3.1. Redispersibility of Nanoparticles from Granules
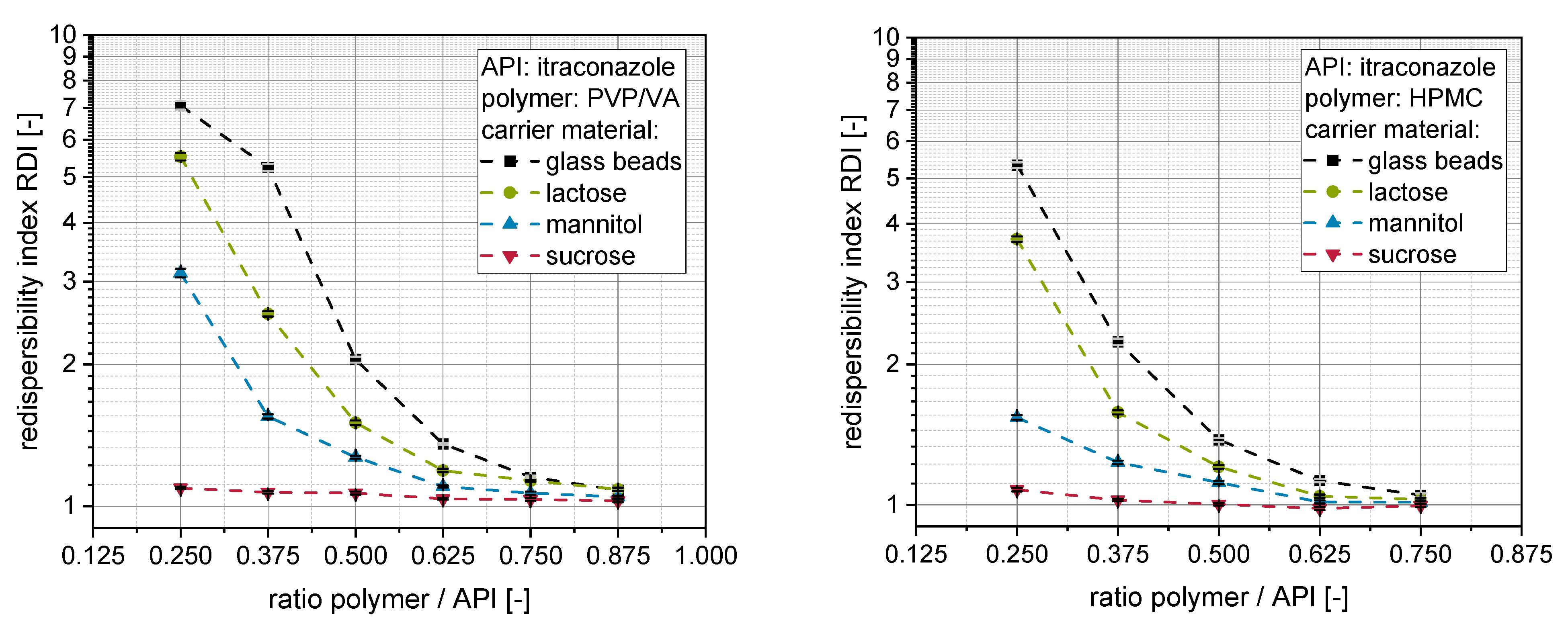
The outcome of the redispersibility studies for granules produced with itraconazole nanosuspensions stabilized either with PVP/VA (left) or HPMC (right) and different carrier materials (glass beads, lactose, mannitol, sucrose), as well as different amounts of additional polymeric excipient, can be seen in
Figure 2.
Distinct dependencies of the redispersibility of the nanoparticles on the applied carrier material can be observed for both nanosuspensions. In accordance with previous investigations, sucrose performed best as a carrier material with the highest intrinsic dissolution rate (1.62 mg·cm
−2·min
−1), followed by mannitol (0.64 mg·cm
−2·min
−1) and lactose (0.29 mg·cm
−2·min
−1) [
20]. In the present study, it can be additionally observed that granules produced with insoluble glass beads performed worst in the redispersibility studies compared to soluble carrier materials at a given polymer to API ratio. This further strengthens our hypothesis that the dissolution rate of the carrier material is a crucial material property during fluidized bed granulation of API nanosuspensions. It determines the degree of carrier particle dissolution and subsequent nanoparticle embedding upon resolidification of dissolved fractions during the drying process [
20].
When applying an insoluble carrier material, only the polymeric excipients dissolved in the nanosuspension serve as embedding matrix material in the dry state. Consequently, larger amounts of additional polymeric excipient are necessary to achieve a redispersibility comparable to granules produced with soluble carrier materials. The necessary amount of additional excipient depends on the dissolution rate of the applied carrier material with a higher dissolution rate resulting in lower necessary amounts of additional excipient needed to completely maintain the nanoparticulate size and dispersity after redispersion.
However, besides the carrier material, the necessary amount of additional excipient in the nanosuspension to facilitate complete redispersibility from the granules is also dependent on the type of polymer applied. The redispersibility of granules produced with HPMC is in most cases better than the redispersibility of comparable (i.e., carrier material, ratio polymer to API) granules produced with PVP/VA, when the same carrier material and the same ratio polymer to API is processed. It is highly unlikely that the solid density of the applied polymers is significantly elevated over drying so that changes in dispersity of the nanoparticles could be explained by these. This can especially not explain the differences in redispersibility, as HPMC has a higher solid density (ρ
S,HPMC = 1.283 g·cm
−3) than PVP/VA (ρ
S,PVP/VA = 1.153 g·cm
−3), which accordingly results in a higher nanoparticle volume concentration for HPMC. Consequently, application of HPMC should result in an even less pronounced nanoparticle embedding in the surface layer of the granules. This should result in a higher potential of nanoparticle interaction and, consequently, agglomeration resulting in a compromised redispersibility [
11,
20,
23].
The differences in redispersibility might possibly be attributed to different viscosities of the nanosuspensions prepared with the two different polymers as stabilizers and eventually as additional excipient (i.e., PVP/VA or HPMC). After milling, nanosuspensions stabilized with PVP/VA exhibit a far lower dynamic viscosity (ɳ
PVP/VA = 2.1 mPa·s) compared to the nanosuspension stabilized with HPMC (ɳ
HPMCV = 4.2 mPa·s). These differences might be even more pronounced when additional polymer is added to the nanosuspension as excipient before granulation. This in turn influences the granulation process and/or the formation of the nanoparticle-loaded layer around the carrier particles: Higher viscosities result in the formation of larger droplets upon spraying [
27], which decreases the rate of evaporation of the granulation liquid’s solvent by the fluidization air, resulting in a stronger wetting of the carrier particles/granules by higher viscous granulation liquid [
28]. As a result, material from the surface of the carrier particles may dissolve, depending on its dissolution rate, to a greater extent resulting in an enhanced nanoparticle embedding and redispersibility. Although this hypothesis could apply to the soluble carrier materials, it is not applicable for the insoluble glass beads used in this study and thus cannot explain the observed differences.
However, differences may be further explained by applying principles and mechanisms of particle engineering via spray drying and the concept of Peclet [
29], which were already applied for explaining differences in redispersibility of spray dried nanosuspensions [
30,
31,
32]. Larger droplets and/or a (viscosity-) associated decreased evaporation rate of the water within the granulation liquid may result in a slower recession of the droplet surface. This in turn may result in the different motion (e.g., diffusional) of the nanoparticles within the granulation liquid being fast compared to the receding droplet surface and in a rather homogeneous distribution of the nanoparticles in the solid state. For lower viscosities and higher evaporation rates, the receding droplet surface moves faster, resulting in an at least partial surface enrichment of the nanoparticles, which leads to nanoparticle agglomeration and consequently poor redispersibility [
29]. Although this hypothesis is transferable to other scenarios influencing the effective drying surface of the applied granulation liquid (e.g., due to differences in wettability of the carrier materials by the differently stabilized nanosuspensions), it is challenging to validate it experimentally. However, a different drying behavior of the differently stabilized nanosuspensions may also lead to differences in the structure of the nanoparticle-loaded granules. Consequently, insights in the fundamental relationships of formulation parameter-associated differences in product performance may be extracted from the corresponding relationship of the structure and the quality property of the nanoparticle-loaded granules as discussed in the following.
3.2. Structure of Nanoparticle-Loaded Granules
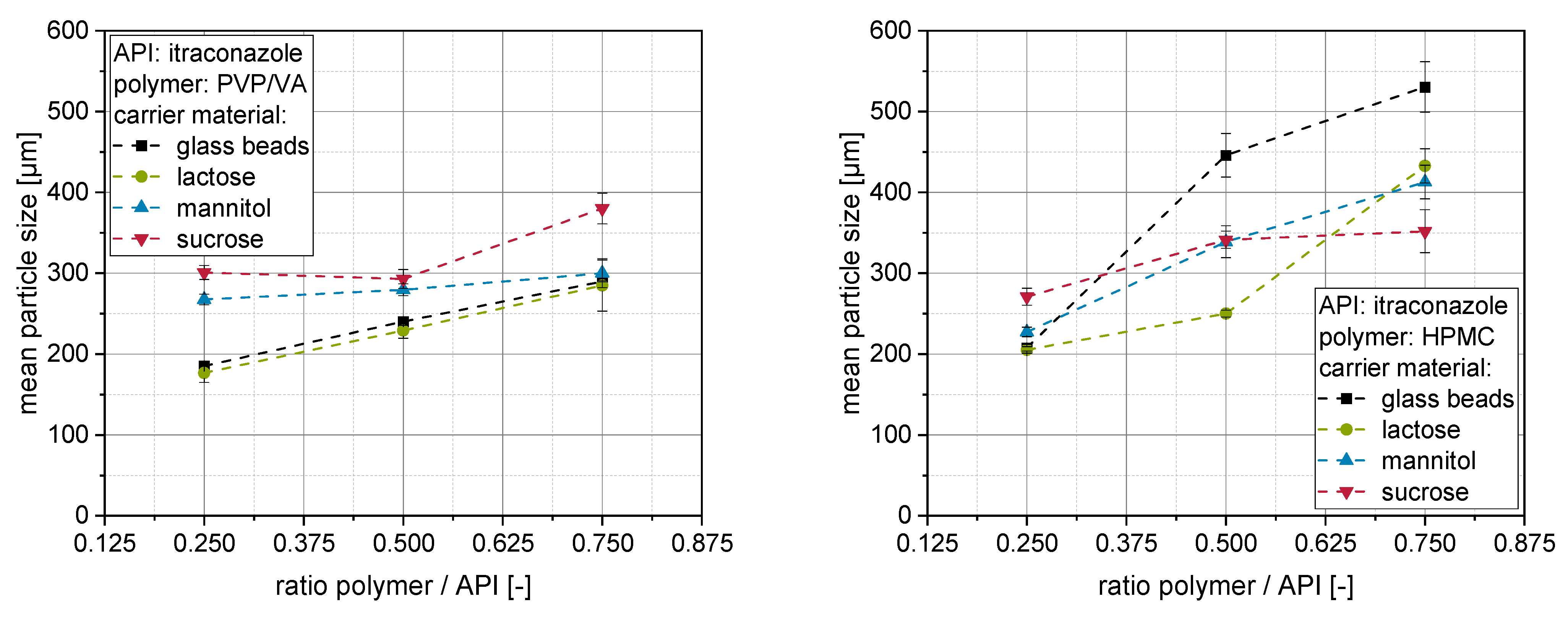
The mean particle sizes of granules produced with differently stabilized itraconazole nanosuspensions and carrier materials can be found in
Figure 3 (left: PVP/VA, right: HPMC) for polymer to API ratios of 0.25, 0.50 and 0.75.
Generally, it can be seen that the mean particle size of the granules increases with increasing polymer to API ratio in the nanosuspension. This observation is in accordance with the literature [
22,
28,
33,
34] and may be attributed to viscosity-related differences in droplet size [
27,
34], adhesiveness of the binder solutions [
28] and/or wetting of the carrier surface by the granulation liquids [
34]. As fluidized bed granulation is a combined coating/layering and granulation process, both mechanisms should be taken into account. The more prominent aspect leading to granule size growth is granulation, which is governed by the viscous properties and the binding properties of the granulation liquid.
For granules produced with nanosuspensions containing PVP/VA as a stabilizer and additional drying excipient, it can be observed that mean particle sizes of granules range between approx. 200 and 400 µm. Larger sizes of these granules generally coincide with higher redispersibilities (compare
Figure 2), also valid between different applied carrier materials. Granules produced with insoluble glass beads or poorly soluble lactose exhibit the lowest mean particle sizes in comparison to mannitol and sucrose carrier particles exhibiting higher intrinsic dissolution rates [
20]. This indicates that the partial surface dissolution and subsequent resolidification of the carrier materials does not only determine the degree of nanoparticle embedding on the product granules [
20] but also may enhance granulation efficiency by promoting the formation and/or strengthening of solid bridges between individual carrier particles [
35].
For granules stabilized with an HPMC-based nanosuspension with higher polymer concentrations, the mean particle sizes were higher when insoluble glass particles or poorly soluble lactose particles were applied as carrier materials (
Figure 3 right). Here, the application of glass beads yielded the largest granules with particle sizes of up to about 500 µm. In contrast to that, granules prepared from mannitol or sucrose with HPMC were in the same size range as with PVP/VA or slightly smaller when the lowest polymer concentration was applied. In summary, the results indicate that HPMC may perform better as a binder by forming larger granules in a fluidized granulation process, which might be attributed to its formation of stronger solid bridges. It additionally indicates that the granulation aspect is more pronounced with a high ratio of HPMC as compared with the layering/coating aspect of fluidized bed granulation. However, the superiority of the granulation capacity of HPMC recedes into the background when the layering aspect, increasing with the solubility of the carrier material, is more pronounced. This results in comparable granule sizes for sugar carriers for both polymers.
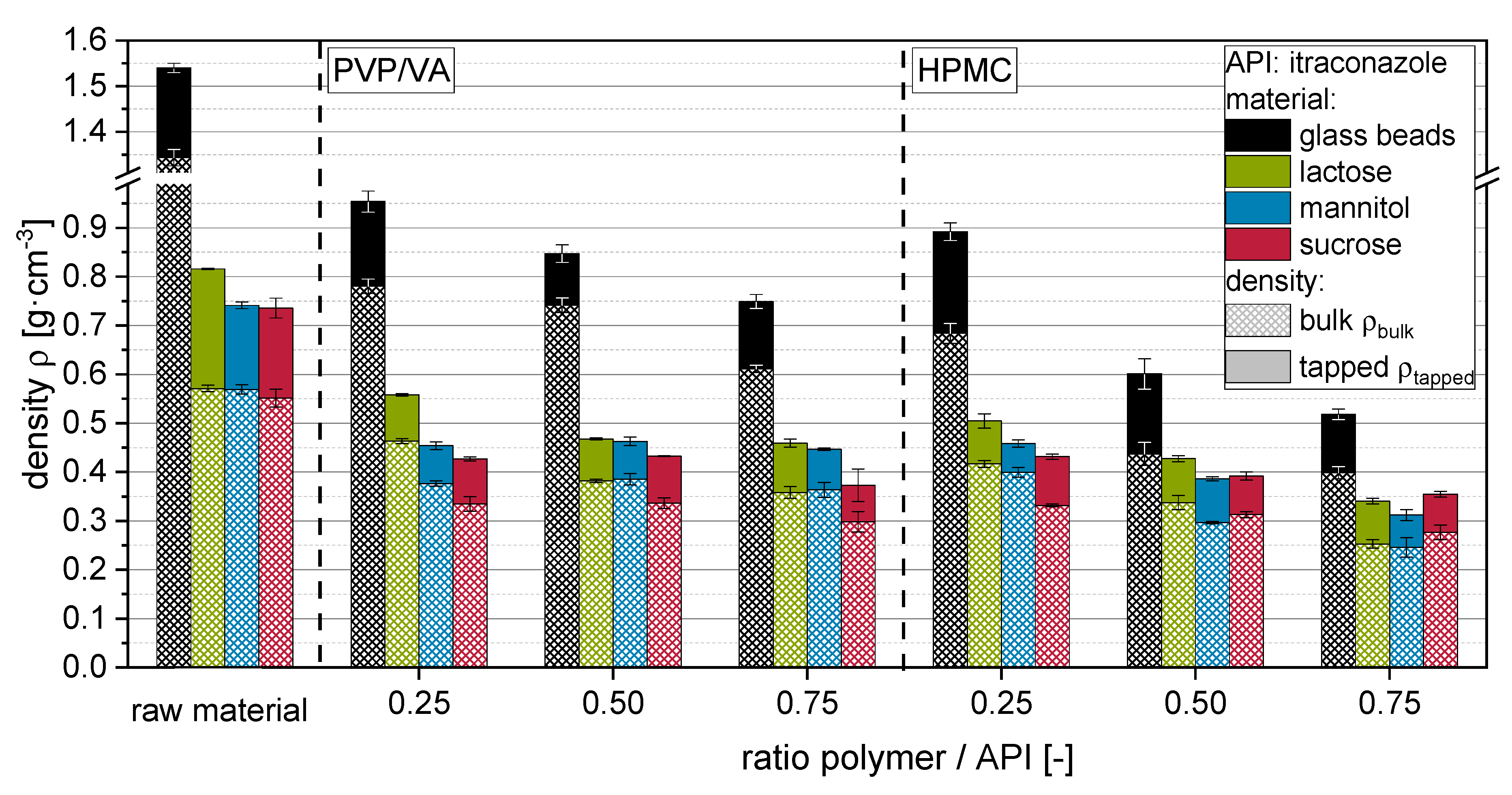
Generally, the bulk density ρ
bulk of product granules correlates with data obtained from particle size measurements (
Figure 4). Bulk densities decreased with increasing granule size (i.e., increasing polymer to API ratio) in comparison to the unprocessed raw materials. Here, an additional influence of the particle shape on the packing behavior and, by that, the interparticulate porosity cannot be excluded. Thus, ρ
tapped should be discussed more explicitly, as here the interparticulate porosity, caused, e.g., by differences in particle size or shape, is reduced to a minimum. Consequently, the corresponding values should at least partly reflect the differences in intraparticulate density/porosity of the granules and the additive contribution of interparticulate porosity originating from the particle size distribution and the deviation of particle morphology from a sphere. In this regard, the porosity increase in the corresponding particles Δε (raw materials or granules) was estimated by the difference in the porosity of the tapped powder bed ε
tapped and the porosity of a densest sphere packing ε
DSP, equal to 0.26 (Equation (1)).
The solid densities ρ
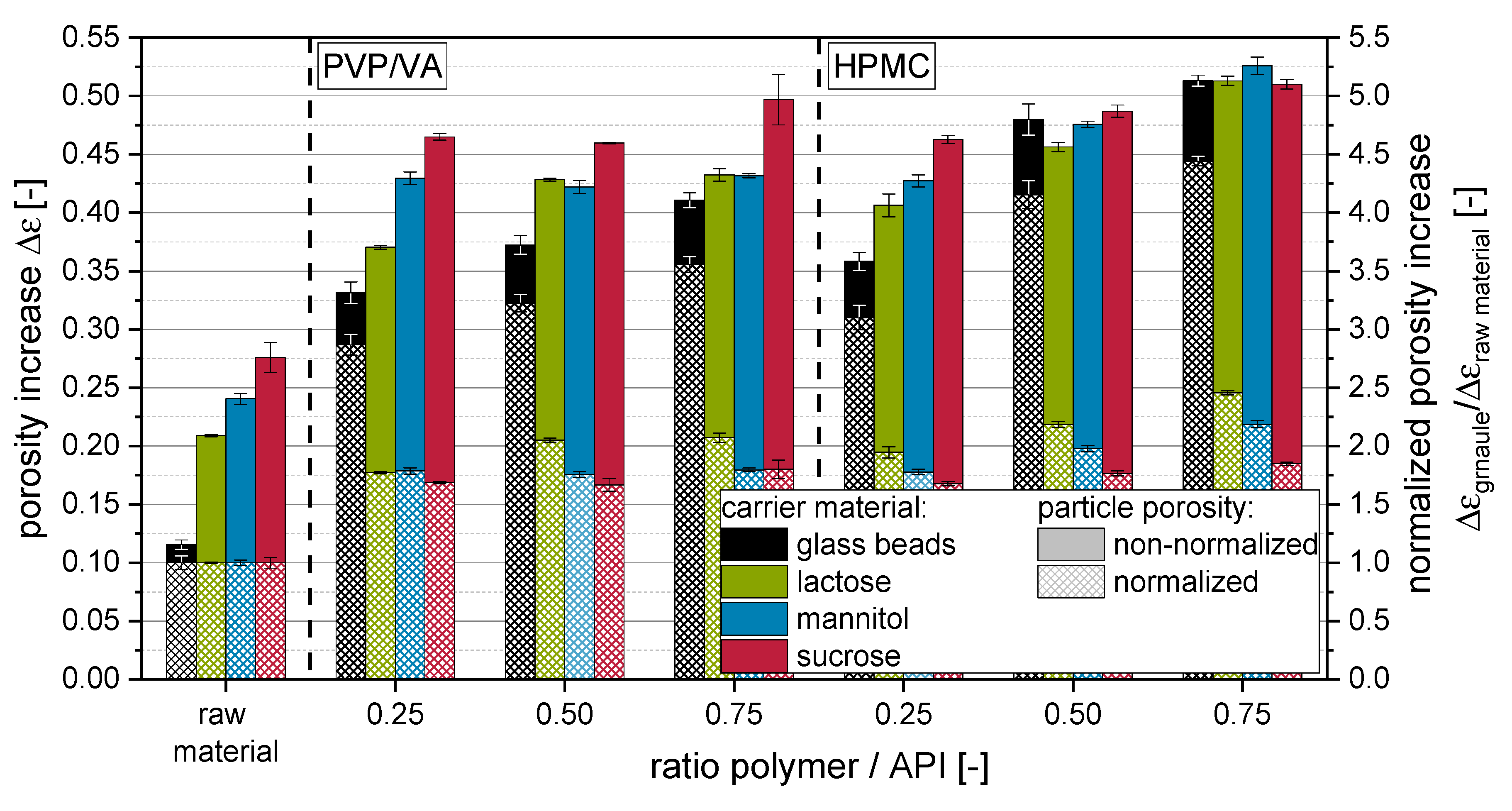
S of the granules were calculated by considering the solid densities of the incorporated raw materials with the corresponding proportions. Values for the porosity increase Δε of the raw materials and granules are depicted in
Figure 5. Additionally, the Δε of the granules is depicted after normalization to the Δε of the raw materials in order to accentuate the influence of the applied amount and type of polymer on the porosity of the granules.
Data for tapped densities and derived porosity increase Δε indicate that more porous and/or irregularly shaped granules are formed when more polymer is applied with the granulation liquid. This is to be expected, as the fluidized bed granulation is generally combining the layering/coating of primary carrier particle surfaces and the granulation, i.e., gluing a number of primary carrier particles together. The latter sub-process is adding to the formation of porous granule structures, as this developing interparticulate porosity is fixed within the granule. Additionally, the outer morphology of the granules is different to that of the starting material, as primary particles are glued together in a structure often compared with that of a raspberry, presenting a rough surface. Changes in the porosity increase are more explicit for HPMC than for PVP/VA. For the latter, especially granules produced with highly soluble carrier materials (i.e., mannitol and sucrose), show minor (ρtapped) or no significant (Δε) dependencies on applied polymer to API ratios. Granules produced with nanosuspensions containing HPMC showed generally lower densities and higher porosities than comparable granules produced with PVP/VA, indicating that application of HPMC results in a more porous granule structure in comparison to granules produced with PVP/VA. However, it cannot be completely excluded for the soluble carrier materials that the dissolution and resolidification processes, which occur in dependence on the applied type and amount of polymer, can superimpose polymer-related differences in the granule porosity. However, data for insoluble glass beads show the same trends. The fact that differences are even more distinctive here may indicate that the dissolution and resolidification of soluble carrier materials during the granulation process may counteract a polymer amount- or type-associated increase in the porosity of granules.
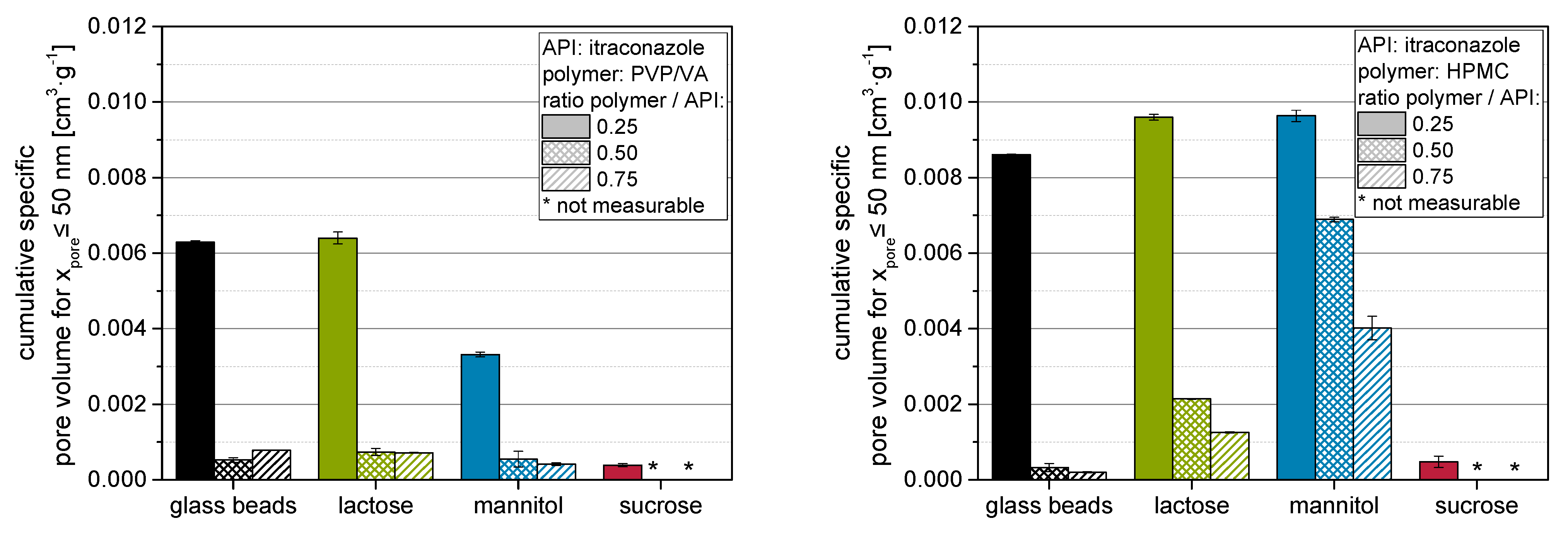
As the porosity within the bulk of granules is constituted of different potential void spaces, it should be differentiated that (a) intragranular porosity may originate from (I) packing of primary particles within the granule as a result of the granulation process and (II) the porosity in the applied layer on the (primary) particle surfaces as a result of the layering/coating process. Conversely, the (b) intergranular porosity is mainly determined by the (III) granule size distribution and by the (IV) granule outer morphology, especially the surface roughness. The above introduced porosity increase takes all these factors cumulatively into account. The intragranular layer porosity (a-II) should possess the most direct effect on nanoparticle embedding, as it is most likely in the size range of the nanoparticles themselves, and it appears in the coating layer where the API nanoparticles are also situated. To this end, the porosity with pore sizes smaller than 50 nm in nanoparticle-loaded granules was evaluated with BJH measurements (
Figure 6 left: PVP/VA; right: HPMC).
It is evident that in most cases, the mesoporosities (<50 nm, as determined by BJH measurements) of granules containing HPMC are considerably higher than those of granules containing PVP/VA for a comparable formulation (i.e., same carrier material and polymer to API ratio), but especially at the lowest polymer to API ratio of 0.25. Comparing these results to results presented in our previous study [
20] and redispersibility of granules (see
Section 2.1), it becomes clear that these differences may not originate from an insufficient nanoparticle embedding. Consequently, differences should be associated with a more pronounced pore structure within the nanoparticle-loaded layer and/or the associated layer formation mechanisms (e.g., drying behavior of the polymer). Contrarily to the porosity data shown above, the mesoporosity of the granules mostly decreases with increasing polymer to API ratio and by that higher polymer content in the granules. This can be attributed to the different size dimensions of the pores under consideration. The decrease in mesoporosity with increasing polymer to API ratio might be attributed to an increase in nanoparticle embedding at the carrier particle surface and/or a densification of the granules [
36]. The BJH method does not access the complete intraparticulate porosity, especially that between the carrier particles, as it is limited to pore sizes in the lower nm range [
25]. In contrast, the calculated porosities represent a more global intraparticulate porosity.
The observed differences in mesoporosity may be used to explain the influence of the polymer on the redispersibility of the nanoparticles from the granules. Higher (meso)porosities of granules produced with HPMC instead of PVP/VA might be related to an increased volume of the nanoparticle-loaded layer for granules produced with HPMC, which result in a better separation of the API nanoparticles within these layers and, consequently, in an enhanced redispersibility [
11,
20,
23]. This hypothesis might also be assigned to a study from Figueroa and Bose [
8], who similarly observed that granules with a higher porosity (determined via mercury intrusion) result in a better redispersibility of nanoparticles. However, when correlating redispersibility with porosity of corresponding granules, it has to be carefully distinguished between the different considered porosities to avoid misinterpretations.
3.3. Exemplary Description of Redispersibility of Nanoparticle-Loaded Granules
Results depicted in the previous sections and in our previous publication [
20] indicate that redispersibility and structure of nanoparticle-loaded granules are highly dependent on the applied formulation parameters. Regarding redispersibility, it might be assumed that the volume, in which the API nanoparticles are dispersed in the dry state, is a critical factor influencing particle agglomeration and stabilization potential of individual nanoparticles after reconstitution [
11,
20,
23]. In a previous study, it was already shown that the apparent thickness of nanoparticle-loaded layers around granules, assessed by means of confocal Raman microscopy, qualitatively differs for different formulations [
20]. By further developing this method in the present study, the thickness of the nanoparticle-loaded layer h
layer was quantified by means of confocal Raman microscopy. Generally, it was observed that the thickness of the nanoparticle-loaded layer on the surface of the granules lies between approx. 1 and 3 µm (data not shown, see
Supplementary Information S3). However, as not the thickness but rather the volume of the nanoparticle-loaded layers is more important regarding the degree of dispersity of the nanoparticles within these layers, microscopically derived nanoparticle-loaded layer volumes V
layer were calculated considering the specific surface area S
m,carrier (derived from particle size measurements, see
Section 2.4.1) and the mass m
carrier of the applied carrier materials (Equation (2)).
Theoretical layer volumes were calculated assuming no dissolution of the carrier material and ideal mixing of stabilizers. Accordingly, the actually applied API, polymer and SDS masses were divided by their respective solids densities to yield their volumes and were summed up to determine the theoretical layer volume.
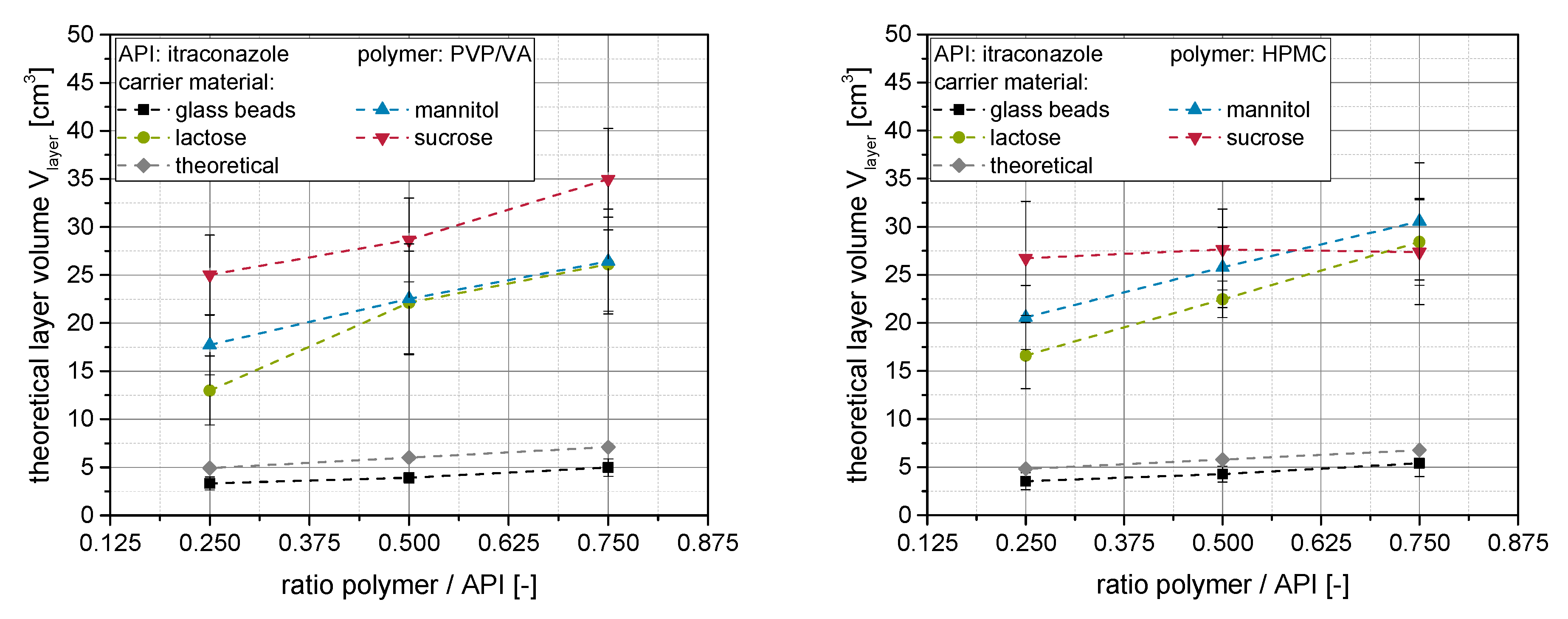
The (actual) layer volumes, calculated using the measured layer thickness and Equation (2), can be seen in
Figure 7 (left: PVP/VA, right: HPMC) as a function of the polymer to API ratio in the product granules.
Generally, it can be seen that the layer volumes increase to a higher or lesser extent with increasing polymer concentration within the granulation liquid, irrespective of the applied carrier material or polymer type. This was expected, as more material is present within the nanosuspension, which participates in the layer formation process and in which the nanoparticles can be dispersed upon resolidification. It can additionally be seen for both polymer types that the layer volume at a given polymer concentration depends on the applied carrier material. For soluble carrier materials, layer volumes are a multitude higher than the theoretical volumes (assuming no carrier dissolution) and align with the dissolution rates of the carrier materials [
20]. The differences diminish at higher polymer to API ratios, indicating a superposition of layer formation processes by dissolved polymer, as well as dissolution and resolidification of the carrier material.
Layer volumes were the lowest on insoluble glass beads for both polymers, as with this carrier, the nanoparticle-layers are only formed by the granulation liquid and no surface dissolution takes place upon granulation. Here, the layer volumes were even slightly lower than theoretical volumes calculated from the applied spraying mass, indicating a slight loss of polymer within the granulation chamber. In principle, however, the layer volumes derived using the two different ways of calculation are in good agreement for this type of carrier underlining the validity of the approach. The application of HPMC tended to result in slightly higher layer volumes at a given polymer content in the granules (except for sucrose), which might be attributed to a higher porosity of the corresponding nanoparticle-loaded layers (see
Section 3.2). However, differences diminish at higher polymer concentrations, further indicating a superposition of the different layer formation processes.
The data obtained for the layer volumes may be further used for exemplarily describing theoretical mean distances between individual nanoparticles within the nanoparticle-loaded layer around the granules. Assuming that the carrier particles and API nanoparticles are spherical with a uniform particle size, that the nanoparticle-loaded layer has no porosity and is homogeneously distributed on the surface of the carrier material, and that the API nanoparticles are uniformly distributed in this layer, the theoretical distances of the surfaces of nanoparticles within the layer a
theor. can be calculated following the study of Steiner et al. [
23] applying Equation (3).
Here, x
50,API refers to the mean particle size (i.e., z-avg.
orig) of the API nanoparticles within the corresponding nanosuspension before granulation (including additional polymeric drying excipient, see
Section 2.4.2.), ε
DSP to the porosity of a densest sphere packing, and V
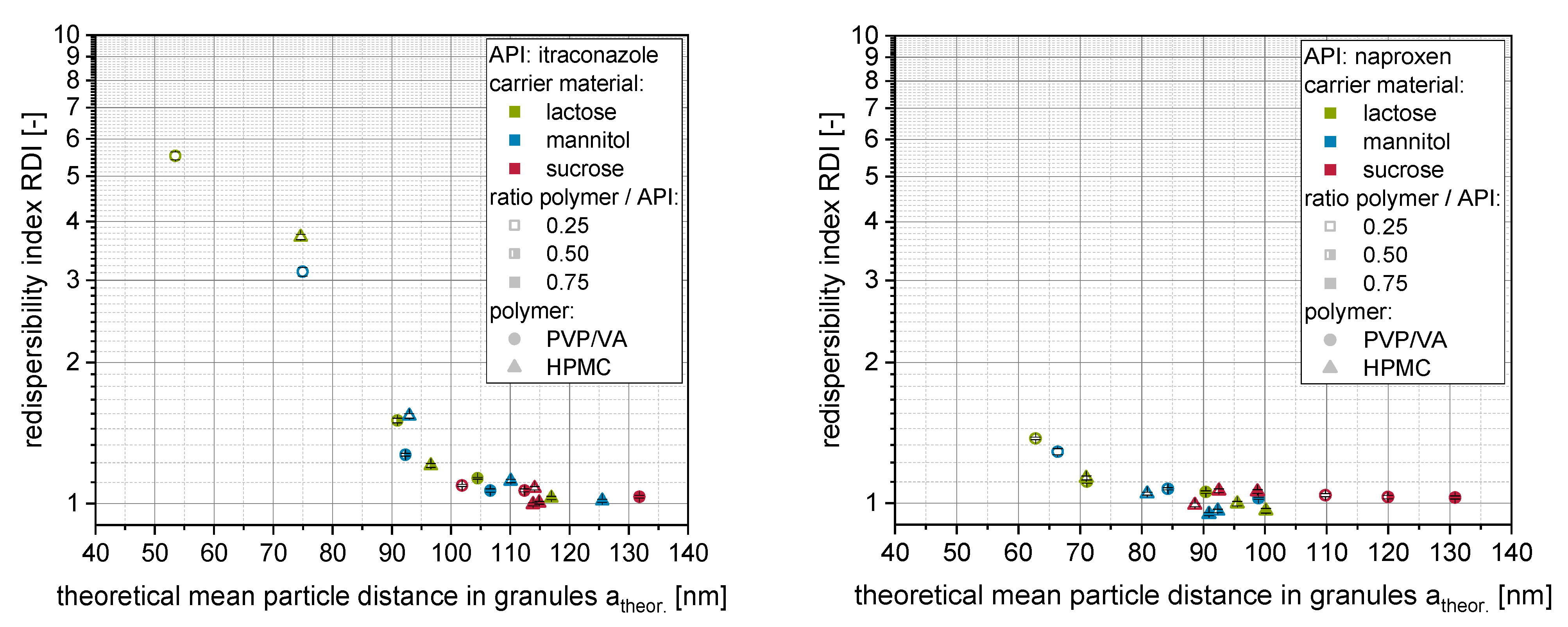
API to the total volume of the API within the granules. The dependency of the redispersibility of nanoparticles from the granules (i.e., RDI) on the theoretical mean distances of the nanoparticles within the surface layer of the granules is presented in
Figure 8 (left: itraconazole) for the soluble carrier materials. Additionally, granules with naproxen from our previous publication [
20] were re-evaluated (
Figure 8 right).
For soluble carrier materials (i.e., lactose, mannitol, sucrose), for each API, one consistent dependency of the redispersibility on the calculated mean nanoparticle distances can be observed irrespective of the exact formulation (i.e., type of polymer, ratio polymer to API). The granules were completely redispersible when theoretical mean distances of nanoparticles within the granules were larger than approx. 100 nm (itraconazole) and 80 nm (naproxen), respectively. These differences indicate that API-specific characteristics (e.g., particle shape, particle size distribution, physicochemical properties) influence the minimum mean nanoparticle distance that is sufficient for an appropriate redispersion. However, the minimum nanoparticle distances are in the same order of magnitude as those shown in the study of Steiner et al. [
23] (particle distance of 150 nm for complete redispersibility considering the factor of ½ as particle center distances were calculated), highlighting the applicability of the corresponding approach to other nanoparticle-containing composites.
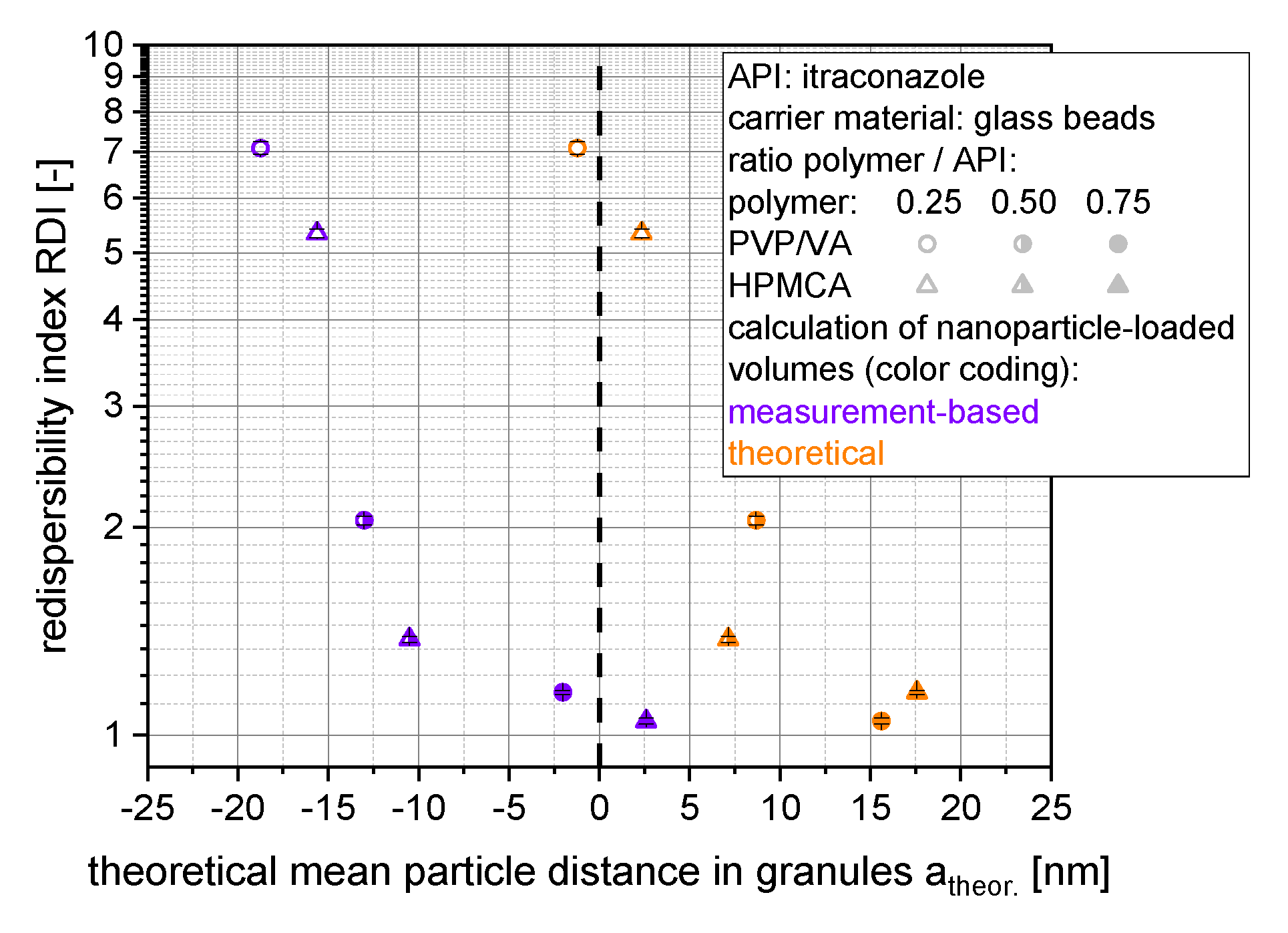
The correlation of the redispersibility of nanoparticles from granules and the theoretical mean distances of the nanoparticles within the granules based on insoluble glass beads as carrier material can be seen in
Figure 9.
Here, even negative distances were obtained when measured layer thickness/volumes were considered, which indicates deficient assumptions regarding the structure of the nanoparticle morphology, the nanoparticle-loaded granules and/or related choice of selected material parameters (e.g., specific surface area). Applying theoretically calculated layer volumes, particle distances were shifted toward positive particle distances in the lower nm range. However, similar dependency of the redispersibility on the theoretically calculated particle distances (irrespective of the applied layer volumes) highlights the importance of the degree of dispersity of the nanoparticles within the granules for their redispersibility.