Non-Invasive Nasal Discharge Fluid and Other Body Fluid Biomarkers in Alzheimer’s Disease
Abstract
1. Introduction
2. Clinical Diagnosis of AD
3. Conventional AD Body Fluid Biomarkers
3.1. CSF
3.2. Blood (Plasma and Serum)
| Body Fluid | Acquisition | Procedure | Reference | |
|---|---|---|---|---|
| CSF | Lumbar puncture | 1. The subject lies on their side and bends knees toward the chest and chin. 2. An atraumatic spinal needle is injected into the vertebral body L3–L5. 3. CSF is collected in polypropylene tubes about 1–2 mL. | [23] | |
| Blood | Plasma | Venipuncture | 1. Clean the venipuncture site and insert the needle. 2. The blood is collected in blood collection tubes, including anticoagulant (EDTA or heparin). 3. Within 1–2 h, collecting tubes are centrifuged, and then supernatant is transferred into new tubes, including protease inhibitor cocktail. | [33,34] |
| Serum | Venipuncture | 1. Clean the venipuncture site and insert the needle. 2. The blood is collected in blood collection tubes, including a silica clot activator. 3. After clotting for 30 min, samples are centrifuged, and then supernatant is transferred into new tubes, including a protease inhibitor cocktail. | ||
3.3. Limitations of Current CSF and Blood AD Biomarkers
| AD Pathology Mechanism | Core Biomarkers | CSF | Blood | ||
|---|---|---|---|---|---|
| Change | Reference | Change | Reference | ||
| Aβ pathology | Aβ42 | Reduced | [29,46,47] | Reduced | [48,49,50] |
| Aβ42/Aβ40 | Reduced | [51,52,53] | Reduced | [48,50,54] | |
| Tau pathology | P-tau | Increased | [55,56,57] | Increased | [58,59,60] |
| T-tau | Increased | [61,62,63] | Increased | [60,64,65] | |
4. Novel Peripheral Body Fluid Biomarkers
4.1. Nasal Discharge
4.2. Tears
4.3. Saliva
4.4. Urine
4.5. Limitations and Future Perspectives of Novel Peripheral Body Fluid Biomarkers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Sevigny, J.; Chiao, P.; Bussiere, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Musiek, E.S.; Bennett, D.A. Aducanumab and the “post-amyloid” era of Alzheimer research? Neuron 2021, 109, 3045–3047. [Google Scholar] [CrossRef] [PubMed]
- Davda, N.; Corkill, R. Biomarkers in the diagnosis and prognosis of Alzheimer’s disease. J. Neurol. 2020, 267, 2475–2477. [Google Scholar] [CrossRef]
- Paraskevaidi, M.; Martin-Hirsch, P.L.; Martin, F.L. Progress and Challenges in the Diagnosis of Dementia: A Critical Review. ACS Chem. Neurosci. 2018, 9, 446–461. [Google Scholar] [CrossRef]
- Guest, F.L.; Rahmoune, H.; Guest, P.C. Early Diagnosis and Targeted Treatment Strategy for Improved Therapeutic Outcomes in Alzheimer’s Disease. In Reviews on New Drug Targets in Age-Related Disorders; Guest, P.C., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 175–191. [Google Scholar]
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 2018, 7, 1161. [Google Scholar] [CrossRef]
- Thal, L.J.; Kantarci, K.; Reiman, E.M.; Klunk, W.E.; Weiner, M.W.; Zetterberg, H.; Galasko, D.; Pratico, D.; Griffin, S.; Schenk, D.; et al. The role of biomarkers in clinical trials for Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2006, 20, 6–15. [Google Scholar] [CrossRef]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef]
- Juva, K.; Sulkava, R.; Erkinjuntti, T.; Ylikoski, R.; Valvanne, J.; Tilvis, R. Staging the severity of dementia: Comparison of clinical (CDR, DSM-III-R), functional (ADL, IADL) and cognitive (MMSE) scales. Acta Neurol. Scand. 1994, 90, 293–298. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. DSM-5 Task Force. In Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; p. xliv. 947p. [Google Scholar]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Feldman, H.H.; Frisoni, G.B.; Hampel, H.; Jagust, W.J.; Johnson, K.A.; Knopman, D.S.; et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016, 87, 539–547. [Google Scholar] [CrossRef]
- Baker, M. In biomarkers we trust? Nat. Biotechnol. 2005, 23, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.; Le Manach, Y.; Riou, B.; Houle, T.T. Statistical evaluation of a biomarker. Anesthesiology 2010, 112, 1023–1040. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; Hampel, H.; Weiner, M.; Zetterberg, H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 131–144. [Google Scholar] [CrossRef]
- Sakka, L.; Coll, G.; Chazal, J. Anatomy and physiology of cerebrospinal fluid. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 309–316. [Google Scholar] [CrossRef]
- van Gool, A.J.; Hendrickson, R.C. The proteomic toolbox for studying cerebrospinal fluid. Expert Rev. Proteom. 2012, 9, 165–179. [Google Scholar] [CrossRef]
- Lee, J.C.; Kim, S.J.; Hong, S.; Kim, Y. Diagnosis of Alzheimer’s disease utilizing amyloid and tau as fluid biomarkers. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef]
- Rosenberg, G.A. Chapter 4—Cerebrospinal Fluid: Formation, Absorption, Markers, and Relationship to Blood–Brain Barrier. In Primer on Cerebrovascular Diseases, 2nd ed.; Caplan, L.R., Biller, J., Leary, M.C., Lo, E.H., Thomas, A.J., Yenari, M., Zhang, J.H., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 25–31. [Google Scholar]
- Teunissen, C.E.; Petzold, A.; Bennett, J.L.; Berven, F.S.; Brundin, L.; Comabella, M.; Franciotta, D.; Frederiksen, J.L.; Fleming, J.O.; Furlan, R.; et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology 2009, 73, 1914–1922. [Google Scholar] [CrossRef]
- Cummings, J.L.; Dubois, B.; Molinuevo, J.L.; Scheltens, P. International Work Group criteria for the diagnosis of Alzheimer disease. Med. Clin. N. Am. 2013, 97, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Dekosky, S.T.; Barberger-Gateau, P.; Cummings, J.; Delacourte, A.; Galasko, D.; Gauthier, S.; Jicha, G.; et al. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007, 6, 734–746. [Google Scholar] [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef]
- Oddo, S.; Caccamo, A.; Kitazawa, M.; Tseng, B.P.; LaFerla, F.M. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol. Aging 2003, 24, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Zetterberg, H.; Del Tredici, K.; Blennow, K. Intraneuronal tau aggregation precedes diffuse plaque deposition, but amyloid-β changes occur before increases of tau in cerebrospinal fluid. Acta Neuropathol. 2013, 126, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Zetterberg, H.; Wahlund, L.O.; Blennow, K. Cerebrospinal fluid markers for prediction of Alzheimer’s disease. Neurosci. Lett. 2003, 352, 67–69. [Google Scholar] [CrossRef]
- Krebs, H.A. Chemical composition of blood plasma and serum. Annu. Rev. Biochem. 1950, 19, 409–430. [Google Scholar] [CrossRef]
- Jacobs, J.M.; Adkins, J.N.; Qian, W.-J.; Liu, T.; Shen, Y.; Camp, D.G.; Smith, R.D. Utilizing Human Blood Plasma for Proteomic Biomarker Discovery. J. Proteome Res. 2005, 4, 1073–1085. [Google Scholar] [CrossRef]
- Shen, Y.; Kim, J.; Strittmatter, E.F.; Jacobs, J.M.; Camp Ii, D.G.; Fang, R.; Tolié, N.; Moore, R.J.; Smith, R.D. Characterization of the human blood plasma proteome. Proteomics 2005, 5, 4034–4045. [Google Scholar] [CrossRef]
- Rai, A.J.; Gelfand, C.A.; Haywood, B.C.; Warunek, D.J.; Yi, J.; Schuchard, M.D.; Mehigh, R.J.; Cockrill, S.L.; Scott, G.B.; Tammen, H.; et al. HUPO Plasma Proteome Project specimen collection and handling: Towards the standardization of parameters for plasma proteome samples. Proteomics 2005, 5, 3262–3277. [Google Scholar] [CrossRef]
- Tuck, M.K.; Chan, D.W.; Chia, D.; Godwin, A.K.; Grizzle, W.E.; Krueger, K.E.; Rom, W.; Sanda, M.; Sorbara, L.; Stass, S.; et al. Standard Operating Procedures for Serum and Plasma Collection: Early Detection Research Network Consensus Statement Standard Operating Procedure Integration Working Group. J. Proteome Res. 2009, 8, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; O’Bryant, S.E.; Molinuevo, J.L.; Zetterberg, H.; Masters, C.L.; Lista, S.; Kiddle, S.J.; Batrla, R.; Blennow, K. Blood-based biomarkers for Alzheimer disease: Mapping the road to the clinic. Nat. Rev. Neurol. 2018, 14, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Zetterberg, H. Blood-based biomarkers for Alzheimer’s disease-An update. J. Neurosci. Methods 2019, 319, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Milà-Alomà, M.; Suárez-Calvet, M.; Molinuevo, J.L. Latest advances in cerebrospinal fluid and blood biomarkers of Alzheimer’s disease. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419888819. [Google Scholar] [CrossRef]
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L.; et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015, 85, 296–302. [Google Scholar] [CrossRef]
- Zetterberg, H.; Blennow, K. From Cerebrospinal Fluid to Blood: The Third Wave of Fluid Biomarkers for Alzheimer’s Disease. J. Alzheimers Dis. 2018, 64, S271–S279. [Google Scholar] [CrossRef]
- Altuna-Azkargorta, M.; Mendioroz-Iriarte, M. Blood biomarkers in Alzheimer’s disease. Neurologia 2021, 36, 704–710. [Google Scholar] [CrossRef]
- Balasa, R.; Barcutean, L.; Balasa, A.; Motataianu, A.; Roman-Filip, C.; Manu, D. The action of TH17 cells on blood brain barrier in multiple sclerosis and experimental autoimmune encephalomyelitis. Hum. Immunol. 2020, 81, 237–243. [Google Scholar] [CrossRef]
- Hart, I.K.; Bone, I.; Hadley, D.M. Development of neurological problems after lumbar puncture. Br. Med. J. 1988, 296, 51–52. [Google Scholar] [CrossRef]
- Mattsson, N.; Zetterberg, H.; Hansson, O.; Andreasen, N.; Parnetti, L.; Jonsson, M.; Herukka, S.K.; van der Flier, W.M.; Blankenstein, M.A.; Ewers, M.; et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 2009, 302, 385–393. [Google Scholar] [CrossRef]
- Mattsson, N.; Zetterberg, H. What is a certified reference material? Biomark. Med. 2012, 6, 369–370. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Behl, T.A.-O.; Sehgal, A.; Singh, S.; Sharma, N.; Bungau, S. Multifaceted Alzheimer’s Disease: Building a Roadmap for Advancement of Novel Therapies. Neurochem. Res. 2021, 46, 2832–2851. [Google Scholar] [CrossRef]
- Motter, R.; Vigo-Pelfrey, C.; Kholodenko, D.; Barbour, R.; Johnson-Wood, K.; Galasko, D.; Chang, L.; Miller, B.; Clark, C.; Green, R.; et al. Reduction of beta-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer’s disease. Ann. Neurol. 1995, 38, 643–648. [Google Scholar] [CrossRef]
- Andreasen, N.; Hesse, C.; Davidsson, P.; Minthon, L.; Wallin, A.; Winblad, B.; Vanderstichele, H.; Vanmechelen, E.; Blennow, K. Cerebrospinal Fluid β-Amyloid(1-42) in Alzheimer Disease: Differences Between Early- and Late-Onset Alzheimer Disease and Stability During the Course of Disease. Arch. Neurol. 1999, 56, 673–680. [Google Scholar] [CrossRef]
- Lui, J.K.; Laws, S.M.; Li, Q.X.; Villemagne, V.L.; Ames, D.; Brown, B.; Bush, A.I.; De Ruyck, K.; Dromey, J.; Ellis, K.A.; et al. Plasma amyloid-beta as a biomarker in Alzheimer’s disease: The AIBL study of aging. J. Alzheimers Dis. 2010, 20, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Lewczuk, P.; Kornhuber, J.; Vanmechelen, E.; Peters, O.; Heuser, I.; Maier, W.; Jessen, F.; Bürger, K.; Hampel, H.; Frölich, L.; et al. Amyloid beta peptides in plasma in early diagnosis of Alzheimer’s disease: A multicenter study with multiplexing. Exp. Neurol. 2010, 223, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Stomrud, E.; Palmqvist, S.; Zetterberg, H.; van Westen, D.; Jeromin, A.; Song, L.; Hanlon, D.; Tan Hehir, C.A.; Baker, D.; et al. Plasma β-amyloid in Alzheimer’s disease and vascular disease. Sci. Rep. 2016, 6, 26801. [Google Scholar] [CrossRef] [PubMed]
- Dumurgier, J.; Schraen, S.; Gabelle, A.; Vercruysse, O.; Bombois, S.; Laplanche, J.-L.; Peoc’h, K.; Sablonnière, B.; Kastanenka, K.V.; Delaby, C.; et al. Cerebrospinal fluid amyloid-β 42/40 ratio in clinical setting of memory centers: A multicentric study. Alzheimer’s Res. Ther. 2015, 7, 30. [Google Scholar] [CrossRef]
- Lewczuk, P.; Matzen, A.; Blennow, K.; Parnetti, L.; Molinuevo, J.L.; Eusebi, P.; Kornhuber, J.; Morris, J.C.; Fagan, A.M. Cerebrospinal Fluid Aβ 42/40 Corresponds Better than Aβ 42 to Amyloid PET in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 55, 813–822. [Google Scholar] [CrossRef]
- Dorey, A.; Perret-Liaudet, A.; Tholance, Y.; Fourier, A.; Quadrio, I. Cerebrospinal Fluid Aβ40 Improves the Interpretation of Aβ42 Concentration for Diagnosing Alzheimer’s Disease. Front. Neurol. 2015, 6, 247. [Google Scholar] [CrossRef]
- Thijssen, E.H.; Verberk, I.M.W.; Vanbrabant, J.; Koelewijn, A.; Heijst, H.; Scheltens, P.; van der Flier, W.; Vanderstichele, H.; Stoops, E.; Teunissen, C.E. Highly specific and ultrasensitive plasma test detects Abeta(1–42) and Abeta(1–40) in Alzheimer’s disease. Sci. Rep. 2021, 11, 9736. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Stomrud, E.; Smith, R.; Palmqvist, S.; Mattsson, N.; Airey, D.C.; Proctor, N.K.; Chai, X.; Shcherbinin, S.; Sims, J.R.; et al. Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer’s disease. Nat. Commun. 2020, 11, 1683. [Google Scholar] [CrossRef] [PubMed]
- Ashton, N.J.; Benedet, A.L.; Pascoal, T.A.; Karikari, T.K.; Lantero-Rodriguez, J.; Brum, W.S.; Mathotaarachchi, S.; Therriault, J.; Savard, M.; Chamoun, M.; et al. Cerebrospinal fluid p-tau231 as an early indicator of emerging pathology in Alzheimer’s disease. EBioMedicine 2022, 76, 103836. [Google Scholar] [CrossRef] [PubMed]
- Barthélemy, N.R.; Bateman, R.J.; Hirtz, C.; Marin, P.; Becher, F.; Sato, C.; Gabelle, A.; Lehmann, S. Cerebrospinal fluid phospho-tau T217 outperforms T181 as a biomarker for the differential diagnosis of Alzheimer’s disease and PET amyloid-positive patient identification. Alzheimer’s Res. Ther. 2020, 12, 26. [Google Scholar] [CrossRef]
- Fossati, S.; Ramos Cejudo, J.; Debure, L.; Pirraglia, E.; Sone, J.Y.; Li, Y.; Chen, J.; Butler, T.; Zetterberg, H.; Blennow, K.; et al. Plasma tau complements CSF tau and P-tau in the diagnosis of Alzheimer’s disease. Alzheimers Dement. 2019, 11, 483–492. [Google Scholar] [CrossRef]
- Shen, X.-N.; Huang, Y.-Y.; Chen, S.-D.; Guo, Y.; Tan, L.; Dong, Q.; Yu, J.-T.; Weiner, M.W.; Aisen, P.; Petersen, R.; et al. Plasma phosphorylated-tau181 as a predictive biomarker for Alzheimer’s amyloid, tau and FDG PET status. Transl. Psychiatry 2021, 11, 585. [Google Scholar] [CrossRef]
- Barthélemy, N.R.; Horie, K.; Sato, C.; Bateman, R.J. Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer’s disease. J. Exp. Med. 2020, 217, e20200861. [Google Scholar] [CrossRef]
- Mattsson, N.; Insel, P.S.; Palmqvist, S.; Portelius, E.; Zetterberg, H.; Weiner, M.; Blennow, K.; Hansson, O.; Alzheimer’s Disease Neuroimaging Initiative. Cerebrospinal fluid tau, neurogranin, and neurofilament light in Alzheimer’s disease. EMBO Mol. Med. 2016, 8, 1184–1196. [Google Scholar] [CrossRef]
- Andreasen, N.; Vanmechelen, E.; Van de Voorde, A.; Davidsson, P.; Hesse, C.; Tarvonen, S.; Räihä, I.; Sourander, L.; Winblad, B.; Blennow, K. Cerebrospinal fluid tau protein as a biochemical marker for Alzheimer’s disease: A community based follow up study. J. Neurol. Neurosurg. Psychiatry 1998, 64, 298. [Google Scholar] [CrossRef]
- Sjögren, M.; Davidsson, P.; Tullberg, M.; Minthon, L.; Wallin, A.; Wikkelso, C.; Granérus, A.K.; Vanderstichele, H.; Vanmechelen, E.; Blennow, K. Both total and phosphorylated tau are increased in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2001, 70, 624. [Google Scholar] [CrossRef]
- Mattsson, N.; Zetterberg, H.; Janelidze, S.; Insel, P.S.; Andreasson, U.; Stomrud, E.; Palmqvist, S.; Baker, D.; Tan Hehir, C.A.; Jeromin, A.; et al. Plasma tau in Alzheimer disease. Neurology 2016, 87, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Nam, E.; Lee, Y.B.; Moon, C.; Chang, K.A. Serum Tau Proteins as Potential Biomarkers for the Assessment of Alzheimer’s Disease Progression. Int. J. Mol. Sci. 2020, 21, 5007. [Google Scholar] [CrossRef] [PubMed]
- Casado, B.; Iadarola, P.; Pannell, L.K. Preparation of nasal secretions for proteome analysis. Methods Mol. Biol. 2008, 425, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.J.; Son, G.; Bae, J.; Kim, S.Y.; Yoo, Y.K.; Park, D.; Baek, S.Y.; Chang, K.A.; Suh, Y.H.; Lee, Y.B.; et al. Longitudinal profiling of oligomeric Aβ in human nasal discharge reflecting cognitive decline in probable Alzheimer’s disease. Sci. Rep. 2020, 10, 11234. [Google Scholar] [CrossRef]
- Wingrave, W. XXXI. The Nature of Discharges and Douches. Ann. Otol. Rhinol. Laryngol. 1902, 11, 407–417. [Google Scholar] [CrossRef]
- Watelet, J.B.; Gevaert, P.; Holtappels, G.; Van Cauwenberge, P.; Bachert, C. Collection of nasal secretions for immunological analysis. Eur. Arch. Otorhinolaryngol. 2004, 261, 242–246. [Google Scholar] [CrossRef]
- Pipkorn, U.; Enerbäck, L. Nasal mucosal mast cells and histamine in hay fever. Effect of topical glucocorticoid treatment. Int. Arch. Allergy Appl. Immunol. 1987, 84, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Kameshima, N.; Nanjo, T.; Shiino, A.; Kato, T.; Shimizu, S.; Shimizu, T.; Tanaka, S.; Miura, K.; Tooyama, I. Development of a High-Sensitivity Method for the Measurement of Human Nasal Aβ42, Tau, and Phosphorylated Tau. J. Alzheimers Dis. 2018, 62, 737–744. [Google Scholar] [CrossRef]
- Van Haeringen, N.J. Clinical biochemistry of tears. Surv. Ophthalmol. 1981, 26, 84–96. [Google Scholar] [CrossRef]
- Stuchell, R.N.; Feldman, J.J.; Farris, R.L.; Mandel, I.D. The effect of collection technique on tear composition. Investig. Ophthalmol. Vis. Sci. 1984, 25, 374–377. [Google Scholar]
- Saijyothi, A.V.; Angayarkanni, N.; Syama, C.; Utpal, T.; Shweta, A.; Bhaskar, S.; Geetha, I.K.; Vinay, P.S.; Thennarasu, M.; Sivakumar, R.M.; et al. Two dimensional electrophoretic analysis of human tears: Collection method in dry eye syndrome. Electrophoresis 2010, 31, 3420–3427. [Google Scholar] [CrossRef] [PubMed]
- Navazesh, M. Methods for collecting saliva. Ann. N. Y. Acad. Sci. 1993, 694, 72–77. [Google Scholar] [CrossRef] [PubMed]
- White, K.D. Salivation: A review and experimental investigation of major techniques. Psychophysiology 1977, 14, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Rohleder, N.; Nater, U.M. Determinants of salivary alpha-amylase in humans and methodological considerations. Psychoneuroendocrinology 2009, 34, 469–485. [Google Scholar] [CrossRef]
- Mischak, H.; Kolch, W.; Aivaliotis, M.; Bouyssié, D.; Court, M.; Dihazi, H.; Dihazi, G.H.; Franke, J.; Garin, J.; Gonzalez de Peredo, A.; et al. Comprehensive human urine standards for comparability and standardization in clinical proteome analysis. Proteom.-Clin. Appl. 2010, 4, 464–478. [Google Scholar] [CrossRef]
- Yamamoto, T. The 4th Human Kidney and Urine Proteome Project (HKUPP) workshop. 26 September 2009, Toronto, Canada. Proteomics 2010, 10, 2069–2070. [Google Scholar] [CrossRef]
- Yamamoto, T.; Langham, R.G.; Ronco, P.; Knepper, M.A.; Thongboonkerd, V. Towards standard protocols and guidelines for urine proteomics: A report on the Human Kidney and Urine Proteome Project (HKUPP) symposium and workshop, 6 October 2007, Seoul, Korea and 1 November 2007, San Francisco, CA, USA. Proteomics 2008, 8, 2156–2159. [Google Scholar] [CrossRef]
- Zürbig, P.; Dihazi, H.; Metzger, J.; Thongboonkerd, V.; Vlahou, A. Urine proteomics in kidney and urogenital diseases: Moving towards clinical applications. Proteom.-Clin. Appl. 2011, 5, 256–268. [Google Scholar] [CrossRef]
- Murphy, C. Olfactory and other sensory impairments in Alzheimer disease. Nat. Rev. Neurol. 2019, 15, 11–24. [Google Scholar] [CrossRef]
- Waldton, S. Clinical observations of impaired cranial nerve function in senile dementia. Acta Psychiatr. Scand. 1974, 50, 539–547. [Google Scholar] [CrossRef]
- Doty, R.L.; Reyes, P.F.; Gregor, T. Presence of both odor identification and detection deficits in Alzheimer’s disease. Brain Res. Bull. 1987, 18, 597–600. [Google Scholar] [CrossRef]
- Doty, R.L.; Hawkes, C.H.; Good, K.P.; Duda, J.E. Odor Perception and Neuropathology in Neurodegenerative Diseases and Schizophrenia. In Handbook of Olfaction and Gustation, 3rd ed.; Wiley Online Books, Doty, R.L., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 403–452. [Google Scholar]
- Son, G.; Jahanshahi, A.; Yoo, S.J.; Boonstra, J.T.; Hopkins, D.A.; Steinbusch, H.W.M.; Moon, C. Olfactory neuropathology in Alzheimer’s disease: A sign of ongoing neurodegeneration. BMB Rep. 2021, 54, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Rasheed, A.; Yoo, S.J.; Kim, S.Y.; Cho, B.; Son, G.; Yu, S.W.; Chang, K.A.; Suh, Y.H.; Moon, C. Distinct amyloid precursor protein processing machineries of the olfactory system. Biochem. Biophys. Res. Commun. 2018, 495, 533–538. [Google Scholar] [CrossRef]
- Wesson, D.W.; Levy, E.; Nixon, R.A.; Wilson, D.A. Olfactory dysfunction correlates with amyloid-beta burden in an Alzheimer’s disease mouse model. J. Neurosci. 2010, 30, 505–514. [Google Scholar] [CrossRef]
- Yoo, S.J.; Lee, J.H.; Kim, S.Y.; Son, G.; Kim, J.Y.; Cho, B.; Yu, S.W.; Chang, K.A.; Suh, Y.H.; Moon, C. Differential spatial expression of peripheral olfactory neuron-derived BACE1 induces olfactory impairment by region-specific accumulation of beta-amyloid oligomer. Cell Death Dis. 2017, 8, e2977. [Google Scholar] [CrossRef] [PubMed]
- Son, G.; Steinbusch, H.W.; López-Iglesias, C.; Moon, C.; Jahanshahi, A. Severe histomorphological alterations in post-mortem olfactory glomeruli in Alzheimer’s disease. Brain Pathol. 2022, 32, e13033. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol. 1991, 1, 213–216. [Google Scholar] [CrossRef]
- Masurkar, A.V.; Devanand, D.P. Olfactory Dysfunction in the Elderly: Basic Circuitry and Alterations with Normal Aging and Alzheimer’s Disease. Curr. Geriatr. Rep. 2014, 3, 91–100. [Google Scholar] [CrossRef]
- Attems, J.; Lintner, F.; Jellinger, K.A. Olfactory involvement in aging and Alzheimer’s disease: An autopsy study. J. Alzheimers Dis. 2005, 7, 149–157. [Google Scholar] [CrossRef]
- Crino, P.B.; Martin, J.A.; Hill, W.D.; Greenberg, B.; Lee, V.M.; Trojanowski, J.Q. Beta-Amyloid peptide and amyloid precursor proteins in olfactory mucosa of patients with Alzheimer’s disease, Parkinson’s disease, and Down syndrome. Ann. Otol. Rhinol. Laryngol. 1995, 104, 655–661. [Google Scholar] [CrossRef]
- Hock, C.; Golombowski, S.; Muller-Spahn, F.; Peschel, O.; Riederer, A.; Probst, A.; Mandelkow, E.; Unger, J. Histological markers in nasal mucosa of patients with Alzheimer’s disease. Eur. Neurol. 1998, 40, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Attems, J.; Walker, L.; Jellinger, K.A. Olfactory bulb involvement in neurodegenerative diseases. Acta Neuropathol. 2014, 127, 459–475. [Google Scholar] [CrossRef] [PubMed]
- Franks, K.H.; Chuah, M.I.; King, A.E.; Vickers, J.C. Connectivity of Pathology: The Olfactory System as a Model for Network-Driven Mechanisms of Alzheimer’s Disease Pathogenesis. Front. Aging Neurosci. 2015, 7, 234. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, R.M.; Piccirillo, J.F.; Chandrasekhar, S.S.; Brook, I.; Kumar, K.A.; Kramper, M.; Orlandi, R.R.; Palmer, J.N.; Patel, Z.M.; Peters, A.; et al. Clinical practice guideline (update): Adult Sinusitis Executive Summary. Otolaryngol. Head Neck Surg. 2015, 152, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Hermelingmeier, K.E.; Weber, R.K.; Hellmich, M.; Heubach, C.P.; Mösges, R. Nasal irrigation as an adjunctive treatment in allergic rhinitis: A systematic review and meta-analysis. Am. J. Rhinol. Allergy 2012, 26, e119–e125. [Google Scholar] [CrossRef] [PubMed]
- Klimek, L.; Rasp, G. Norm values for eosinophil cationic protein in nasal secretions: Influence of specimen collection. Clin. Exp. Allergy 1999, 29, 367–374. [Google Scholar] [CrossRef]
- World Health, O. Laboratory Testing for Coronavirus Disease (COVID-19) in Suspected Human Cases: Interim Guidance, 19 March 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Struble, R.G.; Clark, H.B. Olfactory bulb lesions in Alzheimer’s disease. Neurobiol. Aging 1992, 13, 469–473. [Google Scholar] [CrossRef]
- Arnold, S.E.; Lee, E.B.; Moberg, P.J.; Stutzbach, L.; Kazi, H.; Han, L.Y.; Lee, V.M.; Trojanowski, J.Q. Olfactory epithelium amyloid-beta and paired helical filament-tau pathology in Alzheimer disease. Ann. Neurol. 2010, 67, 462–469. [Google Scholar] [CrossRef]
- Ayala-Grosso, C.A.; Pieruzzini, R.; Diaz-Solano, D.; Wittig, O.; Abrante, L.; Vargas, L.; Cardier, J. Amyloid-aβ Peptide in olfactory mucosa and mesenchymal stromal cells of mild cognitive impairment and Alzheimer’s disease patients. Brain Pathol. 2015, 25, 136–145. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, S.M.; Cho, S.; Kang, J.H.; Minn, Y.K.; Park, H.; Choi, S.H. Amyloid beta in nasal secretions may be a potential biomarker of Alzheimer’s disease. Sci. Rep. 2019, 9, 4966. [Google Scholar] [CrossRef]
- Lee, J.H.; Goedert, M.; Hill, W.D.; Lee, V.M.; Trojanowski, J.Q. Tau proteins are abnormally expressed in olfactory epithelium of Alzheimer patients and developmentally regulated in human fetal spinal cord. Exp. Neurol. 1993, 121, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Attems, J.; Jellinger, K.A. Olfactory tau pathology in Alzheimer disease and mild cognitive impairment. Clin. Neuropathol. 2006, 25, 265–271. [Google Scholar] [PubMed]
- Passali, G.C.; Politi, L.; Crisanti, A.; Loglisci, M.; Anzivino, R.; Passali, D. Tau Protein Detection in Anosmic Alzheimer’s Disease Patient’s Nasal Secretions. Chemosens. Percept. 2015, 8, 201–206. [Google Scholar] [CrossRef]
- Moon, J.; Lee, S.T.; Kong, I.G.; Byun, J.I.; Sunwoo, J.S.; Shin, J.W.; Shim, J.Y.; Park, J.H.; Jeon, D.; Jung, K.H.; et al. Early diagnosis of Alzheimer’s disease from elevated olfactory mucosal miR-206 level. Sci. Rep. 2016, 6, 20364. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K.; Wang, H.; Jaen, C.; Haneoka, M.; Saito, N.; Nakamura, J.; Adappa, N.D.; Cohen, N.A.; Dalton, P. The human olfactory cleft mucus proteome and its age-related changes. Sci. Rep. 2018, 8, 17170. [Google Scholar] [CrossRef] [PubMed]
- Murray, H.C.; Dieriks, B.V.; Swanson, M.E.V.; Anekal, P.V.; Turner, C.; Faull, R.L.M.; Belluscio, L.; Koretsky, A.; Curtis, M.A. The unfolded protein response is activated in the olfactory system in Alzheimer’s disease. Acta Neuropathol. Commun. 2020, 8, 109. [Google Scholar] [CrossRef]
- Carmona-Abellan, M.; Martinez-Valbuena, I.; Marcilla, I.; DiCaudo, C.; Gil, I.; Nunez, J.; Luquin, M.R. Microglia is associated with p-Tau aggregates in the olfactory bulb of patients with neurodegenerative diseases. Neurol. Sci. 2021, 42, 1473–1482. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, S.Z.; Koh, S.K.; Chen, L.; Vaz, C.; Tanavde, V.; Li, X.R.; Beuerman, R.W. In-depth analysis of the human tear proteome. J. Proteom. 2012, 75, 3877–3885. [Google Scholar] [CrossRef]
- Herber, S.; Grus, F.H.; Sabuncuo, P.; Augustin, A.J. Two-dimensional analysis of tear protein patterns of diabetic patients. Electrophoresis 2001, 22, 1838–1844. [Google Scholar] [CrossRef]
- Comoglu, S.S.; Guven, H.; Acar, M.; Ozturk, G.; Kocer, B. Tear levels of tumor necrosis factor-alpha in patients with Parkinson’s disease. Neurosci. Lett. 2013, 553, 63–67. [Google Scholar] [CrossRef]
- Kapnisis, K.; Doormaal, M.V.; Ross Ethier, C. Modeling aqueous humor collection from the human eye. J. Biomech. 2009, 42, 2454–2457. [Google Scholar] [CrossRef]
- Yamane, K.; Minamoto, A.; Yamashita, H.; Takamura, H.; Miyamoto-Myoken, Y.; Yoshizato, K.; Nabetani, T.; Tsugita, A.; Mishima, H.K. Proteome analysis of human vitreous proteins. Mol. Cell. Proteom. 2003, 2, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Gijs, M.; Nuijts, R.M.; Ramakers, I.; Verhey, F.; Webers, C.A.B. Differences in tear protein biomarkers between patients with Alzheimer’s disease and controls. Investig. Ophth. Vis. Sci. 2019, 60, 1744. [Google Scholar]
- Gijs, M.; Ramakers, I.H.G.B.; Visser, P.J.; Verhey, F.R.J.; van de Waarenburg, M.P.H.; Schalkwijk, C.G.; Nuijts, R.M.M.A.; Webers, C.A.B. Association of tear fluid amyloid and tau levels with disease severity and neurodegeneration. Sci. Rep. 2021, 11, 22675. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Chuang, H.C.; Tripathi, A.; Wang, Y.L.; Ko, M.L.; Chuang, C.C.; Chen, J.C. High-Sensitivity and Trace-Amount Specimen Electrochemical Sensors for Exploring the Levels of beta-Amyloid in Human Blood and Tears. Anal. Chem. 2021, 93, 8099–8106. [Google Scholar] [CrossRef]
- Kallo, G.; Emri, M.; Varga, Z.; Ujhelyi, B.; Tozser, J.; Csutak, A.; Csosz, E. Changes in the Chemical Barrier Composition of Tears in Alzheimer’s Disease Reveal Potential Tear Diagnostic Biomarkers. PLoS ONE 2016, 11, e0158000. [Google Scholar] [CrossRef]
- Kenny, A.; Jimenez-Mateos, E.M.; Zea-Sevilla, M.A.; Rabano, A.; Gili-Manzanaro, P.; Prehn, J.H.M.; Henshall, D.C.; Avila, J.; Engel, T.; Hernandez, F. Proteins and microRNAs are differentially expressed in tear fluid from patients with Alzheimer’s disease. Sci. Rep. 2019, 9, 15437. [Google Scholar] [CrossRef]
- Schenkels, L.C.; Veerman, E.C.; Nieuw Amerongen, A.V. Biochemical composition of human saliva in relation to other mucosal fluids. Crit. Rev. Oral Biol. Med. 1995, 6, 161–175. [Google Scholar] [CrossRef]
- Lamy, E.; Mau, M. Saliva proteomics as an emerging, non-invasive tool to study livestock physiology, nutrition and diseases. J. Proteom. 2012, 75, 4251–4258. [Google Scholar] [CrossRef]
- Vitorino, R.; Guedes, S.; Manadas, B.; Ferreira, R.; Amado, F. Toward a standardized saliva proteome analysis methodology. J. Proteom. 2012, 75, 5140–5165. [Google Scholar] [CrossRef]
- Teresi, L.M.; Kolin, E.; Lufkin, R.B.; Hanafee, W.N. MR imaging of the intraparotid facial nerve: Normal anatomy and pathology. AJR Am. J. Roentgenol. 1987, 148, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Sui, Y.T.; Peskind, E.R.; Li, G.; Hwang, H.; Devic, I.; Ginghina, C.; Edgar, J.S.; Pan, C.; Goodlett, D.R.; et al. Salivary Tau Species are Potential Biomarkers of Alzheimer’s Disease. J. Alzheimers Dis. 2011, 27, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Pareja, F.; Antequera, D.; Vargas, T.; Molina, J.A.; Carro, E. Saliva levels of Abeta1-42 as potential biomarker of Alzheimer’s disease: A pilot study. BMC Neurol. 2010, 10, 108. [Google Scholar] [CrossRef]
- Lee, M.; Guo, J.P.; Kennedy, K.; McGeer, E.G.; McGeer, P.L. A Method for Diagnosing Alzheimer’s Disease Based on Salivary Amyloid-beta Protein 42 Levels. J. Alzheimers Dis. 2017, 55, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, M.N.; Shi, J.; Lee, M.; Arnold, L.; Al-Hasan, Y.; Heim, J.; McGeer, P. Salivary beta amyloid protein levels are detectable and differentiate patients with Alzheimer’s disease dementia from normal controls: Preliminary findings. BMC Neurol. 2018, 18, 155. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; Guo, J.P.; Lee, M.; Kennedy, K.; McGeer, E.G. Alzheimer’s Disease Can Be Spared by Nonsteroidal Anti-Inflammatory Drugs. J. Alzheimers Dis. 2018, 62, 1219–1222. [Google Scholar] [CrossRef]
- Kim, C.B.; Choi, Y.Y.; Song, W.K.; Song, K.B. Antibody-based magnetic nanoparticle immunoassay for quantification of Alzheimer’s disease pathogenic factor. J. Biomed. Opt. 2014, 19, 051205. [Google Scholar] [CrossRef]
- Tvarijonaviciute, A.; Zamora, C.; Ceron, J.J.; Bravo-Cantero, A.F.; Pardo-Marin, L.; Valverde, S.; Lopez-Jornet, P. Salivary biomarkers in Alzheimer’s disease. Clin. Oral Investig. 2020, 24, 3437–3444. [Google Scholar] [CrossRef]
- Pekeles, H.; Qureshi, H.Y.; Paudel, H.K.; Schipper, H.M.; Gornistky, M.; Chertkow, H. Development and validation of a salivary tau biomarker in Alzheimer’s disease. Alzheimers Dement. 2019, 11, 53–60. [Google Scholar] [CrossRef]
- Ashton, N.J.; Ide, M.; Scholl, M.; Blennow, K.; Lovestone, S.; Hye, A.; Zetterberg, H. No association of salivary total tau concentration with Alzheimer’s disease. Neurobiol. Aging 2018, 70, 125–127. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef] [PubMed]
- Mayeur, S.; Spahis, S.; Pouliot, Y.; Levy, E. Lactoferrin, a Pleiotropic Protein in Health and Disease. Antioxid. Redox Signal 2016, 24, 813–836. [Google Scholar] [CrossRef] [PubMed]
- Carro, E.; Bartolomé, F.; Bermejo-Pareja, F.; Villarejo-Galende, A.; Molina, J.A.; Ortiz, P.; Calero, M.; Rabano, A.; Cantero, J.L.; Orive, G. Early diagnosis of mild cognitive impairment and Alzheimer’s disease based on salivary lactoferrin. Alzheimers Dement. 2017, 8, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sanchez, M.; Bartolome, F.; Antequera, D.; Puertas-Martin, V.; Gonzalez, P.; Gomez-Grande, A.; Llamas-Velasco, S.; Herrero-San Martin, A.; Perez-Martinez, D.; Villarejo-Galende, A.; et al. Decreased salivary lactoferrin levels are specific to Alzheimer’s disease. EBioMedicine 2020, 57, 102834. [Google Scholar] [CrossRef]
- Whitehouse, P.J.; Price, D.L.; Clark, A.W.; Coyle, J.T.; DeLong, M.R. Alzheimer disease: Evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann. Neurol. 1981, 10, 122–126. [Google Scholar] [CrossRef]
- Jann, M.W. Rivastigmine, a new-generation cholinesterase inhibitor for the treatment of Alzheimer’s disease. Pharmacotherapy 2000, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rinne, J.O.; Kaasinen, V.; Jarvenpaa, T.; Nagren, K.; Roivainen, A.; Yu, M.; Oikonen, V.; Kurki, T. Brain acetylcholinesterase activity in mild cognitive impairment and early Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2003, 74, 113–115. [Google Scholar] [CrossRef]
- Sayer, R.; Law, E.; Connelly, P.J.; Breen, K.C. Association of a salivary acetylcholinesterase with Alzheimer’s disease and response to cholinesterase inhibitors. Clin. Biochem. 2004, 37, 98–104. [Google Scholar] [CrossRef]
- Bakhtiari, S.; Moghadam, N.B.; Ehsani, M.; Mortazavi, H.; Sabour, S.; Bakhshi, M. Can Salivary Acetylcholinesterase be a Diagnostic Biomarker for Alzheimer? J. Clin. Diagn. Res. 2017, 11, ZC58–ZC60. [Google Scholar] [CrossRef]
- Boston, P.F.; Gopalkaje, K.; Manning, L.; Middleton, L.; Loxley, M. Developing a simple laboratory test for Alzheimer’s disease: Measuring acetylcholinesterase in saliva—A pilot study. Int. J. Geriatr. Psychiatry 2008, 23, 439–440. [Google Scholar] [CrossRef]
- Suzuki, Y.J.; Carini, M.; Butterfield, D.A. Protein Carbonylation. Antioxid Redox. Signal 2009, 12, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Aksenov, M.Y.; Aksenova, M.V.; Butterfield, D.A.; Geddes, J.W.; Markesbery, W.R. Protein oxidation in the brain in Alzheimer’s disease. Neuroscience 2001, 103, 373–383. [Google Scholar] [CrossRef]
- Su, H.; Gornitsky, M.; Geng, G.; Velly, A.M.; Chertkow, H.; Schipper, H.M. Diurnal variations in salivary protein carbonyl levels in normal and cognitively impaired human subjects. Age 2008, 30, 1–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yilmaz, A.; Geddes, T.; Han, B.; Bahado-Singh, R.O.; Wilson, G.D.; Imam, K.; Maddens, M.; Graham, S.F. Diagnostic Biomarkers of Alzheimer’s Disease as Identified in Saliva using 1H NMR-Based Metabolomics. J. Alzheimers Dis. 2017, 58, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Q.; Gao, Y.H. Physiological conditions can be reflected in human urine proteome and metabolome. Expert Rev. Proteomic 2015, 12, 623–636. [Google Scholar] [CrossRef]
- Haubitz, M.; Wittke, S.; Weissinger, E.M.; Walden, M.; Rupprecht, H.D.; Floege, J.; Haller, H.; Mischak, H. Urine protein patterns can serve as diagnostic tools in patients with IgA nephropathy. Kidney Int. 2005, 67, 2313–2320. [Google Scholar] [CrossRef][Green Version]
- Nam, D.; Lee, J.Y.; Lee, M.; Kim, J.; Seol, W.; Son, I.; Ho, D.H. Detection and Assessment of alpha-Synuclein Oligomers in the Urine of Parkinson’s Disease Patients. J. Parkinson’s Dis. 2020, 10, 981–991. [Google Scholar] [CrossRef]
- Wang, C.; Cui, Y.J.; Yang, J.C.; Zhang, J.R.; Yuan, D.C.; Wei, Y.; Li, Y.M.; Duo, Y.L.; Li, S.J.; Zhu, W.Y.; et al. Combining serum and urine biomarkers in the early diagnosis of mild cognitive impairment that evolves into Alzheimer’s disease in patients with the apolipoprotein E epsilon 4 genotype. Biomarkers 2015, 20, 84–88. [Google Scholar] [CrossRef]
- Luan, H.; Liu, L.F.; Meng, N.; Tang, Z.; Chua, K.K.; Chen, L.L.; Song, J.X.; Mok, V.C.; Xie, L.X.; Li, M.; et al. LC-MS-based urinary metabolite signatures in idiopathic Parkinson’s disease. J. Proteome Res. 2015, 14, 467–478. [Google Scholar] [CrossRef]
- Takata, M.; Nakashima, M.; Takehara, T.; Baba, H.; Machida, K.; Akitake, Y.; Ono, K.; Hosokawa, M.; Takahashi, M. Detection of amyloid beta protein in the urine of Alzheimer’s disease patients and healthy individuals. Neurosci. Lett. 2008, 435, 126–130. [Google Scholar] [CrossRef]
- Wongta, A.; Hongsibsong, S.; Chantara, S.; Pattarawarapan, M.; Sapbamrer, R.; Sringarm, K.; Xu, Z.L.; Wang, H. Development of an Immunoassay for the Detection of Amyloid Beta 1-42 and Its Application in Urine Samples. J. Immunol. Res. 2020, 2020, 8821181. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Hong, X.; Li, S.; Zhang, Y.; Zhao, Q.; Du, W.; Wang, Y.; Ni, J. Urine-Based Biomarkers for Alzheimer’s Disease Identified Through Coupling Computational and Experimental Methods. J. Alzheimers Dis. 2018, 65, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Ku, B.D.; Kim, H.; Kim, Y.K.; Ryu, H.U. Comparison of Urinary Alzheimer-Associated Neural Thread Protein (AD7c-NTP) Levels Between Patients With Amnestic and Nonamnestic Mild Cognitive Impairment. Am. J. Alzheimers Dis. 2020, 35, 1533317519880369. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Hirao, Y.; Kasuga, K.; Tokutake, T.; Semizu, Y.; Kitamura, K.; Ikeuchi, T.; Nakamura, K.; Yamamoto, T. Molecular Network Analysis of the Urinary Proteome of Alzheimer’s Disease Patients. Dement. Geriatr. Cogn. Dis. Extra 2019, 9, 53–65. [Google Scholar] [CrossRef]
- Watanabe, Y.; Hirao, Y.; Kasuga, K.; Tokutake, T.; Kitamura, K.; Niida, S.; Ikeuchi, T.; Nakamura, K.; Yamamoto, T. Urinary Apolipoprotein C3 Is a Potential Biomarker for Alzheimer’s Disease. Dement. Geriatr. Cogn. Dis. Extra 2020, 10, 94–104. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Giau, V.V.; Wu, S.Y.; Jamerlan, A.; An, S.S.A.; Kim, S.Y.; Hulme, J. Gut Microbiota and Their Neuroinflammatory Implications in Alzheimer’s Disease. Nutrients 2018, 10, 1765. [Google Scholar] [CrossRef]
- Kurbatova, N.; Garg, M.; Whiley, L.; Chekmeneva, E.; Jimenez, B.; Gomez-Romero, M.; Pearce, J.; Kimhofer, T.; D’Hondt, E.; Soininen, H.; et al. Urinary metabolic phenotyping for Alzheimer’s disease. Sci. Rep. 2020, 10, 21745. [Google Scholar] [CrossRef]
- Pena-Bautista, C.; Vigor, C.; Galano, J.M.; Oger, C.; Durand, T.; Ferrer, I.; Cuevas, A.; Lopez-Cuevas, R.; Baquero, M.; Lopez-Nogueroles, M.; et al. New screening approach for Alzheimer’s disease risk assessment from urine lipid peroxidation compounds. Sci. Rep. 2019, 9, 14244. [Google Scholar] [CrossRef]
- Mall, C.; Rocke, D.M.; Durbin-Johnson, B.; Weiss, R.H. Stability of miRNA in human urine supports its biomarker potential. Biomark. Med. 2013, 7, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Seol, W.; Kim, H.; Son, I. Urinary Biomarkers for Neurodegenerative Diseases. Exp. Neurobiol. 2020, 29, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Sun, X.; Scicluna, B.J.; Coleman, B.M.; Hill, A.F. Characterization and deep sequencing analysis of exosomal and non-exosomal miRNA in human urine. Kidney Int. 2014, 86, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhu, X.C.; Jiang, T.; Yu, J.T.; Tan, L. Body fluid biomarkers in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 70. [Google Scholar] [CrossRef]
- Janigro, D.; Bailey, D.M.; Lehmann, S.; Badaut, J.; O’Flynn, R.; Hirtz, C.; Marchi, N. Peripheral Blood and Salivary Biomarkers of Blood–Brain Barrier Permeability and Neuronal Damage: Clinical and Applied Concepts. Front. Neurol. 2021, 11, 577312. [Google Scholar] [CrossRef] [PubMed]
- Frölich, L.; Peters, O.; Lewczuk, P.; Gruber, O.; Teipel, S.J.; Gertz, H.J.; Jahn, H.; Jessen, F.; Kurz, A.; Luckhaus, C.; et al. Incremental value of biomarker combinations to predict progression of mild cognitive impairment to Alzheimer’s dementia. Alz Res. Ther. 2017, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Walhovd, K.B.; Fjell, A.M.; Brewer, J.; McEvoy, L.K.; Fennema-Notestine, C.; Hagler, D.J.; Jennings, R.G.; Karow, D.; Dale, A.M.; Alzheimer’s Disease Neuroimaging Initiative. Combining MR Imaging, Positron-Emission Tomography, and CSF Biomarkers in the Diagnosis and Prognosis of Alzheimer Disease. Am. J. Neuroradiol. 2010, 31, 347. [Google Scholar] [CrossRef]
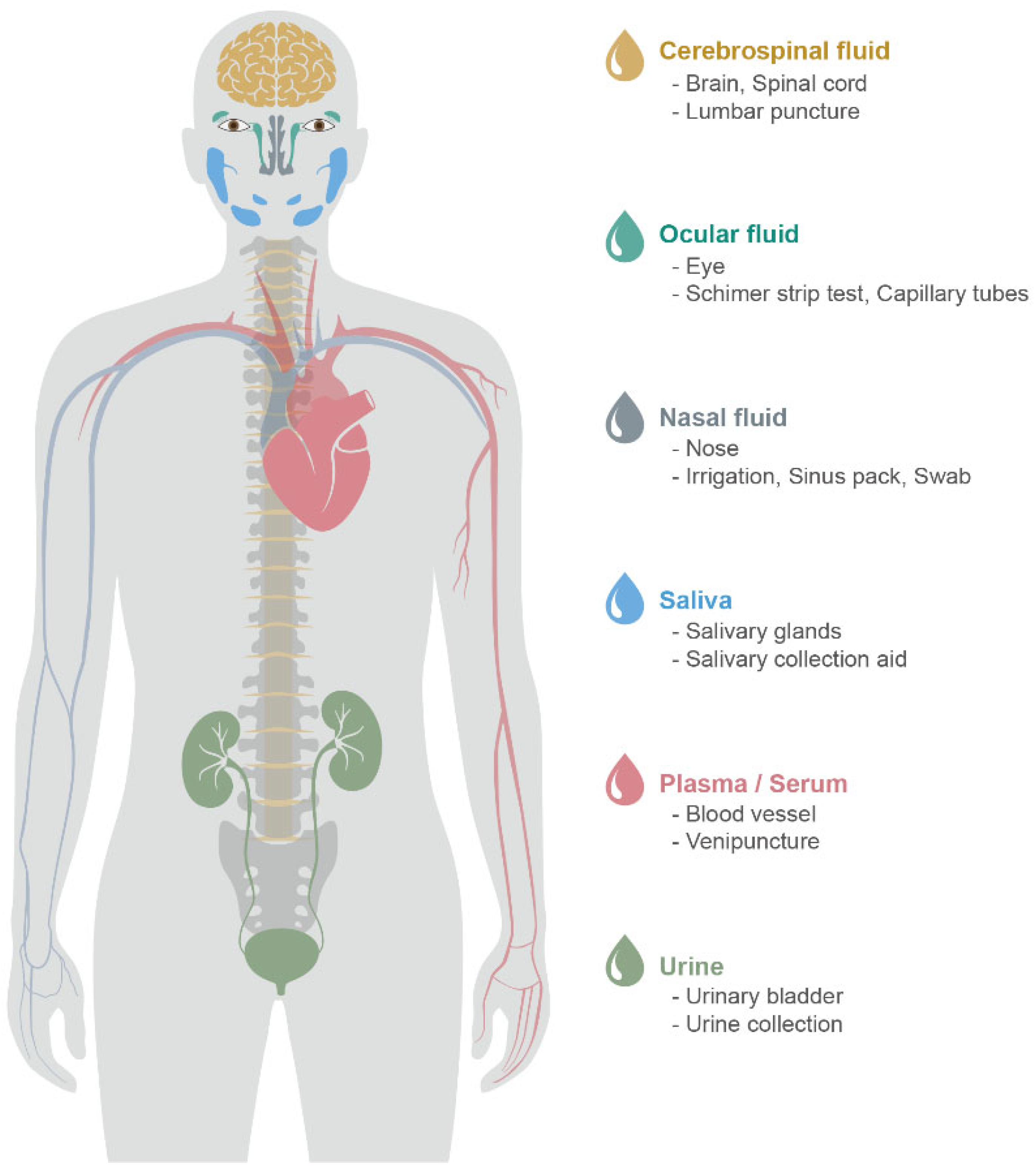
| NINDS-ADRDA+DSM-III a Disease Stage | Dementia | |||||
|---|---|---|---|---|---|---|
| Diagnostic Subgroups | None | Unlikely | Possible AD b | Probable AD | Definite AD | |
| Other comments | − | − | Absence of other diseases capable of producing a dementia syndrome | |||
| Onset age | − | Sudden | Atypical | 40~90 years | 40~90 years | |
| Neuro-psychologicaltest | MMSE c, Blessed dementia scale, etc. | − | ? | +/− | + | + |
| Neuroimage | CT d | − | ? | +/− | + | + |
| Histology | Microscopic examination of brain tissue | − | - | − | − | Confirmed by autopsy or biopsy |
| Others | Other signs | − | Focal neurologic signs, seizures, or gait disturbance | − | Cognitive impairments have to be present in two or more areas of cognition | Cognitive impairments have to be present in two or more areas of cognition |
| NIA-AA+DSM-V a Disease Stage | Preclinical | MCI b | AD c Dementia | Non-AD Dementia | ||||
|---|---|---|---|---|---|---|---|---|
| Diagnostic Subgroups | None | Preclinical AD | Possible AD | Mild AD | Moderate AD | Severe AD | OND d | |
| Neuro-psychological test | MMSE e (30~0) | 30~25 | 24~20 | 19~13 | 12~ | +/− | ||
| CDR f v1-1993 (0~3) | 0 | 0.5 questionable | 1 | 2 | 3 | |||
| CDR v2-1997 (0~3) | 0 | 0.5 questionable | 1 mild CI h | 2 moderate CI | 3 severe CI | |||
| GDS g (1~7) | 1 | 2 very mildCI | 3 mildCI | 4 moderateCI | 5, 6 moderately severe CI | 6, 7 very severe CI | ||
| Neuro-imaging | amyloid-PET i | − | + | + | + | + | + | − |
| tau-PET | − | − | − | + | + | + | +/− | |
| FDG j-PET | − | − | +/− | +/− | +/- | +/− | +/− | |
| Structural MRI | − | − | +/− | +/− | +/- | +/− | +/− | |
| CSF k-biomarker | CSF Aβ42 | − | + | + | + | + | + | n/a |
| CSF P-tau | − | − | − | + | + | + | n/a | |
| CSF T-tau | − | − | +/− | +/− | +/− | +/− | n/a | |
| Type | Characteristics | Goals |
|---|---|---|
| Detectability | Disease specificity | High |
| Biomarker sensitivity | High | |
| Accuracy | High | |
| Accessibility | Repeatability | High |
| Invasiveness | Low | |
| Expense | Low | |
| Stability | Reproducibility | High |
| Reliability | Early detection | |
| Containing pathological correlation | ||
| Body Fluid | Acquisition | Procedure | Reference | |
|---|---|---|---|---|
| Nasal discharge | Nasal irrigation | 1. Subjects are comfortably seated, and sterile normal saline (0.9% NaCl) is administered into each nostril. 2. Subjects must close one nostril and then spray or insert sterile normal saline several times into the other nostril. 3. After raising their head slightly back, let the solution stay as if washing the nasal cavities.4. After inserting sterile normal saline, subjects gently blow the nasal discharge fluids into a cup or tube.5. After a few minutes of rest, do the same for the other nostril.6. Samples are stored at −20 °C until use. | [66,67,68] | |
| Sinus packs | 1. Sinus packs or sponges are placed in nasal cavities between the septum and inferior turbinate along the floor. 2. After 1–10 min, the sinus packs or sponges are removed and placed in tubes. In order to retrieve the secretions from sinus packs or sponges, sterile normal saline (0.9% NaCl) solution is added to the tube and stored at 4 °C for about 2 h. 3. The sinus packs or sponges are then placed into a syringe. Mechanical pressure is applied to them by moving the piston action to squeeze the nasal discharge fluid. 4. After the first pressure, the syringe is replaced with a tube and centrifugation is performed to recover all nasal discharge fluids from the sinus packs or sponges. 5. The nasal discharge fluids are then stored at −80 °C for further analysis. | [69] | ||
| Nasal swab | 1. Subjects are seated in a comfortable bed, placed in a high fowler’s position in bed, supporting the back of the head. 2. Enter a flexible cotton swab several centimeters with a slow and steady motion along the nose floor. Nasal smears are taken from the inferior concha, middle nasal meatus, olfactory cleft, and common nasal meatus. 3. After resistance is met, rotate the cotton swab several times and withdraw the swab. 4. All cotton swabs are placed in a microtube containing sterile normal saline (0.9% NaCl) for a few minutes, and swabs are removed from the microtube. 5. The solutions are then filtered by centrifugation, and then the filtered solutions are stored at −80 °C until further analysis. | [70,71] | ||
| Tear | Capillary tube | 1. Subjects are seated with their head raised and stimulated by a direct light source or airflow. 2. The reflex tears of the subject are collected with tubes. | [72,73,74] | |
| Schirmer strip | 1. A local anesthetic is needed to collect basal tears, not reflex tears. 2. The bent end of the test strip is placed in the lower eyelid and allowed to absorb the tears for several minutes. | |||
| Saliva | Whole saliva | Spitting | 1. Subjects rinse their mouth and then spit the whole saliva into a sterile tube. | [75,76,77] |
| Submandibular saliva | Draining | 1. To block the opening of parotid ducts and sublingual glands, use cotton gauzes, and to dry up, the floor of the mouse is left. 2. Subjects raise the tongue to open the submandibular gland, and saliva is collected using a disposable pipette. | ||
| Sublingual saliva | Draining | 1. To block the opening of parotid ducts and submandibular glands, use cotton gauzes, and to dry up, the floor of the mouse is left. 2. Subjects raise the tongue to open the sublingual gland, and saliva is collected using a disposable pipette. | ||
| Parotid saliva | Draining | 1. To collect parotid saliva, parotid cups or collectors are placed, actively stimulating salivary collection. | ||
| Urine | Collecting | 1. First morning and random collection are not preferred because of increasing variabilities. 2. The mid-stream and second-morning urine is collected in a urine container. | [78,79,80,81] | |
| AD Pathology Mechanism | Specimen | Biomarkers | Analytical Method | Results | Reference |
|---|---|---|---|---|---|
| Aβ pathology | Nasal discharge fluid | Aβ1–16 | Interdigitated microelectrode biosensor | Increased in AD than in OND and CU a | [105] |
| Nasal discharge fluid | Aβ oligomer | Immunoblot | Increased in AD than CU | [67] | |
| Nasal mucosa by nasal swab | Aβ42, Aβ40 | Immunoassay | No differences in median values between AD and CU | [71] | |
| Postmortem olfactory epithelium | Aβ | Histopathology | Increased Aβ aggregates in AD patient | [103] | |
| Postmortem olfactory bulb | Aβ | Histopathology | Increased Aβ load in AD patients | [90,111] | |
| Tau pathology | Nasal discharge fluid | T-tau, P-tau | Immunoassay | Positive T- and P-tau in anosmic AD patients | [108] |
| Nasal mucosa by nasal swab | T-tau, P-tau | Immunoassay | Positive T- and P-tau in AD patients | [71] | |
| Postmortem olfactory epithelium | P-tau | Histopathology | Evident PHF-tau b in AD | [103] | |
| Postmortem olfactory bulb | P-tau | Histopathology | P-tau deposits in the olfactory bulb of AD patients | [112] |
| AD Pathology Mechanism | Biomarker | Analytical Method | Results | Reference |
|---|---|---|---|---|
| Aβ pathology | Aβ42 | Immunoassay | Increased Aβ42 levels in AD patients | [118] |
| Tau pathology | T-tau | Immunoassay | Increased T-tau levels in AD patients | [118] |
| AD Pathology Mechanism | Biomarkers | Analytical Method | Results | Reference |
|---|---|---|---|---|
| Aβ pathology | Aβ42 | Immunoassay | Increased saliva Aβ42 levels in mild AD patients | [128,129,130,131] |
| Aβ42 | Magneto-immunoassay | Salivary Aβ42 levels increase as the AD severity increases | [132] | |
| Aβ42 | Immunoassay | Salivary Aβ42 levels were not detectable | [127] | |
| Aβ42 | Immunoassay (MILLIPLEX) | Lower salivary Aβ42 levels in AD patients | [133] | |
| Aβ40 | Magneto-immunoassay | No statistically significant change | [132] | |
| Tau pathology | T-tau, P-tau | Immunoassay | Increased P-tau/T-tau ratio in AD patients | [127,134] |
| T-tau | Immunoassay | No significant difference in salivary T-tau between AD and healthy control | [135] |
| AD Pathology Mechanism | Biomarker | Analytical Method | Results | Reference |
|---|---|---|---|---|
| Aβ pathology | Aβ42 | Immunoblot | Monomeric Aβ42 levels differed according to cognitive impairment | [155] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, D.H.; Son, G.; Kwon, O.-H.; Chang, K.-A.; Moon, C. Non-Invasive Nasal Discharge Fluid and Other Body Fluid Biomarkers in Alzheimer’s Disease. Pharmaceutics 2022, 14, 1532. https://doi.org/10.3390/pharmaceutics14081532
Jung DH, Son G, Kwon O-H, Chang K-A, Moon C. Non-Invasive Nasal Discharge Fluid and Other Body Fluid Biomarkers in Alzheimer’s Disease. Pharmaceutics. 2022; 14(8):1532. https://doi.org/10.3390/pharmaceutics14081532
Chicago/Turabian StyleJung, Da Hae, Gowoon Son, Oh-Hoon Kwon, Keun-A Chang, and Cheil Moon. 2022. "Non-Invasive Nasal Discharge Fluid and Other Body Fluid Biomarkers in Alzheimer’s Disease" Pharmaceutics 14, no. 8: 1532. https://doi.org/10.3390/pharmaceutics14081532
APA StyleJung, D. H., Son, G., Kwon, O.-H., Chang, K.-A., & Moon, C. (2022). Non-Invasive Nasal Discharge Fluid and Other Body Fluid Biomarkers in Alzheimer’s Disease. Pharmaceutics, 14(8), 1532. https://doi.org/10.3390/pharmaceutics14081532








