The Role of Paracellular Transport in the Intestinal Absorption and Biopharmaceutical Characterization of Minoxidil
Abstract
1. Introduction
2. Methods
2.1. Materials
2.2. Octanol-Aqueous Buffer Partition Coefficient (Log D)
2.3. Physicochemical Analysis
2.4. Parallel Artificial Membrane Permeability Assay (PAMPA)
2.5. Cell Culture and Caco-2 Permeability Measurements
2.6. Rat Single-Pass Intestinal Perfusion
2.7. Analytical Methods
2.8. Statistical Analysis
2.9. Physiologically-Based In-Silico Simulations
3. Results
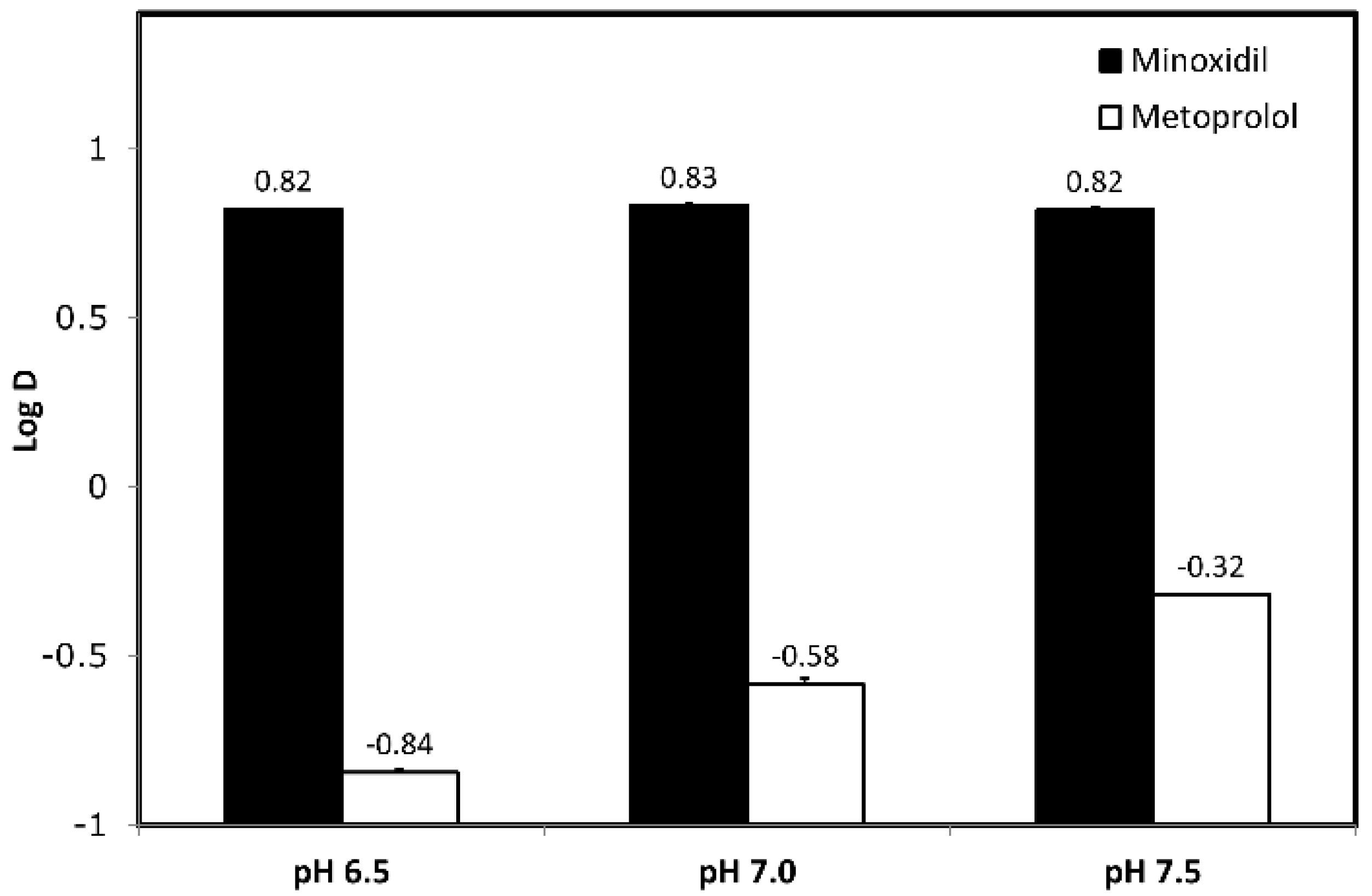
3.1. Octanol-Buffer Partition Coefficients
3.2. Physicochemical Analysis
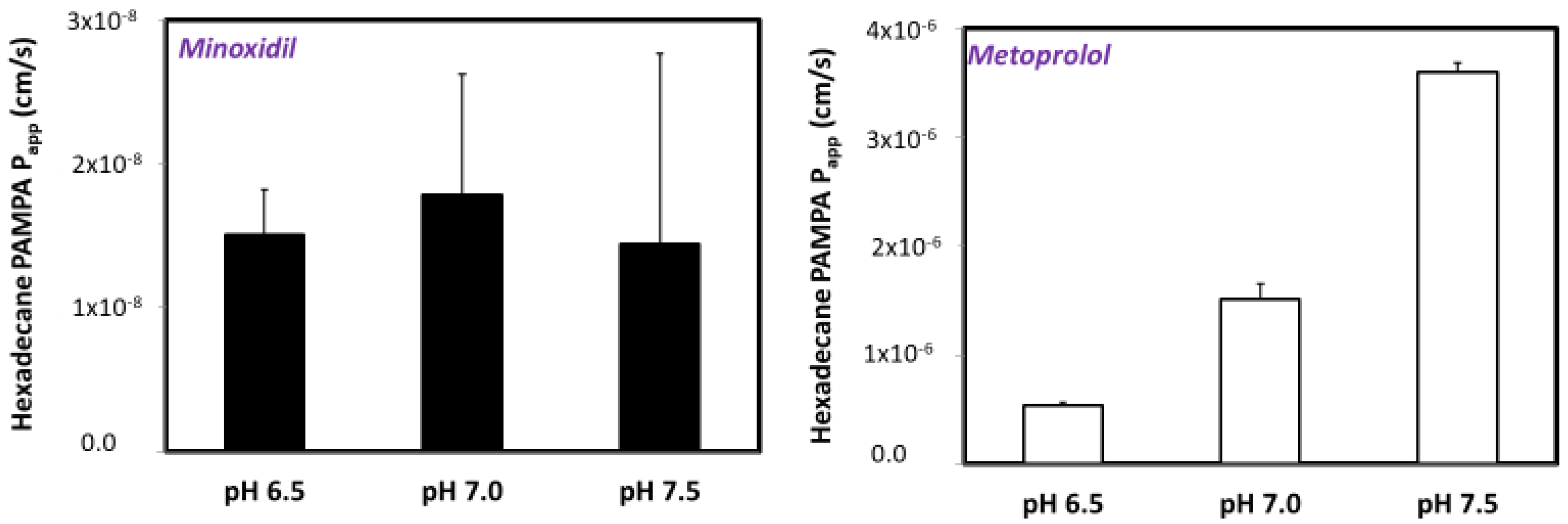
3.3. PAMPA Assay
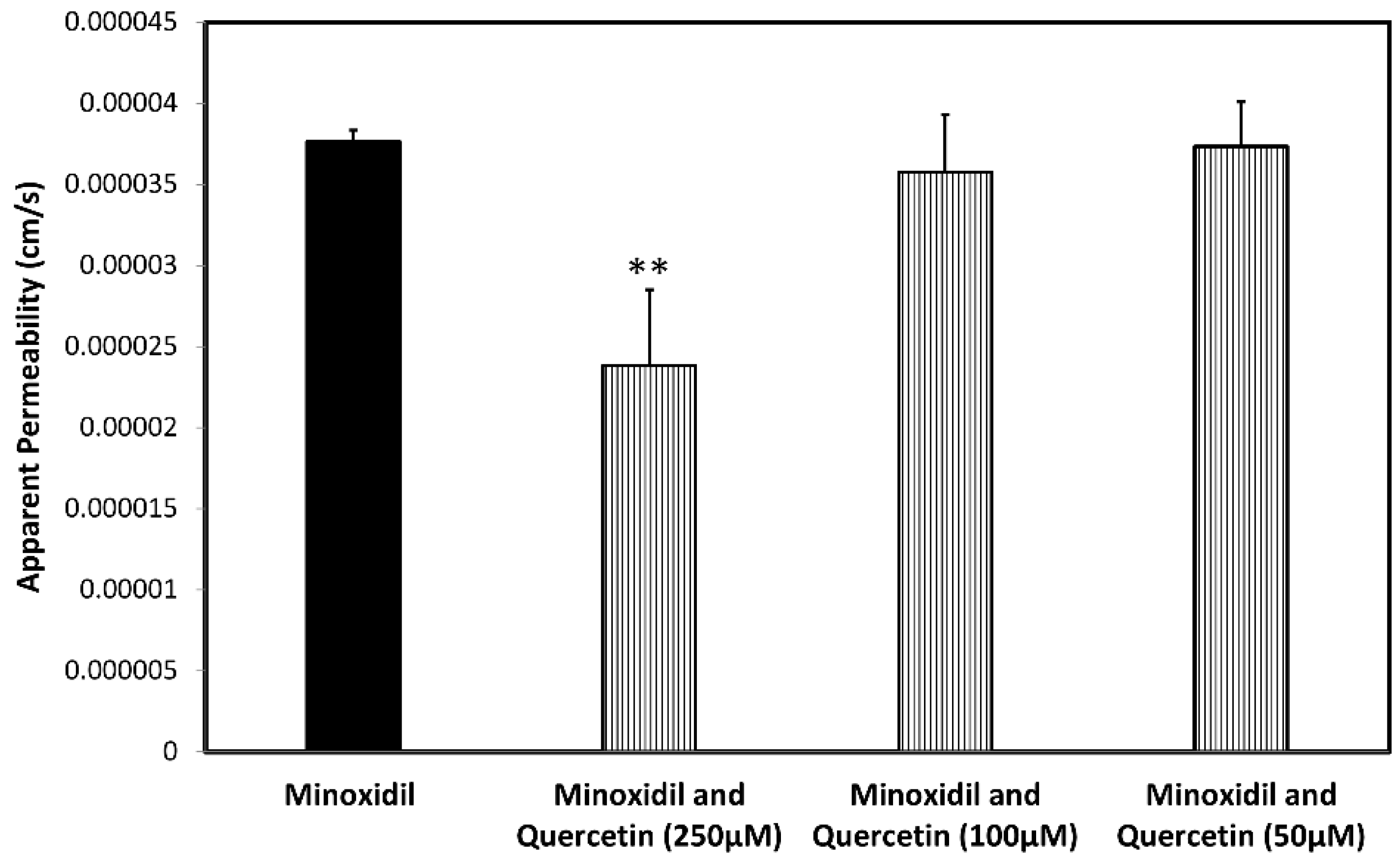
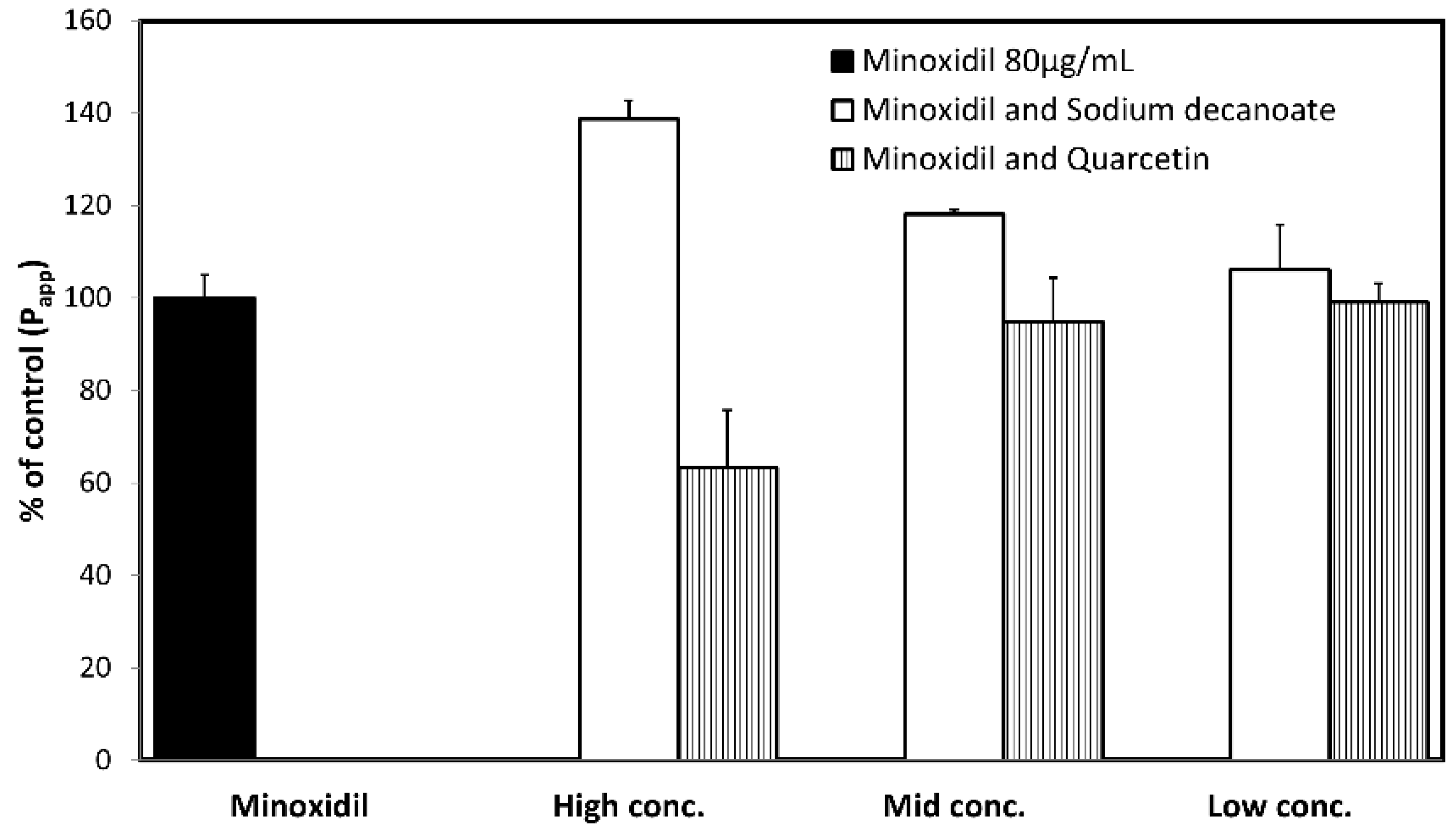
3.4. Permeability Studies across Caco-2 Monolayer
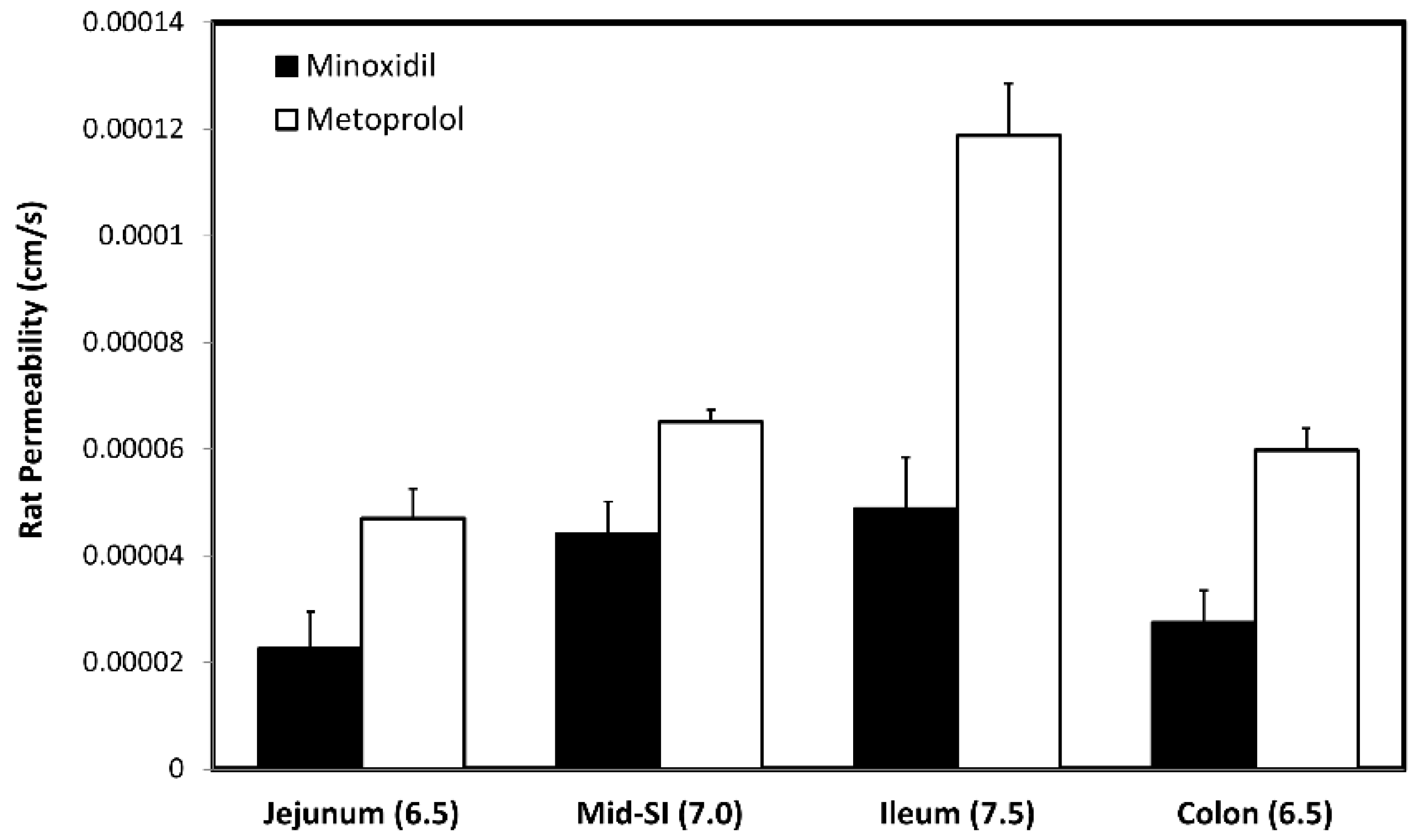
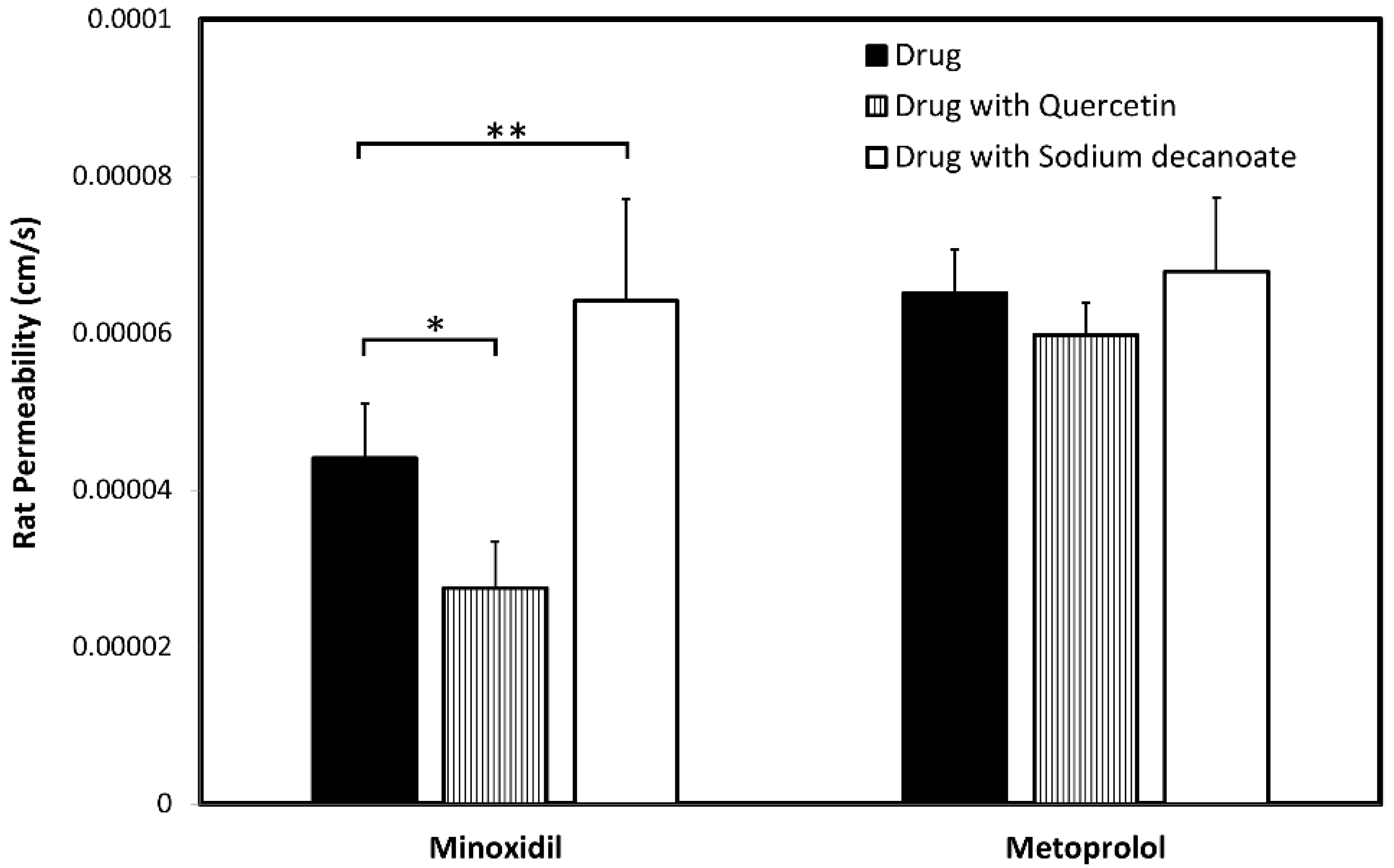
3.5. In-Vivo Rat Intestinal Perfusion (SPIP)
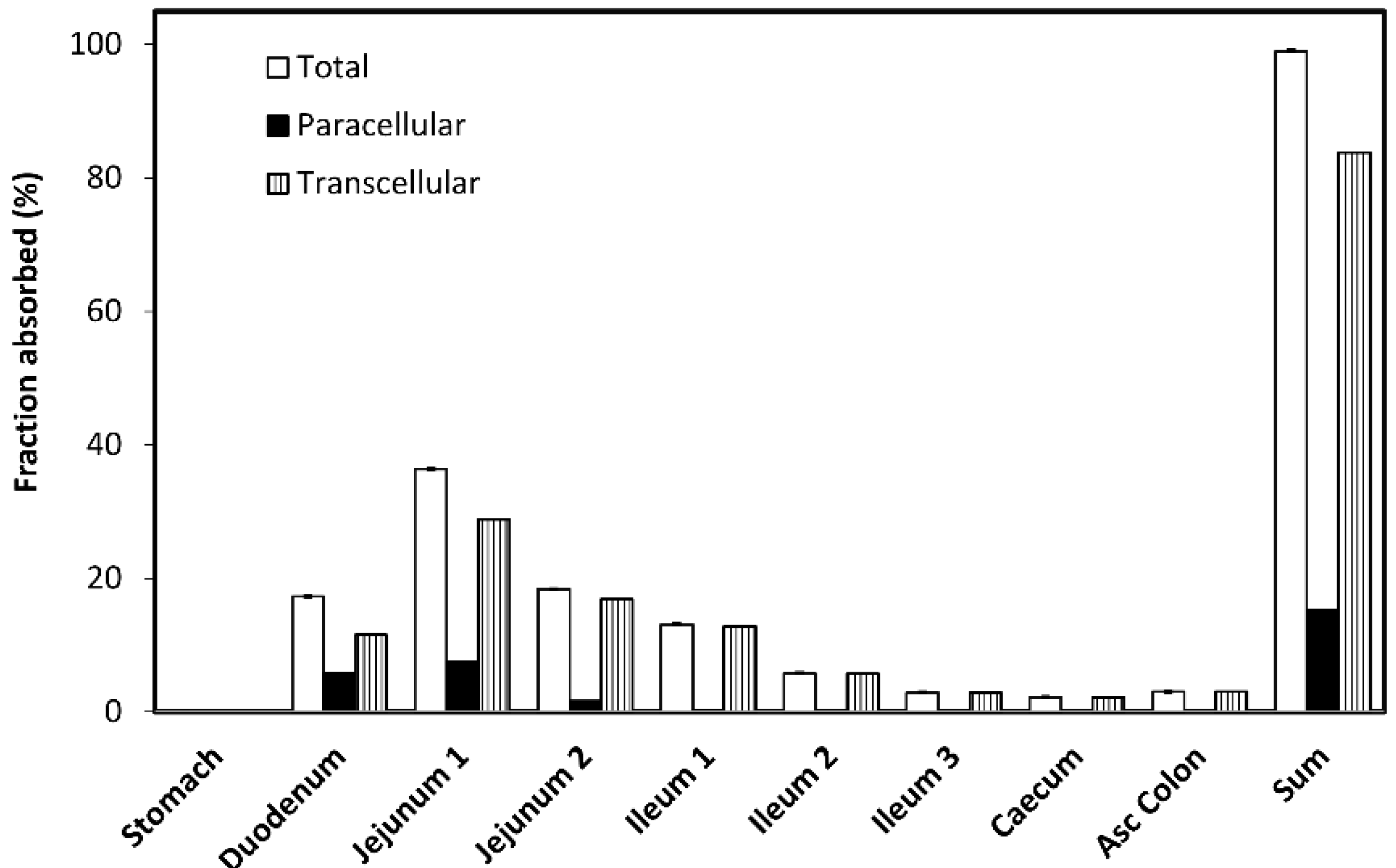
3.6. In-Silico Model Construction
3.7. In-Silico Model Exploration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Amidon, G.L.; Lennernas, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services, Food and Drug Administration, Silver Spring, MD, Center for Drug Evaluation and Research (CDER). Waiver of In-Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System. Guidance for Industry. 2017. Available online: http://resource.nlm.nih.gov/101720038 (accessed on 16 November 2020).
- European Medicines Agency. ICH M9 Guideline on Biopharmaceutics Classification System-Based Biowaivers; European Medicines Agency: Amsterdam, The Netherlands; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-m9-biopharmaceutics-classification-system-based-biowaivers-step-5_en.pdf (accessed on 16 November 2020).
- Cook, J.; Addicks, W.; Wu, Y.H. Application of the biopharmaceutical classification system in clinical drug development--An industrial view. AAPS J. 2008, 10, 306–310. [Google Scholar] [CrossRef]
- Lennernäs, H.; Abrahamsson, B. The use of biopharmaceutic classification of drugs in drug discovery and development: Current status and future extension. J. Pharm. Pharmacol. 2005, 57, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Amidon, K.S.; Langguth, P.; Lennernäs, H.; Yu, L.; Amidon, G.L. Bioequivalence of oral products and the biopharmaceutics classification system: Science, regulation, and public policy. Clin. Pharmacol. Ther. 2011, 90, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Lennernäs, H.; Amidon, G.L. The Fraction Dose Absorbed, in Humans, and High Jejunal Human Permeability Relationship. Mol. Pharm. 2012, 9, 1847–1851. [Google Scholar] [CrossRef]
- Kim, J.S.; Mitchell, S.; Kijek, P.; Tsume, Y.; Hilfinger, J.; Amidon, G.L. The suitability of an in situ perfusion model for permeability determinations: Utility for BCS class I biowaiver requests. Mol. Pharm. 2006, 3, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Regårdh, C.G.; Borg, K.O.; Johansson, R.; Johnsson, G.; Palmer, L. Pharmacokinetic studies on the selective beta1-receptor antagonist metoprolol in man. J. Pharm. Biopharm. 1974, 2, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Fleishaker, J.C.; Andreadis, N.A.; Welshman, I.R.; Wright, C.E., 3rd. The pharmacokinetics of 2.5- to 10-mg oral doses of minoxidil in healthy volunteers. J. Clin. Pharmacol. 1989, 29, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, T.B.; Thomas, R.C.; Chidsey, C.A. Pharmacokinetic studies of minoxidil. Clin. Pharmacol. Ther. 1972, 13, 436–441. [Google Scholar] [CrossRef]
- Thomas, R.C.; Harpootlian, H. Metabolism of minoxidil, a new hypotensive agent II: Biotransformation following oral administration to rats, dogs, and monkeys. J. Pharm. Sci. 1975, 64, 1366–1371. [Google Scholar] [CrossRef]
- Miller, D.D.; Love, D.W. Evaluation of minoxidil. Am. J. Hosp. Pharm. 1980, 37, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Pettinger, W.A. Minoxidil and the treatment of severe hypertension. N. Engl. J. Med. 1980, 303, 922–926. [Google Scholar] [CrossRef] [PubMed]
- Pettinger, W.A.; Mitchell, H.C. Minoxidil—An alternative to nephrectomy for refractory hypertension. N. Engl. J. Med. 1973, 289, 167–171. [Google Scholar] [CrossRef]
- Suchonwanit, P.; Thammarucha, S.; Leerunyakul, K. Minoxidil and its use in hair disorders: A review, drug design. Dev. Ther. 2019, 13, 2777–2786. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Cauhe, J.; Saceda-Corralo, D.; Rodrigues-Barata, R.; Hermosa-Gelbard, A.; Moreno-Arrones, O.M.; Fernandez-Nieto, D.; Vaño-Galvan, S. Effectiveness and safety of low-dose oral minoxidil in male androgenetic alopecia. J. Am. Acad. Dermatol. 2019, 81, 648–649. [Google Scholar] [CrossRef] [PubMed]
- El-Kattan, S.H.A.; Brodfuehrer, J.; Loi, C. Anatomical and physiological factors affecting oral drug bioavailability in rats, dogs, and humans. In Oral Bioavailability: Basic Principles, Advanced Concepts, and Applications; Hu, M., Ed.; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Ozawa, M.; Tsume, Y.; Zur, M.; Dahan, A.; Amidon, G.L. Intestinal permeability study of minoxidil: Assessment of minoxidil as a high permeability reference drug for biopharmaceutics classification. Mol. Pharm. 2015, 12, 204–211. [Google Scholar] [CrossRef]
- Shen, L.; Weber, C.R.; Raleigh, D.R.; Yu, D.; Turner, J.R. Tight junction pore and leak pathways: A dynamic duo. Annu. Rev. Physiol. 2011, 73, 283–309. [Google Scholar] [CrossRef]
- Suzuki, T.; Hara, H. Quercetin enhances intestinal barrier function through the assembly of zonula [corrected] occludens-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells. J. Nutr. 2009, 139, 965–974. [Google Scholar] [CrossRef]
- Valenzano, M.C.; DiGuilio, K.; Mercado, J.; Teter, M.; To, J.; Ferraro, B.; Mixson, B.; Manley, I.; Baker, V.; Moore, B.A.; et al. Remodeling of tight junctions and enhancement of barrier integrity of the CACO-2 intestinal epithelial cell layer by micronutrients. PLoS ONE 2015, 10, e0133926. [Google Scholar] [CrossRef]
- del Vecchio, G.; Tscheik, C.; Tenz, K.; Helms, H.C.; Winkler, L.; Blasig, R.; Blasig, I.E. Sodium caprate transiently opens claudin-5-containing barriers at tight junctions of epithelial and endothelial cells. Mol. Pharm. 2012, 9, 2523–2533. [Google Scholar] [CrossRef]
- Markovic, M.; Zur, M.; Dahan, A.; Cvijić, S. Biopharmaceutical characterization of rebamipide: The role of mucus binding in regional-dependent intestinal permeability. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2020, 152, 105440. [Google Scholar] [CrossRef] [PubMed]
- Markovic, M.; Zur, M.; Fine-Shamir, N.; Haimov, E.; González-Álvarez, I.; Dahan, A. Segmental-dependent solubility and permeability as key factors guiding controlled release drug product development. Pharmaceutics 2020, 12, 295. [Google Scholar] [CrossRef] [PubMed]
- Zur, M.; Cohen, N.; Agbaria, R.; Dahan, A. The biopharmaceutics of successful controlled release drug product: Segmental-dependent permeability of glipizide vs. metoprolol throughout the intestinal tract. Int. J. Pharm. 2015, 489, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Zur, M.; Gasparini, M.; Wolk, O.; Amidon, G.L.; Dahan, A. The low/high BCS permeability class boundary: Physicochemical comparison of metoprolol and labetalol. Mol. Pharm. 2014, 11, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.G.; Sedman, A.J. Quantitaton of rate of gastrointestinal and buccal absorption of acidic and basic drugs based on extraction theory. J. Pharmacokinet. Biopharm. 1973, 1, 23–50. [Google Scholar] [CrossRef][Green Version]
- Winne, D. Shift of pH-absorption curves. J. Pharmacokinet. Biopharm. 1977, 5, 53–94. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Hoekman, D. Exploring QSAR: Hydrophobic, electronic, and steric constants. In ACS Professional Reference Book; Hansch, C., Leo, A., Hoekman, D.H., Eds.; American Chemical Society: Washington, DC, USA, 1995. [Google Scholar]
- Henchoz, Y.; Guillarme, D.; Martel, S.; Rudaz, S.; Veuthey, J.L.; Carrupt, P.A. Fast log P determination by ultra-high-pressure liquid chromatography coupled with UV and mass spectrometry detections. Anal. Bioanal. Chem. 2009, 394, 1919–1930. [Google Scholar] [CrossRef]
- Teksin, Z.S.; Hom, K.; Balakrishnan, A.; Polli, J.E. Ion pair-mediated transport of metoprolol across a three lipid-component PAMPA system. J. Control. Release Off. J. Control. Release Soc. 2006, 116, 50–57. [Google Scholar] [CrossRef]
- Dumanovic, D.; Juranic, I.; Dzeletovic, D.; Vasic, V.M.; Jovanovic, J. Protolytic constants of nizatidine, ranitidine and N,N′-dimethyl-2-nitro-1,1-ethenediamine; spectrophotometric and theoretical investigation. J. Pharm. Biomed. Anal. 1997, 15, 1667–1678. [Google Scholar] [CrossRef]
- Wozniak, T.J. Nizatidine. In Analytical Profiles of Drug Substance; Florey, K., Ed.; Academic Press: Cambridge, MA, USA, 1990; pp. 397–427. [Google Scholar]
- Avdeef, A. Permeability: Caco-2/MDCK. In Absorption and Drug Development; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 499–574. [Google Scholar]
- Gao, J.; Murase, O.; Schowen, R.L.; Aube, J.; Borchardt, R.T. A functional assay for quantitation of the apparent affinities of ligands of P-glycoprotein in Caco-2 cells. Pharm. Res. 2001, 18, 171–176. [Google Scholar] [CrossRef]
- Dahan, A.; West, B.T.; Amidon, G.L. Segmental-dependent membrane permeability along the intestine following oral drug administration: Evaluation of a triple single-pass intestinal perfusion (TSPIP) approach in the rat. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2009, 36, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Lozoya-Agullo, I.; Gonzalez-Alvarez, I.; Zur, M.; Fine-Shamir, N.; Cohen, Y.; Markovic, M.; Garrigues, T.M.; Dahan, A.; Gonzalez-Alvarez, M.; Merino-Sanjuán, M.; et al. Closed-loop doluisio (colon, small intestine) and single-pass intestinal perfusion (colon, jejunum) in rat—biophysical model and predictions based on Caco-2. Pharm. Res. 2017, 35, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Lozoya-Agullo, I.; Zur, M.; Beig, A.; Fine, N.; Cohen, Y.; Gonzalez-Alvarez, M.; Merino-Sanjuan, M.; Gonzalez-Alvarez, I.; Bermejo, M.; Dahan, A. Segmental-dependent permeability throughout the small intestine following oral drug administration: Single-pass vs. Doluisio approach to in-situ rat perfusion. Int. J. Pharm. 2016, 515, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Lozoya-Agullo, I.; Zur, M.; Fine-Shamir, N.; Markovic, M.; Cohen, Y.; Porat, D.; Gonzalez-Alvarez, I.; Gonzalez-Alvarez, M.; Merino-Sanjuan, M.; Bermejo, M.; et al. Investigating drug absorption from the colon: Single-pass vs. Doluisio approaches to in-situ rat large-intestinal perfusion. Int. J. Pharm. 2017, 527, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Lozoya-Agullo, I.; Zur, M.; Wolk, O.; Beig, A.; Gonzalez-Alvarez, I.; Gonzalez-Alvarez, M.; Merino-Sanjuan, M.; Bermejo, M.; Dahan, A. In-situ intestinal rat perfusions for human fabs prediction and BCS permeability class determination: Investigation of the single-pass vs. the doluisio experimental approaches. Int. J. Pharm. 2015, 480, 1–7. [Google Scholar] [CrossRef]
- Dahan, A.; Sabit, H.; Amidon, G.L. Multiple efflux pumps are involved in the transepithelial transport of colchicine: Combined effect of p-glycoprotein and multidrug resistance-associated protein 2 leads to decreased intestinal absorption throughout the entire small intestine. Drug Metab. Dispos. Biol. Fate Chem. 2009, 37, 2028–2036. [Google Scholar] [CrossRef]
- Dahan, A.; Miller, J.M.; Hilfinger, J.M.; Yamashita, S.; Yu, L.X.; Lennernas, H.; Amidon, G.L. High-permeability criterion for BCS classification: Segmental/pH dependent permeability considerations. Mol. Pharm. 2010, 7, 1827–1834. [Google Scholar] [CrossRef]
- Wolk, O.; Markovic, M.; Porat, D.; Fine-Shamir, N.; Zur, M.; Beig, A.; Dahan, A. Segmental-dependent intestinal drug permeability: Development and model validation of in silico predictions guided by in vivo permeability values. J. Pharm. Sci. 2019, 108, 316–325. [Google Scholar] [CrossRef]
- Bolger, M.B.; Agoram, B.; Fraczkiewicz, R.; Steere, B. Simulation of absorption, metabolism, and bioavailability. In Drug Bioavailability; Mannhold, R., Kubinyi, H., Folkers, G., van de Waterbeemd, H., Lennernäs, H., Artursson, P., Eds.; Wiley: Weinheim, Germany, 2003. [Google Scholar]
- Lin, L.; Wong, H. Predicting oral drug absorption: Mini review on physiologically-based pharmacokinetic models. Pharmaceutics 2017, 9, 41. [Google Scholar] [CrossRef]
- Parhi, R.; Terapalli, B.R.; Teja, B.B. Formulation and in vitro evaluation of minoxidil topical gel. Turk. J. Pharm. Sci. 2014, 11, 153–162. [Google Scholar]
- Li, J.; Bukhtiyarov, Y.; Spivey, N.; Force, C.; Hidalgo, C.; Huang, Y.; Owen, A.J.; Hidalgo, I.J. In vitro and in vivo assessment of the potential of supersaturation to enhance the absorption of poorly soluble basic drug. J. Pharm. Innov. 2020, 15, 591–602. [Google Scholar] [CrossRef]
- Wang, S.; Liao, M.; Xia, C. Confidence assessment of an absorption model using limited solubility and permeability data for 21 drugs within a dynamic physiologically-based pharmacokinetic simulator. J. Appl. Biopharm. Pharmacokinet. 2015, 3, 7–17. [Google Scholar] [CrossRef]
- Lombardo, F.; Berellini, G.; Obach, R.S. Trend analysis of a database of intravenous pharmacokinetic parameters in humans for 1352 drug compounds. Drug Metab. Dispos. 2018, 46, 1466–1477. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.H.; Poynor, W.J.; Garnett, W.R.; Karnes, H.T.; Ferry, J.J.; Ryan, K.K.; Sarkar, M.A. Pharmacokinetics of minoxidil in patients with cirrhosis and healthy volunteers. Biopharm. Drug Dispos. 1998, 19, 501–515. [Google Scholar] [CrossRef]
- Loniten®minoxidil Tablets. United States Pharmacopeia. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/018154s026lbl.pdf (accessed on 15 December 2020).
- Lowenthal, D.T.; Affrime, M.B. Pharmacology and pharmacokinetics of minoxidil. J. Cardiovasc. Pharmacol. 1980, 2 (Suppl. S2), S93–S106. [Google Scholar] [CrossRef]
- Garcia-Hernandez, V.; Quiros, M.; Nusrat, A. Intestinal epithelial claudins: Expression and regulation in homeostasis and inflammation. Ann. N. Y. Acad. Sci. 2017, 1397, 66–79. [Google Scholar] [CrossRef]
- Hollander, D.; Kaunitz, J.D. The “Leaky Gut”: Tight junctions but loose associations? Dig. Dis. Sci. 2020, 65, 1277–1287. [Google Scholar] [CrossRef]
- Bjarnason, I.; MacPherson, A.; Hollander, D. Intestinal permeability: An overview. Gastroenterology 1995, 108, 1566–1581. [Google Scholar] [CrossRef]
- Pearce, S.C.; Al-Jawadi, A.; Kishida, K.; Yu, S.; Hu, M.; Fritzky, L.F.; Edelblum, K.L.; Gao, N.; Ferraris, R.P. Marked differences in tight junction composition and macromolecular permeability among different intestinal cell types. BMC Biol. 2018, 16, 1–16. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 4201, Minoxidil. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Minoxidil (accessed on 16 November 2020).



| Parameter | Value | Source |
|---|---|---|
| Molecular weight (g/mol) | 209.25 | / |
| Log D (pH 6.5) | 0.82 | experimental value |
| Solubility (aq) a (mg/mL) | 2.2 | [47] |
| pKa (base) | 4.6 | [48] |
| Human total permeability (cm/s) | see Table 2 | / |
| Diffusion coefficient (cm2/s) | 0.93 × 10−5 | GastroPlus™ calculated value (based on molecular weight) |
| Mean precipitation time (s) | 900 | GastroPlus™ default values |
| Particle density (g/mL) | 1.2 | |
| Particle radius (µm) | 25 | |
| Blood/plasma concentration ratio | 1.39 | [49] |
| Plasma fraction unbound (%) | 1 | [50] |
| Clearance, CL (L/h/kg) | 1.2 | Optimized values |
| Volume of distribution in central compartment, Vc (L/kg) | 0.1 | |
| Distribution constant, k12 (1/h) | 2.0 | |
| Distribution constant, k21 (1/h) | 0.2 | |
| Volume of distribution in peripheral compartment, V2 (L/kg) | 1.0 | GastroPlus™ calculated |
| Elimination half-life, t1/2 (h) | 4.05 | |
| Dosage form | IR tablet | / |
| Dose (mg) | 2.5; 5; 10 | / |
| Experimental (Rat Studies) | Software Default Data | Predicted (Human) a | |
|---|---|---|---|
| Region/pH | Permeability (cm/s) | Region/pH | Permeability (cm/s) |
| Jejunum/pH 6.5 | 2.26 × 10−5 | Duodenum/pH 6.0 Jejunum 1/pH 6.2 Jejunum 2/pH 6.4 | 1.27 × 10−4 |
| Mid-SI/pH 7.0 | 4.41 × 10−5 | Ileum 1/pH 6.6 Ileum 2/pH 6.9 | 2.22 × 10−4 |
| Ileum/pH 7.5 | 4.89 × 10−5 | Ileum 3/pH 7.4 | 2.43 × 10−4 |
| Colon/pH 6.5 | 2.75 × 10−5 | Caecum/pH 6.4 Asc Colon/pH 6.8 | 1.48 × 10−4 |
| Parameter | Optimized Input Value | In-Vivo Values (Range) | References |
|---|---|---|---|
| CL (L/h/kg) | 1.2 | 0.5–1.5 | [10,11,49,50,51] |
| Vtot (L/kg) | 1.1 | 0.9–3.7 | |
| t1/2 (h) | 4.05 | 1.07–4.20 a |
| Parameter | In-Vivo Mean a | Predicted | PE(%) |
|---|---|---|---|
| 2.5 mg dose | |||
| Cmax (µg/mL) | 15.00 | 15.55 | −3.68 |
| tmax (h) | 0.34 | 0.56 | −64.71 |
| AUC0→∞ (µg h/mL) | 30.48 | 29.48 | 3.28 |
| Fabs (%) | / | 99.05 | / |
| 5.0 mg dose | |||
| Cmax (µg/mL) | 35.00 | 31.12 | 11.09 |
| tmax (h) | 0.32 | 0.56 | −75.00 |
| AUC0→∞ (µg h/mL) | 61.46 | 58.99 | 4.02 |
| Fabs (%) | / | 99.09 | / |
| 10 mg dose | |||
| Cmax (µg/mL) | 70.00 | 62.21 | 11.13 |
| tmax (h) | 0.34 | 0.56 | −64.71 |
| AUC0→∞ (µg h/mL) | 116.57 | 117.93 | −1.17 |
| Fabs (%) | / | 99.05 | / |
| Compartment | Drug Permeability | Physiological Characteristics | ||||
|---|---|---|---|---|---|---|
| Total Permeability (cm/s) | Paracellular Permeability (cm/s) | Transcellular Permeability (cm/s) | Length (cm) | Radius (cm) | SEF a | |
| Stomach | 0 | 0 | 28.29 | 9.67 | 1 | |
| Duodenum | 1.27 × 10−4 | 5.82 × 10−5 | 6.87 × 10−5 | 14.13 | 1.53 | 4.23 |
| Jejunum 1 | 1.27 × 10−4 | 3.55 × 10−5 | 9.14 × 10−5 | 58.40 | 1.45 | 3.95 |
| Jejunum 2 | 1.27 × 10−4 | 1.41 × 10−5 | 11.00 × 10−5 | 58.40 | 1.29 | 3.49 |
| Ileum 1 | 2.22 × 10−4 | 0.43 × 10−5 | 22.00 × 10−5 | 58.40 | 1.13 | 3.03 |
| Ileum 2 | 2.22 × 10−4 | 0.09 × 10−5 | 22.00 × 10−5 | 58.40 | 0.98 | 2.57 |
| Ileum 3 | 2.43 × 10−4 | 0 | 24.00 × 10−5 | 58.40 | 0.82 | 2.11 |
| Caecum | 1.48 × 10−4 | 0 | 15.00 × 10−5 | 13.19 | 3.38 | 1.79 |
| Asc Colon | 1.48 × 10−4 | 0 | 15.00 × 10−5 | 27.65 | 2.41 | 2.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markovic, M.; Zur, M.; Garsiani, S.; Porat, D.; Cvijić, S.; Amidon, G.L.; Dahan, A. The Role of Paracellular Transport in the Intestinal Absorption and Biopharmaceutical Characterization of Minoxidil. Pharmaceutics 2022, 14, 1360. https://doi.org/10.3390/pharmaceutics14071360
Markovic M, Zur M, Garsiani S, Porat D, Cvijić S, Amidon GL, Dahan A. The Role of Paracellular Transport in the Intestinal Absorption and Biopharmaceutical Characterization of Minoxidil. Pharmaceutics. 2022; 14(7):1360. https://doi.org/10.3390/pharmaceutics14071360
Chicago/Turabian StyleMarkovic, Milica, Moran Zur, Sapir Garsiani, Daniel Porat, Sandra Cvijić, Gordon L. Amidon, and Arik Dahan. 2022. "The Role of Paracellular Transport in the Intestinal Absorption and Biopharmaceutical Characterization of Minoxidil" Pharmaceutics 14, no. 7: 1360. https://doi.org/10.3390/pharmaceutics14071360
APA StyleMarkovic, M., Zur, M., Garsiani, S., Porat, D., Cvijić, S., Amidon, G. L., & Dahan, A. (2022). The Role of Paracellular Transport in the Intestinal Absorption and Biopharmaceutical Characterization of Minoxidil. Pharmaceutics, 14(7), 1360. https://doi.org/10.3390/pharmaceutics14071360









