Pneumococcal Surface Protein A-Hybrid Nanoparticles Protect Mice from Lethal Challenge after Mucosal Immunization Targeting the Lungs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of NPs and Characterization
2.3. Protein Adsorption or Encapsulation
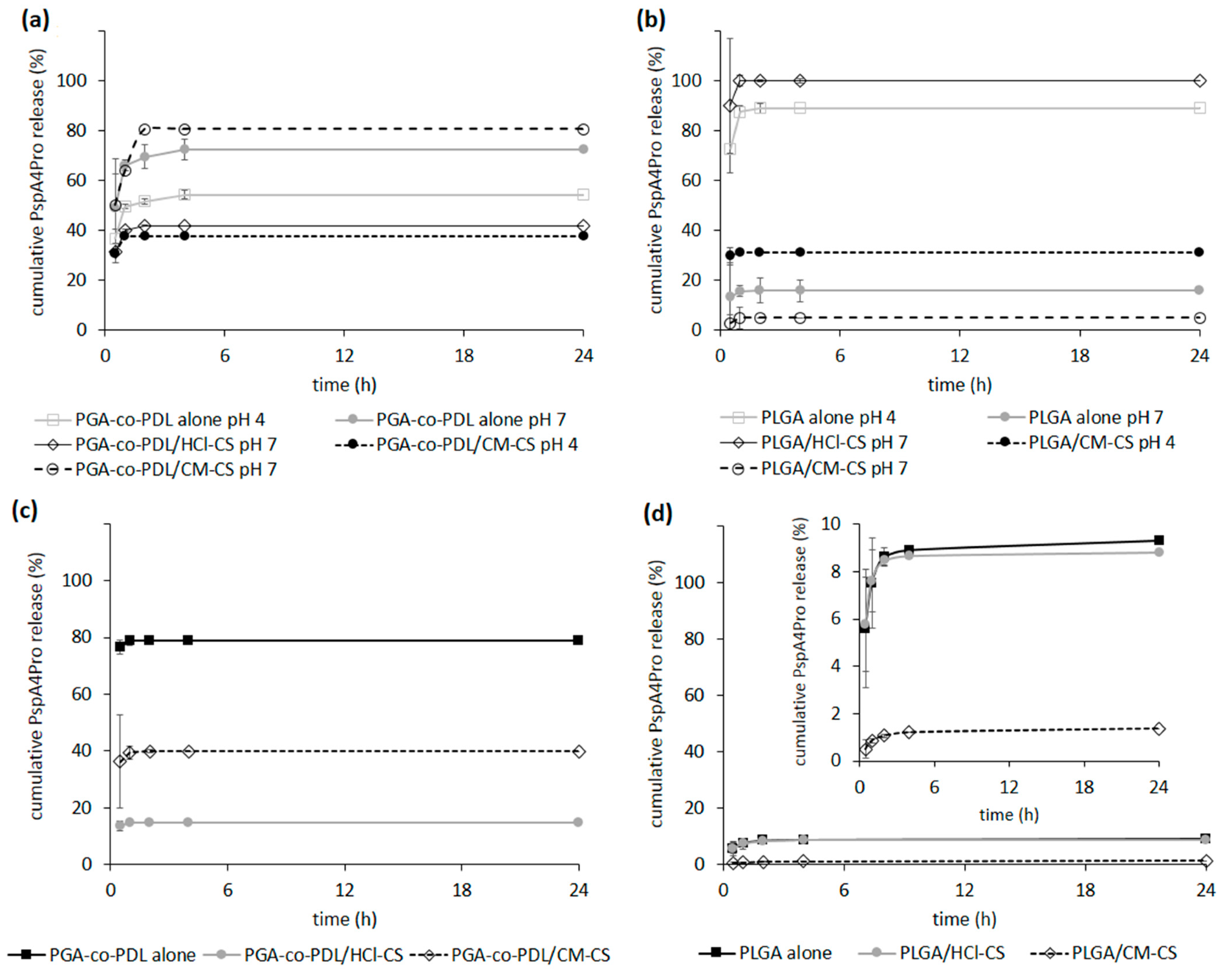
2.4. PspA4Pro In Vitro Release Studies
2.5. PspA4Pro Integrity and Biological Activity
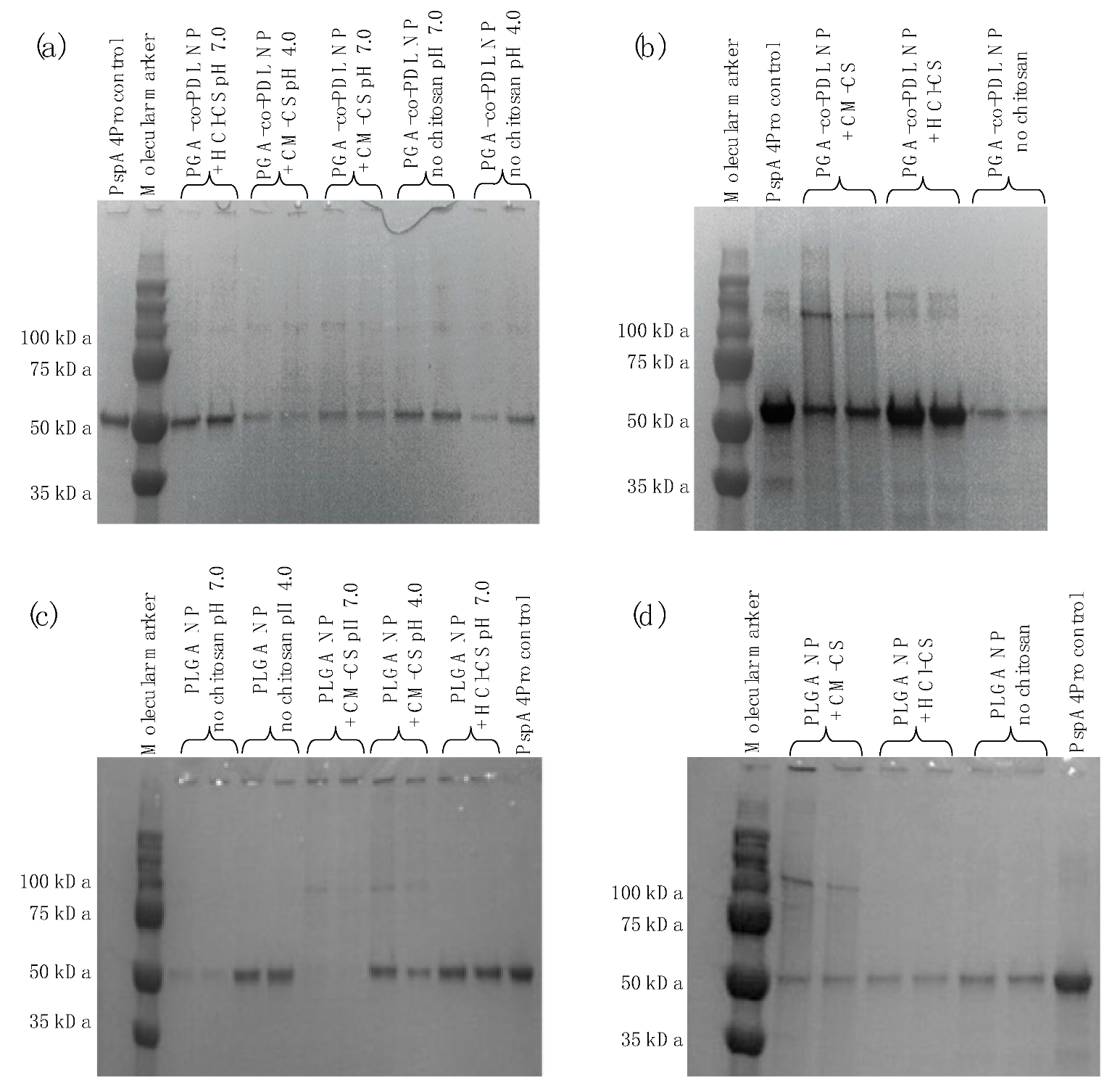
2.5.1. SDS-PAGE
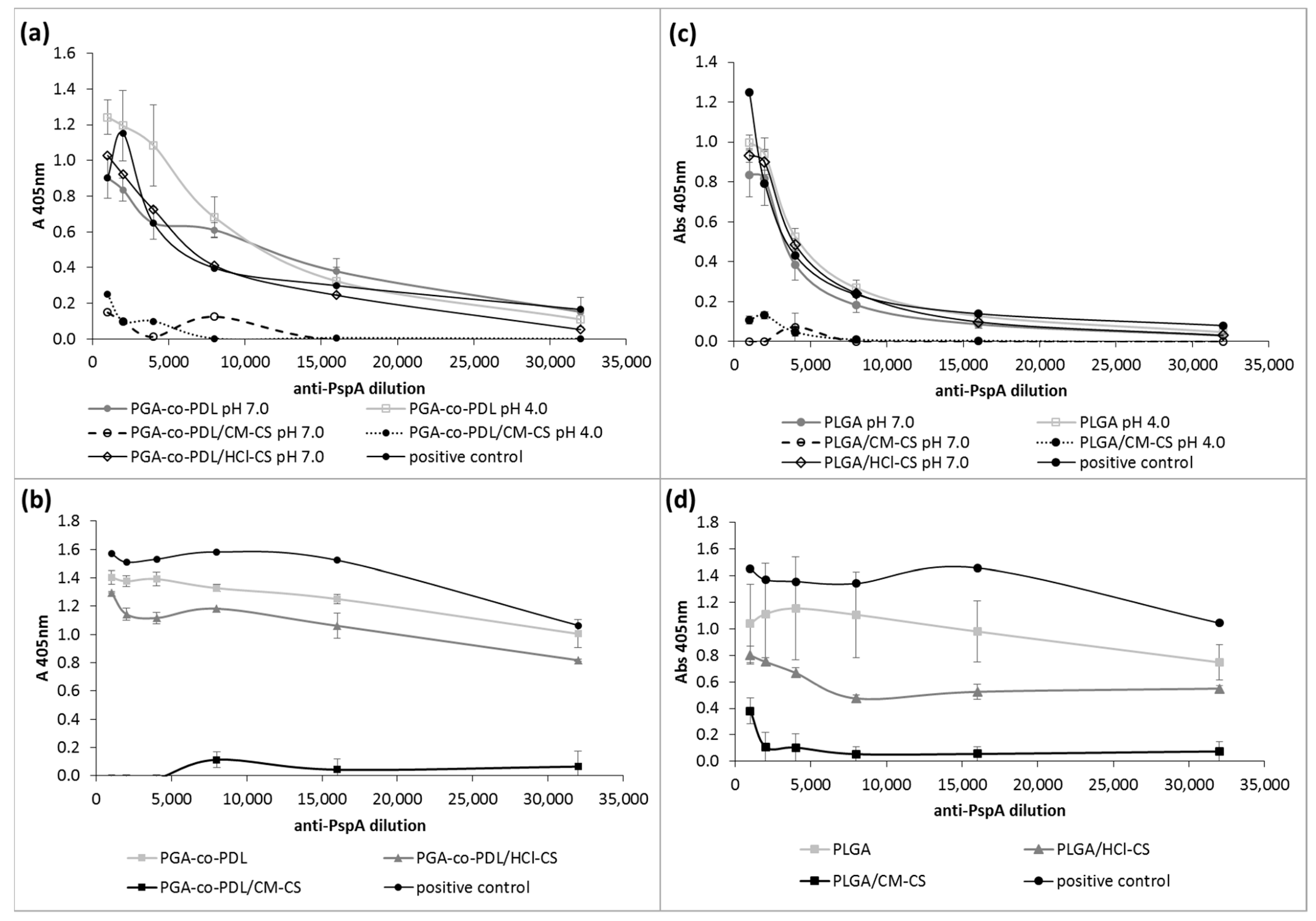
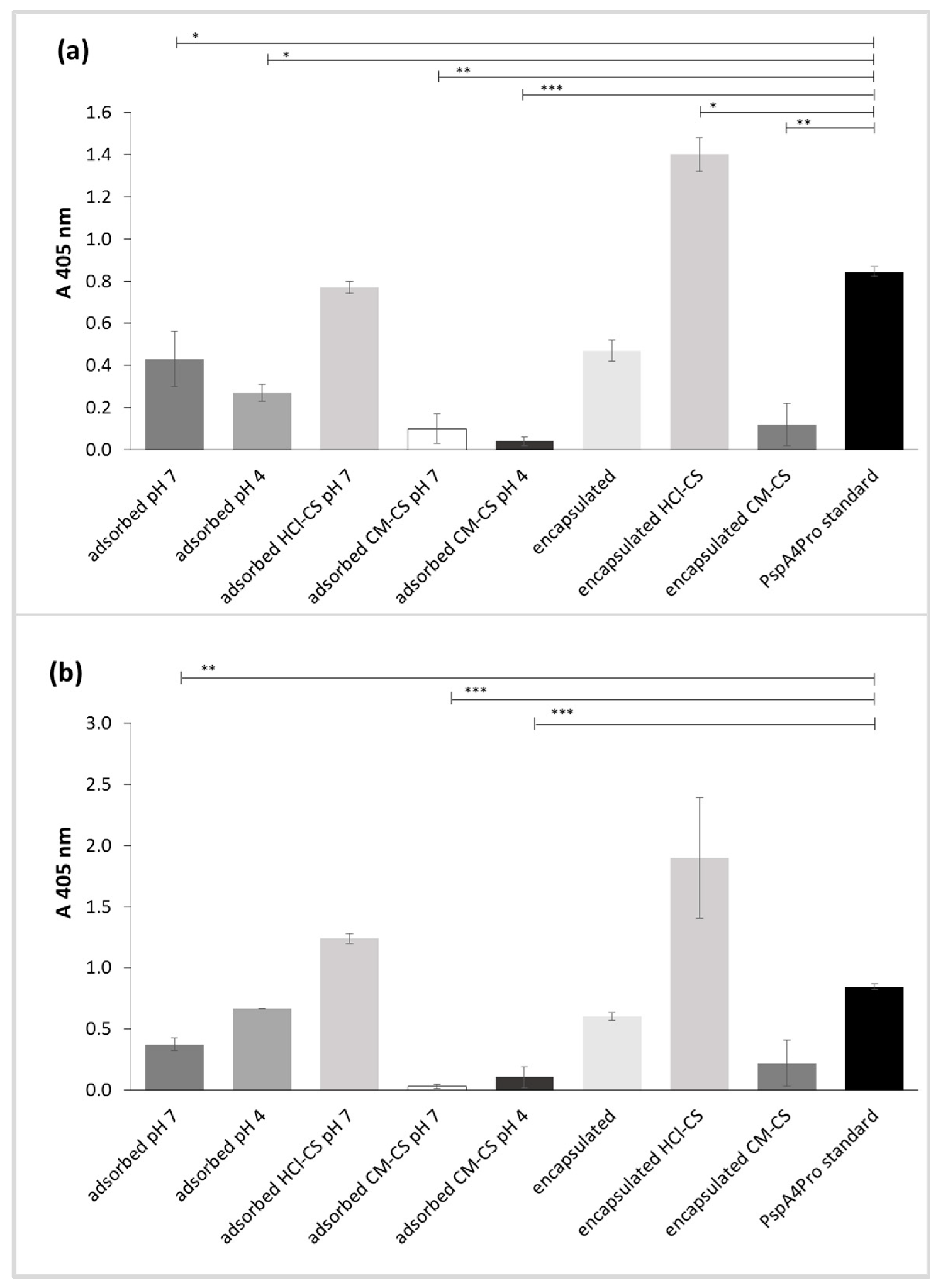
2.5.2. PspA4Pro Antigenicity
2.5.3. Lactoferrin Binding Assay
2.6. Nanocomposite Microparticles
2.7. Activation of DCs
2.8. Mice Immunization
2.9. Measurement of Antibodies by Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Pneumococcal Lethal Challenge
2.11. Statistical Analysis
3. Results
3.1. Conditions for NP Preparation
3.2. Influence of Chitosan on NP Characteristics
3.3. Protein Adsorption or Encapsulation
3.4. PspA4Pro In Vitro Release
3.5. PspA4Pro Integrity and Biological Activity
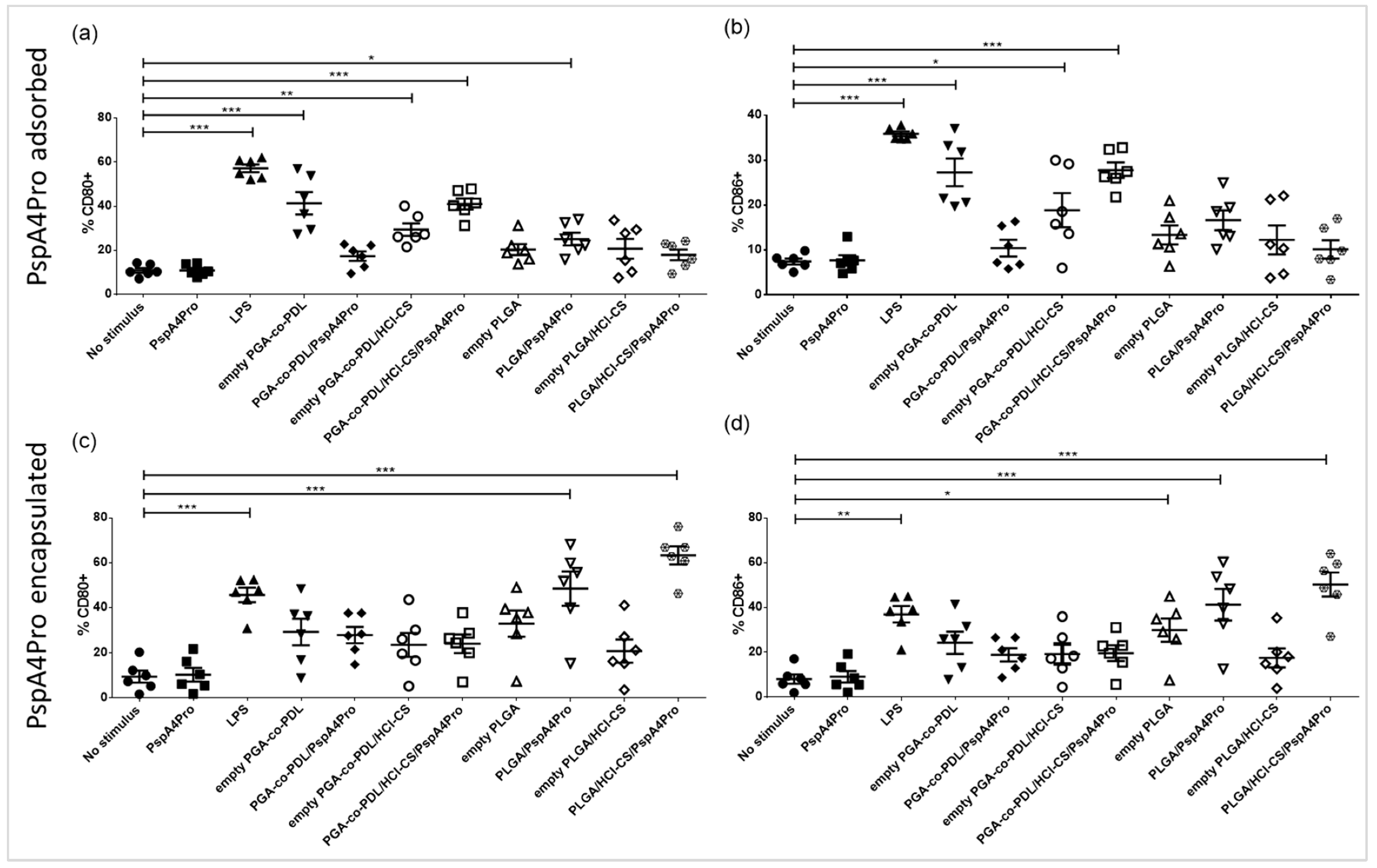
3.6. Activation of DCs
3.7. Induction of Anti-PspA4Pro Antibodies by Immunization with NP/NCMPs
3.8. Pneumococcal Lethal Challenge with Strain ATCC6303 (Serotype 3, PspA5)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Masomian, M.; Ahmad, Z.; Gew, L.T.; Poh, C.L. Development of Next Generation. Vaccines 2020, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Self, W.H.; Wunderink, R.G.; Fakhran, S.; Balk, R.; Bramley, A.M.; Reed, C.; Grijalva, C.G.; Anderson, E.J.; Courtney, D.M.; et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N. Engl. J. Med. 2015, 373, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Cillóniz, C.; Blasi, F.; Chalmers, J.D.; Gaillat, J.; Dartois, N.; Schmitt, H.J.; Welte, T. Burden of pneumococcal community-acquired pneumonia in adults across Europe: A literature review. Respir. Med. 2018, 137, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Benadji, A.; Duval, X.; Danis, K.; Hoen, B.; Page, B.; Béraud, G.; Vernet-Garnier, V.; Strady, C.; Brieu, N.; Maulin, L.; et al. Relationship between serotypes, disease characteristics and 30-day mortality in adults with invasive pneumococcal disease. Infection 2022, 50, 223–233. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, R.; Yokoyama, S.; Boyle, J.; Kwong, J.C.; Mcgeer, A.; To, T.; Sander, B. The impact of acute pneumococcal disease on health state utility values: A systematic review. Qual. Life Res. 2022, 31, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Paton, J.C.; Trappetti, C. Streptococcus pneumoniae capsular polysaccharide. Microbiol. Spectr. 2019, 7, GPP3-0019-2018. [Google Scholar] [CrossRef]
- Almeida, S.C.G.; Lo, S.W.; Hawkins, P.A.; Gladstone, R.A.; Cassiolato, A.P.; Klugman, K.P.; Breiman, R.F.; Bentley, S.D.; Mcgee, L.; Brandileone, M.-C.D.C. Genomic surveillance of invasive Streptococcus pneumoniae isolates in the period pre-PCV10 and post-PCV10 introduction in Brazil. Microb. Genom. 2021, 7, 000635. [Google Scholar] [CrossRef]
- Kaur, R.; Casey, J.R.; Pichichero, M.E. Emerging Streptococcus pneumoniae Strains Colonizing the Nasopharynx in Children After 13-valent Pneumococcal Conjugate Vaccination in Comparison to the 7-valent Era, 2006–2015. Pediatric Infect. Dis. J. 2016, 35, 901–906. [Google Scholar] [CrossRef]
- Zangari, T.; Zafar, M.A.; Lees, J.A.; Abruzzo, A.R.; Bee, G.C.W.; Weiser, J.N. Pneumococcal capsule blocks protection by immunization with conserved surface proteins. NPJ Vaccines 2021, 6, 155. [Google Scholar] [CrossRef]
- Goncalves, V.M.; Kaneko, K.; Solorzano, C.; MacLoughlin, R.; Saleem, I.; Miyaji, E.N. Progress in mucosal immunization for protection against pneumococcal pneumonia. Expert Rev. Vaccines 2019, 18, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Petkar, K.C.; Patil, S.M.; Chavhan, S.S.; Kaneko, K.; Sawant, K.K.; Kunda, N.K.; Saleem, I.Y. An Overview of Nanocarrier-Based Adjuvants for Vaccine Delivery. Pharmaceutics 2021, 13, 455. [Google Scholar] [CrossRef] [PubMed]
- Kheirollahpour, M.; Mehrabi, M.; Dounighi, N.M.; Mohammadi, M.; Masoudi, A. Nanoparticles and Vaccine Development. Pharm. Nanotechnol. 2020, 8, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Sinani, G.; Sessevmez, M.; Gök, M.K.; Özgümüş, S.; Alpar, H.O.; Cevher, E. Modified chitosan-based nanoadjuvants enhance immunogenicity of protein antigens after mucosal vaccination. Int. J. Pharm. 2019, 569, 118592. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.; Yoo, H.S. The Application of Mucoadhesive Chitosan Nanoparticles in Nasal Drug Delivery. Mar. Drugs 2020, 18, 605. [Google Scholar] [CrossRef]
- Turley, J.L.; Moran, H.B.T.; McEntee, C.P.; O’Grady, K.; Muñoz-Wolf, N.; Jin, L.; Follmann, F.; Andersen, P.; Andersson, M.; Lavelle, E.C. Chitin-derived polymer deacetylation regulates mitochondrial reactive oxygen species dependent cGAS-STING and NLRP3 inflammasome activation. Biomaterials 2021, 275, 120961. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, Y.; Xin, Y.; Jiang, C.; Yan, B.; Zhai, S. Interactions Between Nanoparticles and Dendritic Cells: From the Perspective of Cancer Immunotherapy. Front. Oncol. 2018, 8, 404. [Google Scholar] [CrossRef]
- Benson, R.A.; MacLeod, M.K.; Hale, B.G.; Patakas, A.; Garside, P.; Brewer, J.M. Antigen presentation kinetics control T cell/dendritic cell interactions and follicular helper T cell generation in vivo. eLife 2015, 4, e06994. [Google Scholar] [CrossRef]
- Alfagih, I.M.; Kaneko, K.; Kunda, N.K.; Alanazi, F.; Dennison, S.R.; Tawfeek, H.M.; Saleem, I.Y. In Vitro Characterization of Inhalable Cationic Hybrid Nanoparticles as Potential Vaccine Carriers. Pharmaceuticals 2021, 14, 164. [Google Scholar] [CrossRef]
- Chang, T.Z.; Stadmiller, S.S.; Staskevicius, E.; Champion, J.A. Effects of ovalbumin protein nanoparticle vaccine size and coating on dendritic cell processing. Biomater. Sci. 2017, 5, 223–233. [Google Scholar] [CrossRef]
- Tsoras, A.N.; Wong, K.M.; Paravastu, A.K.; Champion, J.A. Rational Design of Antigen Incorporation Into Subunit Vaccine Biomaterials Can Enhance Antigen-Specific Immune Responses. Front. Immunol. 2020, 11, 1547. [Google Scholar] [CrossRef] [PubMed]
- Briles, D.E.; Paton, J.C.; Mukerji, R.; Swiatlo, E.; Crain, M.J. Pneumococcal Vaccines. Microbiol. Spectr. 2019, 7, GPP3-0028-2018. [Google Scholar] [CrossRef] [PubMed]
- Hollingshead, S.K.; Becker, R.; Briles, D.E. Diversity of PspA: Mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect. Immun. 2000, 68, 5889–5900. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.T.; Oliveira, M.L.; Ferreira, D.M.; Ho, P.L.; Darrieux, M.; Leite, L.C.; Ferreira, J.M.; Pimenta, F.C.; Andrade, A.L.; Miyaji, E.N. Immunization of mice with single PspA fragments induces antibodies capable of mediating complement deposition on different pneumococcal strains and cross-protection. Clin. Vaccine Immunol. 2010, 17, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Nakahashi-Ouchida, R.; Uchida, Y.; Yuki, Y.; Katakai, Y.; Yamanoue, T.; Ogawa, H.; Munesue, Y.; Nakano, N.; Hanari, K.; Miyazaki, T.; et al. A nanogel-based trivalent PspA nasal vaccine protects macaques from intratracheal challenge with pneumococci. Vaccine 2021, 39, 3353–3364. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Guo, J.; Wang, D.; Yu, J.; Gu, T.; Jiang, C.; Kong, W.; Wu, Y. Broad protective immune responses elicited by bacterium-like particle-based intranasal pneumococcal particle vaccine displaying PspA2 and PspA4 fragments. Hum. Vaccines Immunother. 2019, 15, 371–380. [Google Scholar] [CrossRef]
- Kye, Y.C.; Park, S.M.; Shim, B.S.; Firdous, J.; Kim, G.; Kim, H.W.; Ju, Y.J.; Kim, C.G.; Cho, C.S.; Kim, D.W.; et al. Intranasal immunization with pneumococcal surface protein A in the presence of nanoparticle forming polysorbitol transporter adjuvant induces protective immunity against the Streptococcus pneumoniae infection. Acta Biomater. 2019, 90, 362–372. [Google Scholar] [CrossRef]
- Tada, R.; Suzuki, H.; Takahashi, S.; Negishi, Y.; Kiyono, H.; Kunisawa, J.; Aramaki, Y. Nasal vaccination with pneumococcal surface protein A in combination with cationic liposomes consisting of DOTAP and DC-chol confers antigen-mediated protective immunity against Streptococcus pneumoniae infections in mice. Int. Immunopharmacol. 2018, 61, 385–393. [Google Scholar] [CrossRef]
- van Beek, L.F.; Langereis, J.D.; van den Berg van Saparoea, H.B.; Gillard, J.; Jong, W.S.P.; van Opzeeland, F.J.; Mesman, R.; van Niftrik, L.; Joosten, I.; Diavatopoulos, D.A.; et al. Intranasal vaccination with protein bodies elicit strong protection against Streptococcus pneumoniae colonization. Vaccine 2021, 39, 6920–6929. [Google Scholar] [CrossRef]
- Kunda, N.K.; Alfagih, I.M.; Miyaji, E.N.; Figueiredo, D.B.; Gonçalves, V.M.; Ferreira, D.M.; Dennison, S.R.; Somavarapu, S.; Hutcheon, G.A.; Saleem, I.Y. Pulmonary dry powder vaccine of pneumococcal antigen loaded nanoparticles. Int. J. Pharm. 2015, 495, 903–912. [Google Scholar] [CrossRef]
- Rodrigues, T.C.; Oliveira, M.L.S.; Soares-Schanoski, A.; Chavez-Rico, S.L.; Figueiredo, D.B.; Gonçalves, V.M.; Ferreira, D.M.; Kunda, N.K.; Saleem, I.Y.; Miyaji, E.N. Mucosal immunization with PspA (Pneumococcal surface protein A)-adsorbed nanoparticles targeting the lungs for protection against pneumococcal infection. PLoS ONE 2018, 13, e0191692. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Miyaji, E.; Goncalves, V.; Ferreira, D.; Solorzano, C.; MacLoughlin, R.; Saleem, I. Evaluation of polymer choice on immunogenicity of chitosan coated PLGA NPs with surface-adsorbed pneumococcal protein antigen PspA4Pro. Int. J. Pharm. 2021, 599, 120407. [Google Scholar] [CrossRef] [PubMed]
- Tawfeek, H.; Khidr, S.; Samy, E.; Ahmed, S.; Murphy, M.; Mohammed, A.; Shabir, A.; Hutcheon, G.; Saleem, I. Poly(glycerol adipate-co-ω-pentadecalactone) spray-dried microparticles as sustained release carriers for pulmonary delivery. Pharm. Res. 2011, 28, 2086–2097. [Google Scholar] [CrossRef]
- Figueiredo, D.B.; Carvalho, E.; Santos, M.P.; Kraschowetz, S.; Zanardo, R.T.; Campani, G.; Silva, G.G.; Sargo, C.R.; Horta, A.C.L.; de C. Giordano, R.; et al. Production and purification of an untagged recombinant pneumococcal surface protein A (PspA4Pro) with high-purity and low endotoxin content. Appl. Microbiol. Biotechnol. 2017, 101, 2305–2317. [Google Scholar] [CrossRef] [PubMed]
- Kunda, N.K.; Alfagih, I.M.; Dennison, S.R.; Tawfeek, H.M.; Somavarapu, S.; Hutcheon, G.A.; Saleem, I.Y. Bovine serum albumin adsorbed PGA-co-PDL nanocarriers for vaccine delivery via dry powder inhalation. Pharm. Res. 2015, 32, 1341–1353. [Google Scholar] [CrossRef]
- Alfagih, I.; Kunda, N.; Alanazi, F.; Dennison, S.R.; Somavarapu, S.; Hutcheon, G.A.; Saleem, I.Y. Pulmonary Delivery of Proteins Using Nanocomposite Microcarriers. J. Pharm. Sci. 2015, 104, 4386–4398. [Google Scholar] [CrossRef]
- Senkovich, O.; Cook, W.J.; Mirza, S.; Hollingshead, S.K.; Protasevich, I.I.; Briles, D.E.; Chattopadhyay, D. Structure of a Complex of Human Lactoferrin N-lobe with Pneumococcal Surface Protein A Provides Insight into Microbial Defense Mechanism. J. Mol. Biol. 2007, 370, 701–713. [Google Scholar] [CrossRef]
- Todoroff, J.; Lemaire, M.M.; Fillee, C.; Jurion, F.; Renauld, J.C.; Huygen, K.; Vanbever, R. Mucosal and systemic immune responses to Mycobacterium tuberculosis antigen 85A following its co-delivery with CpG, MPLA or LTB to the lungs in mice. PLoS ONE 2013, 8, e63344. [Google Scholar] [CrossRef]
- Silva, A.L.; Soema, P.C.; Slütter, B.; Ossendorp, F.; Jiskoot, W. PLGA particulate delivery systems for subunit vaccines: Linking particle properties to immunogenicity. Hum. Vaccines Immunother. 2016, 12, 1056–1069. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, R.K.; Vyas, S.P. Chitosan nanoparticles encapsulated vesicular systems for oral immunization: Preparation, in-vitro and in-vivo characterization. J. Pharm. Pharmacol. 2006, 58, 303–310. [Google Scholar] [CrossRef]
- Araujo, S.C.; Pereira, L.R.; Alves, R.P.S.; Andreata-Santos, R.; Kanno, A.I.; Ferreira, L.C.S.; Gonçalves, V.M. Anti-Flavivirus Vaccines: Review of the Present Situation and Perspectives of Subunit Vaccines Produced in Escherichia coli. Vaccines 2020, 8, 492. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, S.; Kelm, S.; Baker, T.S.; Shi, J.; Martin, A.C.R. B-cell epitopes: Discontinuity and conformational analysis. Mol. Immunol. 2019, 114, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Cook, P.C.; MacDonald, A.S. Dendritic cells in lung immunopathology. Semin. Immunopathol. 2016, 38, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Villadangos, J.A.; Cardoso, M.; Steptoe, R.J.; van Berkel, D.; Pooley, J.; Carbone, F.R.; Shortman, K. MHC class II expression is regulated in dendritic cells independently of invariant chain degradation. Immunity 2001, 14, 739–749. [Google Scholar] [CrossRef]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Osman, N.; Kaneko, K.; Carini, V.; Saleem, I. Carriers for the targeted delivery of aerosolized macromolecules for pulmonary pathologies. Expert Opin. Drug Deliv. 2018, 15, 821–834. [Google Scholar] [CrossRef]



| Condition | DCM 1 (mL) | PVA Volume in 1st Aqueous Phase (mL) | PVA Concentration in 1st Aqueous Phase (%) | Sonicator Amplitude Setting (%) | Time of Sonication (s) | PVA Concentration in 2nd Aqueous Phase (%) |
|---|---|---|---|---|---|---|
| 1 | 1.0 | 4.0 | 2.5 | 65 | 60 | 1.0 |
| 2 | 1.0 | 5.0 | 10.0 | 45 | 300 | 1.0 |
| 3 | 1.5 | 3.0 | 2.5 | 45 | 120 | 1.0 |
| 4 | 1.5 | 3.0 | 2.5 | 60 | 60 | 1.0 |
| 5 | 1.5 | 4.0 | 5.0 | 65 | 300 | 0.5 |
| 6 | 1.5 | 4.0 | 5.0 | 65 | 300 | 0.5 |
| 7 | 2.0 | 4.0 | 10.0 | 45 | 120 | 1.0 |
| 8 | 2.0 | 3.0 | 10.0 | 65 | 120 | 0.5 |
| 9 | 2.0 | 5.0 | 10.0 | 65 | 300 | 0.75 |
| 10 | 2.0 | 5.0 | 10.0 | 65 | 120 | 0.75 |
| Polymer | Chitosan | Antigen Location | pH |
|---|---|---|---|
| PGA-co-PDL | no chitosan | no antigen | 4.0 |
| PLGA | chitosan hydrochloride (HCl-CS) | adsorbed | 7.0 |
| carboxymethyl chitosan (CM-CS) | encapsulated |
| Chitosan Hydrochloride (1 mg/mL) | Carboxymethyl Chitosan * | |||||
|---|---|---|---|---|---|---|
| Size (nm) | Charge (mV) | PDI | Size (nm) | Charge (mV) | PDI | |
| PGA-co-PDL | 291.0 ± 9.2 | 17.4 ± 1.0 | 0.09 ± 0.04 | 280.5 ± 8.8 | −20.1 ± 1.5 | 0.13 ± 0.02 |
| PLGA | 310.2 ± 6.0 | 13.2 ± 0.8 | 0.10 ± 0.03 | 299.4 ± 7.1 | −40.5 ± 0.9 | 0.09 ± 0.07 |
| PGA-co-PDL | PLGA | ||||
|---|---|---|---|---|---|
| Formulation | PspA (µg)/NP (mg) | %LE * | PspA (µg)/NP (mg) | %LE * | |
| Adsorption | without chitosan pH 7.0 | 25.57 ± 8.13 | 63.94 ± 20.33 | 8.24 ± 6.28 | 20.60 ± 15.7 |
| without chitosan pH 4.0 | 9.31 ± 4.34 | 23.26 ± 10.85 | 36.28 ± 0.23 | 90.69 ± 0.57 | |
| HCl-CS pH 7.0 | 36.49 ± 1.15 | 91.23 ± 2.87 | 37.59 ± 1.94 | 93.97 ± 4.84 | |
| CM-CS pH 7.0 | 15.41 ± 2.48 | 38.52 ± 6.21 | 10.55 ± 5.10 | 26.40 ± 12.62 | |
| CM-CS pH 4.0 | 11.39 ± 4.13 | 28.49 ± 10.33 | 29.62 ± 7.80 | 74.04 ± 19.48 | |
| Encapsulation | without chitosan pH 7.0 | 4.14 ± 0.22 | 2.07 ± 0.80 | 29.57 ± 4.9 | 14.78 ± 7.36 |
| HCl-CS pH 7.0 | 22.88 ± 2.65 | 11.44 ± 6.62 | 59.33 ± 1.32 | 29.66 ± 4.65 | |
| CM-CS pH 7.0 | 7.86 ± 1.83 | 3.93 ± 6.71 | 11.88 ± 4.15 | 5.94 ± 1.7 | |
| PspA4Pro Dose | Group | Alive/Total | % Survival | p * |
|---|---|---|---|---|
| 2 µg | saline sc | 0/6 | 0 | - |
| PspA4Pro lungs | 0/6 | 0 | - | |
| PspA4Pro sc | 2/6 | 33.3 | 0.23 | |
| Empty PGA-co-PDL/HCl-CS | 0/6 | 0 | - | |
| PGA-co-PDL/HCl-CS/PspA4Pro adsorbed | 4/6 | 66.7 | 0.03 | |
| Empty PLGA/HCl-CS | 0/6 | 0 | - | |
| PLGA/HCl-CS/PspA4Pro encapsulated | 2/6 | 33.3 | 0.23 | |
| 6 µg | saline sc | 0/6 | 0 | - |
| PspA4Pro lungs | 0/6 | 0 | - | |
| PspA4Pro sc | 2/6 | 33.3 | 0.23 | |
| Empty PGA-co-PDL/HCl-CS | 0/6 | 0 | - | |
| PGA-co-PDL/HCl-CS/PspA4Pro adsorbed | 5/6 | 83.3 | 0.007 | |
| Empty PLGA/HCl-CS | 0/6 | 0 | - | |
| PLGA/HCl-CS/PspA4Pro encapsulated | 6/6 | 100.0 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueiredo, D.B.d.; Kaneko, K.; Rodrigues, T.d.C.; MacLoughlin, R.; Miyaji, E.N.; Saleem, I.; Gonçalves, V.M. Pneumococcal Surface Protein A-Hybrid Nanoparticles Protect Mice from Lethal Challenge after Mucosal Immunization Targeting the Lungs. Pharmaceutics 2022, 14, 1238. https://doi.org/10.3390/pharmaceutics14061238
Figueiredo DBd, Kaneko K, Rodrigues TdC, MacLoughlin R, Miyaji EN, Saleem I, Gonçalves VM. Pneumococcal Surface Protein A-Hybrid Nanoparticles Protect Mice from Lethal Challenge after Mucosal Immunization Targeting the Lungs. Pharmaceutics. 2022; 14(6):1238. https://doi.org/10.3390/pharmaceutics14061238
Chicago/Turabian StyleFigueiredo, Douglas Borges de, Kan Kaneko, Tasson da Costa Rodrigues, Ronan MacLoughlin, Eliane Namie Miyaji, Imran Saleem, and Viviane Maimoni Gonçalves. 2022. "Pneumococcal Surface Protein A-Hybrid Nanoparticles Protect Mice from Lethal Challenge after Mucosal Immunization Targeting the Lungs" Pharmaceutics 14, no. 6: 1238. https://doi.org/10.3390/pharmaceutics14061238
APA StyleFigueiredo, D. B. d., Kaneko, K., Rodrigues, T. d. C., MacLoughlin, R., Miyaji, E. N., Saleem, I., & Gonçalves, V. M. (2022). Pneumococcal Surface Protein A-Hybrid Nanoparticles Protect Mice from Lethal Challenge after Mucosal Immunization Targeting the Lungs. Pharmaceutics, 14(6), 1238. https://doi.org/10.3390/pharmaceutics14061238









