Effects of Cationic Dendrimers and Their Complexes with microRNAs on Immunocompetent Cells
Abstract
1. Introduction
2. Materials and Methods
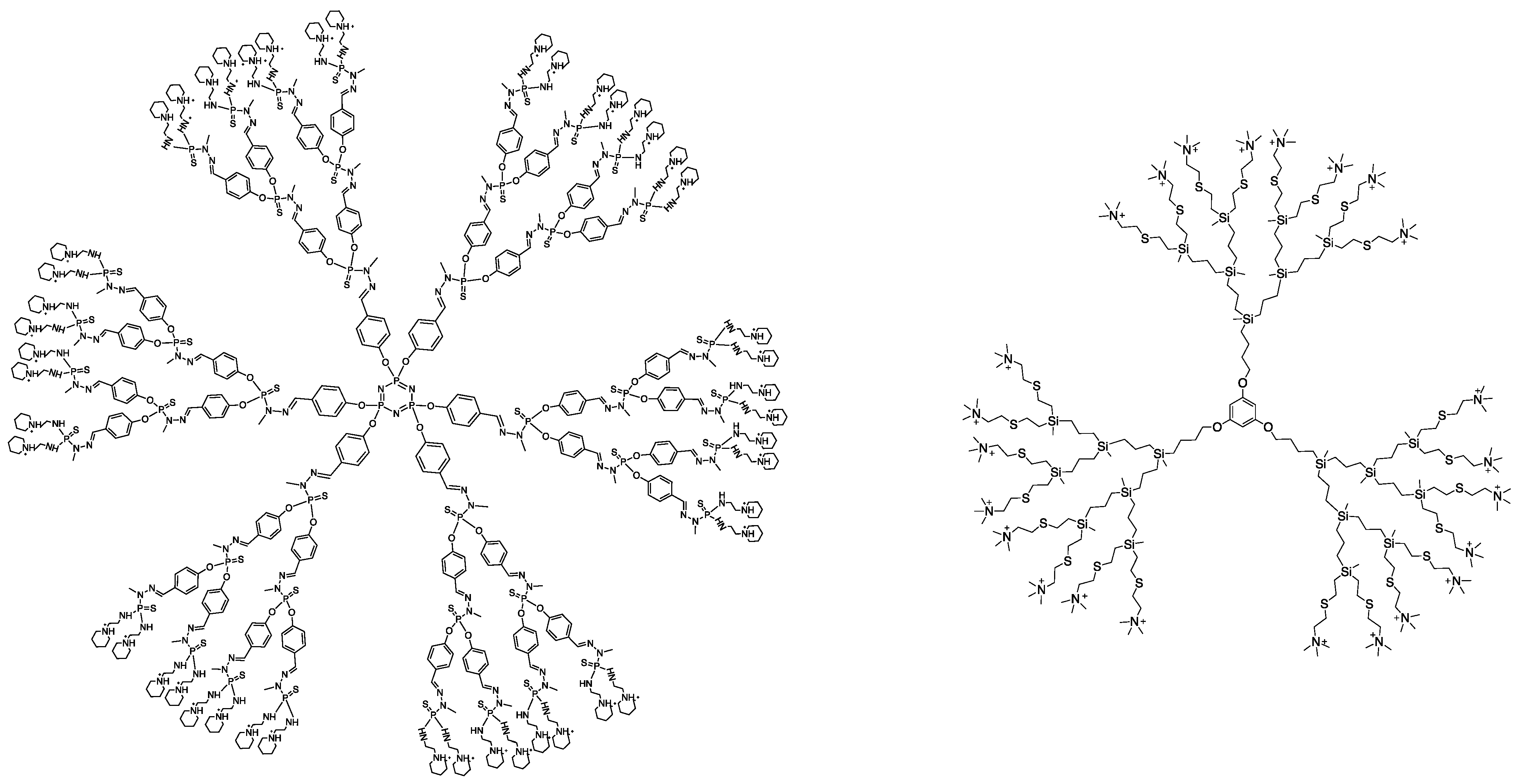
2.1. Dendrimers
2.2. microRNAs
2.3. Therapeutic Formulations
2.4. Blood Samples
2.5. Preparation of the Peripheral Blood Mononuclear Cells (PBMCs)
2.6. Internalization of miR-Containing Complexes
2.7. Fluorescence Microscopy
2.8. WST-Assay
2.9. LDH Activity Assay
2.10. Apoptosis Assay
2.11. Expression of Surface Molecules
2.12. Cell Activation and Proliferation Assay
2.13. Evaluation of Perforin and Granzyme B Production
2.14. Cytokine Secretion Analysis
2.15. Statistical Analysis
3. Results
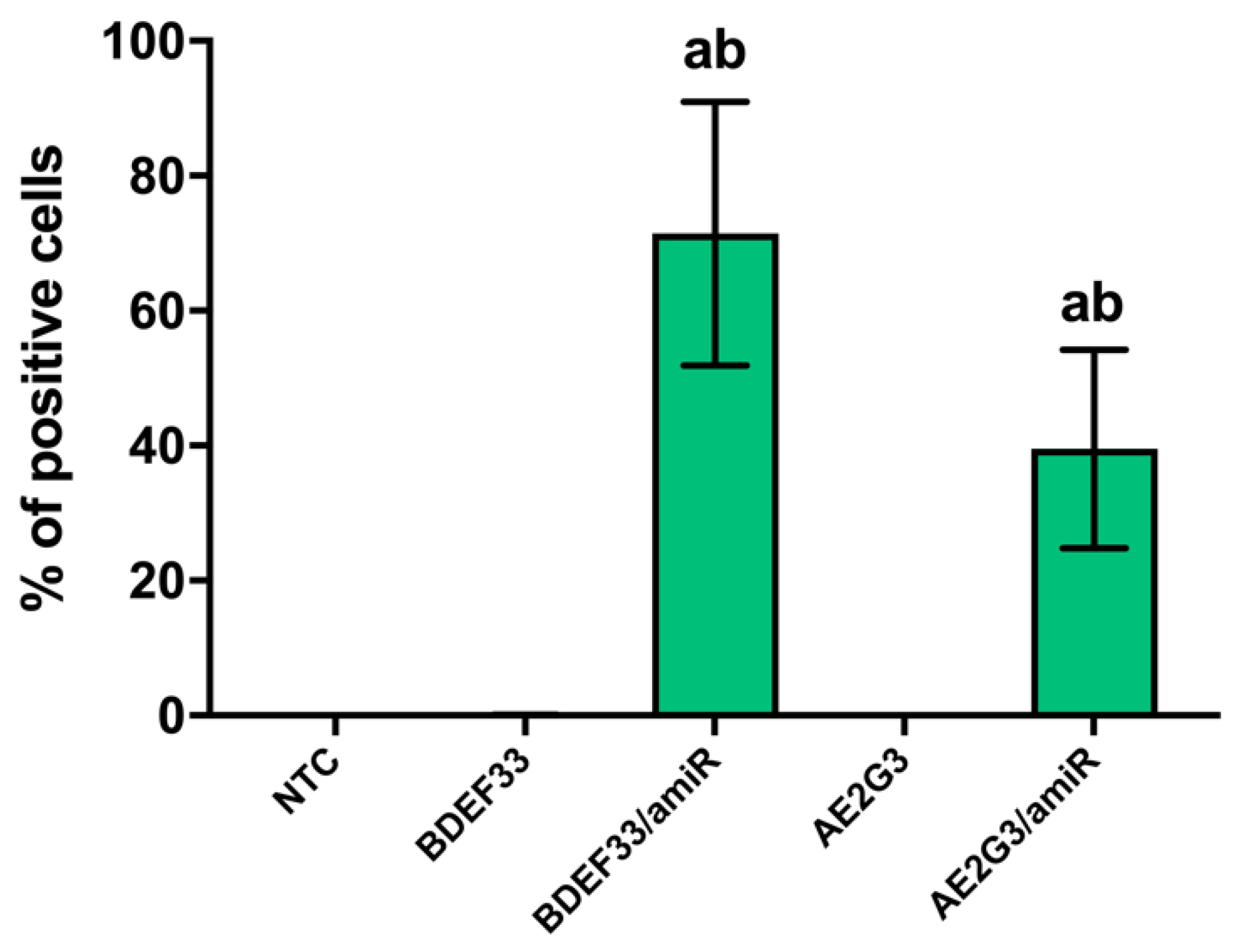
3.1. BDEF33 and AE2G3 Dendrimers Efficiently Transport microRNA into the Cells
3.2. BDEF33- and AE2G3-Based Dendriplexes Did Not Demonstrate Significant Toxicity against PBMCs in General and against T-Cell Subsets Particularly
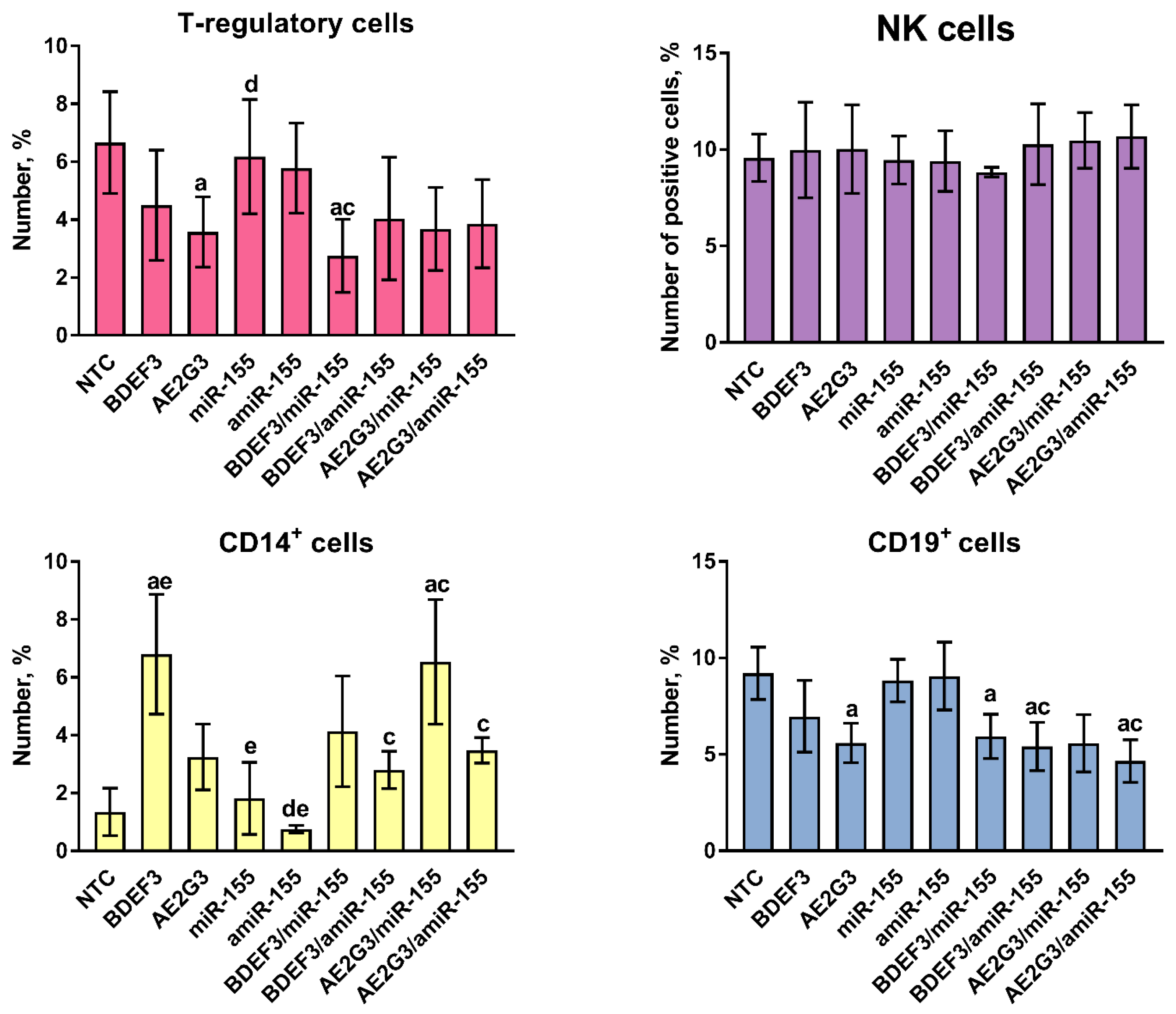
3.3. Treatment by Dendriplexes Did Not Change Neither T-Cells Subsets Ratio Nor Their Proliferative Activity, but Changes in the Number of T-Regulatory Cells, CD14+ and CD19+ Cells Were Found
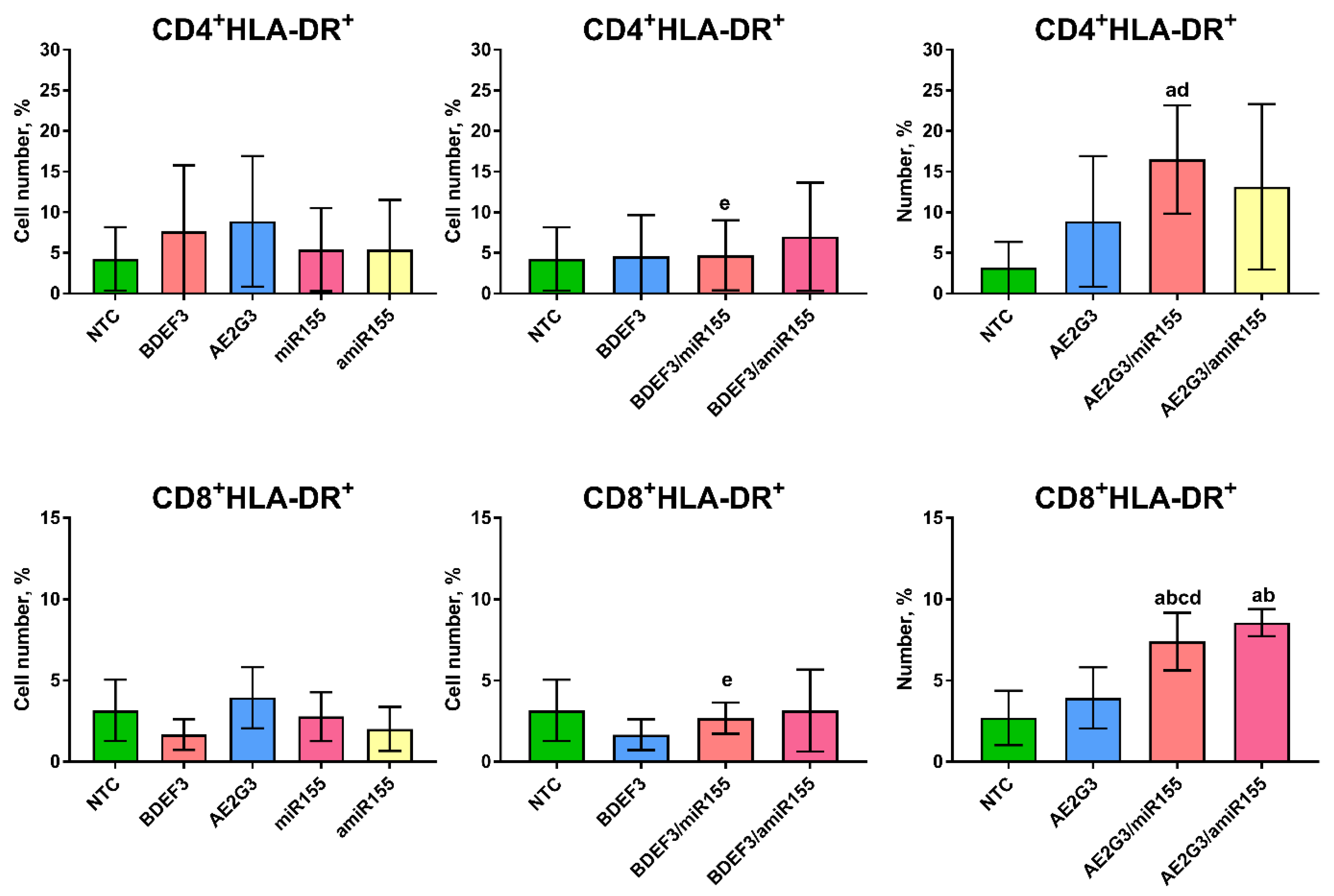
3.4. Expression of CD25+ on T-Cells Was Intact after Treatment but Changes in HLA-DR Expression Were Observed
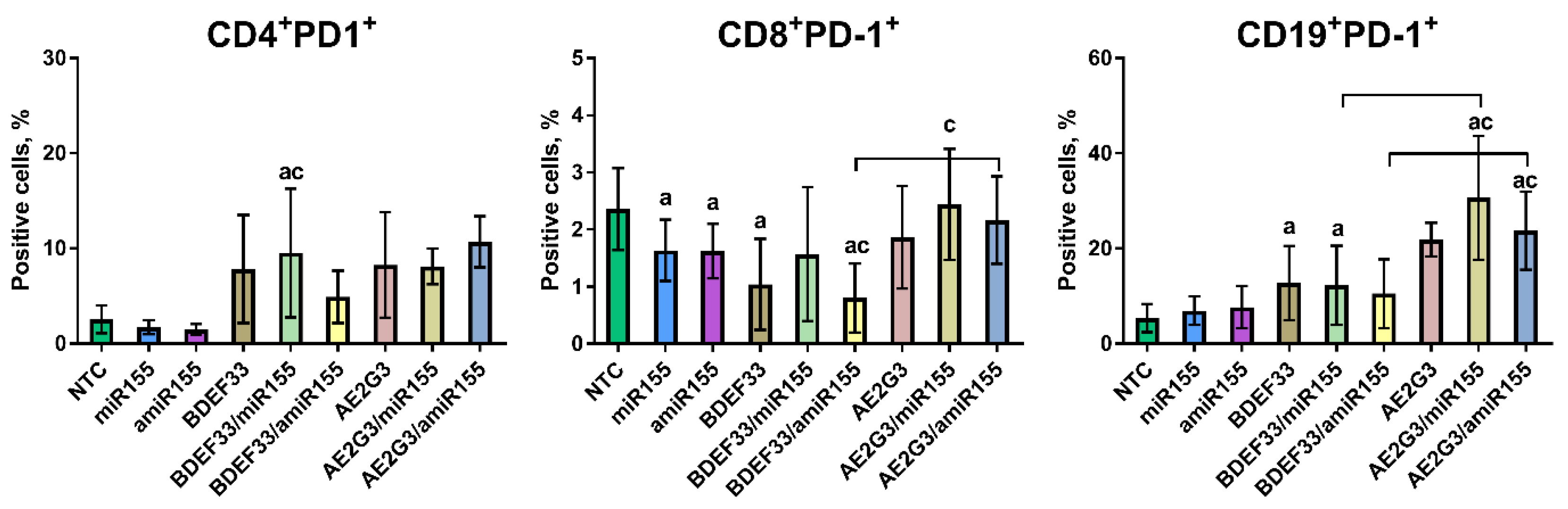
3.5. Dendriplexes and Their Components Can Change PD-1 Expression on T- and B-Lymphocytes
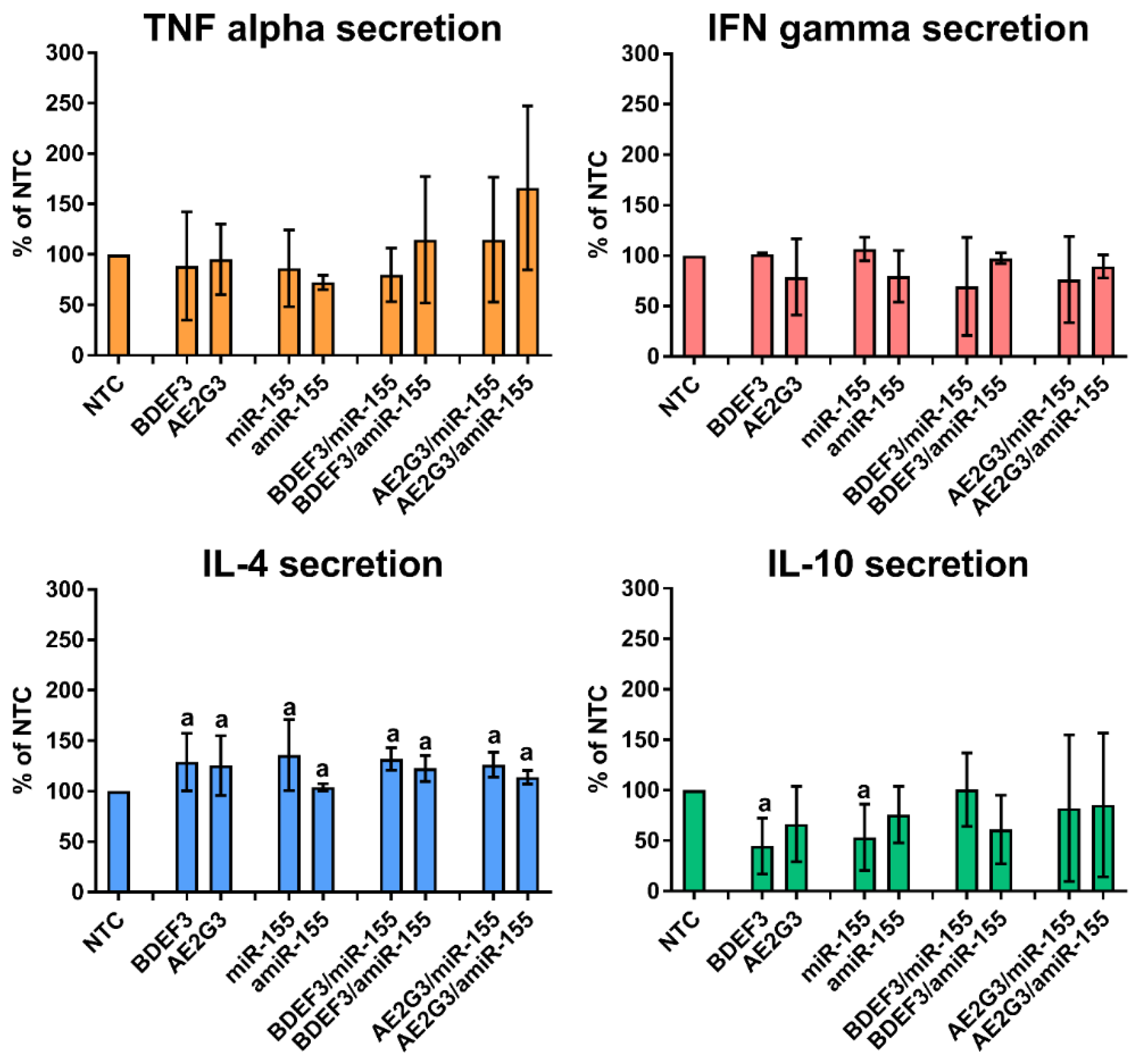
3.6. Dendriplexes Changed Production of IL-4 and IL-10, but Not the Perforin and Granzyme B Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbott, M.; Ustoyev, Y. Cancer and the Immune System: The History and Background of Immunotherapy. Semin. Oncol. Nurs. 2019, 35, 150923. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Haque, F.; Jasinski, D.L.; Binzel, D.W.; Shu, D.; Guo, P. Favorable biodistribution, specific targeting and conditional endosomal escape of RNA nanoparticles in cancer therapy. Cancer Lett. 2018, 414, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, A.J.P.; Burnett, M.E.; Prévot, G.; Klein, E.D.; Bivona, D.; Mulder, W.J.M. Embracing nanomaterials’ interactions with the innate immune system. WIREs Nanomed. Nanobiotechnol. 2021, 13, e1719. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lee, H.Y.; Choi, J.Y.; Hur, J.; Kim, I.K.; Kim, Y.K.; Kang, J.Y.; Lee, S.Y. Inhibition of MicroRNA-21 by an antagomir ameliorates allergic inflammation in a mouse model of asthma. Exp. Lung Res. 2017, 43, 109–119. [Google Scholar] [CrossRef]
- Ji, W.; Sun, B.; Su, C. Targeting microRNAs in cancer gene therapy. Genes 2017, 8, 21. [Google Scholar] [CrossRef]
- Labatut, A.E.; Mattheolabakis, G. Non-viral based miR delivery and recent developments. Eur. J. Pharm. Biopharm. 2018, 128, 82–90. [Google Scholar] [CrossRef]
- Ban, E.; Kwon, T.H.; Kim, A. Delivery of therapeutic miRNA using polymer-based formulation. Drug Deliv. Transl. Res. 2019, 9, 1043–1056. [Google Scholar] [CrossRef]
- Dasgupta, I.; Chatterjee, A. Recent Advances in miRNA Delivery Systems. Methods Protoc. 2021, 4, 10. [Google Scholar] [CrossRef]
- Niccolini, B.; Palmieri, V.; De Spirito, M.; Papi, M. Opportunities offered by graphene nanoparticles for micrornas delivery for amyotrophic lateral sclerosis treatment. Materials 2022, 15, 126. [Google Scholar] [CrossRef]
- Sun, J.; Wang, J.; Yang, Z. Supramolecular assembly models of siRNA delivery systems. Chinese J. Chem. 2015, 33, 79–89. [Google Scholar] [CrossRef]
- Ihnatsyeu-Kachan, A.; Dzmitruk, V.; Apartsin, E.; Krasheninina, O.; Ionov, M.; Loznikova, S.; Venyaminova, A.; Miłowska, K.; Shcharbin, D.; Mignani, S.; et al. Multi-Target Inhibition of Cancer Cell Growth by SiRNA Cocktails and 5-Fluorouracil Using Effective Piperidine-Terminated Phosphorus Dendrimers. Colloids and Interfaces 2017, 1, 6. [Google Scholar] [CrossRef]
- Dzmitruk, V.; Apartsin, E.; Ihnatsyeu-Kachan, A.; Abashkin, V.; Shcharbin, D.; Bryszewska, M. Dendrimers Show Promise for siRNA and microRNA Therapeutics. Pharmaceutics 2018, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Knauer, N.; Pashkina, E.; Apartsin, E. Topological Aspects of the Design of Nanocarriers for Therapeutic Peptides and Proteins. Pharmaceutics 2019, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Aval, S.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.; Joo, S.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef]
- Apartsin, E.; Caminade, A. Supramolecular Self-Associations of Amphiphilic Dendrons and Their Properties. Chem.—A Eur. J. 2021, 27, 17976–17998. [Google Scholar] [CrossRef]
- Cramer, S.A.; Adjei, I.M.; Labhasetwar, V. Advancements in the delivery of epigenetic drugs. Expert Opin. Drug Deliv. 2015, 12, 1501–1512. [Google Scholar] [CrossRef]
- Lazniewska, J.; Milowska, K.; Zablocka, M.; Mignani, S.; Caminade, A.M.; Majoral, J.P.; Bryszewska, M.; Gabryelak, T. Mechanism of cationic phosphorus dendrimer toxicity against murine neural cell lines. Mol. Pharm. 2013, 10, 3484–3496. [Google Scholar] [CrossRef]
- Krasheninina, O.; Apartsin, E.; Fuentes, E.; Szulc, A.; Ionov, M.; Venyaminova, A.; Shcharbin, D.; De la Mata, F.; Bryszewska, M.; Gόmez, R. Complexes of Pro-Apoptotic siRNAs and Carbosilane Dendrimers: Formation and Effect on Cancer Cells. Pharmaceutics 2019, 11, 25. [Google Scholar] [CrossRef]
- Apartsin, E.; Venyaminova, A.; Majoral, J.-P.; Caminade, A.-M. Dendriplex-Impregnated Hydrogels With Programmed Release Rate. Front. Chem. 2022, 9, 780608. [Google Scholar] [CrossRef]
- Knauer, N.; Arkhipova, V.; Li, G.; Hewera, M.; Pashkina, E.; Nguyen, P.-H.; Meschaninova, M.; Kozlov, V.; Zhang, W.; Croner, R.; et al. In Vitro Validation of the Therapeutic Potential of Dendrimer-Based Nanoformulations against Tumor Stem Cells. Int. J. Mol. Sci. 2022, 23, 5691. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wei, Y. Modulators of microrna function in the immune system. Int. J. Mol. Sci. 2020, 21, 2357. [Google Scholar] [CrossRef] [PubMed]
- Giri, B.R.; Mahato, R.I.; Cheng, G. Roles of microRNAs in T cell immunity: Implications for strategy development against infectious diseases. Med. Res. Rev. 2019, 39, 706–732. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Paniagua, E.; Hernández-Ros, J.M.; Sánchez-Milla, M.; Camero, M.A.; Maly, M.; Pérez-Serrano, J.; Copa-Patiño, J.L.; Sánchez-Nieves, J.; Soliveri, J.; Gómez, R.; et al. Carbosilane cationic dendrimers synthesized by thiol–ene click chemistry and their use as antibacterial agents. RSC Adv. 2014, 4, 1256–1265. [Google Scholar] [CrossRef]
- Bellon, L. Oligoribonucleotides with 2′-O-(tert-Butyldimethylsilyl) Groups. Curr. Protoc. Nucleic Acid Chem. 2000, 1. [Google Scholar] [CrossRef] [PubMed]
- Böyum, A. Separation of leukocytes from blood and bone marrow. Introduction. Scand. J. Clin. Lab. Investig. Suppl. 1968, 97, 7. [Google Scholar]
- Ionov, M.; Lazniewska, J.; Dzmitruk, V.; Halets, I.; Loznikova, S.; Novopashina, D.; Apartsin, E.; Krasheninina, O.; Venyaminova, A.; Milowska, K.; et al. Anticancer siRNA cocktails as a novel tool to treat cancer cells. Part (A). Mechanisms of interaction. Int. J. Pharm. 2015, 485, 261–269. [Google Scholar] [CrossRef]
- Babar, I.A.; Cheng, C.J.; Booth, C.J.; Liang, X.; Weidhaas, J.B.; Saltzman, W.M.; Slack, F.J. Nanoparticle-based therapy in an in vivo microRNA-155 (miR-155)-dependent mouse model of lymphoma. Proc. Natl. Acad. Sci. USA 2012, 109, E1695–E1704. [Google Scholar] [CrossRef]
- Trotta, R.; Chen, L.; Ciarlariello, D.; Josyula, S.; Mao, C.; Costinean, S.; Yu, L.; Butchar, J.P.; Tridandapani, S.; Croce, C.M.; et al. miR-155 regulates IFN-γ production in natural killer cells. Blood 2012, 119, 3478–3485. [Google Scholar] [CrossRef]
- Pashangzadeh, S.; Motallebnezhad, M.; Vafashoar, F.; Khalvandi, A.; Mojtabavi, N. Implications the Role of miR-155 in the Pathogenesis of Autoimmune Diseases. Front. Immunol. 2021, 12, 669382. [Google Scholar] [CrossRef]
- Scheideler, M.; Vidakovic, I.; Prassl, R. Lipid nanocarriers for microRNA delivery. Chem. Phys. Lipids 2020, 226, 104837. [Google Scholar] [CrossRef] [PubMed]
- Kheirolomoom, A.; Kim, C.W.; Seo, J.W.; Kumar, S.; Son, D.J.; Gagnon, M.K.J.; Ingham, E.S.; Ferrara, K.W.; Jo, H. Multifunctional Nanoparticles Facilitate Molecular Targeting and miRNA Delivery to Inhibit Atherosclerosis in ApoE –/– Mice. ACS Nano 2015, 9, 8885–8897. [Google Scholar] [CrossRef] [PubMed]
- Placha, D.; Jampilek, J. Chronic Inflammatory Diseases, Anti-Inflammatory Agents and Their Delivery Nanosystems. Pharmaceutics 2021, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Ortega, L.; Rojas, J.M.; Barber, D.F. Improving Tumor Retention of Effector Cells in Adoptive Cell Transfer Therapies by Magnetic Targeting. Pharmaceutics 2020, 12, 812. [Google Scholar] [CrossRef]
- Boosz, P.; Pfister, F.; Stein, R.; Friedrich, B.; Fester, L.; Band, J.; Mühlberger, M.; Schreiber, E.; Lyer, S.; Dudziak, D.; et al. Citrate-Coated Superparamagnetic Iron Oxide Nanoparticles Enable a Stable Non-Spilling Loading of T Cells and Their Magnetic Accumulation. Cancers 2021, 13, 4143. [Google Scholar] [CrossRef]
- Bentley, E.R.; Little, S.R. Local delivery strategies to restore immune homeostasis in the context of inflammation. Adv. Drug Deliv. Rev. 2021, 178, 113971. [Google Scholar] [CrossRef]
- Keshavan, S.; Calligari, P.; Stella, L.; Fusco, L.; Delogu, L.G.; Fadeel, B. Nano-bio interactions: A neutrophil-centric view. Cell Death Dis. 2019, 10, 569. [Google Scholar] [CrossRef]
- Poupot, M.; Griffe, L.; Marchand, P.; Maraval, A.; Rolland, O.; Martinet, L.; L’Faqihi-Olive, F.; Turrin, C.; Caminade, A.; Fournié, J.; et al. Design of phosphorylated dendritic architectures to promote human monocyte activation. FASEB J. 2006, 20, 2339–2351. [Google Scholar] [CrossRef]
- Degboé, Y.; Fruchon, S.; Baron, M.; Nigon, D.; Turrin, C.; Caminade, A.-M.; Poupot, R.; Cantagrel, A.; Davignon, J.-L. Modulation of pro-inflammatory activation of monocytes and dendritic cells by aza-bis-phosphonate dendrimer as an experimental therapeutic agent. Arthritis Res. Ther. 2014, 16, R98. [Google Scholar] [CrossRef]
- Fruchon, S.; Poupot, M.; Martinet, L.; Turrin, C.-O.; Majoral, J.-P.; Fournié, J.-J.; Caminade, A.-M.; Poupot, R.; Fournie, J.-J.; Caminade, A.-M.; et al. Anti-inflammatory and immunosuppressive activation of human monocytes by a bioactive dendrimer. J. Leukoc. Biol. 2009, 85, 553–562. [Google Scholar] [CrossRef]
- Hayder, M.; Poupot, M.; Baron, M.; Nigon, D.; Turrin, C.-O.; Caminade, A.-M.; Majoral, J.-P.; Eisenberg, R.A.; Fournie, J.-J.; Cantagrel, A.; et al. A Phosphorus-Based Dendrimer Targets Inflammation and Osteoclastogenesis in Experimental Arthritis. Sci. Transl. Med. 2011, 3, 81ra35. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.-M. Phosphorus dendrimers for nanomedicine. Chem. Commun. 2017, 53, 9830–9838. [Google Scholar] [CrossRef] [PubMed]
- Gras, R.; García, M.I.; Gómez, R.; de la Mata, F.J.; Muñoz-Fernández, M.A.; López-Fernández, L.A. Carbosilane Dendrimer 2G-NN16 Represses Tc17 Differentiation in Primary T CD8+ Lymphocytes. Mol. Pharm. 2012, 9, 102–110. [Google Scholar] [CrossRef]
- Gras, R.; Relloso, M.; García, M.I.; Javier de la Mata, F.; Gómez, R.; López-Fernández, L.A.; Muñoz-Fernández, M.A. The inhibition of Th17 immune response in vitro and in vivo by the carbosilane dendrimer 2G-NN16. Biomaterials 2012, 33, 4002–4009. [Google Scholar] [CrossRef] [PubMed]
- Gras, R.; Almonacid, L.; Ortega, P.; Serramia, M.J.; Gomez, R.; de la Mata, F.J.; Lopez-Fernandez, L.A.; Muñoz-Fernandez, M.A. Changes in Gene Expression Pattern of Human Primary Macrophages Induced by Carbosilane Dendrimer 2G-NN16. Pharm. Res. 2009, 26, 577–586. [Google Scholar] [CrossRef]
- Fornaguera, C.; Grijalvo, S.; Galán, M.; Fuentes-Paniagua, E.; de la Mata, F.J.; Gómez, R.; Eritja, R.; Calderó, G.; Solans, C. Novel non-viral gene delivery systems composed of carbosilane dendron functionalized nanoparticles prepared from nano-emulsions as non-viral carriers for antisense oligonucleotides. Int. J. Pharm. 2015, 478, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Dzmitruk, V.; Szulc, A.; Shcharbin, D.; Janaszewska, A.; Shcharbina, N.; Lazniewska, J.; Novopashina, D.; Buyanova, M.; Ionov, M.; Klajnert-Maculewicz, B.; et al. Anticancer siRNA cocktails as a novel tool to treat cancer cells. Part (B). Efficiency of pharmacological action. Int. J. Pharm. 2015, 485, 288–294. [Google Scholar] [CrossRef]
- Posadas, I.; López-Hernández, B.; Clemente, M.I.; Jiménez, J.L.; Ortega, P.; de la Mata, J.; Gómez, R.; Muñoz-Fernández, M.A.; Ceña, V. Highly Efficient Transfection of Rat Cortical Neurons Using Carbosilane Dendrimers Unveils a Neuroprotective Role for HIF-1α in Early Chemical Hypoxia-Mediated Neurotoxicity. Pharm. Res. 2009, 26, 1181–1191. [Google Scholar] [CrossRef]
- Gilad, Y.; Eliaz, Y.; Yu, Y.; Han, S.J.; O’Malley, B.W.; Lonard, D.M. Drug-induced PD-L1 expression and cell stress response in breast cancer cells can be balanced by drug combination. Sci. Rep. 2019, 9, 15099. [Google Scholar] [CrossRef]
- Patsoukis, N.; Wang, Q.; Strauss, L.; Boussiotis, V.A. Revisiting the PD-1 pathway. Sci. Adv. 2020, 6, eabd2712. [Google Scholar] [CrossRef]
- Ge, J.; Huang, Z.; Liu, H.; Chen, J.; Xie, Z.; Chen, Z.; Peng, J.; Sun, J.; Hou, J.; Zhang, X. Lower expression of microRNA-155 contributes to dysfunction of natural killer cells in patients with chronic hepatitis B. Front. Immunol. 2017, 8, 1173. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, J.C.; Chen, D.; Lapinski, P.E.; Turner, J.; Grigorova, I.; Swanson, J.A.; King, P.D. Macropinocytosis drives T cell growth by sustaining the activation of mTORC1. Nat. Commun. 2020, 11, 180. [Google Scholar] [CrossRef] [PubMed]
- Dodd, C.H.; Hsu, H.-C.; Chu, W.-J.; Yang, P.; Zhang, H.-G.; Mountz, J.D.; Zinn, K.; Forder, J.; Josephson, L.; Weissleder, R.; et al. Normal T-cell response and in vivo magnetic resonance imaging of T cells loaded with HIV transactivator-peptide-derived superparamagnetic nanoparticles. J. Immunol. Methods 2001, 256, 89–105. [Google Scholar] [CrossRef] [PubMed]
| Concentration of RNA, nM | Cation Excess | Concentration of BDEF33, μM | Concentration of AE2G3, μM |
|---|---|---|---|
| 25 | 10 | 0.22 | 0.11 |
| 50 | 10 | 0.44 | 0.22 |
| 100 | 10 | 0.88 | 0.44 |
| 150 | 10 | 1.31 | 0.66 |
| Dendriplex | Hydrodynamic Diameter (nm) | Zeta Potential (mV) | PDI |
|---|---|---|---|
| AE2G3 | 7.4 ± 0.9 | +19.5 ± 1.1 | 0.18 |
| AE2G3/miR-155 | 48.6 ± 1.6 | +13.8 ± 0.3 | 0.22 |
| AE2G3/anti-miR-155 | 45.2 ± 2.6 | +14.0 ± 0.5 | 0.21 |
| BDEF33 | 3.5 ± 0.5 | +7.2 ± 1.4 | 0.19 |
| BDEF33/miR-155 | 36.7 ± 5.2 | +1.2 ± 0.2 | 0.25 |
| BDEF33/anti-miR-155 | 40.4 ± 5.6 | +1.5 ± 0.4 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knauer, N.; Pashkina, E.; Aktanova, A.; Boeva, O.; Arkhipova, V.; Barkovskaya, M.; Meschaninova, M.; Karpus, A.; Majoral, J.-P.; Kozlov, V.; et al. Effects of Cationic Dendrimers and Their Complexes with microRNAs on Immunocompetent Cells. Pharmaceutics 2023, 15, 148. https://doi.org/10.3390/pharmaceutics15010148
Knauer N, Pashkina E, Aktanova A, Boeva O, Arkhipova V, Barkovskaya M, Meschaninova M, Karpus A, Majoral J-P, Kozlov V, et al. Effects of Cationic Dendrimers and Their Complexes with microRNAs on Immunocompetent Cells. Pharmaceutics. 2023; 15(1):148. https://doi.org/10.3390/pharmaceutics15010148
Chicago/Turabian StyleKnauer, Nadezhda, Ekaterina Pashkina, Alina Aktanova, Olga Boeva, Valeria Arkhipova, Margarita Barkovskaya, Mariya Meschaninova, Andrii Karpus, Jean-Pierre Majoral, Vladimir Kozlov, and et al. 2023. "Effects of Cationic Dendrimers and Their Complexes with microRNAs on Immunocompetent Cells" Pharmaceutics 15, no. 1: 148. https://doi.org/10.3390/pharmaceutics15010148
APA StyleKnauer, N., Pashkina, E., Aktanova, A., Boeva, O., Arkhipova, V., Barkovskaya, M., Meschaninova, M., Karpus, A., Majoral, J.-P., Kozlov, V., & Apartsin, E. (2023). Effects of Cationic Dendrimers and Their Complexes with microRNAs on Immunocompetent Cells. Pharmaceutics, 15(1), 148. https://doi.org/10.3390/pharmaceutics15010148






