Biosynthesis of Silver Nanoparticles Using Seasonal Samples of Sonoran Desert Propolis: Evaluation of Its Antibacterial Activity against Clinical Isolates of Multi-Drug Resistant Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Propolis Samples and Extract Preparation
2.3. Synthesis of Silver Nanoparticles
2.4. Characterization of Silver Nanoparticles
2.5. Cell Culture and Cytotoxic Effect
2.6. Antibacterial Activity
2.7. Anti-Biofilm Assay
2.8. Statistical Analyses
3. Results
3.1. Synthesis and Characterization of Silver Nanoparticles
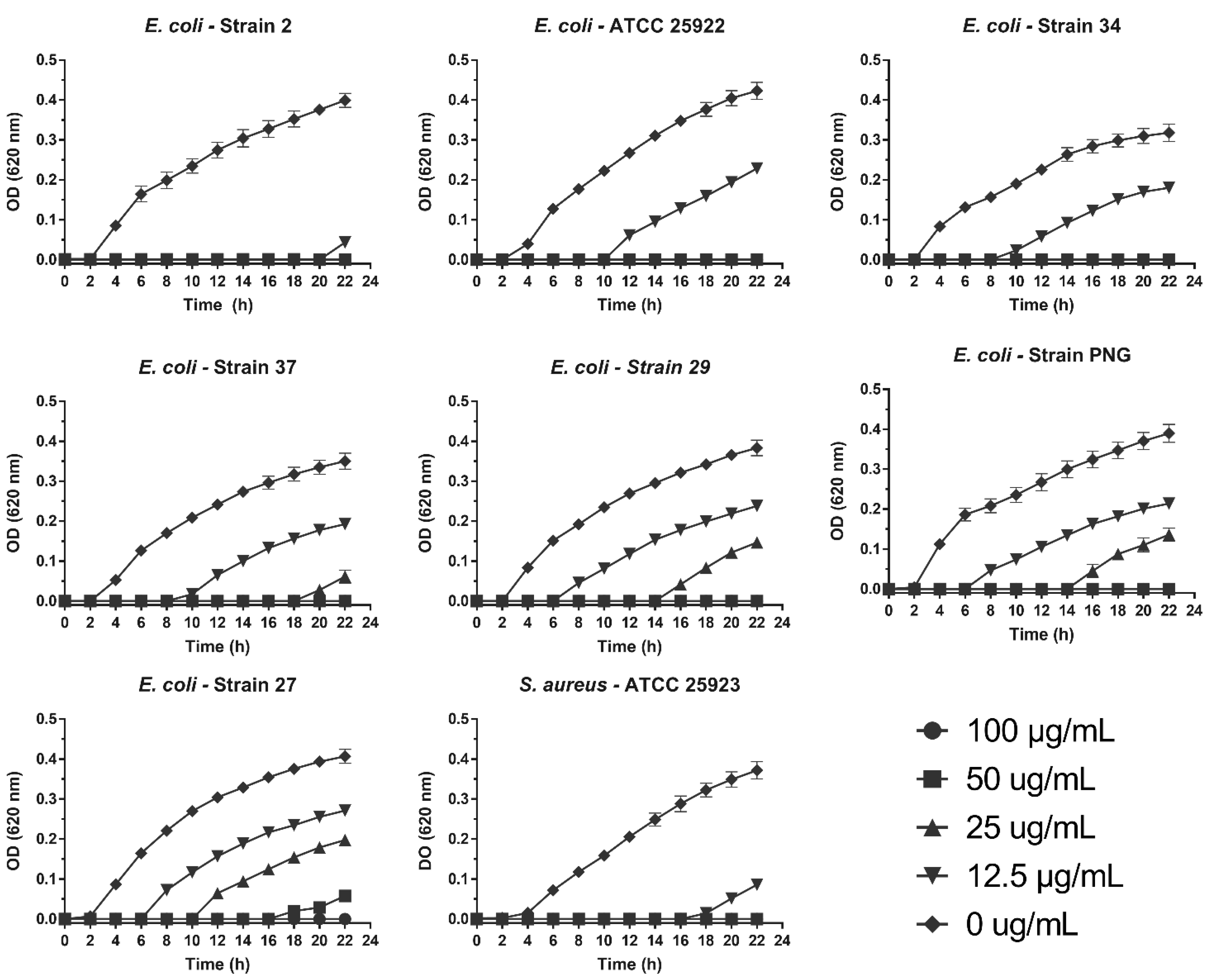
3.2. Antibacterial Activity
3.3. Antibiofilm Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Asokan, G.; Ramadhan, T.; Ahmed, E.; Sanad, H. WHO Global Priority Pathogens List: A Bibliometric Analysis of Medline-PubMed for Knowledge Mobilization to Infection Prevention and Control Practices in Bahrain. Oman Med. J. 2019, 34, 184–193. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef]
- Macovei, I.; Luca, S.V.; Skalicka-Woźniak, K.; Sacarescu, L.; Pascariu, P.; Ghilan, A.; Doroftei, F.; Ursu, E.-L.; Rimbu, C.M.; Horhogea, C.E.; et al. Phyto-Functionalized Silver Nanoparticles Derived from Conifer Bark Extracts and Evaluation of Their Antimicrobial and Cytogenotoxic Effects. Molecules 2021, 27, 217. [Google Scholar] [CrossRef]
- Abada, E.; Galal, T.; Ismail, I. Biosynthesis of silver nanoparticles by Nocardiopsis sp.-MW279108 and its antimicrobial activity. J. Basic Microbiol. 2021, 61, 993–1001. [Google Scholar] [CrossRef]
- Chumpol, J.; Siri, S. Simple green production of silver nanoparticles facilitated by bacterial genomic DNA and their antibacterial activity. Artif. Cells Nanomed. Biotechnol. 2018, 46, 619–625. [Google Scholar] [CrossRef]
- Bankova, V.S.; De Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Bankova, V. Chemical diversity of propolis and the problem of standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef]
- Alday, E.; Valencia, D.; Garibay-Escobar, A.; Domínguez-Esquivel, Z.; Piccinelli, A.L.; Rastrelli, L.; Monribot-Villanueva, J.; Guerrero-Analco, J.A.; Robles-Zepeda, R.E.; Hernandez, J.; et al. Plant origin authentication of Sonoran Desert propolis: An antiproliferative propolis from a semi-arid region. Sci. Nat. 2019, 106, 25. [Google Scholar] [CrossRef]
- Conti, B.J.; Santiago, K.B.; Búfalo, M.C.; Herrera, Y.F.; Alday, E.; Velazquez, C.; Hernandez, J.; Sforcin, J.M. Modulatory effects of propolis samples from Latin America (Brazil, Cuba and Mexico) on cytokine production by human monocytes. J. Pharm. Pharmacol. 2015, 67, 1431–1438. [Google Scholar] [CrossRef]
- Alday, E.; Valencia, D.; Carreño, A.L.; Picerno, P.; Piccinelli, A.L.; Rastrelli, L.; Robles-Zepeda, R.; Hernandez, J.; Velazquez, C. Apoptotic induction by pinobanksin and some of its ester derivatives from Sonoran propolis in a B-cell lymphoma cell line. Chem. Biol. Interact. 2015, 242, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Valencia, D.; Alday, E.; Robles-Zepeda, R.; Garibay-Escobar, A.; Galvez-Ruiz, J.C.; Salas-Reyes, M.; Jiménez-Estrada, M.; Velazquez-Contreras, E.; Hernandez, J.; Velazquez, C. Seasonal effect on chemical composition and biological activities of Sonoran propolis. Food Chem. 2012, 131, 645–651. [Google Scholar] [CrossRef]
- Navarro-Navarro, M.; Ruiz-Bustos, P.; Valencia, D.; Robles-Zepeda, R.; Ruiz-Bustos, E.; Virués, C.; Hernandez, J.; Domínguez, Z.; Velazquez, C. Antibacterial Activity of Sonoran Propolis and Some of Its Constituents Against Clinically Significant Vibrio Species. Foodborne Pathog. Dis. 2013, 10, 150–158. [Google Scholar] [CrossRef]
- Mendez-Pfeiffer, P.; Alday, E.; Carreño, A.L.; Hernández-Tánori, J.; Montaño-Leyva, B.; Ortega-García, J.; Valdez, J.; Garibay-Escobar, A.; Hernandez, J.; Valencia, D.; et al. Seasonality modulates the cellular antioxidant activity and antiproliferative effect of sonoran desert propolis. Antioxidants 2020, 9, 1294. [Google Scholar] [CrossRef]
- Grunberger, D.; Banerjee, R.; Eisinger, K.; Oltz, E.M.; Efros, L.; Caldwell, M.; Estevez, V.; Nakanishi, K. Preferential cytotoxicity on tumor cells by caffeic acid phenethyl ester isolated from propolis. Experientia 1988, 44, 230–232. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef]
- Christensen, G.D.; Simpson, W.A.; Younger, J.J.; Baddour, L.M.; Barrett, F.F.; Melton, D.M.; Beachey, E.H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: A quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 1985, 22, 996–1006. [Google Scholar] [CrossRef]
- Hernandez, J.; Goycoolea, F.M.; Quintero, J.; Acosta, A.; Castañeda, M.; Dominguez, Z.; Robles, R.; Vazquez-Moreno, L.; Velazquez, E.F.; Astiazaran, H.; et al. Sonoran propolis: Chemical composition and antiproliferative activity on cancer cell lines. Planta Med. 2007, 73, 1469–1474. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, 9th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 32, ISBN 1562387839. [Google Scholar]
- Ballesteros-Monrreal, M.G.; Arenas-Hernández, M.M.P.; Barrios-Villa, E.; Juarez, J.; Álvarez-Ainza, M.L.; Taboada, P.; De la Rosa-López, R.; Bolado-Martínez, E.; Valencia, D. Bacterial Morphotypes as Important Trait for Uropathogenic E. coli Diagnostic; a Virulence-Phenotype-Phylogeny Study. Microorganisms 2021, 9, 2381. [Google Scholar] [CrossRef]
- Ballesteros-Monrreal, M.G.; Arenas-Hernández, M.M.P.; Enciso-Martínez, Y.; Martinez de la Peña, C.F.; Rocha-Gracia, R. del C.; Lozano-Zarain, P.; Navarro-Ocaña, A.; Martínez-Laguna, Y.; de la Rosa-López, R. Virulence and Resistance Determinants of Uropathogenic Escherichia coli Strains Isolated from Pregnant and Non-Pregnant Women from Two States in Mexico. Infect. Drug Resist. 2020, 13, 295–310. [Google Scholar] [CrossRef]
- O’Toole, G.A. Microtiter Dish Biofilm Formation Assay. J. Vis. Exp. 2011, 30, e2437. [Google Scholar] [CrossRef]
- İlk, S.; Tan, G.; Emül, E.; Sağlam, N. Investigation the potential use of silver nanoparticles synthesized by propolis extract as N-acyl-homoserine lactone-mediated quorum sensing systems inhibitor. Turk. J. Med. Sci. 2020, 1147–1156. [Google Scholar] [CrossRef]
- Méndez-Pfeiffer, P.A.; Soto Urzúa, L.; Sánchez-Mora, E.; González, A.L.; Romo-Herrera, J.M.; Gervacio Arciniega, J.J.; Martínez Morales, L.J. Damage on Escherichia coli and Staphylococcus aureus using white light photoactivation of Au and Ag nanoparticles. J. Appl. Phys. 2019, 125, 213102. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Velazquez, C.; Navarro, M.; Acosta, A.; Angulo, A.; Dominguez, Z.; Robles, R.; Robles-Zepeda, R.; Lugo, E.; Goycoolea, F.M.; Velazquez, E.F.; et al. Antibacterial and free-radical scavenging activities of Sonoran propolis. J. Appl. Microbiol. 2007, 103, 1747–1756. [Google Scholar] [CrossRef]
- Gregoris, E.; Stevanato, R. Correlations between polyphenolic composition and antioxidant activity of Venetian propolis. Food Chem. Toxicol. 2010, 48, 76–82. [Google Scholar] [CrossRef]
- Frattaruolo, L.; Carullo, G.; Brindisi, M.; Mazzotta, S.; Bellissimo, L.; Rago, V.; Curcio, R.; Dolce, V.; Aiello, F.; Cappello, A.R. Antioxidant and anti-inflammatory activities of flavanones from Glycyrrhiza glabra L. (licorice) leaf phytocomplexes: Identification of licoflavanone as a modulator of NF-kB/MAPK pathway. Antioxidants 2019, 8, 186. [Google Scholar] [CrossRef]
- Roy, N.; Mondal, S.; Laskar, R.A.; Basu, S.; Mandal, D.; Begum, N.A. Biogenic synthesis of Au and Ag nanoparticles by Indian propolis and its constituents. Colloids Surf. B Biointerfaces 2010, 76, 317–325. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, Y. Green Synthetic Nanoarchitectonics of Gold and Silver Nanoparticles Prepared Using Quercetin and Their Cytotoxicity and Catalytic Applications. J. Nanosci. Nanotechnol. 2019, 20, 2781–2790. [Google Scholar] [CrossRef]
- Ahn, M.R.; Kunimasa, K.; Kumazawa, S.; Nakayama, T.; Kaji, K.; Uto, Y.; Hori, H.; Nagasawa, H.; Ohta, T. Correlation between antiangiogenic activity and antioxidant activity of various components from propolis. Mol. Nutr. Food Res. 2009, 53, 643–651. [Google Scholar] [CrossRef]
- Gatea, F.; Teodor, E.D.; Seciu, A.M.; Covaci, O.I.; Mănoiu, S.; Lazăr, V.; Radu, G.L. Antitumour, antimicrobial and catalytic activity of gold nanoparticles synthesized by different pH propolis extracts. J. Nanopart. Res. 2015, 17, 320. [Google Scholar] [CrossRef]
- Al-Rubaye, H.I.; Al-Rubaye, B.K.; Al-Abodi, E.E.; Yousif, E.I. Green Chemistry Synthesis of Modified Silver Nanoparticles. J. Phys. Conf. Ser. 2020, 1664, 012080. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Urnukhsaikhan, E.; Bold, B.-E.; Gunbileg, A.; Sukhbaatar, N.; Mishig-Ochir, T. Antibacterial activity and characteristics of silver nanoparticles biosynthesized from Carduus crispus. Sci. Rep. 2021, 11, 21047. [Google Scholar] [CrossRef]
- Kischkel, B.; Castilho, P.F.D.; de Oliveira, K.M.; Rezende, P.S.; Bruschi, M.L.; Svidzinski, T.I.; Negri, M. Silver nanoparticles stabilized with propolis shows reduced toxicity and potential activity against fungal infections. Future Microbiol. 2020, 15, 521–539. [Google Scholar] [CrossRef]
- Botteon, C.E.A.; Silva, L.B.; Ccana-Ccapatinta, G.V.; Silva, T.S.; Ambrosio, S.R.; Veneziani, R.C.S.; Bastos, J.K.; Marcato, P.D. Biosynthesis and characterization of gold nanoparticles using Brazilian red propolis and evaluation of its antimicrobial and anticancer activities. Sci. Rep. 2021, 11, 1974. [Google Scholar] [CrossRef]
- Barbosa, V.T.; Souza, J.K.C.; Alvino, V.; Meneghetti, M.R.; Florez-Rodriguez, P.P.; Moreira, R.E.; Paulino, G.V.B.; Landell, M.F.; Basílio-Júnior, I.D.; do Nascimento, T.G.; et al. Biogenic synthesis of silver nanoparticles using Brazilian propolis. Biotechnol. Prog. 2019, 35, e2888. [Google Scholar] [CrossRef]
- Zampieri, M.; Enke, T.; Chubukov, V.; Ricci, V.; Piddock, L.; Sauer, U. Metabolic constraints on the evolution of antibiotic resistance. Mol. Syst. Biol. 2017, 13, 917. [Google Scholar] [CrossRef]
- Sulaiman, J.E.; Lam, H. Evolution of Bacterial Tolerance Under Antibiotic Treatment and Its Implications on the Development of Resistance. Front. Microbiol. 2021, 12, 617412. [Google Scholar] [CrossRef]
- Maisonneuve, E.; Gerdes, K. Molecular Mechanisms Underlying Bacterial Persisters. Cell 2014, 157, 539–548. [Google Scholar] [CrossRef] [Green Version]
- Jurėnas, D.; Fraikin, N.; Goormaghtigh, F.; Van Melderen, L. Biology and evolution of bacterial toxin–antitoxin systems. Nat. Rev. Microbiol. 2022, 20, 335–350. [Google Scholar] [CrossRef]
- Harrison, J.J.; Ceri, H.; Roper, N.J.; Badry, E.A.; Sproule, K.M.; Turner, R.J. Persister cells mediate tolerance to metal oxyanions in Escherichia coli. Microbiology 2005, 151, 3181–3195. [Google Scholar] [CrossRef]
- McNeilly, O.; Mann, R.; Hamidian, M.; Gunawan, C. Emerging Concern for Silver Nanoparticle Resistance in Acinetobacter baumannii and Other Bacteria. Front. Microbiol. 2021, 12, 652863. [Google Scholar] [CrossRef]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef]
- Vadillo-Rodríguez, V.; Cavagnola, M.A.; Pérez-Giraldo, C.; Fernández-Calderón, M.C. A physico-chemical study of the interaction of ethanolic extracts of propolis with bacterial cells. Colloids Surf. B Biointerfaces 2021, 200, 111571. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, J.; Wei, W.; Miao, Y.; Liang, C.; Wu, J.; Huang, X.; Yin, L.; Geng, Y.; Chen, D.; et al. A study of the antibacterial mechanism of pinocembrin against multidrug-resistant Aeromonas hydrophila. Int. Microbiol. 2022, 25, 605–613. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Detection of galangin-induced cytoplasmic membrane damage in Staphylococcus aureus by measuring potassium loss. J. Ethnopharmacol. 2005, 101, 243–248. [Google Scholar] [CrossRef]


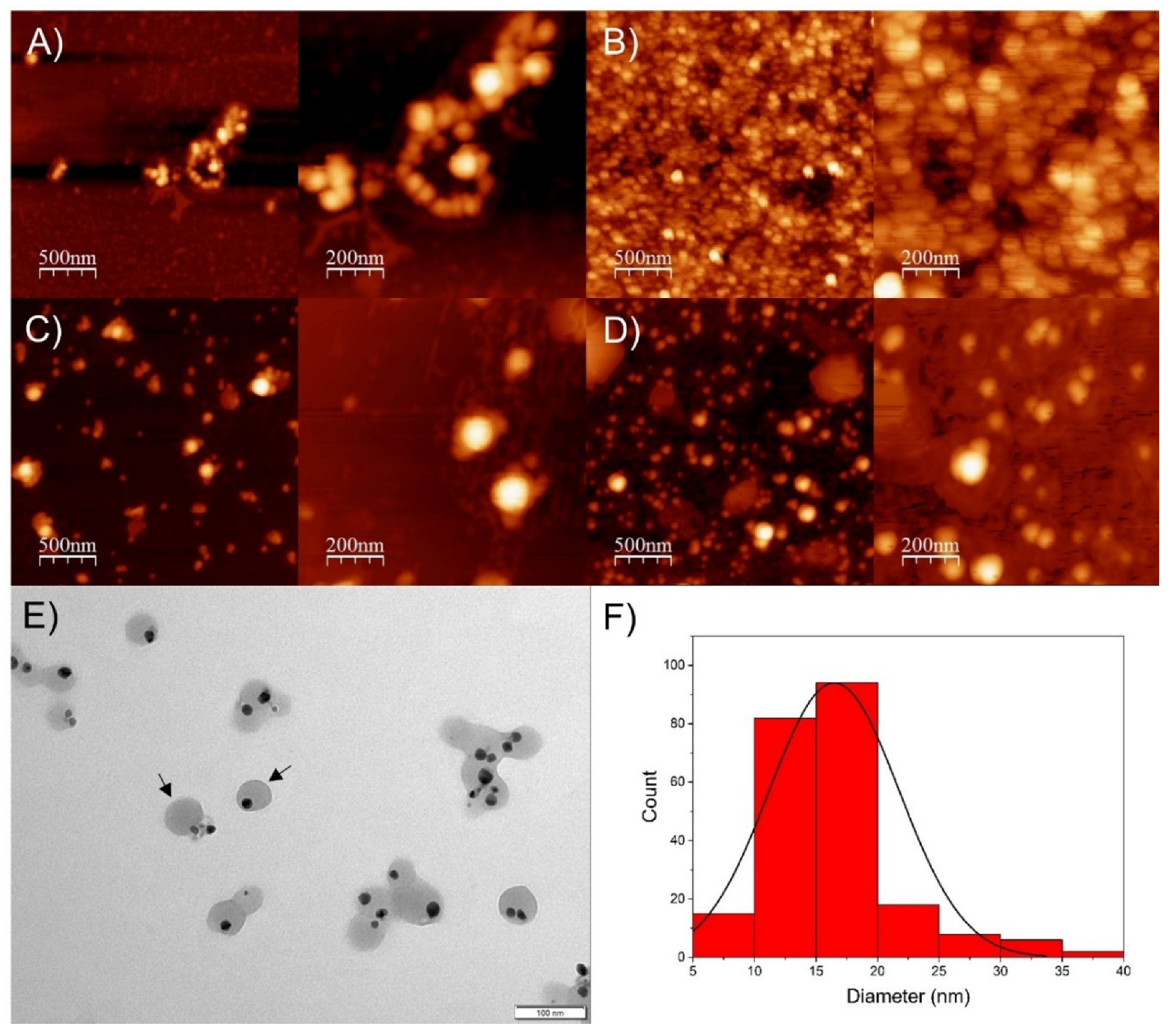
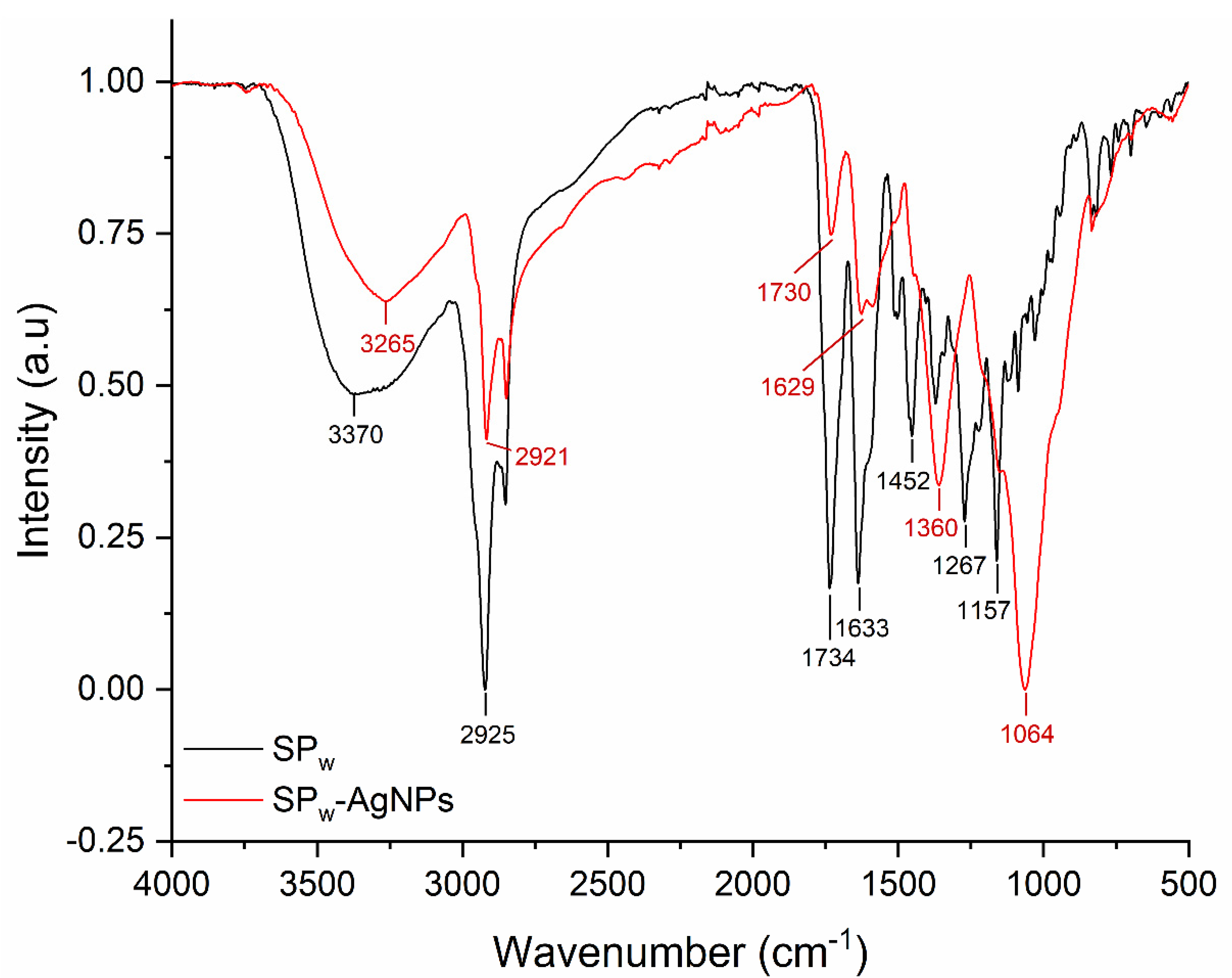

| SP-AgNPs | Size (Hd.nm) | Zeta Potential (mV) |
|---|---|---|
| Spring | 59.9 ± 0.4 | −47.5 ± 3.1 |
| Summer | 58.9 ± 0.2 | −31.6 ± 1.1 |
| Autumn | 60.2 ± 1.1 | −32.4 ± 1.3 |
| Winter | 68.0 ± 1.7 | −52.0 ± 1.5 |
| Bacteria | MIC (µg/mL) | MBC (µg/mL) |
|---|---|---|
| S. aureus ATCC 25923 | 25 | 100 |
| E. coli ATCC 25922 | 25 | 25 |
| E. coli 2 | 25 | 50 |
| E. coli 27 | 100 | 100 |
| E. coli 29 | 50 | 50 |
| E. coli 34 | 25 | 25 |
| E. coli 37 | 50 | 50 |
| E. coli PNG | 50 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendez-Pfeiffer, P.; Ballesteros-Monrreal, M.G.; Gaona-Ochoa, J.; Juarez, J.; Gastelum-Cabrera, M.; Montaño-Leyva, B.; Arenas-Hernández, M.; Caporal-Hernandez, L.; Ortega-García, J.; Barrios-Villa, E.; et al. Biosynthesis of Silver Nanoparticles Using Seasonal Samples of Sonoran Desert Propolis: Evaluation of Its Antibacterial Activity against Clinical Isolates of Multi-Drug Resistant Bacteria. Pharmaceutics 2022, 14, 1853. https://doi.org/10.3390/pharmaceutics14091853
Mendez-Pfeiffer P, Ballesteros-Monrreal MG, Gaona-Ochoa J, Juarez J, Gastelum-Cabrera M, Montaño-Leyva B, Arenas-Hernández M, Caporal-Hernandez L, Ortega-García J, Barrios-Villa E, et al. Biosynthesis of Silver Nanoparticles Using Seasonal Samples of Sonoran Desert Propolis: Evaluation of Its Antibacterial Activity against Clinical Isolates of Multi-Drug Resistant Bacteria. Pharmaceutics. 2022; 14(9):1853. https://doi.org/10.3390/pharmaceutics14091853
Chicago/Turabian StyleMendez-Pfeiffer, Pablo, Manuel G. Ballesteros-Monrreal, Jesus Gaona-Ochoa, Josue Juarez, Marisol Gastelum-Cabrera, Beatriz Montaño-Leyva, Margarita Arenas-Hernández, Liliana Caporal-Hernandez, Jesús Ortega-García, Edwin Barrios-Villa, and et al. 2022. "Biosynthesis of Silver Nanoparticles Using Seasonal Samples of Sonoran Desert Propolis: Evaluation of Its Antibacterial Activity against Clinical Isolates of Multi-Drug Resistant Bacteria" Pharmaceutics 14, no. 9: 1853. https://doi.org/10.3390/pharmaceutics14091853
APA StyleMendez-Pfeiffer, P., Ballesteros-Monrreal, M. G., Gaona-Ochoa, J., Juarez, J., Gastelum-Cabrera, M., Montaño-Leyva, B., Arenas-Hernández, M., Caporal-Hernandez, L., Ortega-García, J., Barrios-Villa, E., Velazquez, C., & Valencia, D. (2022). Biosynthesis of Silver Nanoparticles Using Seasonal Samples of Sonoran Desert Propolis: Evaluation of Its Antibacterial Activity against Clinical Isolates of Multi-Drug Resistant Bacteria. Pharmaceutics, 14(9), 1853. https://doi.org/10.3390/pharmaceutics14091853









