Potential Nanotechnology-Based Therapeutics to Prevent Cancer Progression through TME Cell-Driven Populations
Abstract
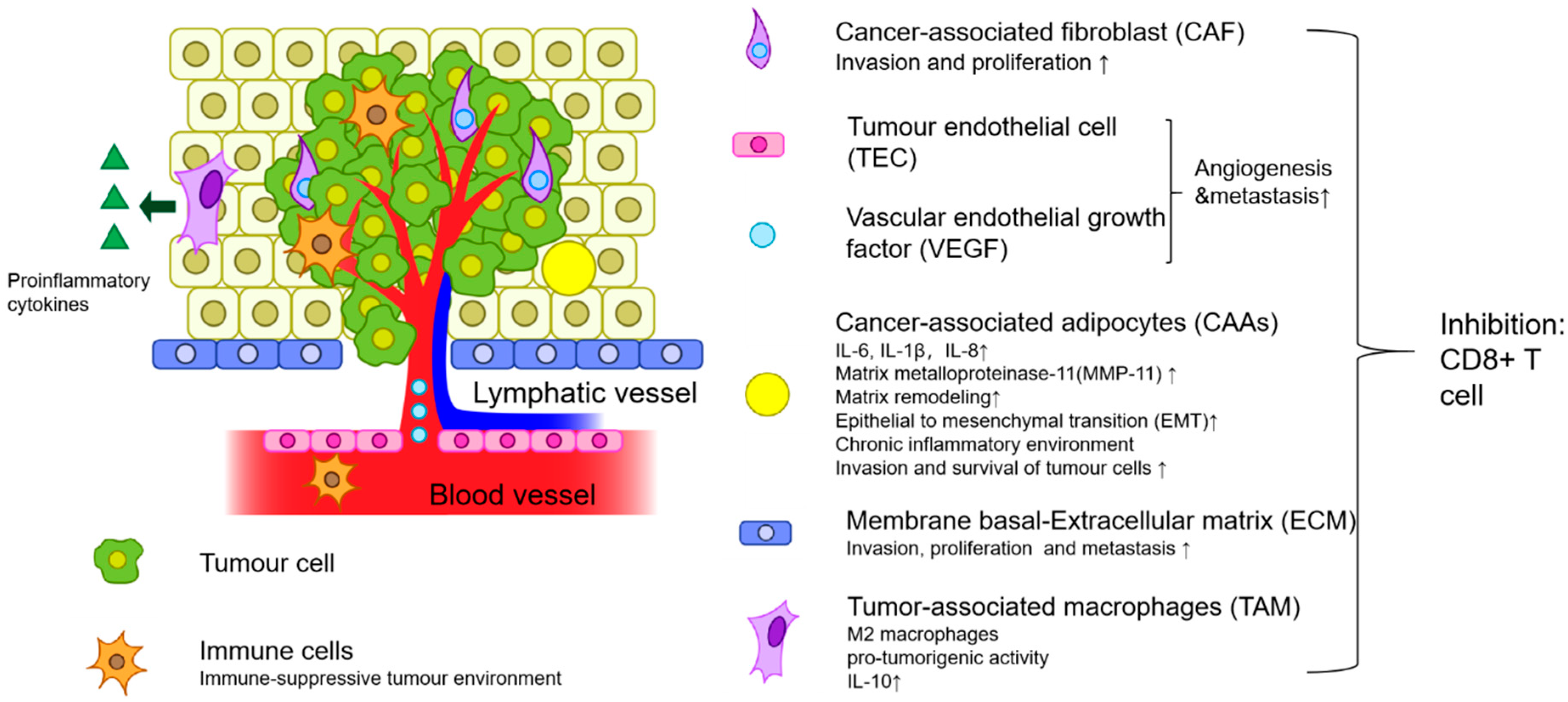
1. Introduction
2. Mesenchymal-Originating Cells
2.1. Cancer-Associated Fibroblasts
2.1.1. H-Ferritin-Based NPs
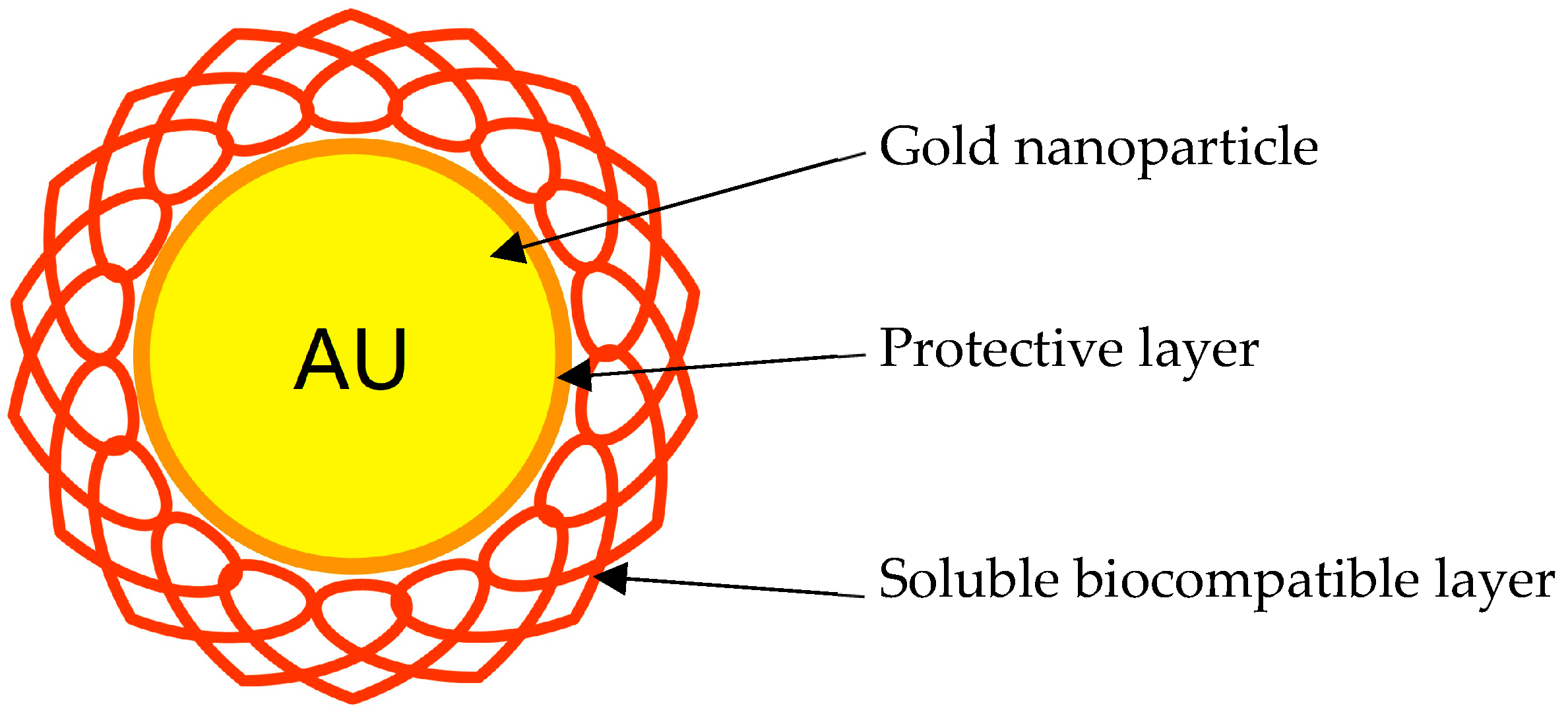
2.1.2. Gold Nanoparticles
2.2. Mesenchymal Stem Cells
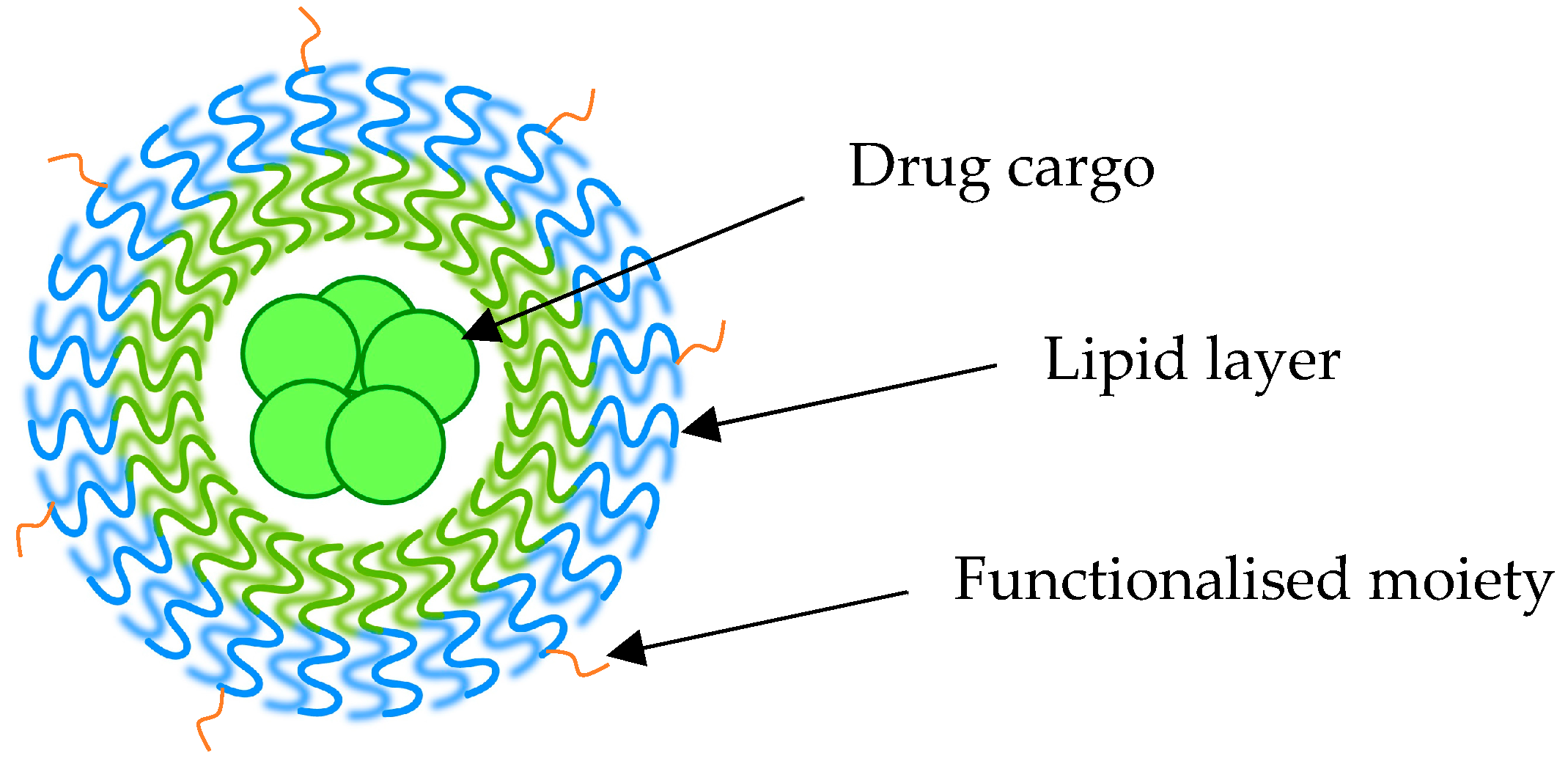
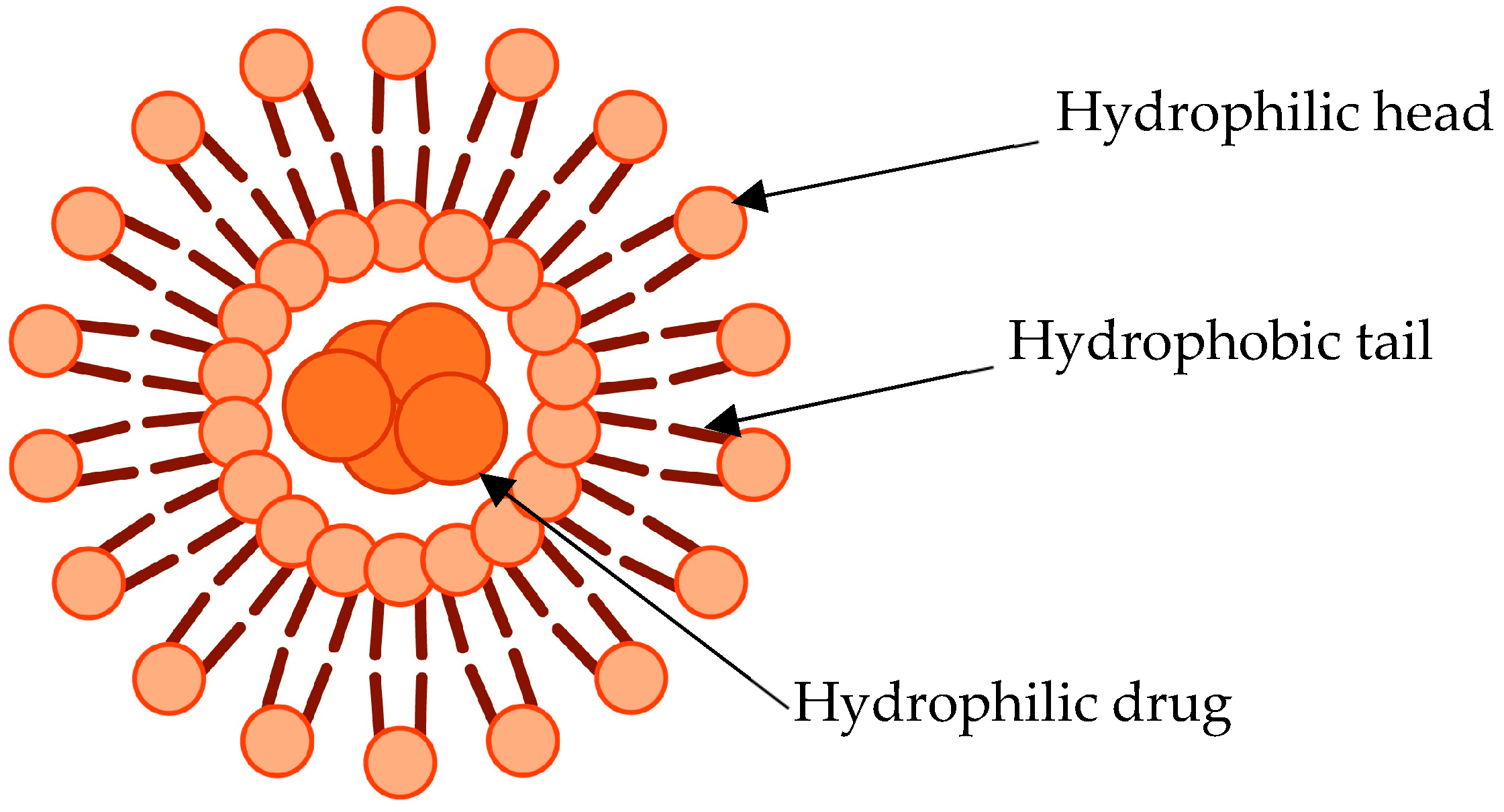
2.2.1. Salinomycin/Doxorubicin-Loaded Liposomes
2.2.2. Targeting CD44+ Receptors
2.3. Tumour-Associated Adipocytes
CCL2 Trap Encapsulated NPs
3. Haematological Cells
3.1. Tumour-Associated Macrophages
Mannose-Targeted PLGA NPs
3.2. Dendritic Cells
Interferon-γ Loaded NPs to Enhance Dendritic Cell Activity
3.3. Myeloid-Derived Suppressor Cells
3.3.1. Ursolic Acid-Loaded Liposomes’ Actions on MDSC
3.3.2. Immune Nanomodulators Targeting MDSCs
3.4. Neutrophils
3.5. Tumour-Associated Lymphocytes
Liposomes Elevating CD8+ Counts
4. Non-Cellular Component
4.1. Extracellular Matrix
Lipid-Based NPs for LOX Inhibition
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fallahpour, S.; Navaneelan, T.; De, P.; Borgo, A. Breast cancer survival by molecular subtype: A population-based analysis of cancer registry data. CMAJ Open 2017, 5, E734–E739. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Valdes, S.; Naguib, Y.W.; Hursting, S.D.; Cui, Z. Tumor-Associated Macrophage-Mediated Targeted Therapy of Triple-Negative Breast Cancer. Mol. Pharm. 2016, 13, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Vagia, E.; Mahalingam, D.; Cristofanilli, M. The Landscape of Targeted Therapies in TNBC. Cancers 2020, 12, 916. [Google Scholar] [CrossRef]
- McCuaig, R.; Wu, F.; Dunn, J.; Rao, S.; Dahlstrom, J.E. The biological and clinical significance of stromal-epithelial interactions in breast cancer. Pathology 2017, 49, 133–140. [Google Scholar] [CrossRef]
- Ribeiro Franco, P.I.; Rodrigues, A.P.; de Menezes, L.B.; Pacheco Miguel, M. Tumor microenvironment components: Allies of cancer progression. Pathol. Res. Pract. 2020, 216, 152729. [Google Scholar] [CrossRef]
- He, Q.; Chen, J.; Yan, J.; Cai, S.; Xiong, H.; Liu, Y.; Peng, D.; Mo, M.; Liu, Z. Tumor microenvironment responsive drug delivery systems. Asian J. Pharm. Sci. 2020, 15, 416–448. [Google Scholar] [CrossRef]
- Truffi, M.; Mazzucchelli, S.; Bonizzi, A.; Sorrentino, L.; Allevi, R.; Vanna, R.; Morasso, C.; Corsi, F. Nano-strategies to target breast cancer-associated fibroblasts: Rearranging the tumor microenvironment to achieve antitumor efficacy. Int. J. Mol. Sci. 2019, 20, 1263. [Google Scholar] [CrossRef]
- Sadeghi Rad, H.; Monkman, J.; Warkiani, M.E.; Ladwa, R.; O’Byrne, K.; Rezaei, N.; Kulasinghe, A. Understanding the tumor microenvironment for effective immunotherapy. Med. Res. Rev. 2021, 41, 1474–1498. [Google Scholar] [CrossRef]
- Musetti, S.; Huang, L. Nanoparticle-Mediated Remodeling of the Tumor Microenvironment to Enhance Immunotherapy. ACS Nano 2018, 12, 11740–11755. [Google Scholar] [CrossRef]
- Cort, A.; Ozben, T.; Saso, L.; De Luca, C.; Korkina, L. Redox Control of Multidrug Resistance and Its Possible Modulation by Antioxidants. Oxid. Med. Cell. Longev. 2016, 2016, 4251912. [Google Scholar] [CrossRef] [PubMed]
- Salama, L.; Pastor, E.R.; Stone, T.; Mousa, S.A. Emerging Nanopharmaceuticals and Nanonutraceuticals in Cancer Management. Biomedicines 2020, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Wang, K.; Oppong-Gyebi, A.; Hu, J. Application of nanotechnology in cancer diagnosis and therapy—A mini-review. Int. J. Med. Sci. 2020, 17, 2964–2973. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Wang, H.; Zhang, M. Nanoparticles for imaging and treatment of metastatic breast cancer. Expert Opin. Drug Deliv. 2017, 14, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Okano, M.; Oshi, M.; Butash, A.L.; Katsuta, E.; Tachibana, K.; Saito, K.; Okayama, H.; Peng, X.; Yan, L.; Kono, K.; et al. Triple-negative breast cancer with high levels of annexin a1 expression is associated with mast cell infiltration, inflammation, and angiogenesis. Int. J. Mol. Sci. 2019, 20, 4197. [Google Scholar] [CrossRef] [PubMed]
- Broad, R.V.; Jones, S.J.; Teske, M.C.; Wastall, L.M.; Hanby, A.M.; Thorne, J.L.; Hughes, T.A. Inhibition of interferon-signalling halts cancer-associated fibroblast-dependent protection of breast cancer cells from chemotherapy. Br. J. Cancer 2021, 124, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, X.H.; Zhao, Y.X.; Chen, C.; Xu, X.Y.; Sun, Q.; Wu, H.Y.; Chen, M.; Sang, J.F.; Su, L.; et al. Cancer-associated fibroblasts correlate with tumor-associated macrophages infiltration and lymphatic metastasis in triple negative breast cancer patients. J. Cancer 2018, 9, 4635–4641. [Google Scholar] [CrossRef]
- Bromma, K.; Bannister, A.; Kowalewski, A.; Cicon, L.; Chithrani, D.B. Elucidating the fate of nanoparticles among key cell components of the tumor microenvironment for promoting cancer nanotechnology. Cancer Nanotechnol. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Bakrania, A.K.; Variya, B.C.; Patel, S.S. Novel targets for paclitaxel nano formulations: Hopes and hypes in triple negative breast cancer. Pharmacol. Res. 2016, 111, 577–591. [Google Scholar] [CrossRef]
- Hu, C.; Liu, X.; Ran, W.; Meng, J.; Zhai, Y.; Zhang, P.; Yin, Q.; Yu, H.; Zhang, Z.; Li, Y. Regulating cancer associated fibroblasts with losartan-loaded injectable peptide hydrogel to potentiate chemotherapy in inhibiting growth and lung metastasis of triple negative breast cancer. Biomaterials 2017, 144, 60–72. [Google Scholar] [CrossRef]
- Sitia, L.; Bonizzi, A.; Mazzucchelli, S.; Negri, S.; Sottani, C.; Grignani, E.; Rizzuto, M.A.; Prosperi, D.; Sorrentino, L.; Morasso, C.; et al. Selective targeting of cancer-associated fibroblasts by engineered h-ferritin nanocages loaded with navitoclax. Cells 2021, 10, 328. [Google Scholar] [CrossRef] [PubMed]
- Rodila, R.; Kim, G.E.; Fan, L.; Chang, M.S.; Zhang, J.; Wu, H.; El-Shourbagy, T.A. HPLC-MS/MS determination of a hardly soluble drug in human urine through drug-albumin binding assisted dissolution. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008, 872, 128–132. [Google Scholar] [CrossRef] [PubMed]
- González-Gualda, E.; Pàez-Ribes, M.; Lozano-Torres, B.; Macias, D.; Wilson, J.R., 3rd; González-López, C.; Ou, H.L.; Mirón-Barroso, S.; Zhang, Z.; Lérida-Viso, A.; et al. Galacto-conjugation of Navitoclax as an efficient strategy to increase senolytic specificity and reduce platelet toxicity. Aging Cell 2020, 19, e13142. [Google Scholar] [CrossRef] [PubMed]
- Kile, B.T. The role of apoptosis in megakaryocytes and platelets. Br. J. Haematol. 2014, 165, 217–226. [Google Scholar] [CrossRef]
- Papaccio, F.; Paino, F.; Regad, T.; Papaccio, G.; Desiderio, V.; Tirino, V. Concise Review: Cancer Cells, Cancer Stem Cells, and Mesenchymal Stem Cells: Influence in Cancer Development. Stem. Cells Transl. Med. 2017, 6, 2115–2125. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Song, Y.; Wang, S.; Huang, X.; Xuan, Q.; Kang, X.; Zhang, Q. CD44(+)/CD24(-) phenotype predicts a poor prognosis in triple-negative breast cancer. Oncol. Lett. 2017, 14, 5890–5898. [Google Scholar] [CrossRef]
- Wang, T.; Narayanaswamy, R.; Ren, H.; Torchilin, V.P. Combination therapy targeting both cancer stem-like cells and bulk tumor cells for improved efficacy of breast cancer treatment. Cancer Biol. Ther. 2016, 17, 698–707. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, F.; Wong, E.T.; Fonkem, E.; Hsieh, T.C.; Wu, J.M.; Wu, E. Salinomycin: A novel anti-cancer agent with known anti-coccidial activities. Curr. Med. Chem. 2013, 20, 4095–4101. [Google Scholar] [CrossRef]
- Kim, Y.J.; Liu, Y.; Li, S.; Rohrs, J.; Zhang, R.; Zhang, X.; Wang, P. Co-Eradication of Breast Cancer Cells and Cancer Stem Cells by Cross-Linked Multilamellar Liposomes Enhances Tumor Treatment. Mol. Pharm. 2015, 12, 2811–2822. [Google Scholar] [CrossRef]
- Chen, D.; Wang, G.; Song, W.; Zhang, Q. Novel CD44 receptor targeting multifunctional “nano-eggs” based on double pH-sensitive nanoparticles for co-delivery of curcumin and paclitaxel to cancer cells and cancer stem cells. J. Nanoparticle Res. 2015, 17, 1–10. [Google Scholar] [CrossRef]
- Hu, K.; Zhou, H.; Liu, Y.; Liu, Z.; Liu, J.; Tang, J.; Li, J.; Zhang, J.; Sheng, W.; Zhao, Y.; et al. Hyaluronic acid functional amphipathic and redox-responsive polymer particles for the co-delivery of doxorubicin and cyclopamine to eradicate breast cancer cells and cancer stem cells. Nanoscale 2015, 7, 8607–8618. [Google Scholar] [CrossRef]
- D’Esposito, V.; Liguoro, D.; Ambrosio, M.R.; Collina, F.; Cantile, M.; Spinelli, R.; Raciti, G.A.; Miele, C.; Valentino, R.; Campiglia, P.; et al. Adipose microenvironment promotes triple negative breast cancer cell invasiveness and dissemination by producing CCL5. Oncotarget 2016, 7, 24495–24509. [Google Scholar] [CrossRef]
- Liu, Y.; Tiruthani, K.; Wang, M.; Zhou, X.; Qiu, N.; Xiong, Y.; Pecot, C.V.; Liu, R.; Huang, L. Tumor-targeted gene therapy with lipid nanoparticles inhibits tumor-associated adipocytes and remodels the immunosuppressive tumor microenvironment in triple-negative breast cancer. Nanoscale Horiz. 2021, 6, 319–329. [Google Scholar] [CrossRef]
- D’Esposito, V.; Ambrosio, M.R.; Giuliano, M.; Cabaro, S.; Miele, C.; Beguinot, F.; Formisano, P. Mammary Adipose Tissue Control of Breast Cancer Progression: Impact of Obesity and Diabetes. Front. Oncol. 2020, 10, 1554. [Google Scholar] [CrossRef]
- G Lahori, D.; Varamini, P. Nanotechnology-based platforms to improve immune checkpoint blockade efficacy in cancer therapy. Future Oncol. 2021, 17, 711–722. [Google Scholar] [CrossRef]
- Lan, M.; Lu, W.; Zou, T.; Li, L.; Liu, F.; Cai, T.; Cai, Y. Role of inflammatory microenvironment: Potential implications for improved breast cancer nano-targeted therapy. Cell. Mol. Life Sci. 2021, 78, 2105–2129. [Google Scholar] [CrossRef]
- Zemek, R.M.; Chin, W.L.; Nowak, A.K.; Millward, M.J.; Lake, R.A.; Lesterhuis, W.J. Sensitizing the Tumor Microenvironment to Immune Checkpoint Therapy. Front. Immunol. 2020, 11, 223. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019, 79, 4557–4567. [Google Scholar] [CrossRef]
- Wu, T.; Qiao, Q.; Qin, X.; Zhang, D.; Zhang, Z. Immunostimulatory cytokine and doxorubicin co-loaded nanovesicles for cancer immunochemotherapy. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 66–77. [Google Scholar] [CrossRef]
- Mediratta, K.; El-Sahli, S.; D’Costa, V.; Wang, L. Current progresses and challenges of immunotherapy in triple-negative breast cancer. Cancers 2020, 12, 3529. [Google Scholar] [CrossRef]
- Dai, X.; Ren, L.; Liu, M.; Cai, H.; Zhang, H.; Gong, Q.; Gu, Z.; Luo, K. Nanomedicines modulating myeloid-derived suppressor cells for improving cancer immunotherapy. Nano Today 2021, 39, 101163. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, S.; Shi, S.; Chen, Y.; Xu, F.; Wei, X.; Xu, Y. Solubilization and delivery of Ursolic-acid for modulating tumor microenvironment and regulatory T cell activities in cancer immunotherapy. J. Control. Release 2020, 320, 168–178. [Google Scholar] [CrossRef]
- Pereira, V.V.; Pereira, N.R.; Pereira, R.C.G.; Duarte, L.P.; Takahashi, J.A.; Silva, R.R. Synthesis and Antimicrobial Activity of Ursolic Acid Ester Derivatives. Chem 2022, 19, e202100566. [Google Scholar] [CrossRef]
- Song, C.; Phuengkham, H.; Kim, Y.S.; Dinh, V.V.; Lee, I.; Shin, I.W.; Shin, H.S.; Jin, S.M.; Um, S.H.; Lee, H.; et al. Syringeable immunotherapeutic nanogel reshapes tumor microenvironment and prevents tumor metastasis and recurrence. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Chen, C.; Li, A.; Sun, P.; Xu, J.; Du, W.; Zhang, J.; Liu, Y.; Zhang, R.; Zhang, S.; Yang, Z.; et al. Efficiently restoring the tumoricidal immunity against resistant malignancies via an immune nanomodulator. J. Control. Release 2020, 324, 574–585. [Google Scholar] [CrossRef]
- Giese, M.A.; Hind, L.E.; Huttenlocher, A. Neutrophil plasticity in the tumor microenvironment. Blood 2019, 133, 2159–2167. [Google Scholar] [CrossRef]
- Yazdi, M.H.; Mahdavi, M.; Varastehmoradi, B.; Faramarzi, M.A.; Shahverdi, A.R. The immunostimulatory effect of biogenic selenium nanoparticles on the 4T1 breast cancer model: An in vivo study. Biol. Trace Elem. Res. 2012, 149, 22–28. [Google Scholar] [CrossRef]
- Yazdi, M.H.; Mahdavi, M.; Kheradmand, E.; Shahverdi, A.R. The preventive oral supplementation of a selenium nanoparticle-enriched probiotic increases the immune response and lifespan of 4T1 breast cancer bearing mice. Arzneimittelforschung 2012, 62, 525–531. [Google Scholar] [CrossRef]
- Mittrücker, H.W.; Visekruna, A.; Huber, M. Heterogeneity in the differentiation and function of CD8+ T cells. Arch. Immunol. Ther. Exp. 2014, 62, 449–458. [Google Scholar] [CrossRef]
- Lowery, F.J.; Krishna, S.; Yossef, R.; Parikh, N.B.; Chatani, P.D.; Zacharakis, N.; Parkhurst, M.R.; Levin, N.; Sindiri, S.; Sachs, A.; et al. Molecular signatures of antitumor neoantigen-reactive T cells from metastatic human cancers. Science 2022, 375, 877–884. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef]
- Mortezaee, K. Enriched cancer stem cells, dense stroma, and cold immunity: Interrelated events in pancreatic cancer. J. Biochem. Mol. Toxicol. 2021, 35, e22708. [Google Scholar] [CrossRef]
- Thommen, D.S.; Schumacher, T.N. T Cell Dysfunction in Cancer. Cancer Cell 2018, 33, 547–562. [Google Scholar] [CrossRef]
- Chen, P.L.; Roh, W.; Reuben, A.; Cooper, Z.A.; Spencer, C.N.; Prieto, P.A.; Miller, J.P.; Bassett, R.L.; Gopalakrishnan, V.; Wani, K.; et al. Analysis of Immune Signatures in Longitudinal Tumor Samples Yields Insight into Biomarkers of Response and Mechanisms of Resistance to Immune Checkpoint Blockade. Cancer Discov. 2016, 6, 827–837. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug. Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Cheng, N.; Watkins-Schulz, R.; Junkins, R.D.; David, C.N.; Johnson, B.M.; Montgomery, S.A.; Peine, K.J.; Darr, D.B.; Yuan, H.; McKinnon, K.P.; et al. A nanoparticle-incorporated STING activator enhances antitumor immunity in PD-L1-insensitive models of triple-negative breast cancer. JCI Insight 2018, 3, e120638. [Google Scholar] [CrossRef]
- Fleming, J.M.; Yeyeodu, S.T.; McLaughlin, A.; Schuman, D.; Taylor, D.K. In Situ Drug Delivery to Breast Cancer-Associated Extracellular Matrix. ACS Chem. Biol. 2018, 13, 2825–2840. [Google Scholar] [CrossRef]
- Nicolas-Boluda, A.; Vaquero, J.; Vimeux, L.; Guilbert, T.; Barrin, S.; Kantari-Mimoun, C.; Ponzo, M.; Renault, G.; Deptula, P.; Pogoda, K.; et al. Tumor stiffening reversion through collagen crosslinking inhibition improves t cell migration and anti-pd-1 treatment. eLife 2021, 10, e58688. [Google Scholar] [CrossRef]
- Vaidya, A.; Wang, H.; Qian, V.; Gilmore, H.; Lu, Z.R. Overexpression of Extradomain-B Fibronectin is Associated with Invasion of Breast Cancer Cells. Cells 2020, 9, 1826. [Google Scholar] [CrossRef]
- De Vita, A.; Liverani, C.; Molinaro, R.; Martinez, J.O.; Hartman, K.A.; Spadazzi, C.; Miserocchi, G.; Taraballi, F.; Evangelopoulos, M.; Pieri, F.; et al. Lysyl oxidase engineered lipid nanovesicles for the treatment of triple negative breast cancer. Sci. Rep. 2021, 11, 5107. [Google Scholar] [CrossRef]
- Ji, T.; Zhao, Y.; Ding, Y.; Wang, J.; Zhao, R.; Lang, J.; Qin, H.; Liu, X.; Shi, J.; Tao, N.; et al. Transformable Peptide Nanocarriers for Expeditious Drug Release and Effective Cancer Therapy via Cancer-Associated Fibroblast Activation. Angew. Chem. Int. Ed. Engl. 2016, 55, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.; Tang, W.; Wang, M.; Zhou, S.; Wang, H.; Wu, Z.; Hao, Z.; Li, Z.; Liu, L.; Xie, J. Protein Nanocage Mediated Fibroblast-Activation Protein Targeted Photoimmunotherapy To Enhance Cytotoxic T Cell Infiltration and Tumor Control. Nano Lett. 2017, 17, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Miao, L.; Goodwin, T.J.; Li, J.; Liu, Q.; Huang, L. Quercetin Remodels the Tumor Microenvironment To Improve the Permeation, Retention, and Antitumor Effects of Nanoparticles. ACS Nano 2017, 11, 4916–4925. [Google Scholar] [CrossRef]
- Miao, L.; Liu, Q.; Lin, C.M.; Luo, C.; Wang, Y.; Liu, L.; Yin, W.; Hu, S.; Kim, W.Y.; Huang, L. Targeting Tumor-Associated Fibroblasts for Therapeutic Delivery in Desmoplastic Tumors. Cancer Res. 2017, 77, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, C.; Qian, P.; Lu, X.; Sun, B.; Zhang, X.; Wang, L.; Gao, X.; Li, H.; Chen, Z.; et al. Gd-metallofullerenol nanomaterial as non-toxic breast cancer stem cell-specific inhibitor. Nat. Commun. 2015, 6, 1–18. [Google Scholar] [CrossRef]
- Sun, T.M.; Wang, Y.C.; Wang, F.; Du, J.Z.; Mao, C.Q.; Sun, C.Y.; Tang, R.Z.; Liu, Y.; Zhu, J.; Zhu, Y.H.; et al. Cancer stem cell therapy using doxorubicin conjugated to gold nanoparticles via hydrazone bonds. Biomaterials 2014, 35, 836–845. [Google Scholar] [CrossRef]
- Emami, F.; Pathak, S.; Nguyen, T.T.; Shrestha, P.; Maharjan, S.; Kim, J.O.; Jeong, J.H.; Yook, S. Photoimmunotherapy with cetuximab-conjugated gold nanorods reduces drug resistance in triple negative breast cancer spheroids with enhanced infiltration of tumor-associated macrophages. J. Control. Release 2021, 329, 645–664. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, N.; Shen, L.; Liu, Q.; Zhang, J.; Cheng, Y.Y.; Lee, K.H.; Huang, L. Nanocarrier-mediated immunogenic chemotherapy for triple negative breast cancer. J. Control. Release 2020, 323, 431–441. [Google Scholar] [CrossRef]
- Zhang, P.; Qin, C.; Liu, N.; Zhou, X.; Chu, X.; Lv, F.; Gu, Y.; Yin, L.; Liu, J.; Zhou, J.; et al. The programmed site-specific delivery of LY3200882 and PD-L1 siRNA boosts immunotherapy for triple-negative breast cancer by remodeling tumor microenvironment. Biomaterials 2022, 284, 121518. [Google Scholar] [CrossRef]
- Schilb, A.L.; Ayat, N.R.; Vaidya, A.M.; Hertz, L.M.; Hall, R.C.; Scheidt, J.H.; Sun, D.; Sun, Z.; Gopalakrishnan, R.; Lu, Z.R. Efficacy of Targeted ECO/miR-200c Nanoparticles for Modulating Tumor Microenvironment and Treating Triple Negative Breast Cancer as Non-invasively Monitored by MR Molecular Imaging. Pharm. Res. 2021, 38, 1405–1418. [Google Scholar] [CrossRef]
- Panagi, M.; Voutouri, C.; Mpekris, F.; Papageorgis, P.; Martin, M.R.; Martin, J.D.; Demetriou, P.; Pierides, C.; Polydorou, C.; Stylianou, A.; et al. TGF-beta inhibition combined with cytotoxic nanomedicine normalizes triple negative breast cancer microenvironment towards anti-tumor immunity. Theranostics 2020, 10, 1910–1922. [Google Scholar] [CrossRef] [PubMed]
| Nanoparticle | Payload | Mechanism of Action | Reference |
|---|---|---|---|
| Cancer-associated fibroblasts | |||
| Spherical nanoparticles engineered with cleavable amphiphilic peptide (CAP) | Paclitaxel | Disruption of stromal barrier and enhanced drug accumulation | [61] |
| Peptide-derived nanofiber | Losartan | Inhibition of collagen I production and increased therapeutic efficacy | [20] |
| Ferritin nanocages engineered with Anti-FAP antibody | Photosensitiser | Suppression of chemokine release from CAFs | [62] |
| Lipid-calcium phosphate (LCP) nanoparticles | Quercetin | Downregulation of Wnt16, CAFs and normalisation of collagen | [63] |
| LCP nanoparticles | Secreted TNF-related apoptosis-induced ligand (sTRAIL) | Changing CAFs to their quiescent state resulting in tumour growth inhibition | [64] |
| Mesenchymal stem cells | |||
| Gd-metallofullerenol (Gd@C82(OH)22) | N/A | Reversal of EMT and depletion of cancer stem cells | [65] |
| Gold nanoparticles coated with poly (ethylene glycol) | Doxorubicin | Inhibition of tumour growth with reductions in stem cells | [66] |
| Tumour-associated macrophages | |||
| Gold nanorods | Cetuximab | In combination with phototherapy, the enhanced temperature at the tumour site resulted in TAM and tumour death | [67] |
| Liposomes | 17-(allylamino)-17-de- methoxygeldanamycin (17-AAG) | Significant reduction in TAMs with an increase in T cell infiltration and overall reductions in tumour volumes | [68] |
| Extracellular Matrix | |||
| Programmed site-specific delivery (PSSD) nanosystem | LY3200882 and siPD-L1 | Remodelling of the extracellular matrix by reversal of immunosuppression and deeper penetration of nano-based drug delivery systems. | [69] |
| RGD-PEG-ECO/miR-200c | N/A | Remodelling of the extracellular matrix by targeting miR-200c. Reduction in fibronectin along with suppression of TNBC proliferation. | [70] |
| Doxil nanoparticle | TGF-β inhibitor tranilast | Reduction of the extracellular matrix density and increased T cell infiltration. | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, R.; Shao, H.; Varamini, P. Potential Nanotechnology-Based Therapeutics to Prevent Cancer Progression through TME Cell-Driven Populations. Pharmaceutics 2023, 15, 112. https://doi.org/10.3390/pharmaceutics15010112
Ali R, Shao H, Varamini P. Potential Nanotechnology-Based Therapeutics to Prevent Cancer Progression through TME Cell-Driven Populations. Pharmaceutics. 2023; 15(1):112. https://doi.org/10.3390/pharmaceutics15010112
Chicago/Turabian StyleAli, Rafia, Huimin Shao, and Pegah Varamini. 2023. "Potential Nanotechnology-Based Therapeutics to Prevent Cancer Progression through TME Cell-Driven Populations" Pharmaceutics 15, no. 1: 112. https://doi.org/10.3390/pharmaceutics15010112
APA StyleAli, R., Shao, H., & Varamini, P. (2023). Potential Nanotechnology-Based Therapeutics to Prevent Cancer Progression through TME Cell-Driven Populations. Pharmaceutics, 15(1), 112. https://doi.org/10.3390/pharmaceutics15010112







