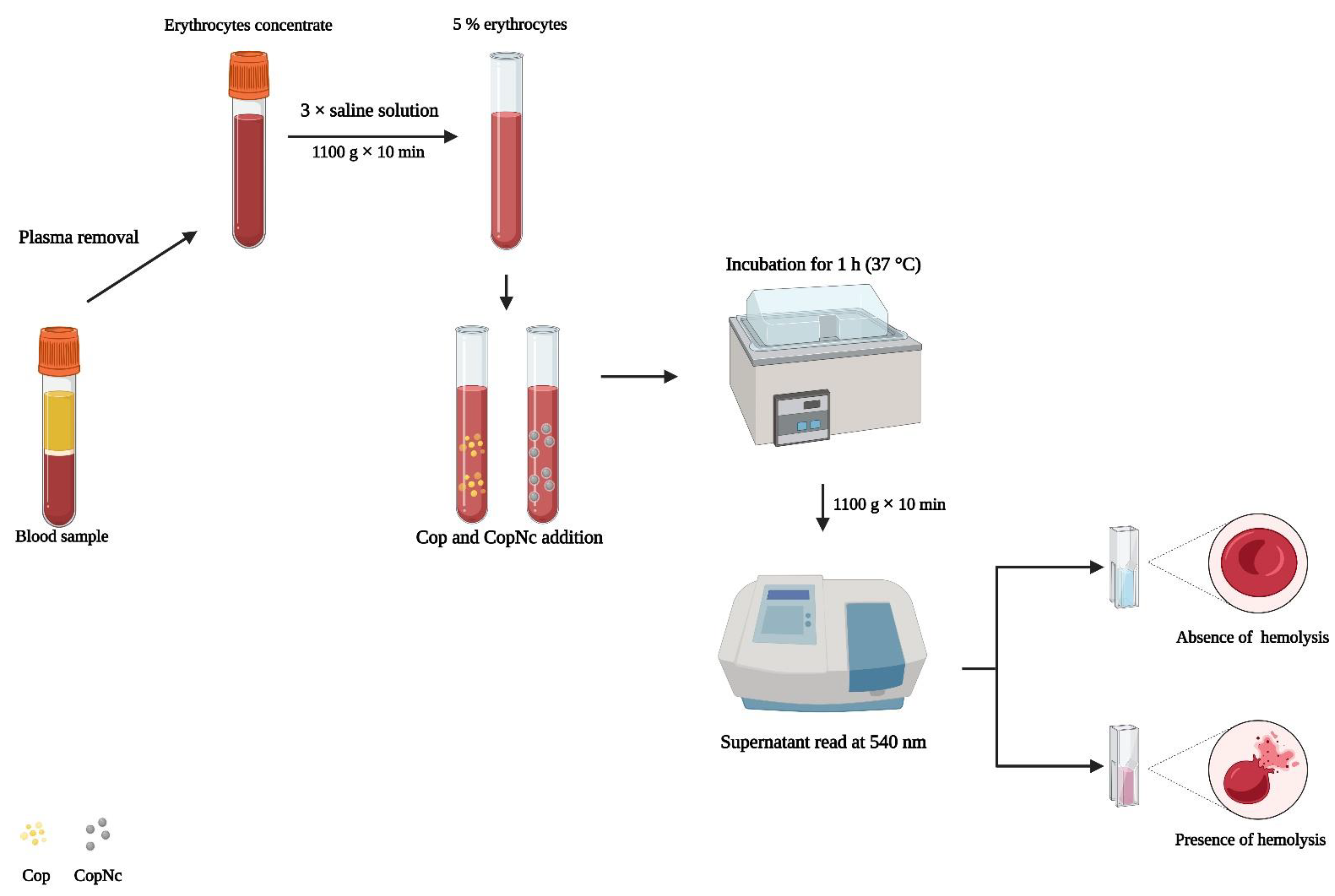
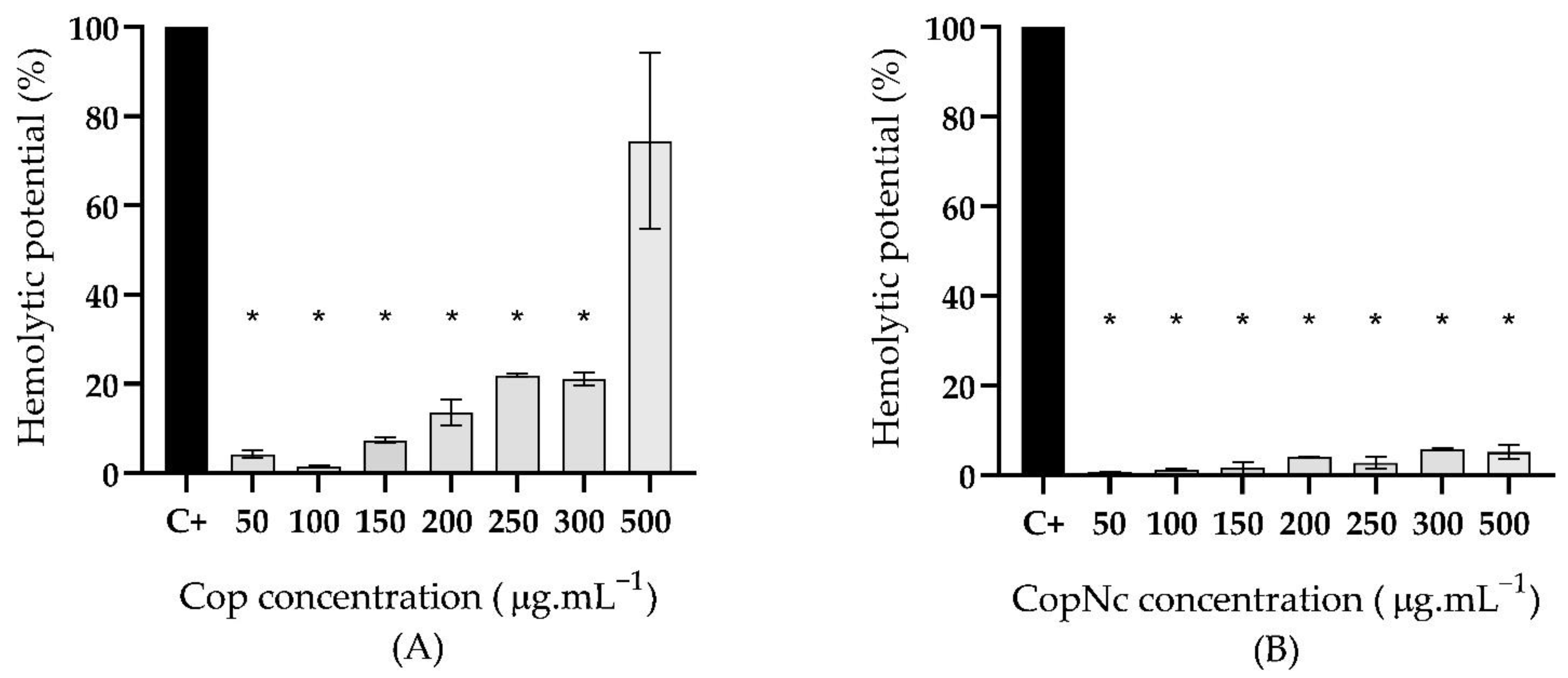
2.5.1. Hemolytic Potential Evaluation
2.5.2. Evaluation of MTT Reduction by Mitochondria Enzymes
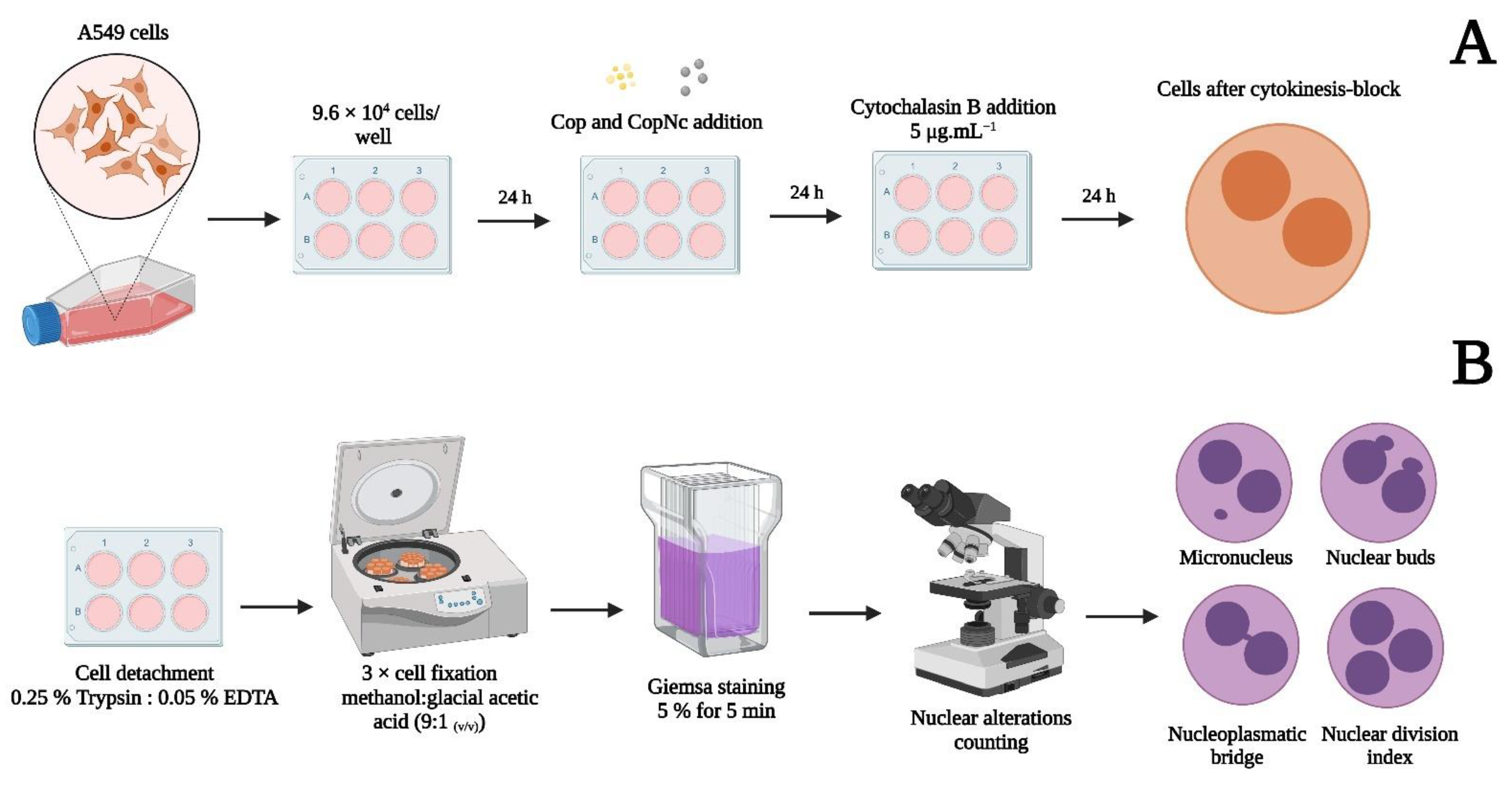
2.5.3. Cytokinesis Block Micronucleus Assay (CBMN)
2.6. Statistical Analyses
3. Results and Discussion
3.1. Copaiba Oil Chemical Characterization
3.2. Copaiba Oil Nanocapsules—Production and Physicochemical Characterization
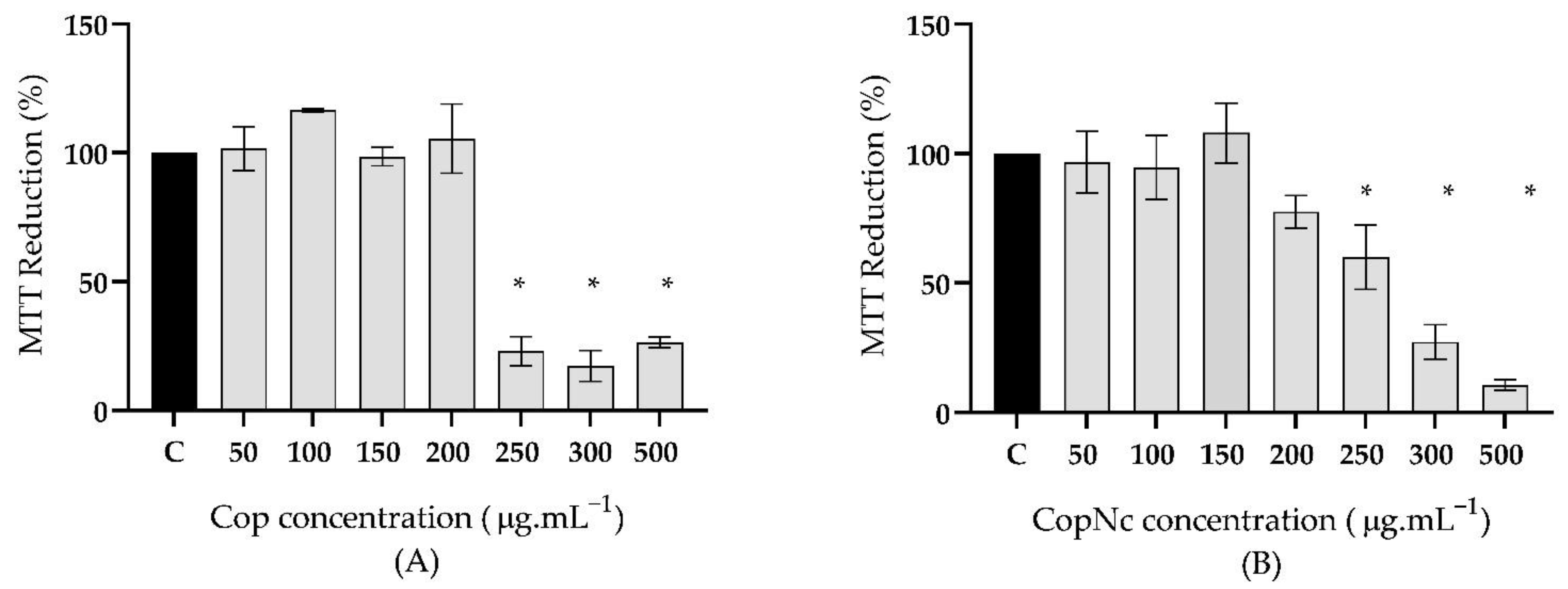
3.3. Biocompatibility Assessment of Cop and CopNc
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leandro, L.M.; Vargas, F.S.; Barbosa, P.C.; Neves, J.K.; Silva, J.A.; Veiga, V.F., Jr. Chemistry and biological activities of terpenoids from copaiba (Copaifera spp.) oleoresins. Molecules 2012, 17, 3866–3889. [Google Scholar] [CrossRef] [PubMed]
- Masson, D.S.; Salvador, S.L.; Polizello, A.C.M.; Frade, M.A.C. Antimicrobial activity of copaíba (Copaifera langsdorffii) oleoresin on bacteria of clinical significance in cutaneous wounds. Rev. Bras. Plantas Med. 2013, 15, 664–669. [Google Scholar] [CrossRef]
- Gomes, N.M.; Rezende, C.M.; Fontes, S.P.; Matheus, M.E.; Fernandes, P.D. Antinociceptive activity of amazonian copaiba oils. J. Ethnopharmacol. 2007, 109, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.C.S.; Alves, L.D.B.; Goldemberg, D.C.; Melo, A.C.; Antunes, H.S. Anti-inflammatory and wound healing effect of Copaiba oleoresin on the oral cavity: A systematic review. Heliyon 2022, 8, e08993. [Google Scholar] [CrossRef]
- Tobouti, P.L.; Andrade-Martins, T.C.; Pereira, T.J.; Mussi, M.C.M. Antimicrobial activity of copaiba oil: A review and a call for further research. Biomed. Pharmacother. 2017, 94, 93–99. [Google Scholar] [CrossRef]
- Paiva, L.A.F.; Rao, V.S.N.; Gramosa, N.V.; Silveira, E.R. Gastroprotective effect of Copaifera langsdorffii oleo-resin on experimental gastric ulcer models in rats. J. Ethnopharmacol. 1998, 62, 73–78. [Google Scholar] [CrossRef]
- Lima, L.R.; Andrade, F.K.; Alves, D.R.; Morais, S.M.; Vieira, R.S. Anti-acetylcholinesterase and toxicity against Artemia salina of chitosan microparticles loaded with essential oils of Cymbopogon flexuosus, Pelargonium x ssp and Copaifera officinalis. Int. J. Biol. Macromol. 2021, 167, 1361–1370. [Google Scholar] [CrossRef]
- Rutckeviski, R.; Xavier-Jr, F.H.; Morais, A.R.V.; Amaral-Machado, L.; Alencar, E.N.; Genre, J.; Araujo, A.A.S.; Egito, E.S.T. Therapeutic bullfrog oil-based nanoemulsion for oral application: Development, characterization and stability. Acta Pharm. 2019, 69, 33–48. [Google Scholar] [CrossRef]
- Oliveira, W.N.; Alencar, E.N.; Rocha, H.A.O.; Amaral-Machado, L.; Egito, E.S.T. Nanostructured systems increase the in vitro cytotoxic effect of bullfrog oil in human melanoma cells (A2058). Biomed. Pharmacother. 2022, 145, 112438. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Edalat, F.; Raymond, K.; Khademhosseini, A.; Chen, P. Biocompatibility of engineered nanoparticles for drug delivery. J. Control. Release 2013, 166, 182–194. [Google Scholar] [CrossRef]
- Nair, A.; Mallya, R.; Suvarna, V.; Khan, T.A.; Momin, M.; Omri, A. Nanoparticles-attractive carriers of antimicrobial essential oils. Antibiotics 2022, 11, 108. [Google Scholar] [CrossRef]
- Filho, G.P.C.; Sousa, A.F.G.; Câmara, R.B.G.; Rocha, H.A.O.; Medeiros, S.R.B.; Moreira, S.M.G. Genotoxicity and osteogenic potential of sulfated polysaccharides from Caulerpa prolifera seaweed. Int. J. Biol. Macromol. 2018, 114, 565–571. [Google Scholar] [CrossRef] [PubMed]
- OECD. Guideline for the Testing of Chemicals: Test No. 487: In Vitro Mammalian Cell Micronucleus Test; Organization for Economic Co-operation Development: Paris, France, 2016. [Google Scholar]
- Cavalcanti, B.C.; Costa-lotufo, L.V.; Moraes, M.O.; Burbano, R.R.; Silveira, E.R.; Cunha, K.M.A.; Rao, V.S.N.; Moura, D.J.; Rosa, R.M.; Henriques, J.A.P.; et al. Genotoxicity evaluation of kaurenoic acid, a bioactive diterpenoid present in Copaiba oil. Food Chem. Toxicol. 2006, 44, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Xavier, F.H., Jr.; Maciuk, A.; Rochelle, V.M.A.; Alencar, E.D.N.; Garcia, V.L.; Egito, E.S.T.; Vauthier, C. Development of a gas chromatography method for the analysis of copaiba oil. J. Chromatogr. Sci. 2017, 55, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Oliveira, W.N.; Amaral-Machado, L.; Alencar, E.N.; Marcelino, H.R.; Genre, J.; Silva-Rocha, W.P.; Gondim, A.D.; Chaves, G.M.; Fernandes-Pedrosa, M.F.; Egito, E.S.T. Getting the jump on the development of bullfrog oil microemulsions: A nanocarrier for amphotericin B intended for antifungal treatment. AAPS PharmSciTech 2018, 19, 2585–2597. [Google Scholar] [CrossRef]
- Fenech, M. Cytokinesis-block micronucleus cytome assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef]
- Wesołowska, A.; Jadczak, P.; Kulpa, D.; Przewodowski, W. Gas chromatography-mass spectrometry (GC-MS) analysis of essential oils from AgNPs and AuNPs elicited Lavandula angustifolia in vitro cultures. Molecules 2019, 24, 606. [Google Scholar] [CrossRef] [PubMed]
- Trindade, R.; Silva, J.K.; Setzer, W.N. Copaifera of the neotropics: A review of the phytochemistry and pharmacology. Int. J. Mol. Sci. 2018, 19, 1511. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.S.; Fontes, L.B.A.; Crotti, A.E.M.; Aarestrup, B.J.V.; Aarestrup, F.M.; Silva, F.A.A.; Corrêa, J.O.A. Copaiba oil suppresses inflammatory cytokines in splenocytes of C57Bl/6 mice induced with experimental autoimmune encephalomyelitis (EAE). Molecules 2014, 19, 12814–12826. [Google Scholar] [CrossRef]
- Cascon, V.; Gilbert, B. Characterization of the chemical composition of oleoresins of Copaifera guianensis Desf., Copaifera duckei Dwyer and Copaifera multijuga Hayne. Phytochemistry 2000, 55, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, D.R.C.; Cabral-Albuquerque, E.C.M.; Velozo, E.S.; Sousa, H.C.; Melo, S.A.B.V.; Braga, M.E.M. Copaiba oil-loaded commercial wound dressings using supercritical CO2: A potential alternative topical antileishmanial treatment. J. Supercrit. Fluids 2017, 129, 106–115. [Google Scholar] [CrossRef]
- Alencar, E.N.; Xavier, F.H., Jr.; Morais, A.R.V.; Dantas, T.R.F.; Dantas-Santos, N.; Verissimo, L.M.; Rehder, V.L.G.; Chaves, G.M.; Oliveira, A.G.; Egito, E.S.T. Chemical characterization and antimicrobial activity evaluation of natural oil nanostructured emulsions. J. Nanosci. Nanotechnol. 2015, 15, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Caputo, L.S.; Campos, M.I.C.; Dias, H.J.; Crotti, A.E.M.; Fajardo, J.B.; Vanelli, C.P.; Presto, A.C.D.; Alves, M.S.; Aarestrup, F.M.; Paula, A.C.C.; et al. Copaiba oil suppresses inflammation in asthmatic lungs of BALB/c mice induced with ovalbumin. Int. Immunopharmacol. 2020, 80, 106177. [Google Scholar] [CrossRef] [PubMed]
- Ghizoni, C.V.C.; Ames, A.P.A.; Lameira, O.A.; Amado, C.A.B.; Nakanishi, A.B.S.; Bracht, L.; Natali, M.R.M.; Peralta, R.M.; Bracht, A.; Comar, J.F. Anti-inflammatory and antioxidant actions of copaiba oil are related to liver cell modifications in arthritic rats. J. Cell Biochem. 2017, 118, 3409–3423. [Google Scholar] [CrossRef]
- Souza, T.; Olenka, L.; Peternella, W. A study of degradation in vegetable oils by exposure to sunlight using fourier transform infrared spectroscopy. Mater. Sci. Appl. 2020, 11, 678–691. [Google Scholar] [CrossRef]
- Amaral-Machado, L.; Oliveira, W.N.; Alencar, E.N.; Cruz, A.K.M.; Rocha, H.A.O.; Ebeid, K.; Salem, A.K.; Egito, E.S.T. Bullfrog oil (Rana catesbeiana Shaw) induces apoptosis, in A2058 human melanoma cells by mitochondrial dysfunction triggered by oxidative stress. Biomed. Pharmacother. 2019, 117, 1–9. [Google Scholar] [CrossRef]
- Barbosa, P.C.S.; Wiedemann, L.S.M.; Medeiros, R.S.; Sampaio, P.T.B.; Vieira, G.; Veiga-Junior, V.F. Phytochemical fingerprints of copaiba oils (Copaifera multijuga Hayne) determined by multivariate analysis. Chem. Biodivers. 2013, 10, 1350–1360. [Google Scholar] [CrossRef]
- Santiago, K.B.; Conti, B.J.; Andrade, B.F.M.T.; Silva, J.J.M.; Rogez, H.L.G.; Crevelin, E.J.; Moraes, L.A.B.; Veneziani, R.; Ambrósio, S.R.; Bastos, J.K.; et al. Immunomodulatory action of Copaifera spp oleoresins on cytokine production by human monocytes. Biomed. Pharmacother. 2015, 70, 12–18. [Google Scholar] [CrossRef]
- Amaral-Machado, L.; Oliveira, W.N.; Torres-Rêgo, M.; Furtado, A.A.; Alencar, E.N.; Fernandes-Pedrosa, M.F.; Rocha, H.A.O.; Egito, E.S.T. Anti-inflammatory activity of bullfrog oil polymeric nanocapsules: From the design to preclinical trials. Int. J. Nanomed. 2021, 16, 7353–7367. [Google Scholar] [CrossRef]
- Preisler, A.C.; Guariz, H.R.; Carvalho, L.B.; Pereira, A.E.S.; Oliveira, J.L.; Fraceto, L.F.; Dalazen, G.; Oliveira, H.C. Phytotoxicity evaluation of poly (ε-caprolactone) nanocapsules prepared using different methods and compositions in Brassica juncea seeds. Plant Nano Biol. 2022, 1, 100003. [Google Scholar] [CrossRef]
- Xavier, F.H., Jr.; Egito, E.S.T.; Morais, A.R.V.; Alencar, E.N.; Maciuk, A.; Vauthier, C. Experimental design approach applied to the development of chitosan coated poly(isobutylcyanoacrylate) nanocapsules encapsulating copaiba oil. Colloids Surf. A Physicochem. Eng. Asp. 2018, 536, 251–258. [Google Scholar] [CrossRef]
- Pramod, K.; Suneesh, C.V.; Shanavas, S.; Ansari, S.H.; Ali, J. Unveiling the compatibility of eugenol with formulation excipients by systematic drug-excipient compatibility studies. J. Anal. Sci. Technol. 2015, 6, 34. [Google Scholar] [CrossRef]
- Farmoudeh, A.; Akbari, J.; Saeedi, M.; Ghasemi, M.; Asemi, N.; Nokhodchi, A. Methylene blue-loaded niosome: Preparation, physicochemical characterization, and in vivo wound healing assessment. Drug Deliv. Transl. Res. 2020, 10, 1428–1441. [Google Scholar] [CrossRef]
- Andrade, L.L.; Pereira, A.E.S.; Fraceto, L.F.; Martinez, C.B.R. Can atrazine loaded nanocapsules reduce the toxic effects of this herbicide on the fish Prochilodus lineatus? A multibiomarker approach. Sci. Total Environ. 2019, 663, 548–559. [Google Scholar] [CrossRef]
- Camargo, G.A.; Ferreira, L.; Schebelski, D.J.; Lyra, A.M.; Barboza, F.M.; Carletto, B.; Koga, A.Y.; Semianko, B.C.; Dias, D.T.; Lipinski, L.C.; et al. Characterization and in vitro and in vivo evaluation of tacrolimus-loaded poly(ε-caprolactone) nanocapsules for the management of atopic dermatitis. Pharmaceutics 2021, 13, 2013. [Google Scholar] [CrossRef] [PubMed]
- Łukasiewicz, S.; Mikołajczyk, A.; Błasiak, E.; Fic, E.; Dziedzicka-Wasylewska, M. Polycaprolactone nanoparticles as promising candidates for nanocarriers in novel nanomedicines. Pharmaceutics 2021, 13, 191. [Google Scholar] [CrossRef]
- Abriata, J.P.; Turatti, R.C.; Luiz, M.T.; Raspantini, G.L.; Tofani, L.B.; Amaral, R.L.F.; Swiech, K.; Marcato, P.D.; Marchetti, J.M. Development, characterization and biological in vitro assays of paclitaxel-loaded PCL polymeric nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 347–355. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Fan, Y.; Feng, Q.; Cui, F. Biocompatibility and toxicity of nanoparticles and nanotubes. J. Nanomater. 2012, 2012, 548389. [Google Scholar] [CrossRef]
- Paur, H.; Cassee, F.R.; Teeguarden, J.; Fissan, H.; Diabate, S.; Aufderheide, M.; Kreyling, W.G.; Hänninen, O.; Kasper, G.; Riediker, M.; et al. In-vitro cell exposure studies for the assessment of nanoparticle toxicity in the lung—A dialog between aerosol science and biology. J. Aerosol. Sci. 2011, 42, 668–692. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Persp. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Tsapis, N.; Bennett, D.; Jackson, B.; Weitz, D.A.; Edwards, D.A. Trojan particles: Large porous carriers of nanoparticles for drug delivery. Proc. Natl. Acad. Sci. USA 2002, 99, 12001–12005. [Google Scholar] [CrossRef]
- Fischer, T.; Tschernig, T.; Drews, F.; Brix, K.; Meier, C.; Simon, M.; Kautenburger, R.; Schneider, M. siRNA delivery to macrophages using aspherical, nanostructured microparticles as delivery system for pulmonary administration. Eur. J. Pharm. Biophar. 2021, 158, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Khayata, N.; Abdelwahed, W.; Chehna, M.F.; Charcosset, C.; Fessi, H. Preparation of vitamin E loaded nanocapsules by the nanoprecipitation method: From laboratory scale to large scale using a membrane contactor. Int. J. Pharm. 2012, 423, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Byun, Y.; Hwang, J.B.; Bang, S.H.; Darby, D.; Cooksey, K.; Dawson, P.L.; Park, H.J.; Whiteside, S. Formulation and characterization of α-tocopherol loaded poly ε-caprolactone (PCL) nanoparticles. LWT-Food Sci. Technol. 2011, 44, 24–28. [Google Scholar] [CrossRef]
- Paranjpe, M.; Müller-Goymann, C.C. Nanoparticle-mediated pulmonary drug delivery: A review. Int. J. Mol. Sci. 2014, 15, 5852–5873. [Google Scholar] [CrossRef]
- Lee, D.; Powers, K.; Baney, R. Physicochemical properties and blood compatibility of acylated chitosan nanoparticles. Carbohydr. Polym. 2004, 58, 371–377. [Google Scholar] [CrossRef]
- Izumi, E.; Ueda-Nakamura, T.; Veiga, V.F.; Pinto, A.C.; Nakamura, C.V. Terpenes from Copaifera demonstrated in vitro antiparasitic and synergic activity. J. Med. Chem. 2012, 55, 2994–3001. [Google Scholar] [CrossRef]
- Vega-Avila, E.; Pugsley, M.K. An overview of colorimetric assay methods used to assess survival or proliferation of mammalian cells. Proc. West Pharmacol. Soc. 2011, 54, 10–14. [Google Scholar]
- Sakagami, M. In vivo, in vitro and ex vivo models to assess pulmonary absorption and disposition of inhaled therapeutics for systemic delivery. Adv. Drug Deliv. Rev. 2006, 58, 1030–1060. [Google Scholar] [CrossRef] [PubMed]
- Veiga-Junior, V.F.; Rosas, E.C.; Carvalho, M.V.; Henriques, M.G.; Pinto, A.C. Chemical composition and anti-inflammatory activity of copaiba oils from Copaifera cearensis Huber ex Ducke, Copaifera reticulata Ducke and Copaifera multijuga Hayne—A comparative study. J. Ethnopharmacol. 2007, 112, 248–254. [Google Scholar] [CrossRef]
- Nigro, F.; Cerqueira, C.; Rossi, A.; Cardoso, V.; Vermelho, A.B.; Ricci, E., Jr.; Santos, E.P.; Mansur, C.R.E. Development, characterization and in vitro toxicity evaluation of nanoemulsion-loaded hydrogel based on copaiba oil and coenzyme Q10. Colloid Surf. A Physicochem. Eng. Asp. 2020, 586, 124132. [Google Scholar] [CrossRef]
- Moras, M.; Lefevre, S.D.; Ostuni, M.A. From erythroblasts to mature red blood cells: Organelle clearance in mammals. Front. Physiol. 2017, 8, 1076. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M.; Kirsch-Volders, M.; Natarajan, A.T.; Surralles, J.; Crott, J.W.; Parry, J.; Norppa, H.; Eastmond, D.A.; Tucker, J.D.; Thomas, P. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis 2011, 26, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.R.; Darin, J.D.; Hernandes, L.C.; Souza-Ramos, M.F.; Antunes, L.M.; Freitas, O. Genotoxicity assessment of Copaiba oil and its fractions in Swiss mice. Genet. Mol. Biol. 2012, 35, 664–672. [Google Scholar] [CrossRef]
- Kapustová, M.; Puškárová, A.; Bučková, M.; Granata, G.; Napoli, E.; Annušová, A.; Mesárošová, M.; Kozics, K.; Pangallo, D.; Geraci, C. Biofilm inhibition by biocompatible poly(ε-caprolactone) nanocapsules loaded with essential oils and their cyto/genotoxicity to human keratinocyte cell line. Int. J. Pharm. 2021, 606, 120846. [Google Scholar] [CrossRef]
- Dalcin, A.J.F.; Vizzotto, B.S.; Bochi, G.V.; Guarda, N.S.; Nascimento, K.; Sagrillo, M.R.; Moresco, R.N.; Schuch, A.P.; Ourique, A.F.; Gomes, P. Nanoencapsulation of the flavonoid dihydromyricetin protects against the genotoxicity and cytotoxicity induced by cationic nanocapsules. Colloids Surf. B Biointerfaces 2019, 173, 798–805. [Google Scholar] [CrossRef]



| Compound | SiCop Area (%) | SiCop RT (min) | Cop Area (%) | Cop RT (min) |
|---|---|---|---|---|
| (-)-beta-Santalene | - | - | 0.2 | 24.9 |
| (-)-Cyperene | - | - | 0.3 | 23.0 |
| (-)-Isocaryophyllene | - | - | 3.6 | 26.4 |
| (-)-α-Panasinsen | 0.1 | 24.5 | - | - |
| (+)-b-Funebrene | - | - | 0.2 | 24.8 |
| I-β-Famesene | 2.1 | 22.9 | - | - |
| (E)-β-Famesene | - | - | 0.9 | 25.4 |
| (E)-γ-Bisabolene | 0.1 | 24.7 | - | - |
| (Z)-Oleic acid | 3.2 | 38.6 | - | - |
| 6,9-Guaiadene | 0.4 | 22.6 | - | - |
| Annonene | 0.2 | 37.0 | - | - |
| Caryophyllene oxide | 0.7 | 26.0 | 4.8 | 30.2 |
| cis-(-)-ª,4a,5,ª,9a-Hexahydro-3,5,5,9-tetramethyl(1H)benzoc | - | - | 0.3 | 32.6 |
| cis-α-Bergamotene | - | - | 23.1 | 24.5 |
| cis-α-Bisabolene | 2.5 | 24.0 | 1.1 | 27.2 |
| Copaene | 0.2 | 20.8 | 0.2 | 22.0 |
| Cyclosativene | 0.9 | 20.6 | - | - |
| Humulene | - | - | 0.7 | 25.2 |
| Humulene epoxide II | - | - | 1.2 | 31.2 |
| Kaur-16-en-18-al, (4α)- | 3.3 | 39.5 | - | - |
| Lanceol, cis | 0.1 | 26.8 | - | - |
| Limona ketone | - | - | 0.1 | 11.9 |
| Linoleic acid | 4.8 | 38.5 | - | - |
| Methyl kolavenate | 0.3 | 41.0 | - | - |
| ni | - | - | 0.7 | 32.9 |
| ni | - | - | 0.7 | 33.5 |
| ni | - | - | 0.5 | 31.5 |
| ni | - | - | 0.3 | 31.7 |
| ni | - | - | 0.3 | 27.6 |
| ni | - | - | 0.2 | 32.2 |
| ni | 0.2 | 28.7 | - | - |
| ni | - | - | 0.2 | 34.3 |
| ni | - | - | 0.2 | 28.2 |
| ni | - | - | 0.2 | 31.4 |
| ni | - | - | 0.1 | 30.8 |
| ni | - | - | 0.1 | 31.0 |
| ni | - | - | 0.1 | 45.6 |
| ni | 0.1 | 27.3 | - | - |
| ni | 0.1 | 30.0 | - | - |
| Sesquiphellandrene | - | - | 0.8 | 28.0 |
| Sesquithujene | - | - | 0.4 | 22.7 |
| trans-α-Bisabolene | 3.8 | 25.0 | - | - |
| α-Bergamotene | 22.3 | 22.4 | 0.1 | 23.7 |
| α-Bisabolene | - | - | 1.8 | 28.7 |
| α-Bisabolol | - | - | 0.7 | 34.0 |
| α-Cyprene | 0.8 | 21.5 | - | - |
| α-Selinene | 3.6 | 23.9 | 0.4 | 26.8 |
| β-Bisabolene | 36.9 | 24.2 | 50.2 | 27.4 |
| β-Caryophyllene | 2.9 | 22.0 | 5.1 | 23.8 |
| β-Elemene | 3.1 | 21.3 | - | - |
| β-Selinene | 5.2 | 23.7 | - | - |
| β-Sesquiphellandrene | 2.0 | 24.6 | - | - |
| γ-Muurolene | - | - | 0.1 | 26.1 |
| δ-Elemene | 0.1 | 19.8 | - | - |
| Day | Size (nm) ± SD | PdI ± SD | Zeta Potential (mV) ± SD |
|---|---|---|---|
| 1 | 215 ± 10 | 0.15 ± 0.01 | −18 ± 1 |
| 5 | 216 ± 12 | 0.14 ± 0.01 | −16 ± 3 |
| 10 | 216 ± 4 | 0.17 ± 0.02 | −17 ± 1 |
| 15 | 209 ± 7 | 0.15 ± 0.03 | −21 ± 1 |
| 30 | 219 ± 4 | 0.17 ± 0.02 | −23 ± 2 |
| Treatment | MN | NPB | NBUD | NDI |
|---|---|---|---|---|
| NC | 8 ± 2.0 | 6 ± 2 | 13 ± 2 | 1.9 ± 0.01 |
| PC | 41 ± 3 * | 18 ± 4 * | 54 ± 6 * | 1.8 ± 0.10 |
| Cop | 9 ± 2 | 7 ± 2 | 17 ± 5 | 1.9 ± 0.02 |
| CopNc | 7 ± 2 | 4 ± 1 | 14 ± 4 | 1.9 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, V.M.; Oliveira, W.N.; Pereira, D.T.; Alencar, É.N.; Porto, D.L.; Aragão, C.F.S.; Moreira, S.M.G.; Rocha, H.A.O.; Amaral-Machado, L.; Egito, E.S.T. Copaiba Oil-Loaded Polymeric Nanocapsules: Production and In Vitro Biosafety Evaluation on Lung Cells as a Pre-Formulation Step to Produce Phytotherapeutic Medicine. Pharmaceutics 2023, 15, 161. https://doi.org/10.3390/pharmaceutics15010161
Rodrigues VM, Oliveira WN, Pereira DT, Alencar ÉN, Porto DL, Aragão CFS, Moreira SMG, Rocha HAO, Amaral-Machado L, Egito EST. Copaiba Oil-Loaded Polymeric Nanocapsules: Production and In Vitro Biosafety Evaluation on Lung Cells as a Pre-Formulation Step to Produce Phytotherapeutic Medicine. Pharmaceutics. 2023; 15(1):161. https://doi.org/10.3390/pharmaceutics15010161
Chicago/Turabian StyleRodrigues, Victor M., Wógenes N. Oliveira, Daniel T. Pereira, Éverton N. Alencar, Dayanne L. Porto, Cícero F. S. Aragão, Susana M. G. Moreira, Hugo A. O. Rocha, Lucas Amaral-Machado, and Eryvaldo S. T. Egito. 2023. "Copaiba Oil-Loaded Polymeric Nanocapsules: Production and In Vitro Biosafety Evaluation on Lung Cells as a Pre-Formulation Step to Produce Phytotherapeutic Medicine" Pharmaceutics 15, no. 1: 161. https://doi.org/10.3390/pharmaceutics15010161
APA StyleRodrigues, V. M., Oliveira, W. N., Pereira, D. T., Alencar, É. N., Porto, D. L., Aragão, C. F. S., Moreira, S. M. G., Rocha, H. A. O., Amaral-Machado, L., & Egito, E. S. T. (2023). Copaiba Oil-Loaded Polymeric Nanocapsules: Production and In Vitro Biosafety Evaluation on Lung Cells as a Pre-Formulation Step to Produce Phytotherapeutic Medicine. Pharmaceutics, 15(1), 161. https://doi.org/10.3390/pharmaceutics15010161