Abstract
Objectives: The objective of this study was to explore the relationship between pharmacokinetic/pharmacodynamic (PK/PD) target attainment of continuous-infusion (CI) meropenem and microbiological outcome in critical COVID-19 patients with documented Gram-negative superinfections. Methods: Patients receiving CI meropenem for documented Gram-negative infections at the COVID ICU of the IRCCS Azienda Ospedaliero-Universitaria di Bologna and undergoing therapeutic drug monitoring from January 2021 to February 2022 were retrospectively assessed. Average steady-state meropenem concentrations (Css) were calculated and the Css/MIC ratio was selected as a pharmacodynamic parameter of meropenem efficacy. The Css/MIC ratio was defined as optimal if ≥4, quasi-optimal if between 1 and 4, and suboptimal if <1. The relationship between Css/MIC and microbiological outcome was assessed. Results: Overall, 43 critical COVID-19 patients with documented Gram-negative infections were retrieved. Combination therapy was implemented in 26 cases. Css/MIC ratios were optimal in 27 (62.8%), quasi-optimal in 7 (16.3%), and suboptimal in 9 cases (20.9%). Microbiological failure occurred in 21 patients (48.8%), with no difference between monotherapy and combination therapy (43.8% vs. 53.8%; p = 0.53). The microbiological failure rate was significantly lower in patients with an optimal Css/MIC ratio compared to those with a quasi-optimal or suboptimal Css/MIC ratio (33.3% vs. 75.0%; p = 0.01). Conclusion: Suboptimal attainment of meropenem PK/PD targets may be a major determinant impacting on microbiological failure in critical COVID-19 patients with Gram-negative superinfections.
1. Introduction
The SARS-CoV-2 pandemic has been responsible for most intensive care unit (ICU) admissions in the last two years, accounting for high morbidity and mortality [1]. Bacterial colonization and superinfections have been described in critically ill COVID-19 patients with an incidence ranging from 27% to 40% [2]. A remarkable proportion of these superinfections were caused by Gram-negative pathogens, including multidrug-resistant (MDR) Enterobacterales and non-fermenting isolates, leading to a significant rise in antibiotic consumption in the ICU setting [3,4,5].
Although the novel approved beta-lactams have significantly enhanced the available therapeutic options against MDR Gram-negative pathogens [6], meropenem still remains the first-line therapy for the management of ICU patients affected by extended-spectrum beta-lactamase (ESBL)-producing Enterobacterales, and a valuable alternative for non-fermenting isolates exhibiting a permissive minimum inhibitory concentration (MIC) [7,8].
The minimum pharmacodynamic (PD) target of efficacy for meropenem is considered a time of 40% of the dosing interval during which the plasma concentrations exceed the pathogen MIC (40%t > MIC) [9]. However, recent evidence proposed the achievement of aggressive pharmacokinetic/pharmacodynamic (PK/PD) targets (namely, up to 100%t > 4–8×MIC) in order to maximize clinical efficacy and minimize resistance development in critically ill patients [10,11,12].
Considering that sepsis-related pathophysiological alterations may significantly affect both the volume of distribution and the clearance of meropenem in critically septic patients, achieving the optimal PK/PD target may be challenging [13]. In this scenario, administering meropenem by continuous infusion (CI) may maximize the achievement of aggressive PK/PD targets while avoiding undesirable fluctuations in serum concentrations and preventing high peak levels that could be potentially associated with toxicity. CI administration has been shown to be superior compared to intermittent infusion in attaining a given pharmacodynamic (PD) target of efficacy and in improving clinical outcomes with beta-lactams among critically ill patients [14,15]. However, real-world data assessing the attainment of the PK/PD target of CI meropenem in critically ill COVID-19 patients are currently limited.
The purpose of this study was to assess the relationship between PK/PD target attainment and microbiological outcome in a cohort of critically ill COVID-19 patients affected by documented Gram-negative superinfections, treated with CI meropenem.
2. Materials and Methods
All the critically ill COVID-19 patients who were treated with CI meropenem because of suspected or documented Gram-negative superinfections at the COVID ICU of the IRCCS Azienda Ospedaliero-Universitaria di Bologna from 1 January 2021 to 28 February 2022 were retrospectively retrieved. Inclusion criteria were: (1) use of CI meropenem for at least 72 h; (2) at least one meropenem therapeutic drug monitoring (TDM) performed during treatment; (3) isolation of Gram-negative pathogens from microbiological cultures and determination of meropenem susceptibility.
Meropenem was prescribed at the discretion of the treating physician or infectious disease consultant according to current clinical practice implemented at the IRCCS Azienda Ospedaliero-Universitaria di Bologna. Treatment with meropenem was always started with a loading dose (LD) of 2 g over a 2 h infusion followed by a maintenance dose (MD) administered by CI. Considering that meropenem (both brand and generic formulations) is stable in aqueous solution for no more than 8 h [16,17], in order to grant active moiety during CI, the total daily MD was divided into three or four doses that were reconstituted every 6–8 h and infused over 6–8 h. MD regimens were initially selected according to renal function and underlying pathophysiological conditions and subsequently optimized by means of TDM.
Blood samples were collected in the first 48–72 h from the beginning of the treatment to determine meropenem Css. Meropenem total blood concentrations were measured at the hospital’s Unique Metropolitan Laboratory and analyzed by means of a commercially available liquid chromatography-tandem mass spectrometry (LC–MS/MS) method (Chromsystems Instruments & Chemicals GmbH, Munich, Germany) [18]. TDM results were made available via the intranet to the MD clinical pharmacologist who provided expert clinical pharmacological advice for prompt dosing adaptation by ICU physicians within 6 h of blood collection.
Demographic (age, sex, weight, body mass index (BMI)) and clinical/laboratory data (need for mechanical ventilation and vasopressors, implementation of continuous renal replacement therapy (CRRT) or extracorporeal membrane oxygenation (ECMO), creatinine clearance (CLCr), site/type of infections, bacterial clinical isolate, MIC for meropenem, average meropenem Css, implementation of antibiotic combination therapy, microbiological and clinical outcome, ICU and 30-day mortality rates) were extracted for each patient. Combination therapy was defined as the concomitant use of other antibiotics active against Gram-negative pathogens (aminoglycosides, colistin, fosfomycin, fluoroquinolones, tigecycline, and cotrimoxazole).
All of the patients undergoing CRRT received continuous venovenous hemodiafiltration (CVVHDF) with regional citrate anticoagulation by a Prismaflex Gambro machine and Prismaflex set ST 150 (AN 69 ST membrane with a surface area of 1.5 m2). The blood flow rate was maintained between 100 and 150 mL/min. The predilution citrate solution flow rate was automatically set for ensuring a circuit blood citrate concentration of 2.5–3 mmol/L. The predilution infusion flow rate/dialysate flow rate ratio was fixed at 1:1. The postdilution solution flow was fixed at 300 mL/h. A dialysis dose of 30–35 mL/Kg/h of the total effluent volume was prescribed.
Documented bloodstream infection (BSI) was defined as the isolation of Gram-negative pathogens from blood cultures. Documented ventilator-associated pneumonia (VAP) was defined as the presence of a Gram-negative bacterial load ≥104 CFU/mL in the bronchoalveolar lavage (BAL) fluid culture documented after more than 48 h of endotracheal intubation and initiation of mechanical ventilation [19]. Documented complicated urinary tract infection (cUTI) was defined as the presence of a Gram-negative bacterial load ≥105 CFU/mL in the urine culture [20].
The MIC of the identified Gram-negative pathogens was determined by means of the semi-automated broth microdilution method (Microscan Beckman NMDRM1) for Enterobacterales and Pseudomonas aeruginosa, or by a broth microdilution panel (ITGN10) for Acinetobacter baumannii. MICs were interpreted according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) clinical breakpoints. The percentage of time during which meropenem concentrations were above the MIC was selected as the best pharmacodynamic parameter for describing efficacy in terms of microbiological outcome and was expressed as the Css/MIC ratio according to CI administration. In patients having multiple Gram-negative isolates, the Css/MIC ratio was calculated using the higher MIC value. The Css/MIC ratio was defined as optimal if ≥4, quasi-optimal if between 1 and 4, and suboptimal if <1. Thresholds were selected according to preclinical models showing that a Css/MIC ≥ 4 may be associated with suppression of emergence of resistance to β-lactams [21]. Microbiological failure was defined as the persistence of the same bacterial pathogen in blood, BAL, or urine cultures after ≥7 days from starting meropenem treatment, as previously reported [22]. Microbiological eradication was defined as the occurrence of negativity of blood, BAL, or urine cultures in at least one subsequent assessment.
Descriptive statistics were used. Continuous data were presented as the mean ± standard deviation (S.D.) or median and interquartile range (IQR), whereas categorial variables were expressed as the count and percentage. Univariate analysis was performed by applying the chi-square test or Fisher’s exact test as appropriate. This study was approved by the Ethics Committee of the IRCCS Azienda Ospedaliero-Universitaria di Bologna (n. 442/2021/Oss/AOUBo approved on 28 June 2021).
3. Results
In the study period, a total of 212 critically ill patients who underwent TDM-guided meropenem in the IRCSS Azienda Ospedaliero-Universitaria di Bologna were screened. Among them, 67 were admitted to the COVID ICU owing to acute respiratory distress syndrome caused by severe COVID-19 pneumonia. Finally, forty-three patients had documented Gram-negative superinfections and were included in the study (Figure 1).
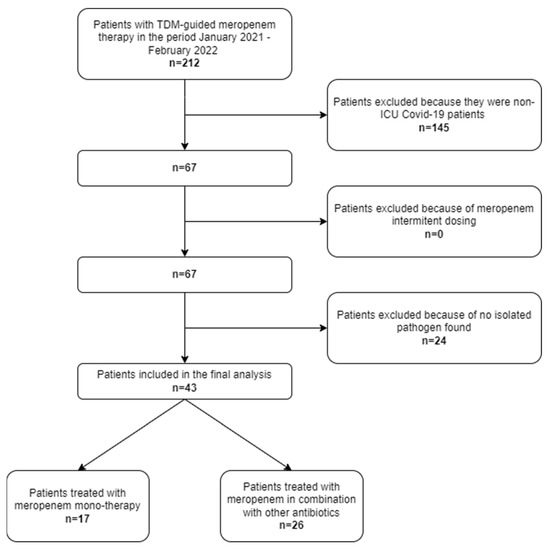
Figure 1.
Flowchart of patient inclusion and exclusion criteria.
The demographics and clinical features of the included patients are reported in Table 1.

Table 1.
Demographics and clinical characteristics of patients included in the PK/PD analysis classified according to the administration of monotherapy or combination therapy with meropenem.
The mean ± S.D. age was 59.0 ± 13.8 years with a slight male preponderance (60.5%). The mean ± S.D. BMI was 30.7 ± 14.9 kg/m2. All patients required mechanical ventilation. Most patients (81.4%) had septic shock. Seventeen patients (39.5%) underwent CRRT and eight (18.6%) required ECMO. The overall ICU and 30-day mortality rates were 46.5%. The median (IQR) SOFA score was 9 (7–11) points.
The types of infection were VAP (38/43; 88.4%), BSI (13/43; 30.2%), and cUTI (4/43; 9.3%). In eight patients (18.6%), BSI and VAP occurred simultaneously. In two cases, concomitant VAP and cUTI were documented, while in one patient BSI, VAP, and cUTI occurred simultaneously. Overall, 47 Gram-negative pathogens were isolated, with Pseudomonas aeruginosa (29.8%), Acinetobacter baumannii (21.3%), and Klebsiella pneumoniae (12.8%) being the most frequent. Enterobacterales accounted for 48.9% of the overall Gram-negative isolates.
Combination therapy was adopted in 26 out of 43 cases (60.5%). Fosfomycin (15/26; 57.8%) and colistin (7/26; 27.0%) were the concomitant agents most frequently used, followed by one case each for ceftazidime-avibactam, ciprofloxacin, cotrimoxazole, and colistin + fosfomycin (Table 1). A total of 138 TDMs of meropenem were determined, with a median (IQR) of 3 (2–4) per patient. The median (IQR) meropenem Css average was 22.4 mg/L (12.3–32.5 mg/L). Overall, meropenem dosing adjustments were recommended in 51 out of 138 TDM assessments (37.0%, divided into 25.4% decreases and 11.6% increases). The starting meropenem dosing regimen was adjusted after the first TDM assessment in 17 out of 43 patients (39.5%, divided into 30.2% decreases and 9.3% increases). Among patients undergoing CVVHDF, meropenem dosing adjustments at the first TDM assessment were recommended in 10 out of 17 cases (58.8%, divided into 41.2% decreases and 17.6% increases). The Css/MIC ratio was optimal in 27 cases (62.8%), quasi-optimal in 7 cases (16.3%), and suboptimal in 9 cases (20.9%).
Microbiological failure occurred in 21 patients (48.8%) and concerned 17 VAP, 2 BSI, and 2 VAP plus BSI cases. Overall, the microbiological failure rate was significantly lower in patients with an optimal Css/MIC ratio compared to those with a quasi-optimal or suboptimal Css/MIC ratio (33.3% vs. 75.0%; p = 0.01). No difference in microbiological failure emerged between patients treated with monotherapy and those receiving meropenem in combination therapy (43.8% vs. 53.8%; p = 0.53). Among the 17 patients who received meropenem monotherapy, the microbiological failure rate was significantly higher in those with a suboptimal Css/MIC ratio compared to those with an optimal or a quasi-optimal Css/MIC ratio (100.0% vs. 28.6%, p = 0.05; Figure 2a).
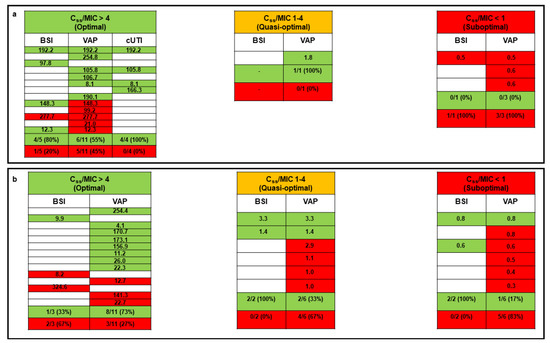
Figure 2.
Relationship between pharmacokinetic/pharmacodynamic target attainment (expressed as the average Css/MIC ratio) and microbiological outcome for critically ill COVID-19 patients treated with CI meropenem in monotherapy (panel a) or combination therapy (panel b). Green box, microbiological eradication; red box, microbiological failure; white box, absence of specific type of infection. Each row corresponds to a single patient. The Css/MIC ratio is shown for each patient and defined as optimal if ≥4, quasi-optimal if between 1 and 4, and suboptimal if <1. BSI: bloodstream infection; Css: meropenem average steady-state concentrations; cUTI: complicated urinary tract infection; MIC: minimum inhibitory concentration; VAP: ventilator-associated pneumonia.
Among the 26 patients treated with meropenem combination therapy, a trend toward a higher microbiological failure rate occurred in those with a suboptimal Css/MIC ratio compared to those with an optimal or a quasi-optimal Css/MIC ratio (83.3% vs. 45.0%, p = 0.16; Figure 2b).
The microbiological failure rates among patients with VAP compared to those with BSIs were higher both in the monotherapy (53.3% vs. 33.3%) and combination therapy groups (52.2% vs. 28.6%). In regard to Gram-negative pathogens, the microbiological failure rate was significantly higher in patients with infections caused by non-fermenting Gram-negative pathogens (66.7%) compared to those with infections caused by Enterobacterales (21.7%, p = 0.002; Figure 3).
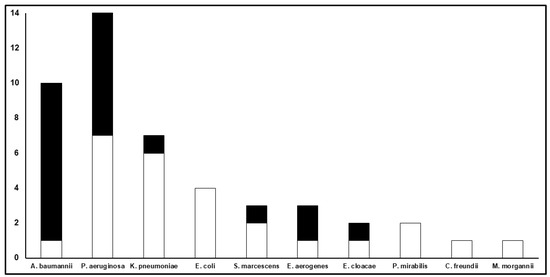
Figure 3.
Microbiological outcome according to specific Gram-negative isolates. The Y-axis refers to the total number of clinical isolates for each Gram-negative pathogen described on the X-axis. The white part of each bar refers to the number of microbiological eradications, whereas the black part refers to the number of microbiological failures.
4. Discussion
Our study assessed the relationship between the PK/PD target attainment of CI meropenem and microbiological outcome in the novel scenario of critically ill COVID-19 patients with documented Gram-negative superinfections. The findings suggest the remarkable role that the achievement of an optimal and/or a quasi-optimal PK/PD target by means of a real-time TDM-guided approach may play in enabling microbiological cure with CI meropenem.
Critically ill COVID-19 patients are at high risk of bacterial superinfections, which in most cases are caused by MDR Gram-negative pathogens [2,3]. In this setting, CI meropenem may be a valuable therapeutic strategy for the management of infections caused by ESBL-producing Enterobacterales and/or non-fermenting pathogens, either as a monotherapy or combination therapy [8,24]. The achievement of Css > 4–5-fold above the MIC was shown to be helpful in minimizing the risk of microbiological failure and of carbapenem resistance development [12,21].
Notably, our findings show that suboptimal meropenem PK/PD target attainment accounted for most of the cases with microbiological failure. Furthermore, there was a trend toward a proportional increase in microbiological failure of meropenem therapy when PK/PD target attainment shifted from optimal to quasi-optimal and suboptimal, as previously reported with other traditional and novel beta-lactams [12,25]. Overall, these findings may support the utility of a TDM-guided approach in promptly assessing the achievement of the optimal meropenem PK/PD target in critically ill COVID-19 patients with documented Gram-negative superinfections.
It is noteworthy that no major difference in the microbiological failure rate was observed between patients receiving meropenem monotherapy and patients receiving combination therapy. Conversely, in both groups, there was a trend toward a proportional increase in the microbiological failure rate when PK/PD target attainment of meropenem shifted from optimal to suboptimal. These findings may offer further support to the contention that PK/PD target maximization of meropenem monotherapy may play a role in enabling microbiological cure that is more determinant than that played by the use of combination therapy. Indeed, combination therapy provided no benefits over monotherapy in terms of clinical and microbiological outcomes, as previously reported [26,27,28].
In our cohort, fosfomycin was the agent most frequently combined with meropenem. Although in vitro studies showed that fosfomycin may be synergic with meropenem against Pseudomonas aeruginosa [29], no real-world evidence currently supports the superiority of this combination therapy over monotherapy. This supports the idea that combination therapy with fosfomycin should not be pursued routinely, but should be reserved only when in the presence of difficult-to-treat strains [30,31].
It is noteworthy that most of the microbiological failures occurred in patients with VAP. This suggests that microbiological eradication may be especially difficult in deep-seated infections compared to bloodstream or urinary infections. This may be due to the limited penetration rate of meropenem into the epithelial lining fluid (ELF), which is approximatively 30% [32,33]. Overall, these findings may support the rationale for administering higher doses of CI meropenem in patients with VAP. This approach, by increasing lung exposure, may allow the achievement of aggressive PK/PD targets even at this difficult-to-treat infection site. In this regard, approaches focused on assessing the antibiotic concentration at the infection site could be helpful [34].
It was not unexpected that patients with infections caused by Gram-negative non-fermenting pathogens had a worse microbiological outcome compared to those having infections caused by Enterobacterales (most of which produce ESBLs). This could be related either to higher MICs for meropenem, which make the achievement of optimal meropenem PK/PD targets more challenging, or to the presence of multiple and complex mechanisms of resistance [34]. We could also speculate that the PD target of meropenem needed for eradicating non-fermenting pathogens could be higher than that needed for eradicating Enterobacterales strains according to preclinical data [35,36,37]. However, even in this challenging scenario, it appeared that maximization of PK/PD targets may be the major determinant of microbiological outcome, as achieving quasi-optimal PK/PD targets allowed the eradication of infections due to Acinetobacter baumannii or Pseudomonas aeruginosa even when in the presence of an MIC of up to 16–32 mg/L.
Our study has some limitations. The retrospective monocentric study design and the limited sample size of patients should be acknowledged. The assessment of bacterial susceptibility by means of the semi-automated broth microdilution method for Enterobacterales and Pseudomonas aeruginosa must be recognized. However, the good trend of the relationship between PK/PD target attainment and microbiological outcome is a major strength.
In conclusion, our findings suggest that achieving optimal PK/PD targets may be a major determinant of microbiological cure of Gram-negative superinfections treated with CI meropenem in critically ill COVID-19 patients. Higher PK/PD targets may be desirable in VAP sustained by non-fermenting Gram-negative pathogens for maximizing efficacy in this difficult-to-access site. Additionally, our findings highlight the potentially relevant role that a real-time TDM-guided strategy may play in this challenging scenario. Obviously, this study is simply a proof of concept, and large prospective clinical studies are warranted for confirming our hypothesis.
Author Contributions
M.S.C.: conceptualization, data curation, formal analysis, and writing original draft; M.G.: conceptualization, data curation, formal analysis, and writing original draft; C.T.: data curation; G.F.: data curation; Z.P.: data curation; F.T.: data curation; A.Z.: review and editing of manuscript; F.C.: review and editing of manuscript; P.V.: conceptualization, review and editing of manuscript; F.P.: conceptualization, review and editing of manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This project received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie Grant agreement No 861323.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the IRCCS Azienda Ospedaliero-Universitaria di Bologna (n. 442/2021/Oss/AOUBo approved on 28 June 2021).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
M.G. reports grants from Angelini S.p.A., outside the submitted work. F.P. reports personal fees from Angelini, Basilea Pharmaceutica, Gilead, Hikma, MSD, Pfizer, Sanofi-Aventis, Shionogi, Thermo Fisher, and Accelerate Diagnostics, outside the submitted work, and has participated in the speaker’s bureaus for Accelerate Diagnostics, Angelini, Basilea Pharmaceutica, Gilead, Hikma, MSD, Pfizer, Sanofi-Aventis, Shionogi, and Thermo Fisher, and as a consultant for Angelini, Basilea Pharmaceutica, Gilead, MSD, Pfizer, and Shionogi, outside the submitted work. P.V. has served as a consultant for bioMérieux, Gilead, Merck Sharp & Dohme, Nabriva, Nordic Pharma, Pfizer, Thermo-Fisher, and Venatorx, and received payment for serving on the speaker’s bureaus for Correvio, Gilead, Merck Sharp & Dohme, Nordic Pharma, and Pfizer, outside the submitted work. The other authors report no potential conflict of interest for this work.
References
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Bardi, T.; Pintado, V.; Gomez-Rojo, M.; Escudero-Sanchez, R.; Azzam Lopez, A.; Diez-Remesal, Y.; Martinez Castro, N.; Ruiz-Garbajosa, P.; Pestaña, D. Nosocomial infections associated to COVID-19 in the intensive care unit: Clinical characteristics and outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Emerick, M.; Cabunoc, M.K.; Williams, M.H.; Preas, M.A.; Schrank, G.; Rabinowitz, R.; Luethy, P.; Johnson, J.K.; Leekha, S. Rapid spread and control of multidrug-resistant gram-negative bacteria in COVID-19 patient care units. Emerg. Infect. Dis. 2021, 27, 1234–1237. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Giordano, C.; Leonildi, A.; Menichini, M.; Vecchione, A.; Pistello, M.; Guarracino, F.; Ghiadoni, L.; Forfori, F.; et al. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: A prospective observational study. J. Antimicrob. Chemother. 2021, 76, 1078–1084. [Google Scholar] [CrossRef]
- Ripa, M.; Galli, L.; Poli, A.; Oltolini, C.; Spagnuolo, V.; Mastrangelo, A.; Muccini, C.; Monti, G.; De Luca, G.; Landoni, G.; et al. Secondary infections in patients hospitalized with COVID-19: Incidence and predictive factors. Clin. Microbiol. Infect. 2021, 27, 451–457. [Google Scholar] [CrossRef]
- Rodríguez-Baño, J.; Gutiérrez-Gutiérrez, B.; Machuca, I.; Pascual, A. Treatment of infections caused by extended-spectrum-beta-lactamase-, AmpC-, and carbapenemase-producing Enterobacteriaceae. Clin. Microbiol. Rev. 2018, 31, e00079-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, P.N.A.; Tambyah, P.A.; Lye, D.C.; Mo, Y.; Lee, T.H.; Yilmaz, M.; Alenazi, T.H.; Arabi, Y.; Falcone, M.; Bassetti, M.; et al. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: A randomized clinical trial. JAMA 2018, 320, 984–994. [Google Scholar] [CrossRef] [Green Version]
- Gatti, M.; Viaggi, B.; Rossolini, G.M.; Pea, F.; Viale, P. An evidence-based multidisciplinary approach focused at creating algorithms for targeted therapy of infection-related ventilator associated complications (IVACs) caused by Enterobacterales in critically ill adult patients. Expert Rev. Anti-Infect. Ther. 2022, 20, 331–352. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.M.; Kuti, J.L.; Nicolau, D.P. Use of Monte Carlo simulation to assess the pharmacodynamics of beta-lactams against Pseudomonas aeruginosa infections in children: A report from the OPTAMA program. Clin. Ther. 2005, 27, 1820–1830. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A position paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef] [PubMed]
- Guilhaumou, R.; Benaboud, S.; Bennis, Y.; Dahyot-Fizelier, C.; Dailly, E.; Gandia, P.; Goutelle, S.; Lefeuvre, S.; Mongardon, N.; Roger, C.; et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients—guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique—SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation—SFAR). Crit. Care 2019, 23, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Gatti, M.; Cojutti, P.G.; Pascale, R.; Tonetti, T.; Laici, C.; Dell’Olio, A.; Siniscalchi, A.; Giannella, M.; Viale, P.; Pea, F. Assessment of a PK/PD target of continuous infusion beta-lactams useful for preventing microbiological failure and/or resistance development in critically ill patients affected by documented gram-negative infections. Antibiotics 2021, 10, 1311. [Google Scholar] [CrossRef]
- Blot, S.I.; Pea, F.; Lipman, J. The effect of pathophysiology on pharmacokinetics in the critically ill patient--concepts appraised by the example of antimicrobial agents. Adv. Drug Deliv. Rev. 2014, 77, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Abdul-Aziz, M.-H.; Davis, J.S.; Dulhunty, J.M.; Cotta, M.O.; Myburgh, J.; Bellomo, R.; Lipman, J. Continuous versus Intermittent β-Lactam Infusion in Severe Sepsis. A meta-analysis of individual patient data from randomized trials. Am. J. Respir. Crit. Care Med. 2016, 194, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Sime, F.B.; Lipman, J.; Roberts, J.A. How do we use therapeutic drug monitoring to improve outcomes from severe infections in critically ill patients? BMC Infect. Dis. 2014, 14, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, L.; Cojutti, P.; Baraldo, M.; Pea, F. Stability of generic meropenem solutions for administration by continuous infusion at normal and elevated temperatures. Ther. Drug Monit. 2014, 36, 674–676. [Google Scholar] [CrossRef]
- Fawaz, S.; Barton, S.; Whitney, L.; Swinden, J.; Nabhani-Gebara, S. Stability of meropenem after reconstitution for administration by prolonged infusion. Hosp. Pharm. 2018, 54, 190–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chromsystems Instruments & Chemicals GmbH. MassTox TDM Series, A. 2022. Available online: https://chromsystems.com/en/products/therapeutic-drug-monitoring.html (accessed on 27 May 2022).
- Chastre, J.; Fagon, J.-Y. Ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 2002, 165, 867–903. [Google Scholar] [CrossRef]
- Miller, J.M.; Binnicker, M.J.; Campbell, S.; Carroll, K.C.; Chapin, K.C.; Gilligan, P.H.; Gonzalez, M.D.; Jerris, R.C.; Kehl, S.C.; Patel, R.; et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin. Infect. Dis. 2018, 67, e1–e94. [Google Scholar] [CrossRef]
- Sumi, C.D.; Heffernan, A.J.; Lipman, J.; Roberts, J.A.; Sime, F.B. What antibiotic exposures are required to suppress the emergence of resistance for Gram-negative bacteria? A systematic review. Clin. Pharmacokinet. 2019, 58, 1407–1443. [Google Scholar] [CrossRef]
- Shields, R.K.; Nguyen, M.H.; Chen, L.; Press, E.G.; Kreiswirth, B.N.; Clancy, C.J. Pneumonia and renal replacement therapy are risk factors for ceftazidime-avibactam treatment failures and resistance among patients with carbapenem-resistant Enterobacteriaceae infections. Antimicrob. Agents Chemother. 2018, 62, e02497-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatti, M.; Viaggi, B.; Rossolini, G.M.; Pea, F.; Viale, P. An evidence-based multidisciplinary approach focused on creating algorithms for targeted therapy of Infection-Related Ventilator-Associated Complications (IVACs) caused by Pseudomonas aeruginosa and Acinetobacter baumannii in critically ill adult patients. Antibiotics 2021, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Bartoletti, M.; Cojutti, P.G.; Gaibani, P.; Conti, M.; Giannella, M.; Viale, P.; Pea, F. A descriptive case series of PK/PD target attainment and microbiological outcome in critically ill patients with documented severe XDR Acinetobacter baumannii BSI and/or VAP treated with cefiderocol. J. Glob. Antimicrob. Resist. 2021, 27, 294–298. [Google Scholar] [CrossRef]
- Paul, M.; Lador, A.; Grozinsky-Glasberg, S.; Leibovici, L. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst. Rev. 2014, 1, CD003344. [Google Scholar] [CrossRef] [PubMed]
- Heyland, D.K.; Dodek, P.; Muscedere, J.; Day, A.; Cook, D. Canadian Critical Care Trials Group Randomized trial of combination versus monotherapy for the empiric treatment of suspected ventilator-associated pneumonia. Crit. Care Med. 2008, 36, 737–744. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Voulgaris, G.L.; Maliaros, A.; Samonis, G.; Falagas, M.E. Prolonged versus short-term intravenous infusion of antipseudomonal β-lactams for patients with sepsis: A systematic review and meta-analysis of randomised trials. Lancet Infect. Dis. 2018, 18, 108–120. [Google Scholar] [CrossRef]
- Drusano, G.L.; Neely, M.N.; Yamada, W.M.; Duncanson, B.; Brown, D.; Maynard, M.; Vicchiarelli, M.; Louie, A. The combination of fosfomycin plus meropenem is synergistic for Pseudomonas aeruginosa PAO1 in a hollow-fiber infection model. Antimicrob. Agents Chemother. 2018, 62, e01682-18. [Google Scholar] [CrossRef] [Green Version]
- Albiero, J.; Mazucheli, J.; Barros, J.P.D.R.; Szczerepa, M.M.D.A.; Nishiyama, S.A.B.; Carrara-Marroni, F.E.; Sy, S.; Fidler, M.; Sy, S.K.B.; Tognim, M.C.B. Pharmacodynamic attainment of the synergism of meropenem and fosfomycin combination against Pseudomonas aeruginosa producing metallo-β-lactamase. Antimicrob. Agents Chemother. 2019, 63, e00126-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadri, S.S.; Adjemian, J.; Lai, Y.L.; Spaulding, A.B.; Ricotta, E.; Prevots, D.R.; Palmore, T.N.; Rhee, C.; Klompas, M.; Dekker, J.P.; et al. Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: Retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin. Infect. Dis. 2018, 67, 1803–1814. [Google Scholar] [CrossRef] [Green Version]
- Paal, M.; Scharf, C.; Denninger, A.K.; Ilia, L.; Kloft, C.; Kneidinger, N.; Liebchen, U.; Michel, S.; Schneider, C.; Schröpf, S.; et al. Target site pharmacokinetics of meropenem: Measurement in human explanted lung tissue by bronchoalveolar lavage, microdialysis, and homogenized lung tissue. Antimicrob. Agents Chemother. 2021, 65, e01564-21. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Cano, A.; Luque, S.; Sorlí, L.; Carazo, J.; Ramos, I.; Campillo, N.; Curull, V.; Sánchez-Font, A.; Vilaplana, C.; Horcajada, J.P.; et al. Intrapulmonary concentrations of meropenem administered by continuous infusion in critically ill patients with nosocomial pneumonia: A randomized pharmacokinetic trial. Crit Care 2020, 24, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz Codina, M.; Zeitlinger, M. Biomarkers predicting tissue pharmacokinetics of antimicrobials in sepsis: A review. Clin. Pharmacokinet. 2022, 61, 593–617. [Google Scholar] [CrossRef] [PubMed]
- Potron, A.; Poirel, L.; Nordmann, P. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: Mechanisms and epidemiology. Int. J. Antimicrob. Agents 2015, 45, 568–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeRyke, C.A.; Banevicius, M.A.; Fan, H.W.; Nicolau, D.P. Bactericidal activities of meropenem and ertapenem against extended-spectrum-beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a neutropenic mouse thigh model. Antimicrob. Agents Chemother. 2007, 51, 1481–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macvane, S.H.; Crandon, J.L.; Nicolau, D.P. Characterizing in vivo pharmacodynamics of carbapenems against Acinetobacter baumannii in a murine thigh infection model to support breakpoint determinations. Antimicrob. Agents Chemother. 2014, 58, 599–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).