Identification of Transferrin Receptor 1 (TfR1) Overexpressed in Lung Cancer Cells, and Internalization of Magnetic Au-CoFe2O4 Core-Shell Nanoparticles Functionalized with Its Ligand in a Cellular Model of Small Cell Lung Cancer (SCLC)
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Sample Processing for Mass Spectrometry
2.3. Relative Quantification by Label-Free DIA Mass Spectrometry
2.4. Data Analysis
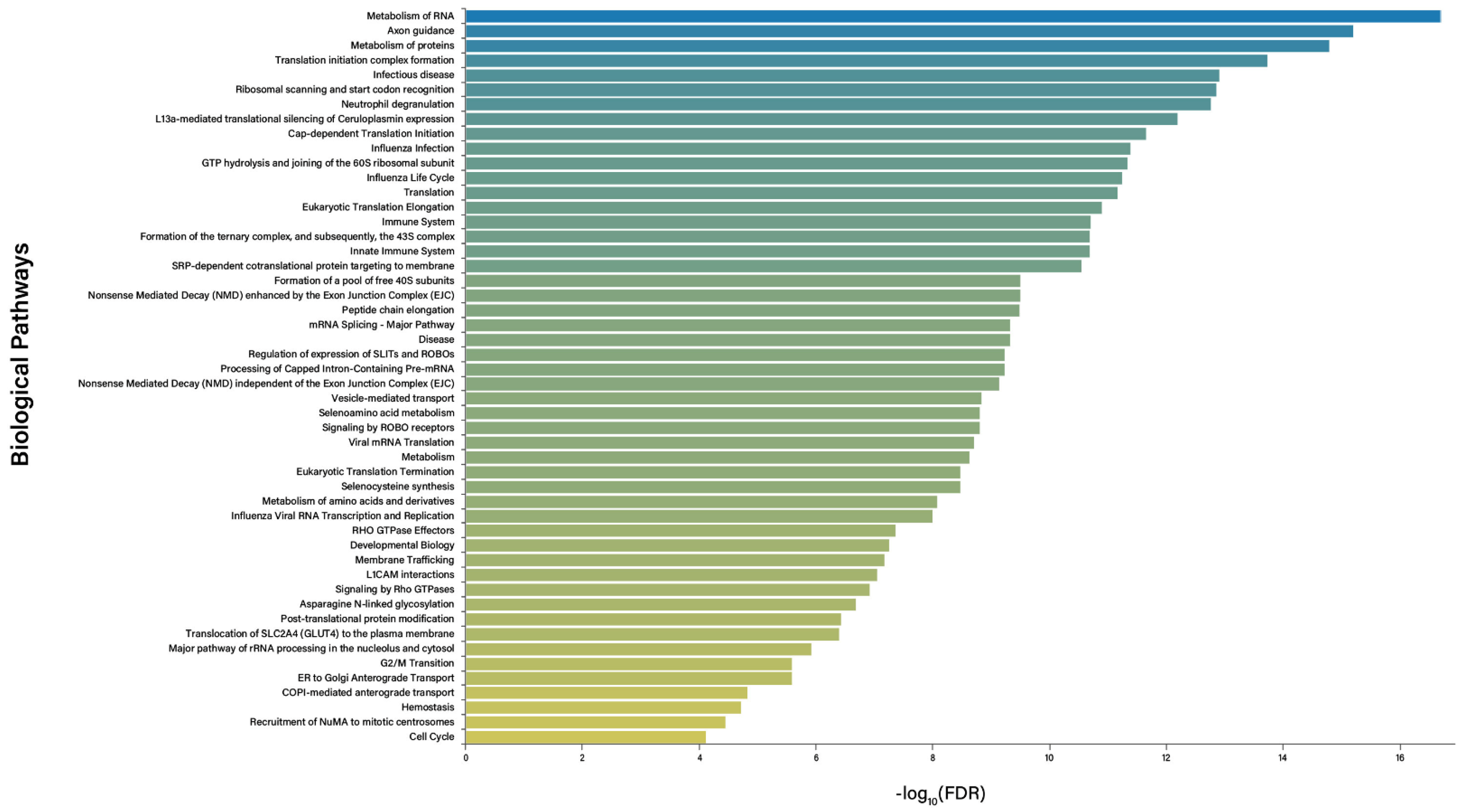
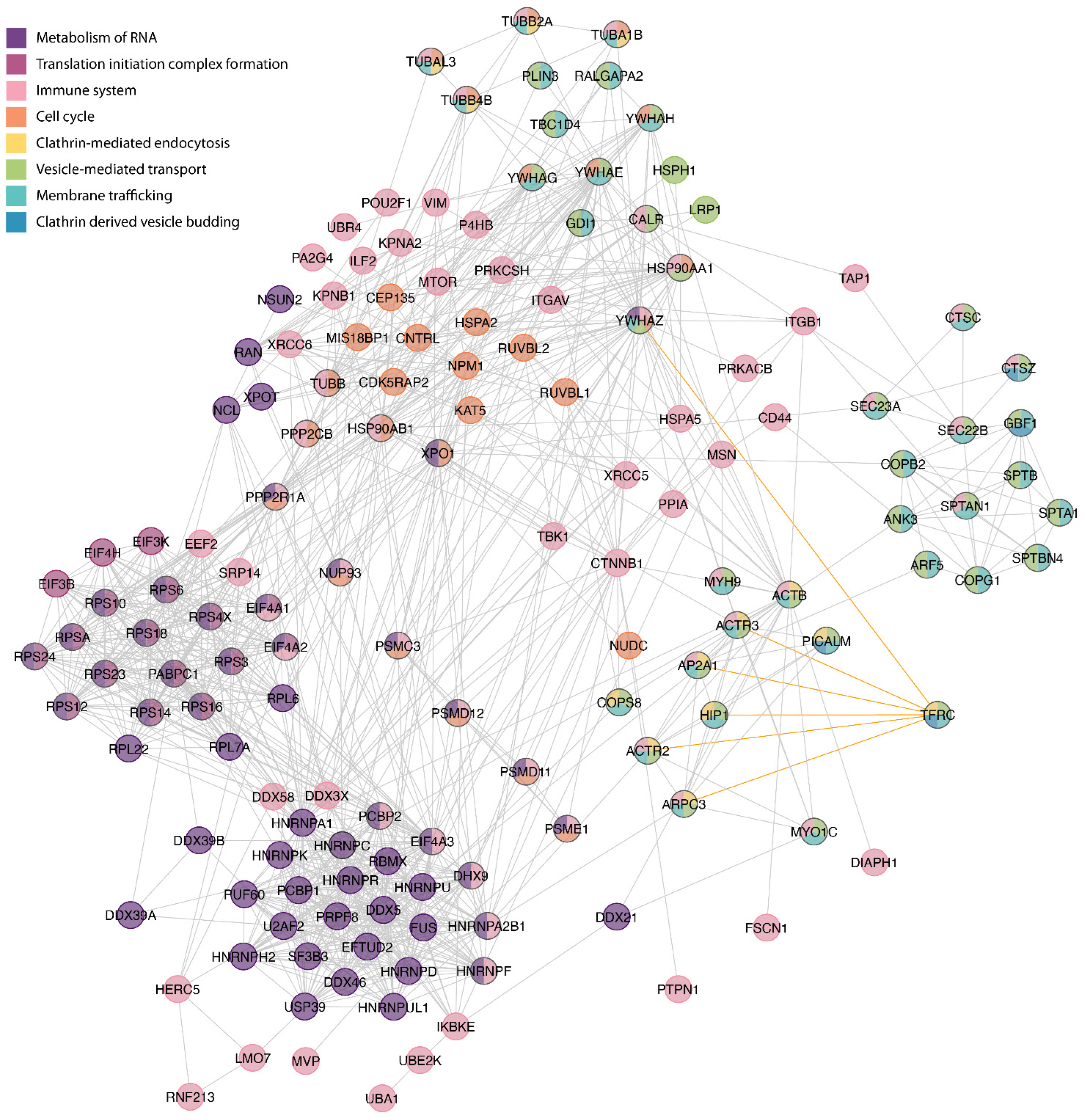
2.5. Bioinformatic Analysis
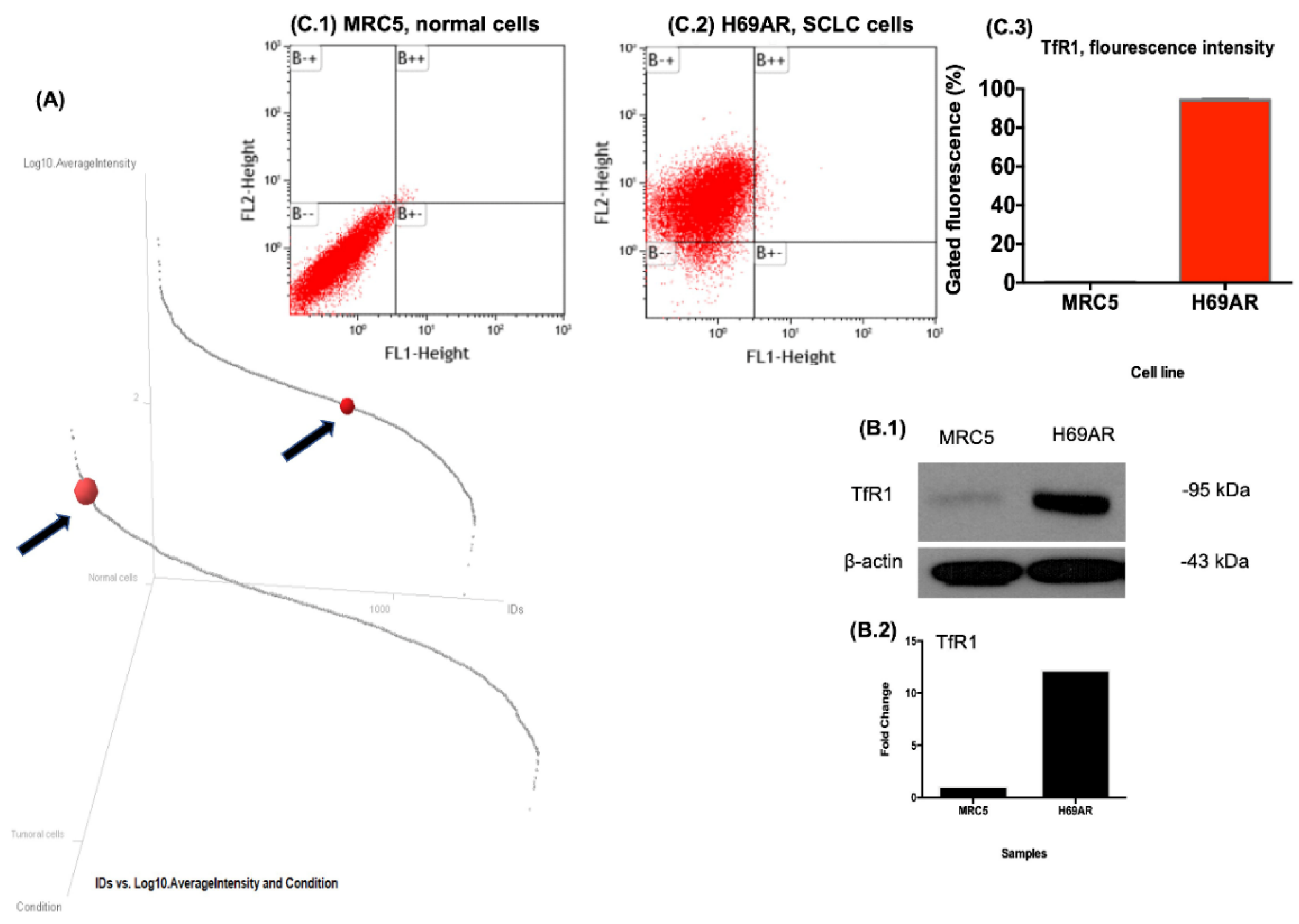
2.6. Western Blot
2.7. Flow Cytometry
2.7.1. For the Overexpressed Receptor
2.7.2. For the Endocytosis Studies
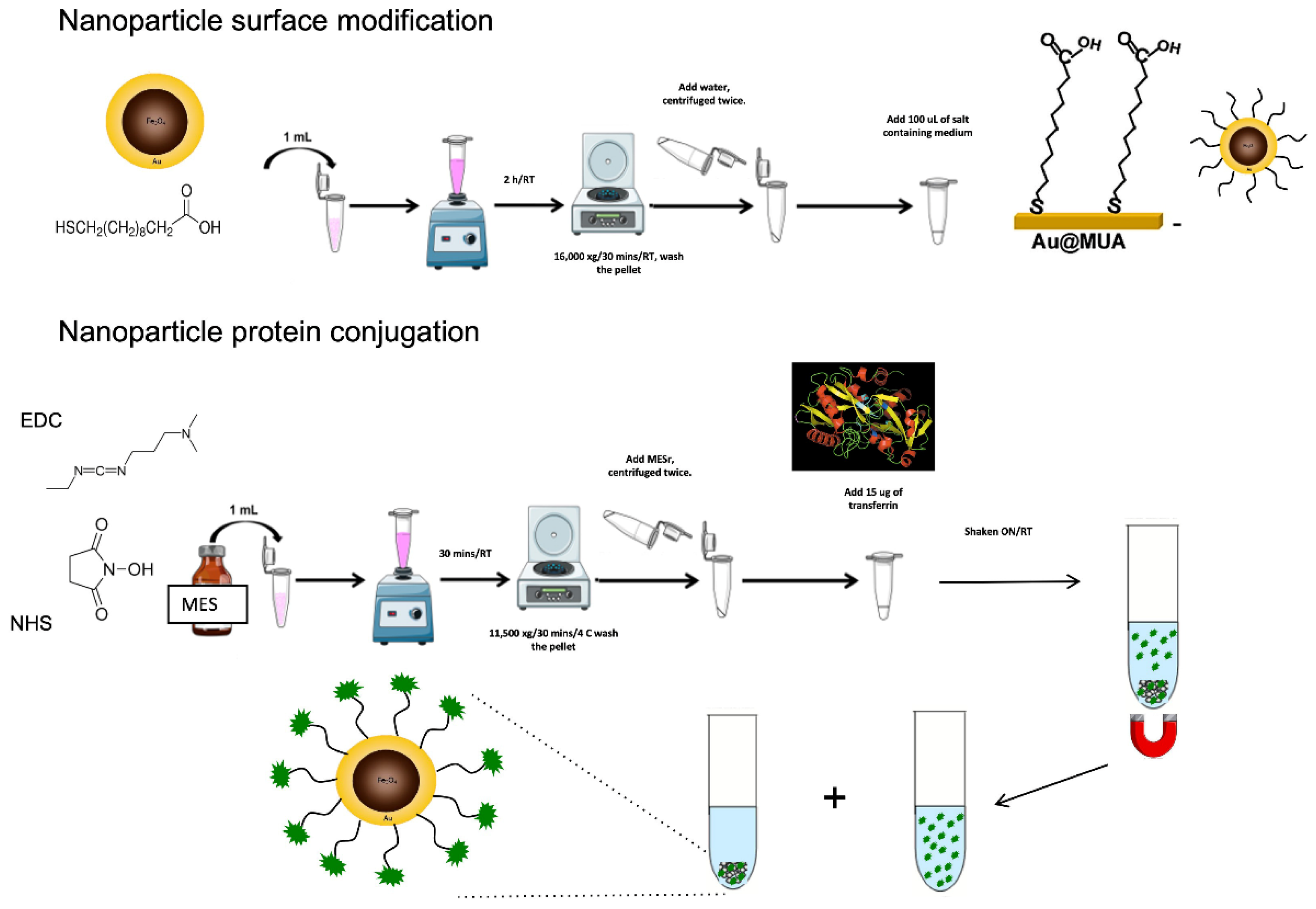
2.8. Synthesis of Nanoparticles
2.8.1. Synthesis of Monodispersed Cobalt Ferrite Nanoparticles
2.8.2. Synthesis of Magnetic Au-CoFe2O4 Core-Shell Nanoparticles (mCSNP’s)
2.9. Functionalization of Au Core-Shell Magnetic Nanoparticles
2.10. Immunofluorescence of TfR1 in Cells
3. Results
3.1. Proteomic Analysis
3.2. Bioinformatics
3.3. Validation of Differential Expression of TfR1
3.4. Immunofluorescence of Transferrin Receptor
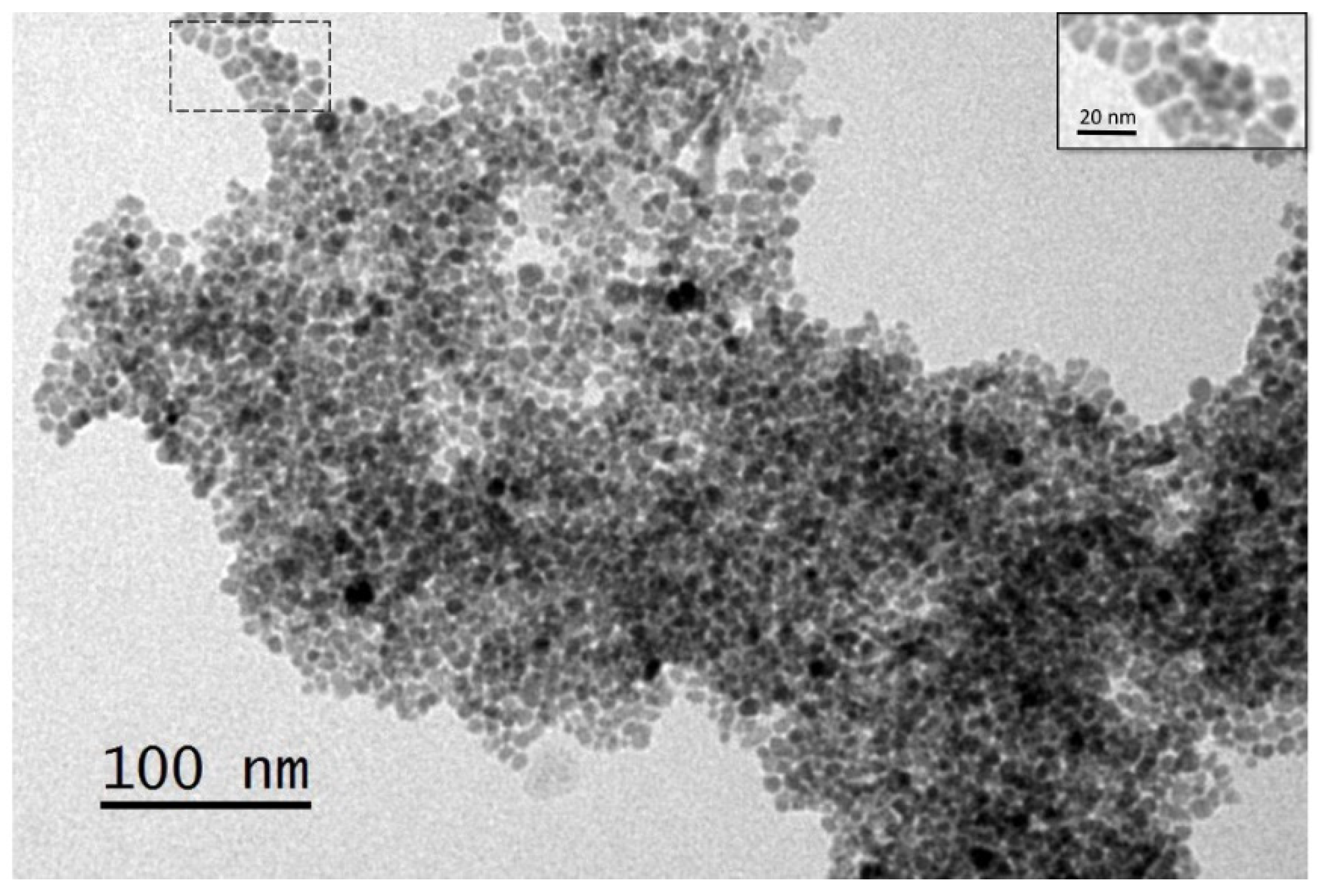
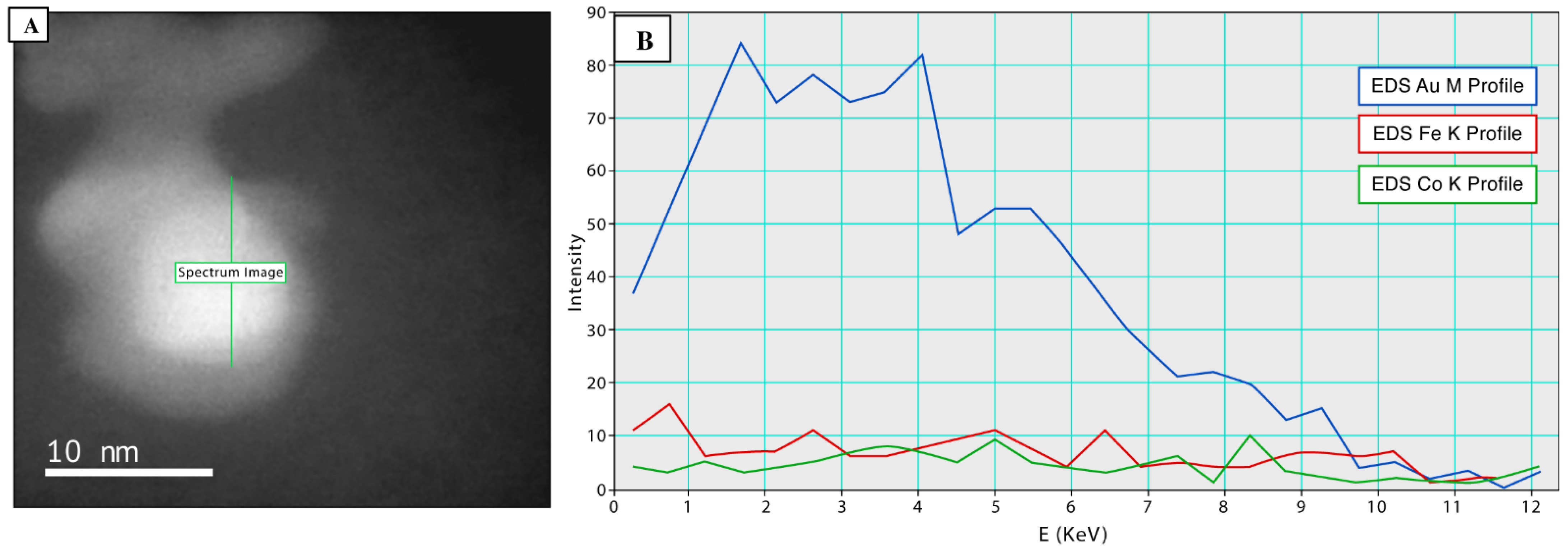
3.5. Synthesis and Functionalization of mSCNP’s
3.6. Endocytosis Assays
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Cancer Observatory: Cancer Today; World Health Organization. Available online: https://gco.iarc.fr/today (accessed on 12 June 2022).
- Yang, X.; Man, J.; Chen, H.; Zhang, T.; Yin, X.; He, Q.; Lu, M. Temporal trends of the lung cancer mortality attributable to smoking from 1990 to 2017: A global, regional and national analysis. Lung Cancer 2021, 152, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of lung cancer. Contemp. Oncol. 2021, 25, 45–52. [Google Scholar] [CrossRef]
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-cell lung cancer. Nat. Rev. Dis. Primers 2021, 7, 3. [Google Scholar] [CrossRef]
- Wistuba, I.I.; Brambilla, E.; Noguchi, M. Classic Anatomic Pathology and Lung Cancer. In IASLC Thoracic Oncology; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Herbst, R.S.; Heymach, J.V.; Lippman, S.M. Lung Cancer. N. Engl. J. Med. 2008, 359, 1367–1380. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Müller-Hermelink, H.K. Pathology and Genetics: Tumours of the Lung, Pleura, Thymus and Heart. Int. Agency Res. Cancer 2004. Available online: https://www.researchgate.net/publication/285709260_Pathology_and_Genetics_of_Tumors_of_the_Lung_Pleura_Thymus_and_Heart (accessed on 8 June 2022).
- Larsen, J.E.; Minna, J.D. Molecular Biology of Lung Cancer: Clinical Implications. Clin. Chest Med. 2011, 32, 703–740. [Google Scholar] [CrossRef] [PubMed]
- Olak, J.; Colson, Y. Gender differences in lung cancer: Have we really come a long way, baby? J. Thorac. Cardiovasc. Surg. 2004, 128, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Müller-Hermelink, H.K.; Harris, C. World Health Organization classification of tumours. Pathol. Genet. Tumours Lung Pleura Thymus Heart 2004, 10, 179–184. [Google Scholar]
- Hayashi, R.; Inomata, M. Small cell lung cancer; recent advances of its biology and therapeutic perspective. Respir. Investig. 2022, 60, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Melosky, B.; Cheema, P.K.; Brade, A.; McLeod, D.; Liu, G.; Price, P.W.; Jao, K.; Schellenberg, D.D.; Juergens, R.; Leighl, N.; et al. Prolonging Survival: The Role of Immune Checkpoint Inhibitors in the Treatment of Extensive-Stage Small Cell Lung Cancer. Oncologist 2020, 25, 981–992. [Google Scholar] [CrossRef]
- Alvarado-Luna, G.; Morales-Espinosa, D. Treatment for small cell lung cancer, where are we now?—A review. Transl. Lung Cancer Res. 2016, 5, 26. [Google Scholar] [PubMed]
- Bae, Y.H. Drug targeting and tumor heterogeneity. J. Control. Release 2009, 133, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Shevchenko, A.; Tomas, H.; Havlis, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Castro, E.; Souza, G.H.M.F.; Delgadillo-Álvarez, D.M.; Ramírez-Reyes, L.; Torres-Huerta, A.L.; Velasco-Suárez, A.; Cruz-Cruz, C.; Hernández-Hernández, J.M.; Tapia-Ramírez, J. Quantitative Proteomic Analysis of MARC-145 Cells Infected with a Mexican Porcine Reproductive and Respiratory Syndrome Virus Strain Using a Label-Free Based DIA approach. J. Am. Soc. Mass Spectrom. 2020, 31, 1302–1312. [Google Scholar] [CrossRef]
- Vásquez-Procopio, J.; Osorio, B.; Cortés-Martínez, L.; Hernández-Hernández, F.; Medina-Contreras, O.; Ríos-Castro, E.; Comjean, A.; Li, F.; Hu, Y.; Mohr, S.; et al. Intestinal response to dietary manganese depletion in Drosophila. Metallomics 2020, 12, 218–240. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef]
- Medina, M.A.; Oza, G.; Ángeles-Pascual, A.; González M., M.; Antaño-López, R.; Vera, A.; Leija, L.; Reguera, E.; Arriaga, L.G.; Hernández Hernández, J.M.; et al. Synthesis, Characterization and Magnetic Hyperthermia of Monodispersed Cobalt Ferrite Nanoparticles for Cancer Therapeutics. Molecules 2020, 25, 4428. [Google Scholar] [CrossRef]
- Craig, G.A.; Allen, P.J.; Mason, M.D. Synthesis, Characterization, and Functionalization of Gold Nanoparticles for Cancer Imaging. In Cancer Nanotechnology. In Methods in Molecular Biology; Grobmyer, S., Moudgil, B., Eds.; Humana Press: Totowa, NJ, USA, 2010; Volume 624. [Google Scholar] [CrossRef]
- Mirski, S.E.; Gerlach, J.H.; Cole, S.P. Multidrug resistance in a human small cell lung cancer cell line selected in adriamycin. Cancer Res. 1987, 47, 2594–2598. [Google Scholar]
- Yamamoto, R.; Lin, L.S.; Lowe, R.; Warren, M.K.; White, T.J. The human lung fibroblast cell line, MRC-5, produces multiple factors involved with megakaryocytopoiesis. J. Immunol. 1990, 144, 1808–1816. [Google Scholar] [PubMed]
- Hughson, F.M. Copy Coats: COPI Mimics Clathrin and COPII. Cell 2010, 142, 19–21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kawabata, H. Transferrin and transferrin receptors update. Free Radic. Biol. Med. 2019, 133, 46–54. [Google Scholar] [CrossRef]
- Legendre-Guillemin, V.; Metzler, M.; Lemaire, J.-F.; Philie, J.; Gan, L.; Hayden, M.; McPherson, P.S. Huntingtin Interacting Protein 1 (HIP1) Regulates Clathrin Assembly through Direct Binding to the Regulatory Region of the Clathrin Light Chain. J. Biol. Chem. 2005, 280, 6101–6108. [Google Scholar] [CrossRef] [PubMed]
- Fuller-Pace, F.V. DEAD box RNA helicase functions in cancer. RNA Biol. 2013, 10, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Shanas, R.; Nelson, M.A.; Bhattacharyya, A.; Shi, J. Increased expression of the heterogeneous nuclear ribonucleoprotein K in pancreatic cancer and its association with the mutant p53. Int. J. Cancer 2010, 126, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Li, W.; Zhang, M. The function of the RNA-binding protein hnRNP in cancer metastasis. J. Cancer Res. Ther. 2013, 9, 129. [Google Scholar] [CrossRef]
- Ali, M.U.; Ur Rahman, M.S.; Jia, Z.; Jiang, C. Eukaryotic translation initiation factors and cancer. Tumor Biol. 2017, 39, 1010428317709805. [Google Scholar] [CrossRef]
- Bhat, M.; Robichaud, N.; Hulea, L.; Sonenberg, N.; Pelletier, J.; Topisirovic, I. Targeting the translation machinery in cancer. Nat. Rev. Drug Discov. 2015, 14, 261–278. [Google Scholar] [CrossRef]
- Lindqvist, L.M.; Tandoc, K.; Topisirovic, I.; Furic, L. Cross-talk between protein synthesis, energy metabolism and autophagy in cancer. Curr. Opin. Genet. Dev. 2018, 48, 104–111. [Google Scholar] [CrossRef]
- Statello, L.; Maugeri, M.; Garre, E.; Nawaz, M.; Wahlgren, J.; Papadimitriou, A.; Lundqvist, C.; Lindfors, L.; Collén, A.; Sunnerhagen, P.; et al. Identification of RNA-binding proteins in exosomes capable of interacting with different types of RNA: RBP-facilitated transport of RNAs into exosomes. PLoS ONE 2018, 13, e0195969. [Google Scholar] [CrossRef] [PubMed]
- Février, B.; Raposo, G. Exosomes: Endosomal-derived vesicles shipping extracellular messages. Curr. Opin. Cell Biol. 2004, 16, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Camussi, G.; Valadi, H.; Nazarenko, I.; Ekström, K.; Wang, X.; Principe, S.; Shah, N.; Ashraf, N.M.; Fatima, F.; et al. The emerging role of extracellular vesicles as biomarkers for urogenital cancers. Nat. Rev. Urol. 2014, 11, 688–701. [Google Scholar] [CrossRef]
- Cahill, M.A.; Medlock, A.E. Thoughts on interactions between PGRMC1 and diverse attested and potential hydrophobic ligands. J. Steroid Biochem. Mol. Biol. 2017, 171, 11–33. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, M.; Oza, G.; Velumani, S.; Ramirez, J.T.; Vera, A.; Leija, L. Design and evaluation of surface functionalized superparamagneto-plasmonic nanoparticles for cancer therapeutics. Int. J. Pharm. 2017, 524, 16–29. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Adam, J.K.; Odhav, B.; Bhoola, K.D. Immune responses in cancer. Pharmacol. Ther. 2003, 99, 113–132. [Google Scholar] [CrossRef]
- Critchley-Thorne, R.J.; Simons, D.L.; Yan, N.; Miyahira, A.K.; Dirbas, F.M.; Johnson, D.L.; Swetter, S.M.; Carlson, R.W.; Fisher, G.A.; Koong, A.; et al. Impaired interferon signaling is a common immune defect in human cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 9010–9015. [Google Scholar] [CrossRef]
- Syedbasha, M.; Egli, A. Interferon Lambda: Modulating Immunity in Infectious Diseases. Front. Immunol. 2017, 8, 119. [Google Scholar] [CrossRef]
- Lasfar, A.; Zloza, A.; Silk, A.W.; Lee, L.Y.; Cohen-Solal, K.A. Interferon Lambda: Toward a Dual Role in Cancer. J. Interf. Cytokine Res. 2019, 39, 22–29. [Google Scholar] [CrossRef]
- Setiadi, A.F.; Omilusik, K.; David, M.D.; Seipp, R.P.; Hartikainen, J.; Gopaul, R.; Choi, K.B.; Jefferies, W.A. Epigenetic Enhancement of Antigen Processing and Presentation Promotes Immune Recognition of Tumors. Cancer Res. 2008, 68, 9601–9607. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Gagarin, D.; St. Laurent, G., III; Hammell, N.; Toma, I.; Hu, C.A.; Iwasa, A.; McCaffrey, T.A. Cardiovascular inflammation and lesion cell apoptosis: A novel connection via the interferon-inducible immunoproteasome. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.H.; Hood, B.L.; Beck, H.C.; Conrads, T.P.; Ditzel, H.J.; Leth-Larsen, R. Downregulation of antigen presentation-associated pathway proteins is linked to poor outcome in triple-negative breast cancer patient tumors. OncoImmunology 2017, 6, e1305531. [Google Scholar] [CrossRef]
- Chen, H.L.; Gabrilovich, D.; Tampé, R.; Girgis, K.R.; Nadaf, S.; Carbone, D.P. A functionally defective allele of TAP1 results in loss of MHC class I antigen presentation in a human lung cancer. Nat. Genet. 1996, 13, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Hermeking, H.; Benzinger, A. 14-3-3 proteins in cell cycle regulation. Semin. Cancer Biol. 2006, 16, 183–192. [Google Scholar] [CrossRef]
- Yaffe, M.B.; Rittinger, K.; Volinia, S.; Caron, P.R.; Aitken, A.; Leffers, H.; Gamblin, S.J.; Smerdon, S.J.; Cantley, L.C. The Structural Basis for 14-3-3: Phosphopeptide Binding Specificity. Cell 1997, 91, 961–971. [Google Scholar] [CrossRef]
- Sluchanko, N.N.; Gusev, N.B. 14-3-3 Proteins and regulation of cytoskeleton. Biochemistry 2010, 75, 1528–1546. [Google Scholar] [CrossRef]
- Katsetos, C.D.; Dráber, P. Tubulins as therapeutic targets in cancer: From bench to bedside. Curr. Pharm. Des. 2012, 18, 2778–2792. [Google Scholar] [CrossRef]
- Khoriaty, R.; Hesketh, G.G.; Bernard, A.; Weyand, A.C.; Mellacheruvu, D.; Zhu, G.; Hoenerhoff, M.J.; McGee, B.; Everett, L.; Adams, E.J.; et al. Functions of the COPII gene paralogs SEC23A and SEC23B are interchangeable in vivo. Proc. Natl. Acad. Sci. USA 2018, 115, E7748–E7757. [Google Scholar] [CrossRef]
- Mancias, J.D.; Goldberg, J. The Transport Signal on Sec22 for Packaging into COPII-Coated Vesicles Is a Conformational Epitope. Mol. Cell 2007, 26, 403–414. [Google Scholar] [CrossRef]
- Ge, L.; Zhang, M.; Schekman, R. Phosphatidylinositol 3-kinase and COPII generate LC3 lipidation vesicles from the ER-Golgi intermediate compartment. eLife 2014, 3, e04135. [Google Scholar] [CrossRef] [PubMed]
- Popoff, V.; Langer, J.D.; Reckmann, I.; Hellwig, A.; Kahn, R.A.; Brügger, B.; Wieland, F.T. Several ADP-ribosylation Factor (Arf) Isoforms Support COPI Vesicle Formation. J. Biol. Chem. 2011, 286, 35634–35642. [Google Scholar] [CrossRef]
- Gurel, P.S.; Hatch, A.L.; Higgs, H.N. Connecting the Cytoskeleton to the Endoplasmic Reticulum and Golgi. Curr. Biol. 2014, 24, R660–R672. [Google Scholar] [CrossRef] [PubMed]
- Qualmann, B.; Kessels, M.M.; Kelly, R.B. Molecular Links between Endocytosis and the Actin Cytoskeleton. J. Cell Biol. 2000, 150, F111–F116. [Google Scholar] [CrossRef] [PubMed]
- Capmany, A.; Yoshimura, A.; Kerdous, R.; Caorsi, V.; Lescure, A.; Del Nery, E.; Coudrier, E.; Goud, B.; Schauer, K. MYO1C stabilizes actin and facilitates the arrival of transport carriers at the Golgi complex. J. Cell Sci. 2019, 132, jcs225029. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.S.; Pal, K.; Nowak, R.B.; Demenko, A.; Zaninetti, C.; Da Costa, L.; Favier, R.; Pecci, A.; Fowler, V.M. MYH9-related disease mutations cause abnormal red blood cell morphology through increased myosin-actin binding at the membrane. Am. J. Hematol. 2019, 94, 667–677. [Google Scholar] [CrossRef]
- Hehnly, H.; Stamnes, M. Regulating cytoskeleton-based vesicle motility. FEBS Lett. 2007, 581, 2112–2118. [Google Scholar] [CrossRef]
- Mayle, K.M.; Le, A.M.; Kamei, D.T. The intracellular trafficking pathway of transferrin. Biochim. Biophys. Acta BBA Gen. Subj. 2012, 1820, 264–281. [Google Scholar] [CrossRef]
- Shtutman, M.; Roninson, I.B. A subunit of coatomer protein complex offers a novel tumor-specific target through a surprising mechanism. Autophagy 2011, 7, 1551–1552. [Google Scholar] [CrossRef]
- Mellman, I.; Yarden, Y. Endocytosis and Cancer. Cold Spring Harb. Perspect. Biol. 2013, 5, a016949. [Google Scholar] [CrossRef]
- Wang, Y.; Chai, Z.; Wang, M.; Jin, Y.; Yang, A.; Li, M. COPB2 suppresses cell proliferation and induces cell cycle arrest in human colon cancer by regulating cell cycle-related proteins. Exp. Ther. Med. 2018, 15, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Wessling-Resnick, M. Iron transport. Annu. Rev. Nutr. 2000, 20, 129. [Google Scholar] [CrossRef] [PubMed]
- Gammella, E.; Buratti, P.; Cairo, G.; Recalcati, S. The transferrin receptor: The cellular iron gate. Metallomics 2017, 9, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Low, P.S. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv. Drug Deliv. Rev. 2002, 54, 675–693. [Google Scholar] [CrossRef]
- Chen, F.; Fan, Y.; Hou, J.; Liu, B.; Zhang, B.; Shang, Y.; Chang, Y.; Cao, P.; Tan, K. Integrated analysis identifies TfR1 as a prognostic biomarker which correlates with immune infiltration in breast cancer. Aging 2021, 13, 21671–21699. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Wei, Y.; Cheng, Y.; Wang, X.; Wang, Q.; Chen, D.; Li, W. Iron metabolism protein transferrin receptor 1 involves in cervical cancer progression by affecting gene expression and alternative splicing in HeLa cells. Genes Genom. 2022, 44, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.P.M.; Chen, A.L.; Foster, A.; Drezek, R. In vivo biodistribution of nanoparticles. Nanomedicine 2011, 6, 815–835. [Google Scholar] [CrossRef]
- Shen, Y.; Li, X.; Dong, D.; Zhang, B.; Xue, Y.; Shang, P. Transferrin receptor 1 in cancer: A new sight for cancer therapy. Am. J. Cancer Res. 2018, 8, 916–931. [Google Scholar]
- Jiao, Y.; Wilkinson, J., 4th; Pietsch, E.C.; Buss, J.L.; Wang, W.; Planalp, R.; Torti, F.M.; Torti, S.V. Iron chelation in the biological activity of curcumin. Free Radic. Biol. Med. 2006, 40, 1152–1160. [Google Scholar] [CrossRef]
- Yang, C.; Ma, X.; Wang, Z.; Zeng, X.; Hu, Z.; Ye, Z.; Shen, G. Curcumin induces apoptosis and protective autophagy in castration-resistant prostate cancer cells through iron chelation. Drug Des. Dev. Ther. 2017, ume11, 431–439. [Google Scholar] [CrossRef]
- Moura, I.C.; Lepelletier, Y.; Arnulf, B.; England, P.; Baude, C.; Beaumont, C.; Bazarbachi, A.; Benhamou, M.; Monteiro, R.C.; Hermine, O. A neutralizing monoclonal antibody (mAb A24) directed against the transferrin receptor induces apoptosis of tumor T lymphocytes from ATL patients. Blood 2004, 103, 1838–1845. [Google Scholar] [CrossRef] [PubMed]
- Callens, C.; Moura, I.C.; Lepelletier, Y.; Coulon, S.; Renand, A.; Dussiot, M.; Ghez, D.; Benhamou, M.; Monteiro, R.; Bazarbachi, A.; et al. Recent advances in adult T-cell leukemia therapy: Focus on a new anti-transferrin receptor monoclonal antibody. Leukemia 2008, 22, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Daniels-Wells, T.R.; Widney, D.P.; Leoh, L.S.; Martínez-Maza, O.; Penichet, M.L. 2015. Efficacy of an Anti-transferrin Receptor Antibody Against AIDS-related non-Hodgkin Lymphoma: A Brief Communication. J. Immunother. 2015, 38, 307. [Google Scholar] [CrossRef] [PubMed]
- Shimosaki, S.; Nakahata, S.; Ichikawa, T.; Kitanaka, A.; Kameda, T.; Hidaka, T.; Kubuki, Y.; Kurosawa, G.; Zhang, L.; Sudo, Y.; et al. Development of a complete human IgG monoclonal antibody to transferrin receptor 1 targeted for adult T-cell leukemia/lymphoma. Biochem. Biophys. Res. Commun. 2017, 485, 144–151. [Google Scholar] [CrossRef]
- Schaar, D.G.; Medina, D.J.; Moore, D.F.; Strair, R.K.; Ting, Y. miR-320 targets transferrin receptor 1 (CD71) and inhibits cell proliferation. Exp. Hematol. 2009, 37, 245–255. [Google Scholar] [CrossRef]
- Camp, E.R.; Wang, C.; Little, E.C.; Watson, P.M.; Pirollo, K.F.; Rait, A.; Cole, D.J.; Chang, E.H.; Watson, D.K. Transferrin receptor targeting nanomedicine delivering wild-type p53 gene sensitizes pancreatic cancer to gemcitabine therapy. Cancer Gene Ther. 2013, 20, 222–228. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhai, M.; Chen, Z.; Han, X.; Yu, F.; Li, Z.; Xie, X.; Han, C.; Yu, L.; Yang, Y.; et al. Dual-modified liposome codelivery of doxorubicin and vincristine improve targeting and therapeutic efficacy of glioma. Drug Deliv. 2017, 24, 1045–1055. [Google Scholar] [CrossRef]
- Seidu, T.A.; Kutoka, P.T.; Asante, D.O.; Farooq, M.A.; Alolga, R.N.; Bo, W. Functionalization of Nanoparticulate Drug Delivery Systems and Its Influence in Cancer Therapy. Pharmaceutics 2022, 14, 1113. [Google Scholar] [CrossRef]
- Scheeren, L.E.; Nogueira-Librelotto, D.R.; Macedo, L.B.; de Vargas, J.M.; Mitjans, M.; Vinardell, M.P.; Rolim, C. Transferrin-conjugated doxorubicin-loaded PLGA nanoparticles with pH-responsive behavior: A synergistic approach for cancer therapy. J. Nanoparticle Res. 2020, 22, 1–18. [Google Scholar] [CrossRef]
- Candelaria, P.V.; Leoh, L.S.; Penichet, M.L.; Daniels-Wells, T.R. Antibodies targeting the transferrin receptor 1 (TfR1) as direct anti-cancer agents. Front. Immunol. 2021, 12, 607692. [Google Scholar] [CrossRef]
- Xie, Y.; Killinger, B.; Moszczynska, A.; Merkel, O.M. Targeted Delivery of siRNA to Transferrin Receptor Overexpressing Tumor Cells via Peptide Modified Polyethylenimine. Molecules 2016, 21, 1334. [Google Scholar] [CrossRef] [PubMed]
- Soni, V.; Kohli, D.V.; Jain, S.K. Transferrin-conjugated liposomal system for improved delivery of 5-fluorouracil to brain. J. Drug Target. 2008, 16, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Akinc, A.; Battaglia, G. Exploiting Endocytosis for Nanomedicines. Cold Spring Harb. Perspect. Biol. 2013, 5, a016980. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.M.S. Artificial cell evolves into nanomedicine, biotherapeutics, blood substitutes, drug delivery, enzyme/gene therapy, cancer therapy, cell/stem cell therapy, nanoparticles, liposomes, bioencapsulation, replicating synthetic cells, cell encapsulation/scaffold, biosorbent/immunosorbent haemoperfusion/plasmapheresis, regenerative medicine, encapsulated microbe, nanobiotechnology, nanotechnology. Artif. Cells Nanomed. Biotechnol. 2019, 47, 997–1013. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villalobos-Manzo, R.; Ríos-Castro, E.; Hernández-Hernández, J.M.; Oza, G.; Medina, M.A.; Tapia-Ramírez, J. Identification of Transferrin Receptor 1 (TfR1) Overexpressed in Lung Cancer Cells, and Internalization of Magnetic Au-CoFe2O4 Core-Shell Nanoparticles Functionalized with Its Ligand in a Cellular Model of Small Cell Lung Cancer (SCLC). Pharmaceutics 2022, 14, 1715. https://doi.org/10.3390/pharmaceutics14081715
Villalobos-Manzo R, Ríos-Castro E, Hernández-Hernández JM, Oza G, Medina MA, Tapia-Ramírez J. Identification of Transferrin Receptor 1 (TfR1) Overexpressed in Lung Cancer Cells, and Internalization of Magnetic Au-CoFe2O4 Core-Shell Nanoparticles Functionalized with Its Ligand in a Cellular Model of Small Cell Lung Cancer (SCLC). Pharmaceutics. 2022; 14(8):1715. https://doi.org/10.3390/pharmaceutics14081715
Chicago/Turabian StyleVillalobos-Manzo, Rocío, Emmanuel Ríos-Castro, José Manuel Hernández-Hernández, Goldie Oza, Mauricio A. Medina, and José Tapia-Ramírez. 2022. "Identification of Transferrin Receptor 1 (TfR1) Overexpressed in Lung Cancer Cells, and Internalization of Magnetic Au-CoFe2O4 Core-Shell Nanoparticles Functionalized with Its Ligand in a Cellular Model of Small Cell Lung Cancer (SCLC)" Pharmaceutics 14, no. 8: 1715. https://doi.org/10.3390/pharmaceutics14081715
APA StyleVillalobos-Manzo, R., Ríos-Castro, E., Hernández-Hernández, J. M., Oza, G., Medina, M. A., & Tapia-Ramírez, J. (2022). Identification of Transferrin Receptor 1 (TfR1) Overexpressed in Lung Cancer Cells, and Internalization of Magnetic Au-CoFe2O4 Core-Shell Nanoparticles Functionalized with Its Ligand in a Cellular Model of Small Cell Lung Cancer (SCLC). Pharmaceutics, 14(8), 1715. https://doi.org/10.3390/pharmaceutics14081715







