3D-Printing Graphene Scaffolds for Bone Tissue Engineering
Abstract
:1. Introduction
2. Material Properties for Tissue Engineering
3. Carbon Nanomaterials
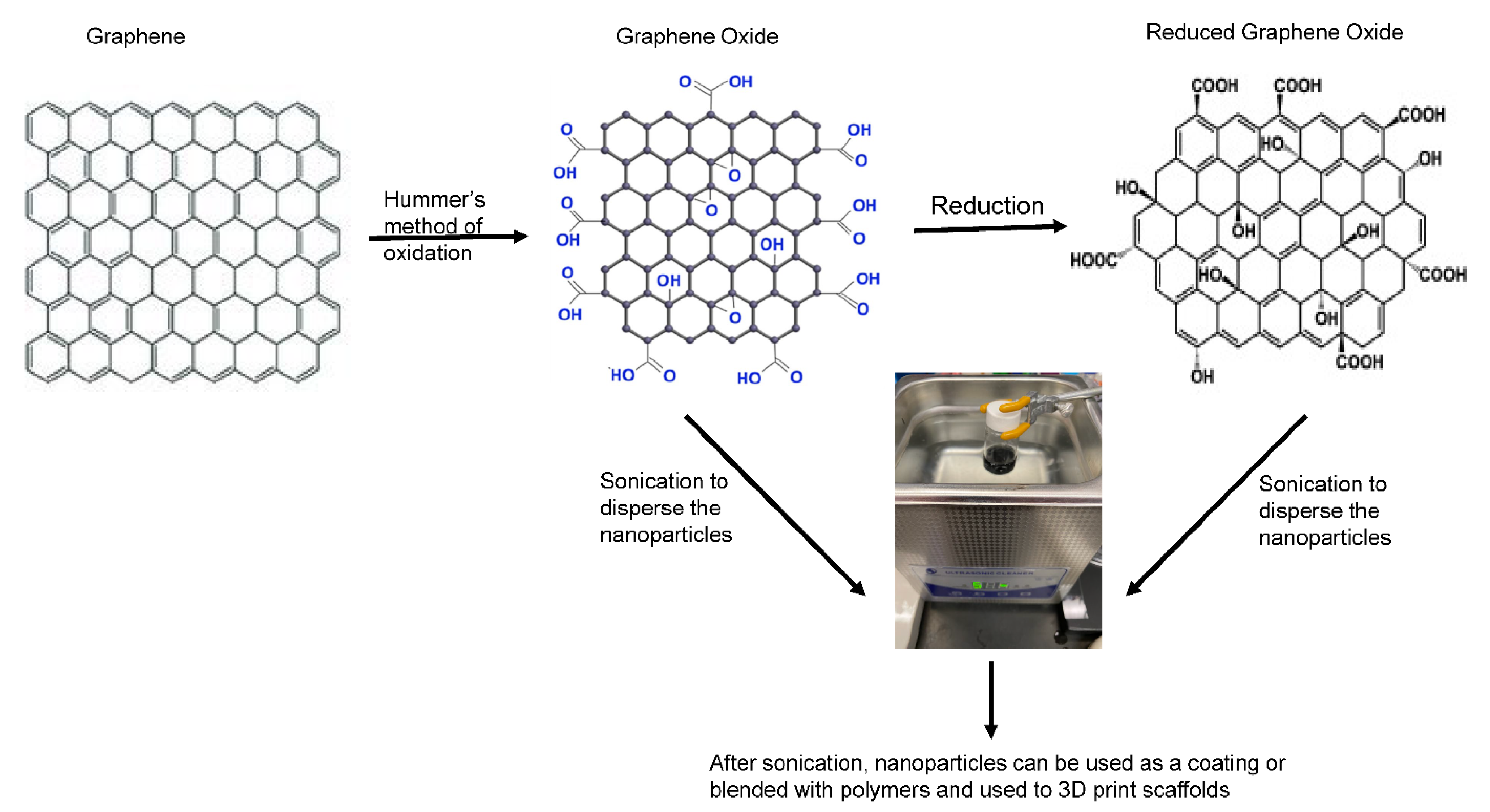
Graphene Materials
4. Material Fabrication Techniques
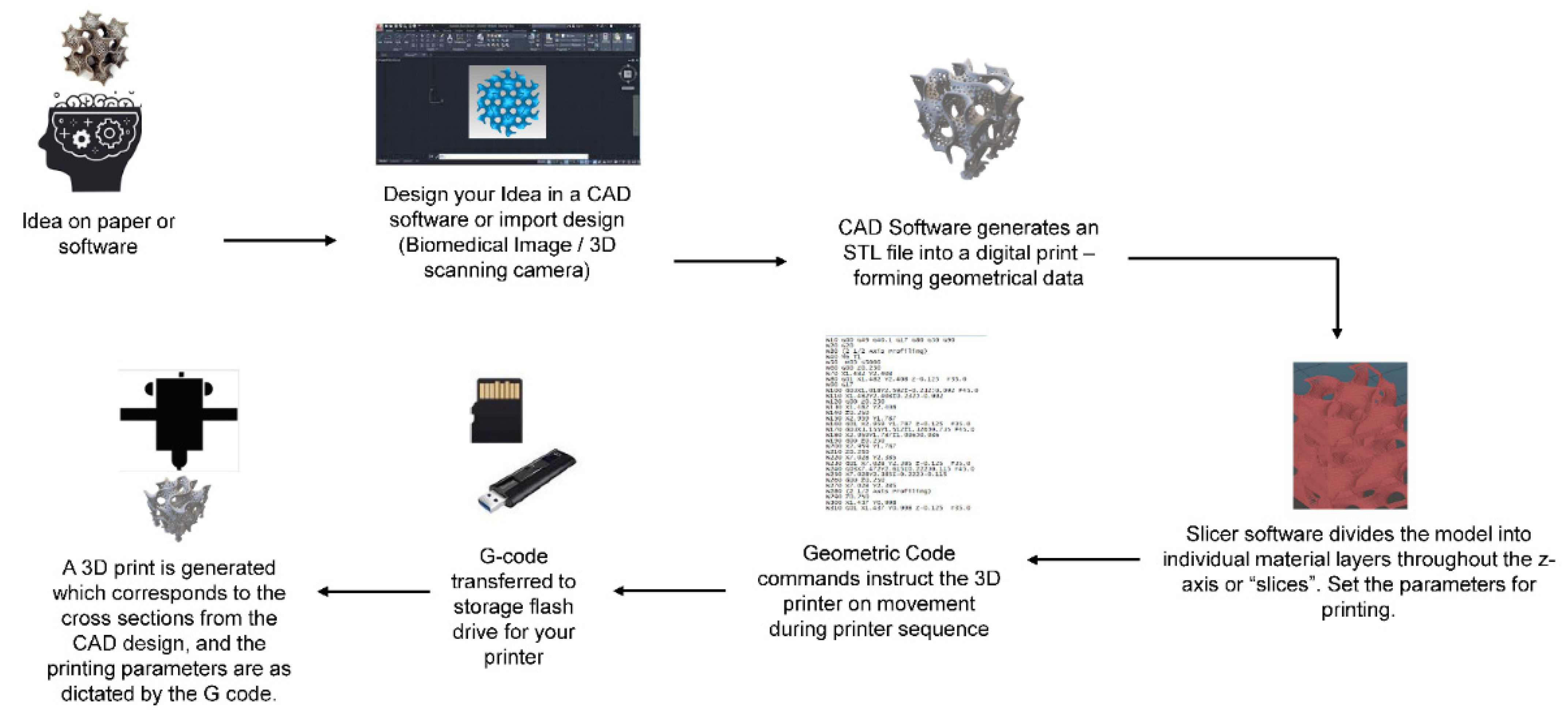
5. 3D Printing of Graphene Scaffolds
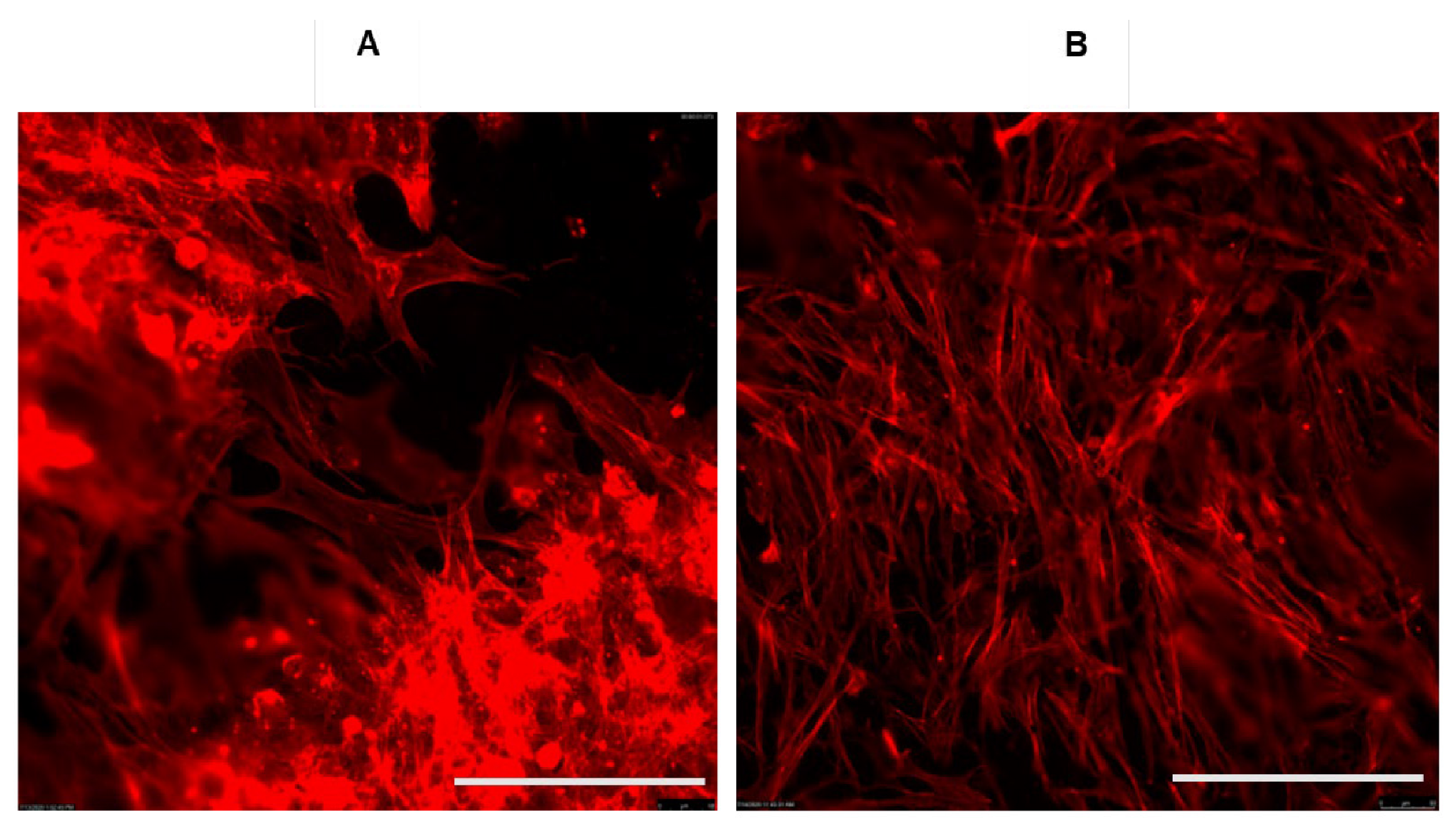
6. Graphene and Bone Regeneration
7. Challenges in 3D Printing Graphene
8. Toxicity Challenges of Graphene Materials
9. Future Perspective and Conclusions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [PubMed]
- Sharma, R. Stem Cells and Tissue Engineering in Medical Practice: Ethical and Regulatory Policies. Curr. Drug Targets 2019, 20, 388–398. [Google Scholar] [PubMed]
- Grimm, D.; Egli, M.; Krüger, M.; Riwaldt, S.; Corydon, T.J.; Kopp, S.; Wehland, M.; Wise, P.; Infanger, M.; Mann, V.; et al. Tissue Engineering Under Microgravity Conditions–Use of Stem Cells and Specialized Cells. Stem Cells Dev. 2018, 27, 787–804. [Google Scholar]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—A review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar]
- Oberweis, C.V.; Marchal, J.A.; Ruiz, E.L.; Galvez-Martin, M.P. A Worldwide Overview of Regulatory Frameworks for Tissue-Based Products. Tissue Eng. Part B Rev. 2020, 26, 181–196. [Google Scholar] [PubMed]
- Carpenter, M.K. Regulatory considerations for pluripotent stem cell therapies. Prog. Brain Res. 2017, 230, 151–163. [Google Scholar]
- George, B. Regulations and guidelines governing stem cell based products: Clinical considerations. Perspect. Clin. Res. 2011, 2, 94–99. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, D.A.; Wu, D.; He, F.; Wang, H.; Huang, L.; Shi, D.; Liu, Q.; Ni, N.; Pakvasa, M.; et al. Applications of Biocompatib le Scaffold Materials in Stem Cell-Based Cartilage Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 603444. [Google Scholar]
- Crapo, P.M.; Tottey, S.; Slivka, P.F.; Badylak, S.F. Effects of Biologic Scaffolds on Human Stem Cells and Implications for CNS Tissue Engineering. Tissue Eng. Part A 2014, 20, 313–323. [Google Scholar]
- Rana, D.; Zreiqat, H.; Benkirane-Jessel, N.; Ramakrishna, S.; Ramalingam, M. Development of decellularized scaffolds for stem cell-driven tissue engineering. J. Tissue Eng. Regen. Med. 2015, 11, 942–965. [Google Scholar]
- Kenry, L.W.; Loh, K.P.; Lim, C.T. When stem cells meet graphene: Opportunities and challenges in regenerative medicine. Biomaterials 2018, 155, 236–250. [Google Scholar] [PubMed]
- Shang, L.; Qi, Y.; Lu, H.; Pei, H.; Li, Y.; Qu, L.; Wu, Z.; Zhang, W. Graphene and Graphene Oxide for Tissue Engineering and Regeneration. In Theranostic Bionanomaterials; Cui, W., Zhao, X., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 165–185. [Google Scholar]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [PubMed]
- Grijalvo, S.; Díaz, D.D. Graphene-based hybrid materials as promising scaffolds for peripheral nerve regeneration. Neurochem. Int. 2021, 147, 105005. [Google Scholar] [PubMed]
- MacDonald, A.F.; Trotter, R.D.; Griffin, C.D.; Bow, A.J.; Newby, S.D.; King, W.J.; Amelse, L.L.; Masi, T.J.; Bourdo, S.E.; Dhar, M.S. Genetic profiling of human bone marrow and adipose tissue-derived mesenchymal stem cells reveals differences in osteogenic signaling mediated by graphene. J. Nanobiotechnol. 2021, 19, 285. [Google Scholar]
- Newby, S.D.; Masi, T.; Griffin, C.D.; King, W.J.; Chipman, A.; Stephenson, S.; Anderson, D.E.; Biris, A.S.; Bourdo, S.E.; Dhar, M. Functionalized Graphene Nanoparticles Induce Human Mesenchymal Stem Cells to Express Distinct Extracellular Matrix Proteins Mediating Osteogenesis. Int. J. Nanomed. 2020, 15, 2501–2513. [Google Scholar]
- Liu, S.; Li, Z.; Wang, Q.; Han, J.; Wang, W.; Li, S.; Liu, H.; Guo, S.; Zhang, J.; Ge, K.; et al. Graphene Oxide/Chitosan/Hydroxyapatite Composite Membranes Enhance Osteoblast Adhesion and Guided Bone Regeneration. ACS Appl. Bio Mater. 2021, 4, 8049–8059. [Google Scholar]
- Xie, H.; Cao, T.; Franco-Obregón, A.; Rosa, V. Graphene-Induced Osteogenic Differentiation Is Mediated by the Integrin/FAK Axis. Int. J. Mol. Sci. 2019, 20, 574. [Google Scholar]
- Qiu, X.; Liu, S.; Zhang, H.; Zhu, B.; Su, Y.; Zheng, C.; Tian, R.; Wang, M.; Kuang, H.; Zhao, X.; et al. Mesenchymal stem cells and extracellular matrix scaffold promote muscle regeneration by synergistically regulating macrophage polarization toward the M2 phenotype. Stem Cell Res. Ther. 2018, 9, 88. [Google Scholar]
- Zhu, C.; Du, D.; Lin, Y. Graphene-like 2D nanomaterial-based biointerfaces for biosensing applications. Biosens. Bioelectron. 2017, 89 Pt 1, 43–55. [Google Scholar]
- Luo, Y.; Li, Z.; Zhu, C.; Cai, X.; Qu, L.; Du, D.; Lin, Y. Graphene-like Metal-Free 2D Nanosheets for Cancer Imaging and Theranostics. Trends Biotechnol. 2018, 36, 1145–1156. [Google Scholar]
- Xia, B.; Deng, Y.; Lv, Y.; Chen, G. Stem cell recruitment based on scaffold features for bone tissue engineering. Biomater. Sci. 2020, 9, 1189–1203. [Google Scholar]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—Different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [PubMed]
- Oh, S.H.; Park, I.K.; Kim, J.M.; Lee, J.H. In vitro and in vivo characteristics of PCL scaffolds with pore size gradient fabricated by a centrifugation method. Biomaterials 2007, 28, 1664–1671. [Google Scholar] [PubMed]
- Li, J.; Liu, X.; Crook, J.M.; Wallace, G.G. 3D Printing of Cytocompatible Graphene/Alginate Scaffolds for Mimetic Tissue Constructs. Front. Bioeng. Biotechnol. 2020, 8, 824. [Google Scholar] [PubMed]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar]
- Bonnier, F.; Keating, M.; Wróbel, T.; Majzner, K.; Baranska, M.; Garcia-Munoz, A.; Blanco, A.; Byrne, H. Cell viability assessment using the Alamar blue assay: A comparison of 2D and 3D cell culture models. Toxicol. In Vitro 2014, 29, 124–131. [Google Scholar]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension: How 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125 Pt 13, 3015–3024. [Google Scholar]
- Harrison, B.S.; Atala, A. Carbon nanotube applications for tissue engineering. Biomaterials 2007, 28, 344–353. [Google Scholar]
- Maiti, D.; Tong, X.; Mou, X.; Yang, K. Carbon-Based Nanomaterials for Biomedical Applications: A Recent Study. Front. Pharmacol. 2019, 9, 1401. [Google Scholar]
- Veetil, J.V.; Ye, K. Tailored carbon nanotubes for tissue engineering applications. Biotechnol. Prog. 2009, 25, 709–721. [Google Scholar]
- Tabish, T.A.; Pranjol, M.Z.I.; Jabeen, F.; Abdullah, T.; Latif, A.; Khalid, A.; Ali, M.; Hayat, H.; Winyard, P.G.; Whatmore, J.L.; et al. Investigation into the toxic effects of graphene nanopores on lung cancer cells and biological tissues. Appl. Mater. Today 2018, 12, 389–401. [Google Scholar] [CrossRef]
- Patel, K.; Singh, R.; Kim, H.-W. Carbon based-nanomaterials as an emerging platform for theranostics. Mater. Horiz. 2018, 6, 434–469. [Google Scholar]
- Bai, R.G.; Muthoosamy, K.; Manickam, S.; Hilal-Alnaqbi, A. Graphene-based 3D scaffolds in tissue engineering: Fabrication, applications, and future scope in liver tissue engineering. Int. J. Nanomed. 2019, 14, 5753–5783. [Google Scholar]
- Bahrami, S.; Baheiraei, N.; Shahrezaee, M. Biomimetic reduced graphene oxide coated collagen scaffold for in situ bone regeneration. Sci. Rep. 2021, 11, 16783. [Google Scholar]
- Arnold, A.M.; Holt, B.D.; Daneshmandi, L.; Laurencin, C.T.; Sydlik, S.A. Phosphate graphene as an intrinsically osteoinductive scaffold for stem cell-driven bone regeneration. Proc. Natl. Acad. Sci. USA 2019, 116, 4855–4860. [Google Scholar] [CrossRef] [PubMed]
- Daneshmandi, L.; Barajaa, M.; Rad, A.T.; Sydlik, S.A.; Laurencin, C.T. Graphene-Based Biomaterials for Bone Regenerative Engineering: A Comprehensive Review of the Field and Considerations Regarding Biocompatibility and Biodegradation. Adv. Healthc. Mater. 2020, 10, e2001414. [Google Scholar]
- Prasadh, S.; Suresh, S.; Wong, R. Osteogenic Potential of Graphene in Bone Tissue Engineering Scaffolds. Materials 2018, 11, 1430. [Google Scholar] [CrossRef]
- Gong, M.; Sun, J.; Liu, G.; Li, L.; Wu, S.; Xiang, Z. Graphene oxide–modified 3D acellular cartilage extracellular matrix scaffold for cartilage regeneration. Mater. Sci. Eng. C 2020, 119, 111603. [Google Scholar] [CrossRef]
- Zhou, M.; Lozano, N.; Wychowaniec, J.K.; Hodgkinson, T.; Richardson, S.M.; Kostarelos, K.; Hoyland, J.A. Graphene oxide: A growth factor delivery carrier to enhance chondrogenic differentiation of human mesenchymal stem cells in 3D hydrogels. Acta Biomater. 2019, 96, 271–280. [Google Scholar]
- Shamekhi, M.A.; Mirzadeh, H.; Mahdavi, H.; Rabiee, A.; Mohebbi-Kalhori, D.; Eslaminejad, M.B. Graphene oxide containing chitosan scaffolds for cartilage tissue engineering. Int. J. Biol. Macromol. 2019, 127, 396–405. [Google Scholar]
- Bei, H.P.; Yang, Y.; Zhang, Q.; Tian, Y.; Luo, X.; Yang, M.; Zhao, X. Graphene-Based Nanocomposites for Neural Tissue Engineering. Molecules 2019, 24, 658. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Wang, X.; Song, J.; Chen, W.; Chen, S.; Jin, Y.; Ouyang, Y.; Yuan, W.-E.; Fan, C. Preclinical assessment on neuronal regeneration in the injury-related microenvironment of graphene-based scaffolds. NPJ Regen. Med. 2021, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Safina, I.; Bourdo, S.E.; AlGazali, K.M.; Kannarpady, G.; Watanabe, F.; Vang, K.B.; Biris, A.S. Graphene-based 2D constructs for enhanced fibroblast support. PLoS ONE 2020, 15, e0232670. [Google Scholar] [CrossRef]
- Hussein, K.H.; Abdelhamid, H.N.; Zou, X.; Woo, H.-M. Ultrasonicated graphene oxide enhances bone and skin wound regeneration. Mater. Sci. Eng. C 2018, 94, 484–492. [Google Scholar] [CrossRef]
- Lasocka, I.; Jastrzębska, E.; Szulc-Dąbrowska, L.; Skibniewski, M.; Pasternak, I.; Kalbacova, M.H.; Skibniewska, E.M. The effects of graphene and mesenchymal stem cells in cutaneous wound healing and their putative action mechanism. Int. J. Nanomed. 2019, 14, 2281–2299. [Google Scholar] [CrossRef] [PubMed]
- Sekuła-Stryjewska, M.; Noga, S.; Dźwigońska, M.; Adamczyk, E.; Karnas, E.; Jagiełło, J.; Szkaradek, A.; Chytrosz, P.; Boruczkowski, D.; Madeja, Z.; et al. Graphene-based materials enhance cardiomyogenic and angiogenic differentiation capacity of human mesenchymal stem cells in vitro—Focus on cardiac tissue regeneration. Mater. Sci. Eng. C 2020, 119, 111614. [Google Scholar] [CrossRef]
- Hajishoreh, N.K.; Baheiraei, N.; Naderi, N.; Salehnia, M. Reduced graphene oxide facilitates biocompatibility of alginate for cardiac repair. J. Bioact. Compat. Polym. 2020, 35, 363–377. [Google Scholar] [CrossRef]
- Bahrami, S.; Baheiraei, N.; Mohseni, M.; Razavi, M.; Ghaderi, A.; Azizi, B.; Rabiee, N.; Karimi, M. Three-dimensional graphene foam as a conductive scaffold for cardiac tissue engineering. J. Biomater. Appl. 2019, 34, 74–85. [Google Scholar] [CrossRef]
- Kozbial, A.; Li, Z.; Sun, J.; Gong, X.; Zhou, F.; Wang, Y.; Xu, H.; Liu, H.; Li, L. Understanding the intrinsic water wettability of graphite. Carbon 2014, 74, 218–225. [Google Scholar] [CrossRef]
- Brodie, B.C. XIII. On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar]
- Halim, A.; Luo, Q.; Ju, Y.; Song, G. A Mini Review Focused on the Recent Applications of Graphene Oxide in Stem Cell Growth and Differentiation. Nanomaterials 2018, 8, 736. [Google Scholar] [CrossRef]
- Tiginyanu, I.; Ursaki, V.; Popa, V. Ultra-thin membranes for sensor applications. In Nanocoatings and Ultra-Thin Films; Makhlouf, A.S.H., Tiginyanu, I., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 330–354. [Google Scholar]
- Araújo, M.P.; Soares, O.S.G.P.; Fernandes, A.J.S.; Pereira, M.F.R.; Freire, C. Tuning the surface chemistry of graphene flakes: New strategies for selective oxidation. RSC Adv. 2017, 7, 14290–14301. [Google Scholar] [CrossRef]
- Seyedsalehi, A.; Daneshmandi, L.; Barajaa, M.; Riordan, J.; Laurencin, C.T. Fabrication and characterization of mechanically competent 3D printed polycaprolactone-reduced graphene oxide scaffolds. Sci. Rep. 2020, 10, 22210. [Google Scholar] [CrossRef]
- Wei, X.; Li, D.; Jiang, W.; Gu, Z.; Wang, X.; Zhang, Z.; Sun, Z. 3D Printable Graphene Composite. Sci. Rep. 2015, 5, 11181. [Google Scholar] [CrossRef]
- Shahdeo, D.; Roberts, A.; Abbineni, N.; Gandhi, S. Graphene based sensors. In Comprehensive Analytical Chemistry; Hussain, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 175–199. [Google Scholar]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.-S.; Edwards, J.R.; Jeon, H. Electrospun Fibrous Scaffolds for Tissue Engineering: Viewpoints on Architecture and Fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef]
- Sola, A.; Bertacchini, J.; D’Avella, D.; Anselmi, L.; Maraldi, T.; Marmiroli, S.; Messori, M. Development of solvent-casting particulate leaching (SCPL) polymer scaffolds as improved three-dimensional supports to mimic the bone marrow niche. Mater. Sci. Eng. C 2018, 96, 153–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehterami, A.; Masoomikarimi, M.; Bastami, F.; Jafarisani, M.; Alizadeh, M.; Mehrabi, M.; Salehi, M. Fabrication and Characterization of Nanofibrous Poly (L-Lactic Acid)/Chitosan-Based Scaffold by Liquid-Liquid Phase Separation Technique for Nerve Tissue Engineering. Mol. Biotechnol. 2021, 63, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Pati, F.; Shim, J.H.; Cho, D.W. Rapid prototyping technology for bone regeneration. In Rapid Prototyping of Biomaterials; Narayan, R., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 254–284. [Google Scholar]
- Manjunath, K.S.; Sridhar, K.; Gopinath, V.; Sankar, K.; Sundaram, A.; Gupta, N.; Shiek, A.S.S.J.; Shantanu, P.S. Facile manufacturing of fused-deposition modeled composite scaffolds for tissue engineering—An embedding model with plasticity for incorporation of additives. Biomed. Mater. 2020, 16, 015028. [Google Scholar] [CrossRef]
- Percoco, G.; Uva, A.E.; Fiorentino, M.; Gattullo, M.; Manghisi, V.M.; Boccaccio, A. Mechanobiological Approach to Design and Optimize Bone Tissue Scaffolds 3D Printed with Fused Deposition Modeling: A Feasibility Study. Materials 2020, 13, 648. [Google Scholar] [CrossRef]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An Overview on 3D Printing Technology: Technological, Materials, and Applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Lai, J.H.; Wang, C.; Wang, M. 3D printing in biomedical engineering: Processes, materials, and applications. Appl. Phys. Rev. 2021, 8, 021322. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Seo, T.H.; Lee, S.; Jang, W.; Kim, M.J.; Sung, J.-S. Neuronal differentiation of human mesenchymal stem cells in response to the domain size of graphene substrates. J. Biomed. Mater. Res. Part A 2017, 106, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.A.; Kwak, S.-Y.; Yang, J.-K.; Lee, Y.-S.; Kim, J.-H.; Kim, H.D.; Hwang, N.S. Graphene oxide film guided skeletal muscle differentiation. Mater. Sci. Eng. C 2021, 126, 112174. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Q.; Gao, S.; Song, Q.; Huang, R.; Wang, L.; Liu, L.; Dai, J.; Tang, M.; Cheng, G. Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Sci. Rep. 2013, 3, 1604. [Google Scholar] [CrossRef]
- Crowder, S.W.; Prasai, D.; Rath, R.; Balikov, D.; Bae, H.; Bolotin, K.I.; Sung, H.-J. Three-dimensional graphene foams promote osteogenic differentiation of human mesenchymal stem cells. Nanoscale 2013, 5, 4171–4176. [Google Scholar] [CrossRef]
- Tasnim, N.; Thakur, V.; Chattopadhyay, M.; Joddar, B. The Efficacy of Graphene Foams for Culturing Mesenchymal Stem Cells and Their Differentiation into Dopaminergic Neurons. Stem Cells Int. 2018, 2018, 3410168. [Google Scholar] [CrossRef]
- Amani, H.; Mostafavi, E.; Arzaghi, H.; Davaran, S.; Akbarzadeh, A.; Akhavan, O.; Pazoki-Toroudi, H.; Webster, T.J. Three-Dimensional Graphene Foams: Synthesis, Properties, Biocompatibility, Biodegradability, and Applications in Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 5, 193–214. [Google Scholar] [CrossRef]
- Tolou, N.B.; Salimijazi, H.; Kharaziha, M.; Faggio, G.; Chierchia, R.; Lisi, N. A three-dimensional nerve guide conduit based on graphene foam/polycaprolactone. Mater. Sci. Eng. C 2021, 126, 112110. [Google Scholar] [CrossRef]
- Shin, Y.C.; Kang, S.H.; Lee, J.H.; Kim, B.; Hong, S.W.; Han, D.-W. Three-dimensional graphene oxide-coated polyurethane foams beneficial to myogenesis. J. Biomater. Sci. Polym. Ed. 2017, 29, 762–774. [Google Scholar] [CrossRef]
- Zhu, C.; Han, T.Y.-J.; Duoss, E.B.; Golobic, A.M.; Kuntz, J.; Spadaccini, C.M.; Worsley, M.A. Highly compressible 3D periodic graphene aerogel microlattices. Nat. Commun. 2015, 6, 6962. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xu, Z.; Huang, T.; Liu, Y.; Guo, F.; Xi, J.; Gao, W.; Gao, C. Direct 3D Printing of Ultralight Graphene Oxide Aerogel Microlattices. Adv. Funct. Mater. 2018, 28, 1707024. [Google Scholar] [CrossRef]
- VijayaVenkataRaman, S.; Thaharah, S.; Zhang, S.; Lu, W.F.; Fuh, J.Y.H. 3D-Printed PCL/rGO Conductive Scaffolds for Peripheral Nerve Injury Repair. Artif. Organs 2018, 43, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, W.; Bartolo, P. Novel Poly(varepsilon-caprolactone)/Graphene Scaffolds for Bone Cancer Treatment and Bone Regeneration. 3D Print Addit. Manuf. 2020, 7, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.; Locascio, L.E. Polymer microfluidic devices. Talanta 2002, 56, 267–287. [Google Scholar] [CrossRef]
- Nalesso, P.R.L.; Wang, W.; Hou, Y.; Bagne, L.; Pereira, A.T.; Helaehil, J.V.; de Andrade, T.A.M.; Chiarotto, G.B.; Bártolo, P.; Caetano, G.F. In vivo investigation of 3D printed polycaprolactone/graphene electro-active bone scaffolds. Bioprinting 2021, 24, e00164. [Google Scholar] [CrossRef]
- Gasparotto, M.; Bellet, P.; Scapin, G.; Busetto, R.; Rampazzo, C.; Vitiello, L.; Shah, D.I.; Filippini, F. 3D Printed Graphene-PLA Scaffolds Promote Cell Alignment and Differentiation. Int. J. Mol. Sci. 2022, 23, 1736. [Google Scholar] [CrossRef]
- Biscaia, S.; Silva, J.C.; Moura, C.; Viana, T.; Tojeira, A.; Mitchell, G.R.; Pascoal-Faria, P.; Ferreira, F.C.; Alves, N. Additive Manufactured Poly(ε-caprolactone)-graphene Scaffolds: Lamellar Crystal Orientation, Mechanical Properties and Biological Performance. Polymers 2022, 14, 1669. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Crook, J.M.; Wallace, G.G. Development of a porous 3D graphene-PDMS scaffold for improved osseointegration. Colloids Surf. B Biointerfaces 2017, 159, 386–393. [Google Scholar] [CrossRef]
- Yang, S.; Leong, K.-F.; Du, Z.; Chua, C.-K. The Design of Scaffolds for Use in Tissue Engineering. Part II. Rapid Prototyping Techniques. Tissue Eng. 2002, 8, 1–11. [Google Scholar] [CrossRef]
- Murphy, C.M.; O’Brien, F.J. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adhes. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Tupone, M.G.; Panella, G.; D’Angelo, M.; Castelli, V.; Caioni, G.; Catanesi, M.; Benedetti, E.; Cimini, A. An Update on Graphene-Based Nanomaterials for Neural Growth and Central Nervous System Regeneration. Int. J. Mol. Sci. 2021, 22, 13047. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, Y.C.; Jin, O.S.; Kang, S.H.; Hwang, Y.-S.; Park, J.-C.; Hong, S.W.; Han, D.-W. Reduced graphene oxide-coated hydroxyapatite composites stimulate spontaneous osteogenic differentiation of human mesenchymal stem cells. Nanoscale 2015, 7, 11642–11651. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Cao, T.; Gomes, J.V.; Neto, A.H.C.; Rosa, V. Two and three-dimensional graphene substrates to magnify osteogenic differentiation of periodontal ligament stem cells. Carbon 2015, 93, 266–275. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Q.; Chen, Y.; Fu, Y.; Lu, S.; Yu, X.; Yu, D.; Zhao, W. Effects of graphene oxide and graphene oxide quantum dots on the osteogenic differentiation of stem cells from human exfoliated deciduous teeth. Artif. Cells Nanomed. Biotechnol. 2019, 47, 822–832. [Google Scholar] [CrossRef]
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.L.R.; Ahn, J.-H.; Hong, B.H.; Pastorin, G.; et al. Graphene for Controlled and Accelerated Osteogenic Differentiation of Human Mesenchymal Stem Cells. ACS Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.-R.; Kim, Y.; Lim, K.T.; Seonwoo, H.; Park, S.; Cho, S.-P.; Hong, B.H.; Choung, P.-H.; Chung, T.D.; et al. Graphene-incorporated chitosan substrata for adhesion and differentiation of human mesenchymal stem cells. J. Mater. Chem. B 2013, 1, 933–938. [Google Scholar] [CrossRef]
- Mo, X.; Wei, Y.; Zhang, X.; Cai, Q.; Shen, Y.; Dai, X.; Meng, S.; Liu, X.; Liu, Y.; Hu, Z.; et al. Enhanced Stem Cell Osteogenic Differentiation by Bioactive Glass Functionalized Graphene Oxide Substrates. J. Nanomater. 2016, 2016, 5613980. [Google Scholar] [CrossRef]
- Hermenean, A.; Codreanu, A.; Herman, H.; Balta, C.; Rosu, M.; Mihali, C.V.; Ivan, A.; Dinescu, S.; Ionita, M.; Costache, M. Chitosan-Graphene Oxide 3D scaffolds as Promising Tools for Bone Regeneration in Critical-Size Mouse Calvarial Defects. Sci. Rep. 2017, 7, 16641. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, Y.C.; Lee, S.-M.; Jin, O.S.; Kang, S.H.; Hong, S.W.; Jeong, C.-M.; Huh, J.B.; Han, D.-W. Enhanced Osteogenesis by Reduced Graphene Oxide/Hydroxyapatite Nanocomposites. Sci. Rep. 2015, 5, 18833. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, P.; Li, X.; Liu, H.; Ge, S. Bioactivity of periodontal ligament stem cells on sodium titanate coated with graphene oxide. Sci. Rep. 2016, 6, srep19343. [Google Scholar] [CrossRef] [PubMed]
- Lyu, C.-Q.; Lu, J.-Y.; Cao, C.-H.; Luo, D.; Fu, Y.-X.; He, Y.-S.; Zou, D.-R. Induction of Osteogenic Differentiation of Human Adipose-Derived Stem Cells by a Novel Self-Supporting Graphene Hydrogel Film and the Possible Underlying Mechanism. ACS Appl. Mater. Interfaces 2015, 7, 20245–20254. [Google Scholar] [CrossRef] [PubMed]
- Radunovic, M.; De Colli, M.; De Marco, P.; Di Nisio, C.; Fontana, A.; Piattelli, A.; Cataldi, A.; Zara, S. Graphene oxide enrichment of collagen membranes improves DPSCs differentiation and controls inflammation occurrence. J. Biomed. Mater. Res. Part A 2017, 105, 2312–2320. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.D.; Park, J.; Lee, E.-S.; Kim, E.; Lee, S.S.; Yang, J.-K.; Lee, Y.-S.; Hwang, N.S. Enhanced osteogenic commitment of murine mesenchymal stem cells on graphene oxide substrate. Biomater. Res. 2018, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- Shie, M.-Y.; Chiang, W.-H.; Chen, I.-W.P.; Liu, W.-Y.; Chen, Y.-W. Synergistic acceleration in the osteogenic and angiogenic differentiation of human mesenchymal stem cells by calcium silicate–graphene composites. Mater. Sci. Eng. C 2017, 73, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, R.; Di Crescenzo, A.; Pilato, S.; Ventrella, A.; Piattelli, A.; Recinella, L.; Chiavaroli, A.; Giordani, S.; Baldrighi, M.; Camisasca, A.; et al. Osteoblastic Differentiation on Graphene Oxide-Functionalized Titanium Surfaces: An In Vitro Study. Nanomaterials 2020, 10, 654. [Google Scholar] [CrossRef]
- Zhang, J.; Eyisoylu, H.; Qin, X.H.; Rubert, M.; Müller, R. 3D bioprinting of graphene oxide-incorporated cell-laden bone mimicking scaffolds for promoting scaffold fidelity, osteogenic differentiation and mineralization. Acta Biomater. 2021, 121, 637–652. [Google Scholar] [CrossRef]
- Zhong, C.; Feng, J.; Lin, X.; Bao, Q. Continuous release of bone morphogenetic protein-2 through nano-graphene oxide-based delivery influences the activation of the NF-κB signal transduction pathway. Int. J. Nanomed. 2017, 12, 1215–1226. [Google Scholar] [CrossRef]
- La, W.-G.; Jung, M.-J.; Yoon, J.-K.; Bhang, S.H.; Jang, H.-K.; Lee, T.-J.; Yoon, H.-H.; Shin, J.-Y.; Kim, B.-S. Bone morphogenetic protein-2 for bone regeneration—Dose reduction through graphene oxide-based delivery. Carbon 2014, 78, 428–438. [Google Scholar] [CrossRef]
- Wei, C.; Liu, Z.; Jiang, F.; Zeng, B.; Huang, M.; Yu, D. Cellular behaviours of bone marrow-derived mesenchymal stem cells towards pristine graphene oxide nanosheets. Cell Prolif. 2017, 50, e12367. [Google Scholar] [CrossRef]
- Sun, J.; Li, L.; Xing, F.; Yang, Y.; Gong, M.; Liu, G.; Wu, S.; Luo, R.; Duan, X.; Liu, M.; et al. Graphene oxide-modified silk fibroin/nanohydroxyapatite scaffold loaded with urine-derived stem cells for immunomodulation and bone regeneration. Stem Cell Res. Ther. 2021, 12, 591. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, M.; Wang, K.; Sun, Y.; Zhang, H.; Lu, X.; Duan, K. Molecular mechanisms of interactions between BMP-2 and graphene: Effects of functional groups and microscopic morphology. Appl. Surf. Sci. 2020, 525, 146636. [Google Scholar] [CrossRef]
- Fu, C.; Yang, X.; Tan, S.; Song, L. Enhancing Cell Proliferation and Osteogenic Differentiation of MC3T3-E1 Pre-osteoblasts by BMP-2 Delivery in Graphene Oxide-Incorporated PLGA/HA Biodegradable Microcarriers. Sci. Rep. 2017, 7, 12549. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Feng, X.; Wang, Y.; Wang, X.; He, Y. Biomimetic and immunomodulatory baicalin-loaded graphene oxide-demineralized bone matrix scaffold for in vivo bone regeneration. J. Mater. Chem. B 2021, 9, 9720–9733. [Google Scholar] [CrossRef]
- Weaver, C.L.; Cui, X.T. Directed Neural Stem Cell Differentiation with a Functionalized Graphene Oxide Nanocomposite. Adv. Healthc. Mater. 2015, 4, 1408–1416. [Google Scholar] [CrossRef]
- Ghaemi, A.; Javadi, S.; Heidari, M.K.; Rashedi, H.; Yazdian, F.; Omidi, M.; Tavakoli, Z.; Sheikhpour, M. Graphene-based materials in drug delivery and growth factor release: A critical review. Wound Med. 2020, 31, 100193. [Google Scholar] [CrossRef]
- Yoon, H.H.; Bhang, S.H.; Kim, T.; Yu, T.; Hyeon, T.; Kim, B.-S. Dual Roles of Graphene Oxide in Chondrogenic Differentiation of Adult Stem Cells: Cell-Adhesion Substrate and Growth Factor-Delivery Carrier. Adv. Funct. Mater. 2014, 24, 6455–6464. [Google Scholar] [CrossRef]
- EL Magri, A.; Vanaei, S.; Shirinbayan, M.; Vaudreuil, S.; Tcharkhtchi, A. An Investigation to Study the Effect of Process Parameters on the Strength and Fatigue Behavior of 3D-Printed PLA-Graphene. Polymers 2021, 13, 3218. [Google Scholar] [CrossRef]
- Bustillos, J.; Montero, D.; Nautiyal, P.; Loganathan, A.; Boesl, B.; Agarwal, A. Integration of graphene in poly(lactic) acid by 3D printing to develop creep and wear-resistant hierarchical nanocomposites. Polym. Compos. 2017, 39, 3877–3888. [Google Scholar] [CrossRef]
- Vidakis, N.; Maniadi, A.; Petousis, M.; Vamvakaki, M.; Kenanakis, G.; Koudoumas, E. Mechanical and Electrical Properties Investigation of 3D-Printed Acrylonitrile–Butadiene–Styrene Graphene and Carbon Nanocomposites. J. Mater. Eng. Perform. 2020, 29, 1909–1918. [Google Scholar] [CrossRef]
- Ivanov, E.; Kotsilkova, R.; Xia, H.; Chen, Y.; Donato, R.K.; Donato, K.; Godoy, A.P.; Di Maio, R.; Silvestre, C.; Cimmino, S.; et al. PLA/Graphene/MWCNT Composites with Improved Electrical and Thermal Properties Suitable for FDM 3D Printing Applications. Appl. Sci. 2019, 9, 1209. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Savvakis, K.; Maniadi, A.; Koudoumas, E. A comprehensive investigation of the mechanical behavior and the dielectrics of pure polylactic acid (PLA) and PLA with graphene (GnP) in fused deposition modeling (FDM). Int. J. Plast. Technol. 2019, 23, 195–206. [Google Scholar] [CrossRef]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part. Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.-P.; Sun, T.; Junaid, M.; Yang, L.; Ma, Y.-B.; Cui, Z.-S.; Wei, D.-P.; Shi, H.-F.; Pei, D.-S. Nanotoxicity of different sizes of graphene (G) and graphene oxide (GO) in vitro and in vivo. Environ. Pollut. 2019, 247, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Seabra, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Durán, N. Nanotoxicity of Graphene and Graphene Oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef]
- Sharma, G.; Nagpal, A. Hairy intraoral flaps: An uncommon complication of surgical therapy in oral cancer. Saudi J. Oral Sci. 2014, 1, 123–124. [Google Scholar] [CrossRef]
- Ma, Y.; Shen, H.; Tu, X.; Zhang, Z. Assessing in vivo toxicity of graphene materials: Current methods and future outlook. Nanomedicine 2014, 9, 1565–1580. [Google Scholar] [CrossRef]
- Chong, Y.; Ma, Y.; Shen, H.; Tu, X.; Zhou, X.; Xu, J.; Dai, J.; Fan, S.; Zhang, Z. The in vitro and in vivo toxicity of graphene quantum dots. Biomaterials 2014, 35, 5041–5048. [Google Scholar] [CrossRef]
- Zhang, L.; Ouyang, S.; Zhang, H.; Qiu, M.; Dai, Y.; Wang, S.; Wang, Y.; Ou, J. Graphene oxide induces dose-dependent lung injury in rats by regulating autophagy. Exp. Ther. Med. 2021, 21, 462. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Chao, H.-R.; Jiang, J.-J.; Su, Y.-H.; Cortez, M.-S.P.; Tayo, L.L.; Lu, I.-C.; Hsieh, H.; Lin, C.-C.; Lin, S.-L.; et al. Toxicity of Low-dose Graphene Oxide Nanoparticles in an in-vivo Wild Type of Caenorhabditis elegans Model. Aerosol Air Qual. Res. 2021, 21, 200559. [Google Scholar] [CrossRef]
- D’Amora, M.; Alfe, M.; Gargiulo, V.; Giordani, S. Graphene-Like Layers from Carbon Black: In Vivo Toxicity Assessment. Nanomaterials 2020, 10, 1472. [Google Scholar] [CrossRef] [PubMed]
- Fanizza, C.; Stefanelli, M.; Risuglia, A.; Bruni, E.; Ietto, F.; Incoronato, F.; Marra, F.; Preziosi, A.; Mancini, P.; Sarto, M.S.; et al. In Vitro and In Vivo Biocompatibility Studies on Engineered Fabric with Graphene Nanoplatelets. Nanomaterials 2022, 12, 1405. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.-T.; Liu, Z. Graphene in Mice: Ultrahigh In Vivo Tumor Uptake and Efficient Photothermal Therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef] [PubMed]
- Rhazouani, A.; Gamrani, H.; El Achaby, M.; Aziz, K.; Gebrati, L.; Uddin, M.S.; Aziz, F. Synthesis and Toxicity of Graphene Oxide Nanoparticles: A Literature Review of In Vitro and In Vivo Studies. BioMed Res. Int. 2021, 2021, 5518999. [Google Scholar] [CrossRef]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett. 2010, 6, 8. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, J.; Peng, C.; Hu, W.; Zhu, Z.; Li, W.; Fan, C.; Huang, Q. Distribution and biocompatibility studies of graphene oxide in mice after intravenous administration. Carbon 2011, 49, 986–995. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, M.K.; Kulkarni, P.P.; Sonkar, V.K.; Grácio, J.J.A.; Dash, D. Amine-Modified Graphene: Thrombo-Protective Safer Alternative to Graphene Oxide for Biomedical Applications. ACS Nano 2012, 6, 2731–2740. [Google Scholar] [CrossRef]
- Schinwald, A.; Murphy, F.A.; Jones, A.; MacNee, W.; Donaldson, K. Graphene-Based Nanoplatelets: A New Risk to the Respiratory System as a Consequence of Their Unusual Aerodynamic Properties. ACS Nano 2012, 6, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Amrollahi-Sharifabadi, M.; Koohi, M.K.; Zayerzadeh, E.; Hablolvarid, M.H.; Hassan, J.; Seifalian, A.M. In vivo toxicological evaluation of graphene oxide nanoplatelets for clinical application. Int. J. Nanomed. 2018, 13, 4757–4769. [Google Scholar] [CrossRef] [Green Version]

| Author, Year | Graphene Source | 3D-Printer Model | Polymer | Printing Parameters | Overall Purpose |
|---|---|---|---|---|---|
| Zhu, 2015 [75] | GO | Silica | 3-axis positioning stage (ABL 9000, Aerotech) | N/A | To demonstrate a 3D-printing strategy for graphene |
| Wei, 2015 [56] | rGO | ABS Or PLA | HOF1-X1 | rGO-ABS Chamber Temp: 230 °C Platform Temp: 80 °C Nozzle: 130 °C Speed: 20 mm/s rGO-PLA Chamber Temp: 190 °C Platform Temp: 60 °C Nozzle:130 °C Speed: 20 mm/s | To demonstrate graphene is 3D printable |
| Jiang, 2018 [76] | GO | GO was crosslinked with Ca2+ ions to form a hydrogel | TH-206H | Room Temp Pressure: 2–3 bar Speed: 4–10 mm s−1 | To enhance the functionality of 3D-printed graphene structures |
| Vijayavenkataraman, 2019 [77] | rGO | PCL | Electrohydrodynamic jet (EHD-jet) | N/A | To create a nerve guide conduit for neural regeneration |
| Seyedsalehi, 2020 [55] | rGO | PCL | 4th Generation 3D Bioplotter | Temp: 100 °C Platform Temp: 10 °C Pressure: 0.6 MPa Speed: 1.4 mm/s | To evaluate printability, mechanical, and biological properties |
| Hou, 2020 [78] | Graphene | PCL | 3DDisconveryTM Evolution | Temp: 90 °C Screw Rotation Velocity: 8 rpm Deposit velocity: 12 mm/s Pressure: 6 bar | To create a scaffold for osteosarcoma and bone regeneration |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacDonald, A.F.; Harley-Troxell, M.E.; Newby, S.D.; Dhar, M.S. 3D-Printing Graphene Scaffolds for Bone Tissue Engineering. Pharmaceutics 2022, 14, 1834. https://doi.org/10.3390/pharmaceutics14091834
MacDonald AF, Harley-Troxell ME, Newby SD, Dhar MS. 3D-Printing Graphene Scaffolds for Bone Tissue Engineering. Pharmaceutics. 2022; 14(9):1834. https://doi.org/10.3390/pharmaceutics14091834
Chicago/Turabian StyleMacDonald, Amber F., Meaghan E. Harley-Troxell, Steven D. Newby, and Madhu S. Dhar. 2022. "3D-Printing Graphene Scaffolds for Bone Tissue Engineering" Pharmaceutics 14, no. 9: 1834. https://doi.org/10.3390/pharmaceutics14091834
APA StyleMacDonald, A. F., Harley-Troxell, M. E., Newby, S. D., & Dhar, M. S. (2022). 3D-Printing Graphene Scaffolds for Bone Tissue Engineering. Pharmaceutics, 14(9), 1834. https://doi.org/10.3390/pharmaceutics14091834







