The Role of Toll-like Receptor Agonists and Their Nanomedicines for Tumor Immunotherapy
Abstract
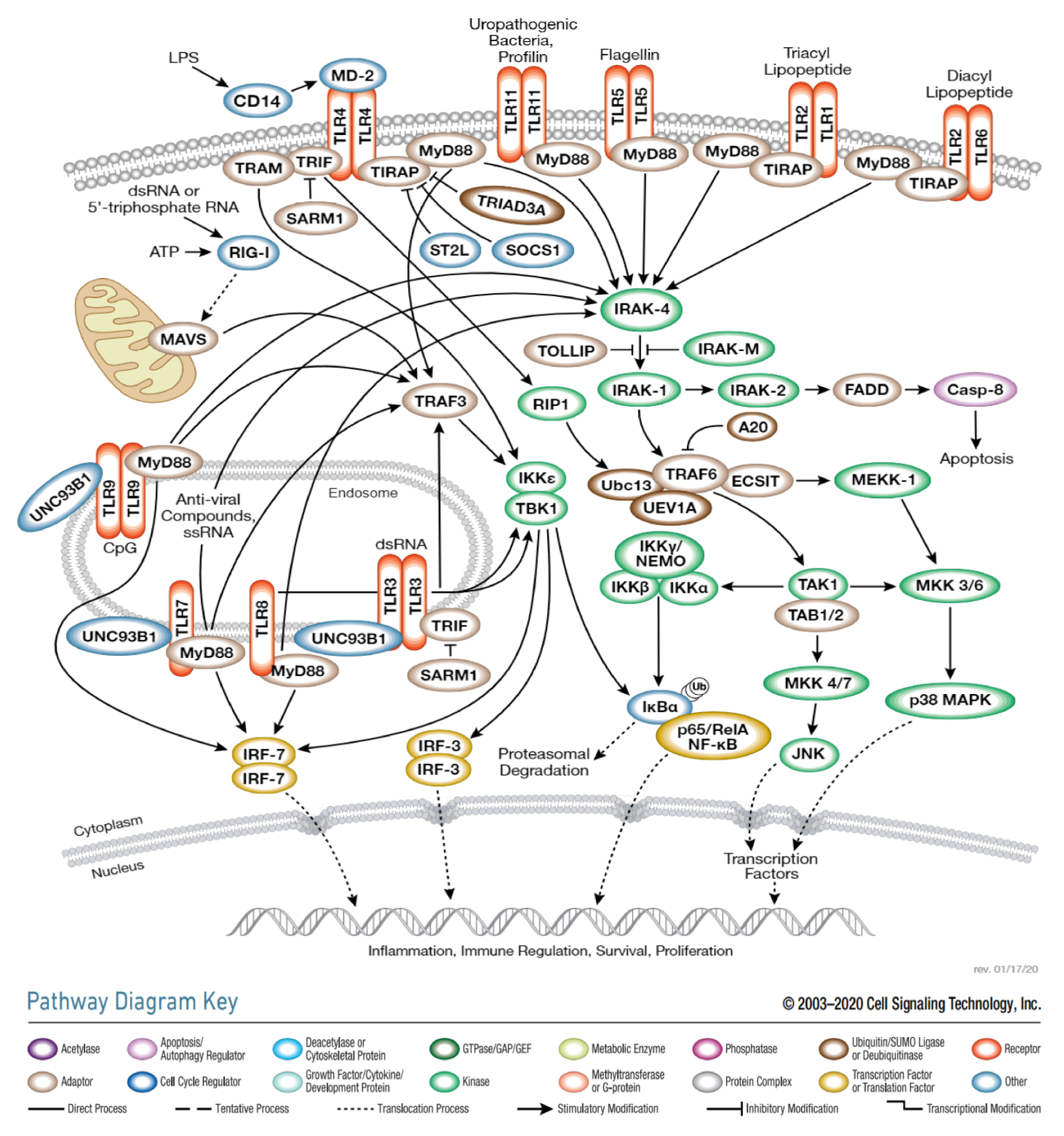
1. Introduction
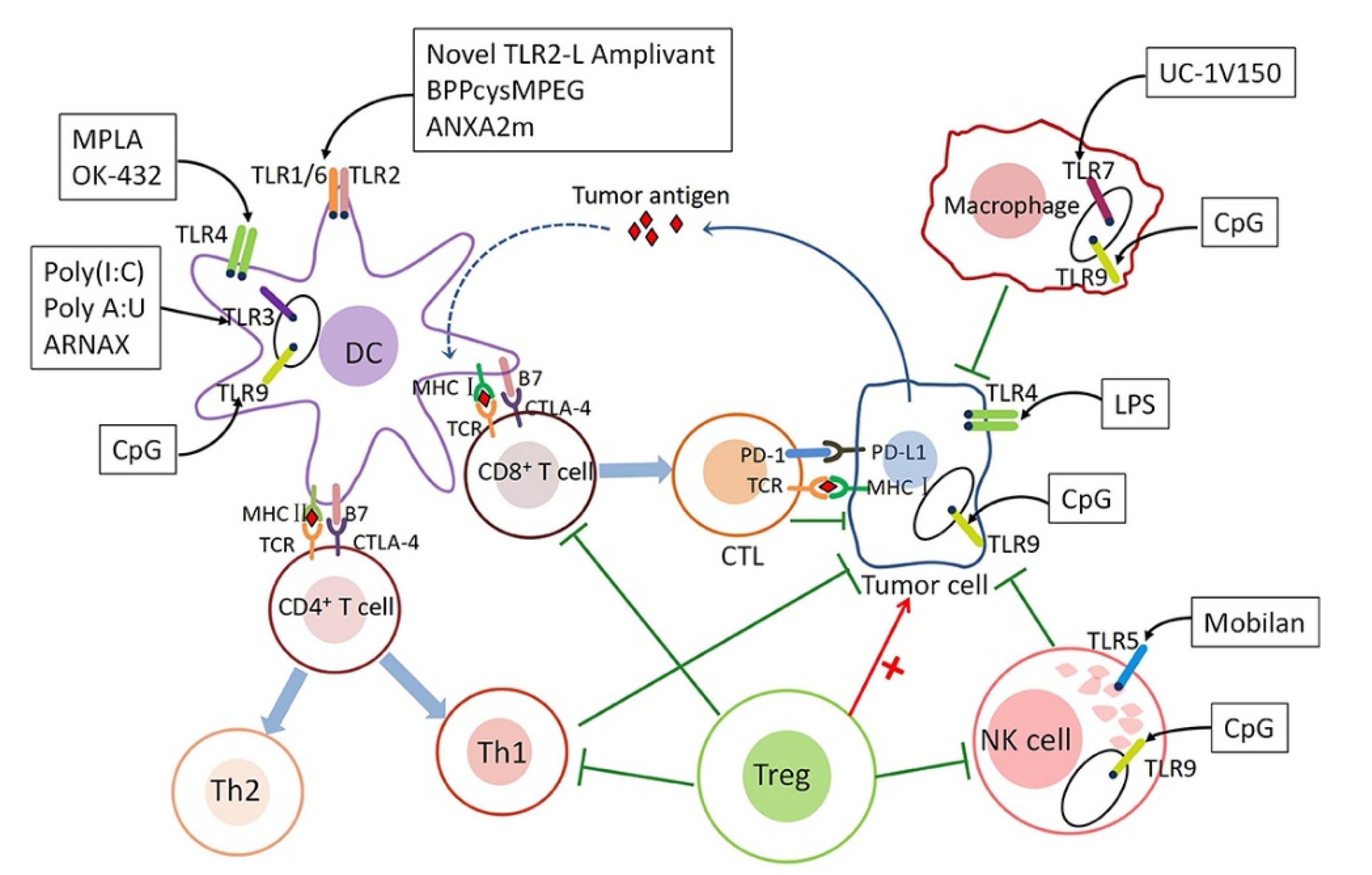
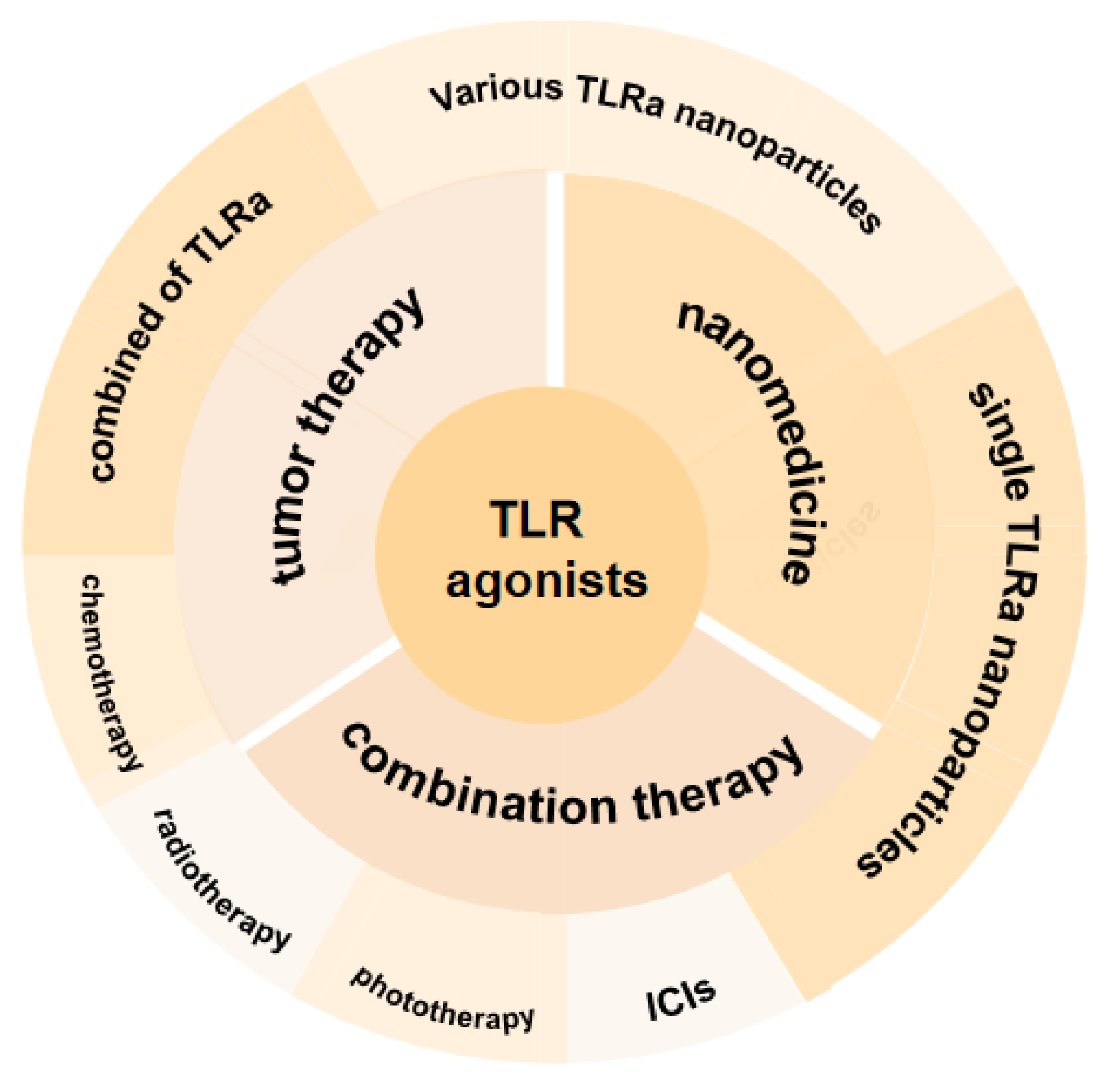
2. TLRa in Tumor Therapy
3. Combination of Multiple TLRa for Tumor Therapy
4. Application of Single TLRa Nanoparticles in Tumor Therapy
4.1. TLR3a
4.2. TLR4a
4.3. TLR7a
4.4. TLR7/8a
4.5. TLR9a
5. Application of Multiple TLRa Nanoparticles in Tumor Therapy
5.1. TLR3a + TLR7a
5.2. TLR3a + TLR9a
5.3. TLR4a + TLR9a
5.4. TLR4a + TLR7/8a
5.5. TLR7/8a + TLR9a
6. TLRa-Based Combination Therapy
6.1. Combination of Immunotherapy and Chemotherapy
6.2. Combination of Immunotherapy and Radiotherapy
6.3. Combination of Immune Adjuvant and Phototherapy
6.4. Combination of Immune Adjuvants with Immune Checkpoint Inhibitors
6.5. Clinical Trials of TLRa Combination Therapy
7. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.; Liu, Y.; Wu, W.; Ling, D.; Zhang, Q.; Zhao, P.; Hu, X. Indoleamine 2,3-dioxygenase (Ido) inhibitors and their nanomedicines for cancer immunotherapy. Biomaterials 2021, 276, 121018. [Google Scholar] [CrossRef] [PubMed]
- Fay, E.K.; Graff, J.N. Immunotherapy in Prostate Cancer. Cancers 2020, 12, 1752. [Google Scholar] [CrossRef] [PubMed]
- Vermaelen, K. Vaccine Strategies to Improve Anti-cancer Cellular Immune Responses. Front. Immunol. 2019, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Hammerich, L.; Binder, A.; Brody, J.D. In situ vaccination: Cancer immunotherapy both personalized and off-the-shelf. Mol. Oncol. 2015, 9, 1966–1981. [Google Scholar] [CrossRef]
- Werling, D.; Jann, O.C.; Offord, V.; Glass, E.J.; Coffey, T.J. Variation matters: TLR structure and species-specific pathogen recognition. Trends Immunol. 2009, 30, 124–130. [Google Scholar] [CrossRef]
- Peng, G.; Guo, Z.; Kiniwa, Y.; Voo, K.S.; Peng, W.; Fu, T.; Wang, D.Y.; Li, Y.; Wang, H.Y.; Wang, R.F. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science 2005, 309, 1380–1384. [Google Scholar] [CrossRef]
- Goulopoulou, S.; McCarthy, C.G.; Webb, R.C. Toll-like Receptors in the Vascular System: Sensing the Dangers Within. Pharmacol. Rev. 2016, 68, 142–167. [Google Scholar] [CrossRef]
- Banstola, A.; Jeong, J.H.; Yook, S. Immunoadjuvants for cancer immunotherapy: A review of recent developments. Acta Biomater. 2020, 114, 16–30. [Google Scholar] [CrossRef]
- Smith, M.; Garcia-Martinez, E.; Pitter, M.R.; Fucikova, J.; Spisek, R.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial Watch: Toll-like receptor agonists in cancer immunotherapy. Oncoimmunology 2018, 7, e1526250. [Google Scholar] [CrossRef]
- Shetab Boushehri, M.A.; Lamprecht, A. TLR4-Based Immunotherapeutics in Cancer: A Review of the Achievements and Shortcomings. Mol. Pharm. 2018, 15, 4777–4800. [Google Scholar] [CrossRef]
- Brodsky, I.; Medzhitov, R. Two modes of ligand recognition by TLRs. Cell 2007, 130, 979–981. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Choi, Y.; Kim, K. Engineering Therapeutic Strategies in Cancer Immunotherapy via Exogenous Delivery of Toll-like Receptor Agonists. Pharmaceutics 2021, 13, 1374. [Google Scholar] [CrossRef] [PubMed]
- Ignacio, B.J.; Albin, T.J.; Esser-Kahn, A.P.; Verdoes, M. Toll-like Receptor Agonist Conjugation: A Chemical Perspective. Bioconjug. Chem. 2018, 29, 587–603. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Li, Y.; Zhao, Q.; Chen, Y.; Fu, S.; Wu, J. Coordinated regulation of immune contexture: Crosstalk between STAT3 and immune cells during breast cancer progression. Cell Commun. Signal. 2021, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- Kaczanowska, S.; Joseph, A.M.; Davila, E. TLR agonists: Our best frenemy in cancer immunotherapy. J. Leukoc. Biol. 2013, 93, 847–863. [Google Scholar] [CrossRef] [PubMed]
- Muller, E.; Christopoulos, P.F.; Halder, S.; Lunde, A.; Beraki, K.; Speth, M.; Oynebraten, I.; Corthay, A. Toll-like Receptor Ligands and Interferon-gamma Synergize for Induction of Antitumor M1 Macrophages. Front. Immunol. 2017, 8, 1383. [Google Scholar] [CrossRef] [PubMed]
- Muller, E.; Speth, M.; Christopoulos, P.F.; Lunde, A.; Avdagic, A.; Oynebraten, I.; Corthay, A. Both Type I and Type II Interferons Can Activate Antitumor M1 Macrophages When Combined with TLR Stimulation. Front. Immunol. 2018, 9, 2520. [Google Scholar] [CrossRef]
- Ohadian Moghadam, S.; Nowroozi, M.R. Toll-like receptors: The role in bladder cancer development, progression and immunotherapy. Scand. J. Immunol. 2019, 90, e12818. [Google Scholar] [CrossRef]
- Chasov, V.; Zaripov, M.; Mirgayazova, R.; Khadiullina, R.; Zmievskaya, E.; Ganeeva, I.; Valiullina, A.; Rizvanov, A.; Bulatov, E. Promising New Tools for Targeting p53 Mutant Cancers: Humoral and Cell-Based Immunotherapies. Front. Immunol. 2021, 12, 707734. [Google Scholar] [CrossRef]
- Liu, R.; Luo, F.; Liu, X.; Wang, L.; Yang, J.; Deng, Y.; Huang, E.; Qian, J.; Lu, Z.; Jiang, X.; et al. Biological Response Modifier in Cancer Immunotherapy. Adv. Exp. Med. Biol. 2016, 909, 69–138. [Google Scholar] [CrossRef]
- Cui, L.; Wang, X.; Zhang, D. TLRs as a Promise Target Along with Immune Checkpoint Against Gastric Cancer. Front. Cell Dev. Biol. 2020, 8, 611444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Hu, Y.; Pang, Z. Modulating the Tumor Microenvironment to Enhance Tumor Nanomedicine Delivery. Front. Pharmacol. 2017, 8, 952. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Gao, J.; Pei, Q.; Xu, H.; Yu, H. Engineering Prodrug Nanomedicine for Cancer Immunotherapy. Adv. Sci. 2020, 7, 2002365. [Google Scholar] [CrossRef] [PubMed]
- Lynn, G.M.; Chytil, P.; Francica, J.R.; Lagova, A.; Kueberuwa, G.; Ishizuka, A.S.; Zaidi, N.; Ramirez-Valdez, R.A.; Blobel, N.J.; Baharom, F.; et al. Impact of Polymer-TLR-7/8 Agonist (Adjuvant) Morphology on the Potency and Mechanism of CD8 T Cell Induction. Biomacromolecules 2019, 20, 854–870. [Google Scholar] [CrossRef]
- Braunstein, M.J.; Kucharczyk, J.; Adams, S. Targeting Toll-Like Receptors for Cancer Therapy. Target. Oncol. 2018, 13, 583–598. [Google Scholar] [CrossRef]
- Lu, Y. Toll-like Receptor Agonists in Cancer Therapy. Nov. Approaches Cancer Study 2020, 4, 402–409. [Google Scholar] [CrossRef]
- Bianchi, F.; Pretto, S.; Tagliabue, E.; Balsari, A.; Sfondrini, L. Exploiting poly(I:C) to induce cancer cell apoptosis. Cancer Biol. Ther. 2017, 18, 747–756. [Google Scholar] [CrossRef]
- Du, B.; Jiang, Q.-L.; Cleveland, J.; Liu, B.-R.; Zhang, D. Targeting Toll-like receptors against cancer. J. Cancer Metastasis Treat. 2016, 2, 463. [Google Scholar] [CrossRef]
- Holl, T.M.; Kelsoe, G. Outside influence: TLRs direct hematopoietic cell fates. Immunity 2006, 24, 667–669. [Google Scholar] [CrossRef][Green Version]
- Walshaw, R.C.; Honeychurch, J.; Choudhury, A.; Illidge, T.M. Toll-like Receptor Agonists and Radiation Therapy Combinations: An Untapped Opportunity to Induce Anticancer Immunity and Improve Tumor control. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 27–37. [Google Scholar] [CrossRef]
- Federico, S.; Pozzetti, L.; Papa, A.; Carullo, G.; Gemma, S.; Butini, S.; Campiani, G.; Relitti, N. Modulation of the Innate Immune Response by Targeting Toll-like Receptors: A Perspective on Their Agonists and Antagonists. J. Med. Chem. 2020, 63, 13466–13513. [Google Scholar] [CrossRef] [PubMed]
- Lemdani, K.; Seguin, J.; Lesieur, C.; Al Sabbagh, C.; Doan, B.T.; Richard, C.; Capron, C.; Malafosse, R.; Boudy, V.; Mignet, N. Mucoadhesive thermosensitive hydrogel for the intra-tumoral delivery of immunomodulatory agents, in vivo evidence of adhesion by means of non-invasive imaging techniques. Int. J. Pharm. 2019, 567, 118421. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Mu, R.; Wang, Z.; Xing, P.; Zhang, J.; Dong, L.; Wang, C. A toll-like receptor agonist mimicking microbial signal to generate tumor-suppressive macrophages. Nat. Commun. 2019, 10, 2272. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Feng, R.; Wang, Y.Z.; Sun, H.W.; Zou, Q.M.; Li, H.B. Toll-like receptors: Triggers of regulated cell death and promising targets for cancer therapy. Immunol. Lett. 2020, 223, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Camargo, J.A.; Passos, G.R.; Ferrari, K.L.; Billis, A.; Saad, M.J.A.; Reis, L.O. Intravesical Immunomodulatory Imiquimod Enhances Bacillus Calmette-Guerin Downregulation of Nonmuscle-invasive Bladder Cancer. Clin. Genitourin. Cancer 2018, 16, e587–e593. [Google Scholar] [CrossRef]
- Ayari, C.; Besancon, M.; Bergeron, A.; LaRue, H.; Bussieres, V.; Fradet, Y. Poly(I:C) potentiates Bacillus Calmette-Guerin immunotherapy for bladder cancer. Cancer Immunol. Immunother. 2016, 65, 223–234. [Google Scholar] [CrossRef]
- Cho, S.; Kim, S.B.; Lee, Y.; Song, E.C.; Kim, U.; Kim, H.Y.; Suh, J.H.; Goughnour, P.C.; Kim, Y.; Yoon, I.; et al. Endogenous TLR2 ligand embedded in the catalytic region of human cysteinyl-tRNA synthetase 1. J. Immunother. Cancer 2020, 8, e000277. [Google Scholar] [CrossRef]
- Aznar, M.A.; Planelles, L.; Perez-Olivares, M.; Molina, C.; Garasa, S.; Etxeberria, I.; Perez, G.; Rodriguez, I.; Bolanos, E.; Lopez-Casas, P.; et al. Immunotherapeutic effects of intratumoral nanoplexed poly I:C. J. Immunother. Cancer 2019, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Pressnall, M.M.; Huayamares, S.G.; Berkland, C.J. Immunostimulant Complexed with Polylysine Limits Transport and Maintains Immune Cell Activation. J. Pharm. Sci. 2020, 109, 2836–2846. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, A.N.; Contractor, S.; Castaneda, I.; Cathcart, C.S.; Razdan, D.; Klyde, D.; Kisza, P.; Gonzales, S.F.; Salazar, A.M. A Phase I trial using local regional treatment, nonlethal irradiation, intratumoral and systemic polyinosinic-polycytidylic acid polylysine carboxymethylcellulose to treat liver cancer: In search of the abscopal effect. J. Hepatocell. Carcinoma 2017, 4, 111–121. [Google Scholar] [CrossRef]
- Pavlick, A.; Blazquez, A.B.; Meseck, M.; Lattanzi, M.; Ott, P.A.; Marron, T.U.; Holman, R.M.; Mandeli, J.; Salazar, A.M.; McClain, C.B.; et al. Combined Vaccination with NY-ESO-1 Protein, Poly-ICLC, and Montanide Improves Humoral and Cellular Immune Responses in Patients with High-Risk Melanoma. Cancer Immunol. Res. 2020, 8, 70–80. [Google Scholar] [CrossRef]
- Schulke, S.; Flaczyk, A.; Vogel, L.; Gaudenzio, N.; Angers, I.; Loschner, B.; Wolfheimer, S.; Spreitzer, I.; Qureshi, S.; Tsai, M.; et al. MPLA shows attenuated pro-inflammatory properties and diminished capacity to activate mast cells in comparison with LPS. Allergy 2015, 70, 1259–1268. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, S.; Qin, Y.; Fan, F.; Zhang, Z.; Huang, C.; Ji, W.; Lu, L.; Wang, C.; Sun, H.; et al. Targeted Codelivery of an Antigen and Dual Agonists by Hybrid Nanoparticles for Enhanced Cancer Immunotherapy. Nano Lett. 2019, 19, 4237–4249. [Google Scholar] [CrossRef]
- Matzner, P.; Sorski, L.; Shaashua, L.; Elbaz, E.; Lavon, H.; Melamed, R.; Rosenne, E.; Gotlieb, N.; Benbenishty, A.; Reed, S.G.; et al. Perioperative treatment with the new synthetic TLR-4 agonist GLA-SE reduces cancer metastasis without adverse effects. Int. J. Cancer 2016, 138, 1754–1764. [Google Scholar] [CrossRef]
- Reintjens, N.R.M.; Tondini, E.; de Jong, A.R.; Meeuwenoord, N.J.; Chiodo, F.; Peterse, E.; Overkleeft, H.S.; Filippov, D.V.; van der Marel, G.A.; Ossendorp, F.; et al. Self-Adjuvanting Cancer Vaccines from Conjugation-Ready Lipid A Analogues and Synthetic Long Peptides. J. Med. Chem. 2020, 63, 11691–11706. [Google Scholar] [CrossRef]
- Sciullo, P.D.; Menay, F.; Cocozza, F.; Gravisaco, M.J.; Waldner, C.I.; Mongini, C. Systemic administration of imiquimod as an adjuvant improves immunogenicity of a tumor-lysate vaccine inducing the rejection of a highly aggressive T-cell lymphoma. Clin. Immunol. 2019, 203, 154–161. [Google Scholar] [CrossRef]
- Salazar, L.G.; Lu, H.; Reichow, J.L.; Childs, J.S.; Coveler, A.L.; Higgins, D.M.; Waisman, J.; Allison, K.H.; Dang, Y.; Disis, M.L. Topical Imiquimod Plus Nab-paclitaxel for Breast Cancer Cutaneous Metastases: A Phase 2 Clinical Trial. JAMA Oncol. 2017, 3, 969–973. [Google Scholar] [CrossRef]
- Oyama, S.; Funasaka, Y.; Tsuchiya, S.I.; Kawana, S.; Saeki, H. Increased number of mast cells in the dermis in actinic keratosis lesions effectively treated with imiquimod. J. Dermatol. 2017, 44, 944–949. [Google Scholar] [CrossRef]
- Villamon, E.; Gonzalez-Fernandez, J.; Such, E.; Cervera, J.V.; Gozalbo, D.; Luisa Gil, M. Imiquimod inhibits growth and induces differentiation of myeloid leukemia cell lines. Cancer Cell Int. 2018, 18, 15. [Google Scholar] [CrossRef]
- Mullins, S.R.; Vasilakos, J.P.; Deschler, K.; Grigsby, I.; Gillis, P.; John, J.; Elder, M.J.; Swales, J.; Timosenko, E.; Cooper, Z.; et al. Intratumoral immunotherapy with TLR7/8 agonist MEDI9197 modulates the tumor microenvironment leading to enhanced activity when combined with other immunotherapies. J. Immunother. Cancer 2019, 7, 244. [Google Scholar] [CrossRef]
- Fakhari, A.; Nugent, S.; Elvecrog, J.; Vasilakos, J.; Corcoran, M.; Tilahun, A.; Siebenaler, K.; Sun, J.; Subramony, J.A.; Schwarz, A. Thermosensitive Gel-Based Formulation for Intratumoral Delivery of Toll-Like Receptor 7/8 Dual Agonist, MEDI9197. J. Pharm. Sci. 2017, 106, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Komura, F.; Okuzumi, K.; Takahashi, Y.; Takakura, Y.; Nishikawa, M. Development of RNA/DNA Hydrogel Targeting Toll-Like Receptor 7/8 for Sustained RNA Release and Potent Immune Activation. Molecules 2020, 25, 728. [Google Scholar] [CrossRef] [PubMed]
- Beesu, M.; Caruso, G.; Salyer, A.C.; Shukla, N.M.; Khetani, K.K.; Smith, L.J.; Fox, L.M.; Tanji, H.; Ohto, U.; Shimizu, T.; et al. Identification of a Human Toll-Like Receptor (TLR) 8-Specific Agonist and a Functional Pan-TLR Inhibitor in 2-Aminoimidazoles. J. Med. Chem. 2016, 59, 3311–3330. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.Q.M.; Morishima, C.; Eaton, K.D.; Baik, C.S.; Goulart, B.H.; Anderson, L.N.; Manjarrez, K.L.; Dietsch, G.N.; Bryan, J.K.; Hershberg, R.M.; et al. Phase Ib Trial of the Toll-like Receptor 8 Agonist, Motolimod (VTX-2337), Combined with Cetuximab in Patients with Recurrent or Metastatic SCCHN. Clin. Cancer Res. 2017, 23, 2442–2450. [Google Scholar] [CrossRef] [PubMed]
- Beesu, M.; Salyer, A.C.; Trautman, K.L.; Hill, J.K.; David, S.A. Human Toll-like Receptor (TLR) 8-Specific Agonistic Activity in Substituted Pyrimidine-2,4-diamines. J. Med. Chem. 2016, 59, 8082–8093. [Google Scholar] [CrossRef]
- Zhang, L.; Dewan, V.; Yin, H. Discovery of Small Molecules as Multi-Toll-like Receptor Agonists with Proinflammatory and Anticancer Activities. J. Med. Chem. 2017, 60, 5029–5044. [Google Scholar] [CrossRef]
- Behm, B.; Di Fazio, P.; Michl, P.; Neureiter, D.; Kemmerling, R.; Hahn, E.G.; Strobel, D.; Gress, T.; Schuppan, D.; Wissniowski, T.T. Additive antitumour response to the rabbit VX2 hepatoma by combined radio frequency ablation and toll like receptor 9 stimulation. Gut 2016, 65, 134–143. [Google Scholar] [CrossRef]
- Jo, S.; Fotovati, A.; Duque-Afonso, J.; Cleary, M.L.; van den Elzen, P.; Seif, A.E.; Reid, G.S.D. Differential Depletion of Bone Marrow Resident B-ALL after Systemic Administration of Endosomal TLR Agonists. Cancers 2020, 12, 169. [Google Scholar] [CrossRef]
- Ronsley, R.; Kariminia, A.; Ng, B.; Mostafavi, S.; Reid, G.; Subrt, P.; Hijiya, N.; Schultz, K.R. The TLR9 agonist (GNKG168) induces a unique immune activation pattern in vivo in children with minimal residual disease positive acute leukemia: Results of the TACL T2009-008 phase I study. Pediatr. Hematol. Oncol. 2019, 36, 468–481. [Google Scholar] [CrossRef]
- Melssen, M.M.; Pollack, K.E.; Meneveau, M.O.; Smolkin, M.E.; Pinczewski, J.; Koeppel, A.F.; Turner, S.D.; Sol-Church, K.; Hickman, A.; Deacon, D.H.; et al. Characterization and comparison of innate and adaptive immune responses at vaccine sites in melanoma vaccine clinical trials. Cancer Immunol. Immunother. 2021, 70, 2151–2164. [Google Scholar] [CrossRef]
- Moreno Ayala, M.A.; Gottardo, M.F.; Gori, M.S.; Nicola Candia, A.J.; Caruso, C.; De Laurentiis, A.; Imsen, M.; Klein, S.; Bal de Kier Joffe, E.; Salamone, G.; et al. Dual activation of Toll-like receptors 7 and 9 impairs the efficacy of antitumor vaccines in murine models of metastatic breast cancer. J. Cancer Res. Clin. Oncol. 2017, 143, 1713–1732. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.; Maiti, S.; Shen, J.; Du, W.; Esser-Kahn, A.P. Pathogen-like Nanoassemblies of Covalently Linked TLR Agonists Enhance CD8 and NK Cell-Mediated Antitumor Immunity. ACS Cent. Sci. 2020, 6, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Oosterhoff, D.; Heusinkveld, M.; Lougheed, S.M.; Kosten, I.; Lindstedt, M.; Bruijns, S.C.; van Es, T.; van Kooyk, Y.; van der Burg, S.H.; de Gruijl, T.D. Intradermal delivery of TLR agonists in a human explant skin model: Preferential activation of migratory dendritic cells by polyribosinic-polyribocytidylic acid and peptidoglycans. J. Immunol. 2013, 190, 3338–3345. [Google Scholar] [CrossRef] [PubMed]
- Bogunovic, D.; Manches, O.; Godefroy, E.; Yewdall, A.; Gallois, A.; Salazar, A.M.; Marie, I.; Levy, D.E.; Bhardwaj, N. TLR4 engagement during TLR3-induced proinflammatory signaling in dendritic cells promotes IL-10-mediated suppression of antitumor immunity. Cancer Res. 2011, 71, 5467–5476. [Google Scholar] [CrossRef]
- Nouri-Shirazi, M.; Tamjidi, S.; Nourishirazi, E.; Guinet, E. TLR8 combined withTLR3 or TLR4 agonists enhances DC-NK driven effector Tc1 cells. Immunol. Lett. 2018, 193, 58–66. [Google Scholar] [CrossRef]
- Pearson, F.E.; Chang, K.; Minoda, Y.; Rojas, I.M.L.; Haigh, O.L.; Daraj, G.; Tullett, K.M.; Radford, K.J. Activation of human CD141(+) and CD1c(+) dendritic cells in vivo with combined TLR3 and TLR7/8 ligation. Immunol. Cell Biol. 2018, 96, 390–400. [Google Scholar] [CrossRef]
- Sajadian, A.; Tabarraei, A.; Soleimanjahi, H.; Fotouhi, F.; Gorji, A.; Ghaemi, A. Comparing the effect of Toll-like receptor agonist adjuvants on the efficiency of a DNA vaccine. Arch. Virol. 2014, 159, 1951–1960. [Google Scholar] [CrossRef]
- Gierlich, P.; Lex, V.; Technau, A.; Keupp, A.; Morper, L.; Glunz, A.; Sennholz, H.; Rachor, J.; Sauer, S.; Marcu, A.; et al. Prostaglandin E2 in a TLR3- and 7/8-agonist-based DC maturation cocktail generates mature, cytokine-producing, migratory DCs but impairs antigen cross-presentation to CD8(+) T cells. Cancer Immunol. Immunother. 2020, 69, 1029–1042. [Google Scholar] [CrossRef]
- Caisova, V.; Uher, O.; Nedbalova, P.; Jochmanova, I.; Kvardova, K.; Masakova, K.; Krejcova, G.; Padoukova, L.; Chmelar, J.; Kopecky, J.; et al. Effective cancer immunotherapy based on combination of TLR agonists with stimulation of phagocytosis. Int. Immunopharmacol. 2018, 59, 86–96. [Google Scholar] [CrossRef]
- Julier, Z.; de Titta, A.; Grimm, A.J.; Simeoni, E.; Swartz, M.A.; Hubbell, J.A. Fibronectin EDA and CpG synergize to enhance antigen-specific Th1 and cytotoxic responses. Vaccine 2016, 34, 2453–2459. [Google Scholar] [CrossRef]
- Zhao, B.G.; Vasilakos, J.P.; Tross, D.; Smirnov, D.; Klinman, D.M. Combination therapy targeting toll like receptors 7, 8 and 9 eliminates large established tumors. J. Immunother. Cancer 2014, 2, 12. [Google Scholar] [CrossRef]
- Amos, S.M.; Pegram, H.J.; Westwood, J.A.; John, L.B.; Devaud, C.; Clarke, C.J.; Restifo, N.P.; Smyth, M.J.; Darcy, P.K.; Kershaw, M.H. Adoptive immunotherapy combined with intratumoral TLR agonist delivery eradicates established melanoma in mice. Cancer Immunol. Immunother. 2011, 60, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, A.; Sui, Q.; Zhang, C.; Tian, Z.; Zhang, J. Phosphorothioate modification of the TLR9 ligand CpG ODN inhibits poly(I:C)-induced apoptosis of hepatocellular carcinoma by entry blockade. Cancer Lett. 2014, 355, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Stier, S.; Maletzki, C.; Klier, U.; Linnebacher, M. Combinations of TLR ligands: A promising approach in cancer immunotherapy. Clin. Dev. Immunol. 2013, 2013, 271246. [Google Scholar] [CrossRef] [PubMed]
- Albin, T.J.; Tom, J.K.; Manna, S.; Gilkes, A.P.; Stetkevich, S.A.; Katz, B.B.; Supnet, M.; Felgner, J.; Jain, A.; Nakajima, R.; et al. Linked Toll-Like Receptor Triagonists Stimulate Distinct, Combination-Dependent Innate Immune Responses. ACS Cent. Sci. 2019, 5, 1137–1145. [Google Scholar] [CrossRef]
- Nuhn, L.; De Koker, S.; Van Lint, S.; Zhong, Z.; Catani, J.P.; Combes, F.; Deswarte, K.; Li, Y.; Lambrecht, B.N.; Lienenklaus, S.; et al. Nanoparticle-Conjugate TLR7/8 Agonist Localized Immunotherapy Provokes Safe Antitumoral Responses. Adv. Mater. 2018, 30, e1803397. [Google Scholar] [CrossRef]
- Lee, S.N.; Jin, S.M.; Shin, H.S.; Lim, Y.T. Chemical Strategies to Enhance the Therapeutic Efficacy of Toll-like Receptor Agonist Based Cancer Immunotherapy. Acc. Chem. Res. 2020, 53, 2081–2093. [Google Scholar] [CrossRef]
- Shetab Boushehri, M.A.; Abdel-Mottaleb, M.M.A.; Beduneau, A.; Pellequer, Y.; Lamprecht, A. A nanoparticle-based approach to improve the outcome of cancer active immunotherapy with lipopolysaccharides. Drug Deliv. 2018, 25, 1414–1425. [Google Scholar] [CrossRef]
- Kim, H.; Sehgal, D.; Kucaba, T.A.; Ferguson, D.M.; Griffith, T.S.; Panyam, J. Acidic pH-responsive polymer nanoparticles as a TLR7/8 agonist delivery platform for cancer immunotherapy. Nanoscale 2018, 10, 20851–20862. [Google Scholar] [CrossRef]
- Kim, H.; Niu, L.; Larson, P.; Kucaba, T.A.; Murphy, K.A.; James, B.R.; Ferguson, D.M.; Griffith, T.S.; Panyam, J. Polymeric nanoparticles encapsulating novel TLR7/8 agonists as immunostimulatory adjuvants for enhanced cancer immunotherapy. Biomaterials 2018, 164, 38–53. [Google Scholar] [CrossRef]
- Seth, A.; Lee, H.; Cho, M.Y.; Park, C.; Korm, S.; Lee, J.Y.; Choi, I.; Lim, Y.T.; Hong, K.S. Combining vasculature disrupting agent and Toll-like receptor 7/8 agonist for cancer therapy. Oncotarget 2017, 8, 5371–5381. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klauber, T.C.B.; Laursen, J.M.; Zucker, D.; Brix, S.; Jensen, S.S.; Andresen, T.L. Delivery of TLR7 agonist to monocytes and dendritic cells by DCIR targeted liposomes induces robust production of anti-cancer cytokines. Acta Biomater. 2017, 53, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Luo, T.; Culbert, A.; Kaufmann, M.; Jiang, X.; Lin, W. Nanoscale Metal-Organic Framework Co-delivers TLR-7 Agonists and Anti-CD47 Antibodies to Modulate Macrophages and Orchestrate Cancer Immunotherapy. J. Am. Chem. Soc. 2020, 142, 12579–12584. [Google Scholar] [CrossRef] [PubMed]
- Traini, G.; Ruiz-de-Angulo, A.; Blanco-Canosa, J.B.; Zamacola Bascaran, K.; Molinaro, A.; Silipo, A.; Escors, D.; Mareque-Rivas, J.C. Cancer Immunotherapy of TLR4 Agonist-Antigen Constructs Enhanced with Pathogen-Mimicking Magnetite Nanoparticles and Checkpoint Blockade of PD-L1. Small 2019, 15, e1803993. [Google Scholar] [CrossRef]
- Shen, N.; Wu, J.; Yang, C.; Yu, H.; Yang, S.; Li, T.; Chen, J.; Tang, Z.; Chen, X. Combretastatin A4 Nanoparticles Combined with Hypoxia-Sensitive Imiquimod: A New Paradigm for the Modulation of Host Immunological Responses during Cancer Treatment. Nano Lett. 2019, 19, 8021–8031. [Google Scholar] [CrossRef]
- Xu, J.; Xu, L.; Wang, C.; Yang, R.; Zhuang, Q.; Han, X.; Dong, Z.; Zhu, W.; Peng, R.; Liu, Z. Near-Infrared-Triggered Photodynamic Therapy with Multitasking Upconversion Nanoparticles in Combination with Checkpoint Blockade for Immunotherapy of Colorectal Cancer. ACS Nano 2017, 11, 4463–4474. [Google Scholar] [CrossRef]
- Kim, H.; Khanna, V.; Kucaba, T.A.; Zhang, W.; Ferguson, D.M.; Griffith, T.S.; Panyam, J. Combination of Sunitinib and PD-L1 Blockade Enhances Anticancer Efficacy of TLR7/8 Agonist-Based Nanovaccine. Mol. Pharm. 2019, 16, 1200–1210. [Google Scholar] [CrossRef]
- Huang, Z.; Gan, J.; Long, Z.; Guo, G.; Shi, X.; Wang, C.; Zang, Y.; Ding, Z.; Chen, J.; Zhang, J.; et al. Targeted delivery of let-7b to reprogramme tumor-associated macrophages and tumor infiltrating dendritic cells for tumor rejection. Biomaterials 2016, 90, 72–84. [Google Scholar] [CrossRef]
- Vinod, N.; Hwang, D.; Azam, S.H.; Van Swearingen, A.E.D.; Wayne, E.; Fussell, S.C.; Sokolsky-Papkov, M.; Pecot, C.V.; Kabanov, A.V. High-capacity poly(2-oxazoline) formulation of TLR 7/8 agonist extends survival in a chemo-insensitive, metastatic model of lung adenocarcinoma. Sci. Adv. 2020, 6, eaba5542. [Google Scholar] [CrossRef]
- Bahmani, B.; Gong, H.; Luk, B.T.; Haushalter, K.J.; DeTeresa, E.; Previti, M.; Zhou, J.; Gao, W.; Bui, J.D.; Zhang, L.; et al. Intratumoral immunotherapy using platelet-cloaked nanoparticles enhances antitumor immunity in solid tumors. Nat. Commun. 2021, 12, 1999. [Google Scholar] [CrossRef]
- Rodell, C.B.; Arlauckas, S.P.; Cuccarese, M.F.; Garris, C.S.; Li, R.; Ahmed, M.S.; Kohler, R.H.; Pittet, M.J.; Weissleder, R. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat. Biomed. Eng. 2018, 2, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Rodell, C.B.; Ahmed, M.S.; Garris, C.S.; Pittet, M.J.; Weissleder, R. Development of Adamantane-Conjugated TLR7/8 Agonists for Supramolecular Delivery and Cancer Immunotherapy. Theranostics 2019, 9, 8426–8436. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.M.; Pan, W.Y.; Wu, C.Y.; Yeh, C.Y.; Korupalli, C.; Luo, P.K.; Chou, C.J.; Chia, W.T.; Sung, H.W. Modulation of tumor microenvironment using a TLR-7/8 agonist-loaded nanoparticle system that exerts low-temperature hyperthermia and immunotherapy for in situ cancer vaccination. Biomaterials 2020, 230, 119629. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Groer, C.; Kleindl, P.A.; Moulder, K.R.; Huang, A.; Hunt, J.R.; Cai, S.; Aires, D.J.; Berkland, C.; Forrest, M.L. Formulation and preclinical evaluation of a toll-like receptor 7/8 agonist as an anti-tumoral immunomodulator. J. Control. Release 2019, 306, 165–176. [Google Scholar] [CrossRef]
- Ilyinskii, P.O.; Kovalev, G.I.; O‘Neil, C.P.; Roy, C.J.; Michaud, A.M.; Drefs, N.M.; Pechenkin, M.A.; Fu, F.N.; Johnston, L.P.M.; Ovchinnikov, D.A.; et al. Synthetic vaccine particles for durable cytolytic T lymphocyte responses and anti-tumor immunotherapy. PLoS ONE 2018, 13, e0197694. [Google Scholar] [CrossRef]
- Shan, H.; Dou, W.; Zhang, Y.; Qi, M. Targeted ferritin nanoparticle encapsulating CpG oligodeoxynucleotides induces tumor-associated macrophage M2 phenotype polarization into M1 phenotype and inhibits tumor growth. Nanoscale 2020, 12, 22268–22280. [Google Scholar] [CrossRef]
- Muraoka, D.; Seo, N.; Hayashi, T.; Tahara, Y.; Fujii, K.; Tawara, I.; Miyahara, Y.; Okamori, K.; Yagita, H.; Imoto, S.; et al. Antigen delivery targeted to tumor-associated macrophages overcomes tumor immune resistance. J. Clin. Investig. 2019, 129, 1278–1294. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, S.; Si, X.; Zhao, J.; Yu, H.; Ma, L.; Song, W.; Tang, Z. Polyethyleneimine-CpG Nanocomplex as an In Situ Vaccine for Boosting Anticancer Immunity in Melanoma. Macromol. Biosci. 2021, 21, e2000207. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, H.; Jiang, H.; Yu, J.; Wang, Y.; Ke, H.; Gong, T.; Zhang, Z.; Sun, X. Tailoring polymeric hybrid micelles with lymph node targeting ability to improve the potency of cancer vaccines. Biomaterials 2017, 122, 105–113. [Google Scholar] [CrossRef]
- Caisova, V.; Li, L.; Gupta, G.; Jochmanova, I.; Jha, A.; Uher, O.; Huynh, T.T.; Miettinen, M.; Pang, Y.; Abunimer, L.; et al. The Significant Reduction or Complete Eradication of Subcutaneous and Metastatic Lesions in a Pheochromocytoma Mouse Model after Immunotherapy Using Mannan-BAM, TLR Ligands, and Anti-CD40. Cancers 2019, 11, 654. [Google Scholar] [CrossRef]
- Vindevogel, E.; Baert, T.; Van Hoylandt, A.; Verbist, G.; Vande Velde, G.; Garg, A.D.; Agostinis, P.; Vergote, I.; Coosemans, A.N. The Use of Toll-like Receptor 4 Agonist to Reshape the Immune Signature in Ovarian Cancer. Anticancer Res. 2016, 36, 5781–5792. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Tran, T.T.P.; Truong, D.H.; Nguyen, H.T.; Pham, T.T.; Yong, C.S.; Kim, J.O. Toll-like receptor-targeted particles: A paradigm to manipulate the tumor microenvironment for cancer immunotherapy. Acta Biomater. 2019, 94, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Li, C.; Zhao, F.S.; Zhang, L.; Ng, T.B.; Jin, G.; Sha, O. Anti-tumor Activity of Toll-Like Receptor 7 Agonists. Front. Pharmacol. 2017, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Khanna, V.; Kucaba, T.A.; Zhang, W.; Sehgal, D.; Ferguson, D.M.; Griffith, T.S.; Panyam, J. TLR7/8 Agonist-Loaded Nanoparticles Augment NK Cell-Mediated Antibody-Based Cancer Immunotherapy. Mol. Pharm. 2020, 17, 2109–2124. [Google Scholar] [CrossRef]
- Dowling, D.J. Recent Advances in the Discovery and Delivery of TLR7/8 Agonists as Vaccine Adjuvants. Immunohorizons 2018, 2, 185–197. [Google Scholar] [CrossRef]
- Li, J.; Yu, X.; Jiang, Y.; He, S.; Zhang, Y.; Luo, Y.; Pu, K. Second Near-Infrared Photothermal Semiconducting Polymer Nanoadjuvant for Enhanced Cancer Immunotherapy. Adv. Mater. 2021, 33, e2003458. [Google Scholar] [CrossRef]
- Alam, M.M.; Yang, D.; Trivett, A.; Meyer, T.J.; Oppenheim, J.J. HMGN1 and R848 Synergistically Activate Dendritic Cells Using Multiple Signaling Pathways. Front. Immunol. 2018, 9, 2982. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, Q.; Guo, Z.; Li, M.; Yang, X.; Wan, G.; Chen, H.; Zhang, Q.; Wang, Y. An Intelligent Biomimetic Nanoplatform for Holistic Treatment of Metastatic Triple-Negative Breast Cancer via Photothermal Ablation and Immune Remodeling. ACS Nano 2020, 14, 15161–15181. [Google Scholar] [CrossRef]
- Jia, Y.P.; Shi, K.; Yang, F.; Liao, J.F.; Han, R.X.; Yuan, L.P.; Hao, Y.; Pan, M.; Xiao, Y.; Qian, Z.Y.; et al. Multifunctional Nanoparticle Loaded Injectable Thermoresponsive Hydrogel as NIR Controlled Release Platform for Local Photothermal Immunotherapy to Prevent Breast Cancer Postoperative Recurrence and Metastases. Adv. Funct. Mater. 2020, 30, 2001059. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, S.; Wang, X.Y.; Zhu, G. Nanovaccines for cancer immunotherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1559. [Google Scholar] [CrossRef]
- Zhang, D.; Lin, Z.; Wu, M.; Cai, Z.; Zheng, Y.; He, L.; Li, Z.; Zhou, J.; Sun, L.; Chen, G.; et al. Cytosolic Delivery of Thiolated Neoantigen Nano-Vaccine Combined with Immune Checkpoint Blockade to Boost Anti-Cancer T Cell Immunity. Adv. Sci. 2021, 8, 2003504. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; An, M.; Li, M.; Liu, H. Immunostimulatory Properties of Lipid Modified CpG Oligonucleotides. Mol. Pharm. 2017, 14, 2815–2823. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, B.; Peng, Y.; Huang, J.; Wong, S.H.D.; Bian, L.; Zhu, K.; Shuai, X.; Han, S. Nanomedicine-Boosting Tumor Immunogenicity for Enhanced Immunotherapy. Adv. Funct. Mater. 2021, 31, 2011171. [Google Scholar] [CrossRef]
- Bocanegra Gondan, A.I.; Ruiz-de-Angulo, A.; Zabaleta, A.; Gomez Blanco, N.; Cobaleda-Siles, B.M.; Garcia-Granda, M.J.; Padro, D.; Llop, J.; Arnaiz, B.; Gato, M.; et al. Effective cancer immunotherapy in mice by polyIC-imiquimod complexes and engineered magnetic nanoparticles. Biomaterials 2018, 170, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Bayyurt, B.; Tincer, G.; Almacioglu, K.; Alpdundar, E.; Gursel, M.; Gursel, I. Encapsulation of two different TLR ligands into liposomes confer protective immunity and prevent tumor development. J. Control. Release 2017, 247, 134–144. [Google Scholar] [CrossRef]
- Ebrahimian, M.; Hashemi, M.; Maleki, M.; Hashemitabar, G.; Abnous, K.; Ramezani, M.; Haghparast, A. Co-delivery of Dual Toll-Like Receptor Agonists and Antigen in Poly(Lactic-Co-Glycolic) Acid/Polyethylenimine Cationic Hybrid Nanoparticles Promote Efficient In Vivo Immune Responses. Front. Immunol. 2017, 8, 1077. [Google Scholar] [CrossRef]
- Zhu, D.; Hu, C.; Fan, F.; Qin, Y.; Huang, C.; Zhang, Z.; Lu, L.; Wang, H.; Sun, H.; Leng, X.; et al. Co-delivery of antigen and dual agonists by programmed mannose-targeted cationic lipid-hybrid polymersomes for enhanced vaccination. Biomaterials 2019, 206, 25–40. [Google Scholar] [CrossRef]
- Sainz, V.; Moura, L.I.F.; Peres, C.; Matos, A.I.; Viana, A.S.; Wagner, A.M.; Vela Ramirez, J.E.; Barata, T.S.B.; Gaspar, M.; Brocchini, S.; et al. alpha-Galactosylceramide and peptide-based nano-vaccine synergistically induced a strong tumor suppressive effect in melanoma. Acta Biomater. 2018, 76, 193–207. [Google Scholar] [CrossRef]
- Zhu, M.; Ding, X.; Zhao, R.; Liu, X.; Shen, H.; Cai, C.; Ferrari, M.; Wang, H.Y.; Wang, R.F. Co-delivery of tumor antigen and dual toll-like receptor ligands into dendritic cell by silicon microparticle enables efficient immunotherapy against melanoma. J. Control. Release 2018, 272, 72–82. [Google Scholar] [CrossRef]
- Kuai, R.; Sun, X.; Yuan, W.; Ochyl, L.J.; Xu, Y.; Hassani Najafabadi, A.; Scheetz, L.; Yu, M.Z.; Balwani, I.; Schwendeman, A.; et al. Dual TLR agonist nanodiscs as a strong adjuvant system for vaccines and immunotherapy. J. Control. Release 2018, 282, 131–139. [Google Scholar] [CrossRef]
- Ni, Q.Q.; Zhang, F.W.; Liu, Y.J.; Wang, Z.T.; Yu, G.C.; Liang, B.; Niu, G.; Su, T.; Zhu, G.Z.; Lu, G.M.; et al. A bi-adjuvant nanovaccine that potentiates immunogenicity of neoantigen for combination immunotherapy of colorectal cancer. Sci. Adv. 2020, 6, eaaw6071. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; He, P.; Zhao, C.; Ren, Q.; Dong, Z.; Qiu, J.; Jing, Y.; Liu, S.; Du, Y. A Designed alpha-GalCer Analog Promotes Considerable Th1 Cytokine Response by Activating the CD1d-iNKT Axis and CD11b-Positive Monocytes/Macrophages. Adv. Sci. 2020, 7, 2000609. [Google Scholar] [CrossRef] [PubMed]
- Temizoz, B.; Kuroda, E.; Ishii, K.J. Vaccine adjuvants as potential cancer immunotherapeutics. Int. Immunol. 2016, 28, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Shi, K.; Hao, Y.; Jia, Y.; Liu, Q.; Chen, Y.; Pan, M.; Yuan, L.; Yu, Y.; Qian, Z. Cyclophosphamide loaded thermo-responsive hydrogel system synergize with a hydrogel cancer vaccine to amplify cancer immunotherapy in a prime-boost manner. Bioact. Mater. 2021, 6, 3036–3048. [Google Scholar] [CrossRef] [PubMed]
- Matsiko, A. Cancer immunotherapy making headway. Nat. Mater. 2018, 17, 472. [Google Scholar] [CrossRef]
- Salem, M.L.; El-Naggar, S.A.; Cole, D.J. Cyclophosphamide induces bone marrow to yield higher numbers of precursor dendritic cells in vitro capable of functional antigen presentation to T cells in vivo. Cell. Immunol. 2010, 261, 134–143. [Google Scholar] [CrossRef]
- Dumitru, C.D.; Antonysamy, M.A.; Tomai, M.A.; Lipson, K.E. Potentiation of the anti-tumor effects of imidazoquinoline immune response modifiers by cyclophosphamide. Cancer Biol. Ther. 2010, 10, 155–165. [Google Scholar] [CrossRef]
- Manrique, S.Z.; Dominguez, A.L.; Mirza, N.; Spencer, C.D.; Bradley, J.M.; Finke, J.H.; Lee, J.J.; Pease, L.R.; Gendler, S.J.; Cohen, P.A. Definitive activation of endogenous antitumor immunity by repetitive cycles of cyclophosphamide with interspersed Toll-like receptor agonists. Oncotarget 2016, 7, 42919–42942. [Google Scholar] [CrossRef]
- Kim, W.S.; Kim, H.; Kwon, K.W.; Im, S.H.; Lee, B.R.; Ha, S.J.; Shin, S.J. Cisplatin induces tolerogenic dendritic cells in response to TLR agonists via the abundant production of IL-10, thereby promoting Th2- and Tr1-biased T-cell immunity. Oncotarget 2016, 7, 33765–33782. [Google Scholar] [CrossRef]
- Liu, Z.; Xie, Y.; Xiong, Y.; Liu, S.; Qiu, C.; Zhu, Z.; Mao, H.; Yu, M.; Wang, X. TLR 7/8 agonist reverses oxaliplatin resistance in colorectal cancer via directing the myeloid-derived suppressor cells to tumoricidal M1-macrophages. Cancer Lett. 2020, 469, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Belani, C.P.; Chakraborty, B.C.; Modi, R.I.; Khamar, B.M. A randomized trial of TLR-2 agonist CADI-05 targeting desmocollin-3 for advanced non-small-cell lung cancer. Ann. Oncol. 2017, 28, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; He, S.; Du, J.; Wang, Z.; Li, W.; Chen, X.; Jiang, W.; Zheng, D.; Jin, G. Local administration of a novel Toll-like receptor 7 agonist in combination with doxorubicin induces durable tumouricidal effects in a murine model of T cell lymphoma. J. Hematol. Oncol. 2015, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.G.; Camps, M.G.M.; Li, T.; Zerrillo, L.; Lowik, C.W.; Ossendorp, F.; Cruz, L.J. Effective chemoimmunotherapy by co-delivery of doxorubicin and immune adjuvants in biodegradable nanoparticles. Theranostics 2019, 9, 6485–6500. [Google Scholar] [CrossRef]
- Liu, C.; Han, C.; Liu, J. The Role of Toll-Like Receptors in Oncotherapy. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2019, 27, 965–978. [Google Scholar] [CrossRef]
- Cen, X.; Liu, S.; Cheng, K. The Role of Toll-Like Receptor in Inflammation and Tumor Immunity. Front. Pharmacol. 2018, 9, 878. [Google Scholar] [CrossRef]
- Dudek, A.M.; Garg, A.D.; Krysko, D.V.; De Ruysscher, D.; Agostinis, P. Inducers of immunogenic cancer cell death. Cytokine Growth Factor Rev. 2013, 24, 319–333. [Google Scholar] [CrossRef]
- Scholch, S.; Rauber, C.; Weitz, J.; Koch, M.; Huber, P.E. TLR activation and ionizing radiation induce strong immune responses against multiple tumor entities. Oncoimmunology 2015, 4, e1042201. [Google Scholar] [CrossRef]
- Li, G.; Wu, A.; Qi, D.; Cui, F.; Zeng, Y.; Xie, F.; Wu, H.; Gu, Y.; Chen, Q.; Zhang, X. Differential effects of peptidoglycan on colorectal tumors and intestinal tissue post-pelvic radiotherapy. Oncotarget 2016, 7, 75685–75697. [Google Scholar] [CrossRef][Green Version]
- Rodriguez-Ruiz, M.E.; Perez-Gracia, J.L.; Rodriguez, I.; Alfaro, C.; Onate, C.; Perez, G.; Gil-Bazo, I.; Benito, A.; Inoges, S.; Lopez-Diaz de Cerio, A.; et al. Combined immunotherapy encompassing intratumoral poly-ICLC, dendritic-cell vaccination and radiotherapy in advanced cancer patients. Ann. Oncol. 2018, 29, 1312–1319. [Google Scholar] [CrossRef]
- Cho, J.H.; Lee, H.J.; Ko, H.J.; Yoon, B.I.; Choe, J.; Kim, K.C.; Hahn, T.W.; Han, J.A.; Choi, S.S.; Jung, Y.M.; et al. The TLR7 agonist imiquimod induces anti-cancer effects via autophagic cell death and enhances anti-tumoral and systemic immunity during radiotherapy for melanoma. Oncotarget 2017, 8, 24932–24948. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.I.; Barsoumian, H.B.; Sezen, D.; Verma, V.; Patel, R.; Wasley, M.; Hu, Y.; Dunn, J.D.; He, K.; Chen, D.; et al. Addition of TLR9 agonist immunotherapy to radiation improves systemic antitumor activity. Transl. Oncol. 2021, 14, 100983. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lei, X.; Li, X.; Cai, J.M.; Gao, F.; Yang, Y.Y. Toll-like receptors and radiation protection. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Fan, T.; An, J.; Choi, W.; Duo, Y.; Ge, Y.; Zhang, B.; Nie, G.; Xie, N.; Zheng, T.; et al. Emerging combination strategies with phototherapy in cancer nanomedicine. Chem. Soc. Rev. 2020, 49, 8065–8087. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.W.; Li, J.; Pu, K. Recent Progresses in Phototherapy-Synergized Cancer Immunotherapy. Adv. Funct. Mater. 2018, 28, 1804688. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, M.; Fu, Q.; Li, J.; Sun, H. Targeted near infrared hyperthermia combined with immune stimulation for optimized therapeutic efficacy in thyroid cancer treatment. Oncotarget 2016, 7, 6878–6890. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, L.; Liang, C.; Wang, C.; Peng, R.; Liu, Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 2016, 7, 13193. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Zhao, H.; Hao, Y.; Van Herck, S.; Xu, Z.; Wang, G.; Wang, X.; Zhang, X.; Ge, X.; et al. In Situ Tumor Vaccination with Calcium-Linked Degradable Coacervate Nanocomplex Co-Delivering Photosensitizer and TLR7/8 Agonist to Trigger Effective Anti-Tumor Immune Responses. Adv. Healthc. Mater. 2022, e2102781. [Google Scholar] [CrossRef]
- Hao, Y.; Li, H.; Zhao, H.; Liu, Y.; Ge, X.; Li, X.; Chen, H.; Yang, A.; Zou, J.; Li, X.; et al. An Intelligent Nanovehicle Armed with Multifunctional Navigation for Precise Delivery of Toll-Like Receptor 7/8 Agonist and Immunogenic Cell Death Amplifiers to Eliminate Solid Tumors and Trigger Durable Antitumor Immunity. Adv. Healthc. Mater. 2022, e2102739. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, W.L.; Kheirolomoom, A.; Fite, B.Z.; Wu, B.; Lau, K.; Baikoghli, M.; Raie, M.N.; Tumbale, S.K.; Foiret, J.; et al. Development of thermosensitive resiquimod-loaded liposomes for enhanced cancer immunotherapy. J. Control. Release 2021, 330, 1080–1094. [Google Scholar] [CrossRef]
- Lu, Q.; Qi, S.; Li, P.; Yang, L.; Yang, S.; Wang, Y.; Cheng, Y.; Song, Y.; Wang, S.; Tan, F.; et al. Photothermally activatable PDA immune nanomedicine combined with PD-L1 checkpoint blockade for antimetastatic cancer photoimmunotherapy. J. Mater. Chem. B 2019, 7, 2499–2511. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Li, C.; Xu, N.; Watanabe, M.; Xue, R.; Xu, A.; Araki, M.; Sun, R.; Liu, C.; Nasu, Y.; et al. Dual-Functional PLGA Nanoparticles Co-Loaded with Indocyanine Green and Resiquimod for Prostate Cancer Treatment. Int. J. Nanomed. 2021, 16, 2775–2787. [Google Scholar] [CrossRef]
- Noman, M.Z.; Parpal, S.; Van Moer, K.; Xiao, M.L.; Yu, Y.; Viklund, J.; De Milito, A.; Hasmim, M.; Andersson, M.; Amaravadi, R.K.; et al. Inhibition of Vps34 reprograms cold into hot inflamed tumors and improves anti-PD-1/PD-L1 immunotherapy. Sci. Adv. 2020, 6, eaax7881. [Google Scholar] [CrossRef] [PubMed]
- Widemann, B.C. Immune checkpoint inhibitors for refractory childhood cancers. Lancet Oncol. 2020, 21, 14–15. [Google Scholar] [CrossRef]
- Gaynor, N.; Crown, J.; Collins, D.M. Immune checkpoint inhibitors: Key trials and an emerging role in breast cancer. Semin. Cancer Biol. 2020, 79, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; von Roemeling, C.A.; Chen, Y.; Qie, Y.; Liu, X.; Chen, J.; Kim, B.Y.S. Designing nanomedicine for immuno-oncology. Nat. Biomed. Eng. 2017, 1, 0029. [Google Scholar] [CrossRef]
- Scheetz, L.; Park, K.S.; Li, Q.; Lowenstein, P.R.; Castro, M.G.; Schwendeman, A.; Moon, J.J. Engineering patient-specific cancer immunotherapies. Nat. Biomed. Eng. 2019, 3, 768–782. [Google Scholar] [CrossRef]
- Zamarin, D.; Postow, M.A. Immune checkpoint modulation: Rational design of combination strategies. Pharmacol. Ther. 2015, 150, 23–32. [Google Scholar] [CrossRef]
- Wang, Y.; Su, L.; Morin, M.D.; Jones, B.T.; Mifune, Y.; Shi, H.; Wang, K.W.; Zhan, X.; Liu, A.; Wang, J.; et al. Adjuvant effect of the novel TLR1/TLR2 agonist Diprovocim synergizes with anti-PD-L1 to eliminate melanoma in mice. Proc. Natl. Acad. Sci. USA 2018, 115, E8698–E8706. [Google Scholar] [CrossRef]
- Takeda, Y.; Yoshida, S.; Takashima, K.; Ishii-Mugikura, N.; Shime, H.; Seya, T.; Matsumoto, M. Vaccine immunotherapy with ARNAX induces tumor-specific memory T cells and durable anti-tumor immunity in mouse models. Cancer Sci. 2018, 109, 2119–2129. [Google Scholar] [CrossRef]
- Jeong, Y.; Kim, G.B.; Ji, Y.; Kwak, G.J.; Nam, G.H.; Hong, Y.; Kim, S.; An, J.; Kim, S.H.; Yang, Y.; et al. Dendritic cell activation by an E. coli-derived monophosphoryl lipid A enhances the efficacy of PD-1 blockade. Cancer Lett. 2020, 472, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Kell, S.A.; Kachura, M.A.; Renn, A.; Traquina, P.; Coffman, R.L.; Campbell, J.D. Preclinical development of the TLR9 agonist DV281 as an inhaled aerosolized immunotherapeutic for lung cancer: Pharmacological profile in mice, non-human primates, and human primary cells. Int. Immunopharmacol. 2019, 66, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Reilley, M.J.; Morrow, B.; Ager, C.R.; Liu, A.; Hong, D.S.; Curran, M.A. TLR9 activation cooperates with T cell checkpoint blockade to regress poorly immunogenic melanoma. J. Immunother. Cancer 2019, 7, 323. [Google Scholar] [CrossRef] [PubMed]
- Sato-Kaneko, F.; Yao, S.; Ahmadi, A.; Zhang, S.S.; Hosoya, T.; Kaneda, M.M.; Varner, J.A.; Pu, M.; Messer, K.S.; Guiducci, C.; et al. Combination immunotherapy with TLR agonists and checkpoint inhibitors suppresses head and neck cancer. JCI Insight 2017, 2, e93397. [Google Scholar] [CrossRef]
- Zhu, S.; Lv, X.; Zhang, X.; Li, T.; Zang, G.; Yang, N.; Wang, X.; Wu, J.; Chen, W.; Liu, Y.J.; et al. An effective dendritic cell-based vaccine containing glioma stem-like cell lysate and CpG adjuvant for an orthotopic mouse model of glioma. Int. J. Cancer 2019, 144, 2867–2879. [Google Scholar] [CrossRef]
- Halwani, A.S.; Panizo, C.; Isufi, I.; Herrera, A.F.; Okada, C.Y.; Cull, E.H.; Kis, B.; Chaves, J.M.; Bartlett, N.L.; Ai, W.; et al. Phase 1/2 study of intratumoral G100 (TLR4 agonist) with or without pembrolizumab in follicular lymphoma. Leuk. Lymphoma 2022, 63, 821–833. [Google Scholar] [CrossRef]
- Li, X.; Naylor, M.F.; Le, H.; Nordquist, R.E.; Teague, T.K.; Howard, C.A.; Murray, C.; Chen, W.R. Clinical effects of in situ photoimmunotherapy on late-stage melanoma patients: A preliminary study. Cancer Biol. Ther. 2010, 10, 1081–1087. [Google Scholar] [CrossRef]
- Siu, L.; Brody, J.; Gupta, S.; Marabelle, A.; Jimeno, A.; Munster, P.; Grilley-Olson, J.; Rook, A.H.; Hollebecque, A.; Wong, R.K.S.; et al. Safety and clinical activity of intratumoral MEDI9197 alone and in combination with durvalumab and/or palliative radiation therapy in patients with advanced solid tumors. J. Immunother. Cancer 2020, 8, e001095. [Google Scholar] [CrossRef]
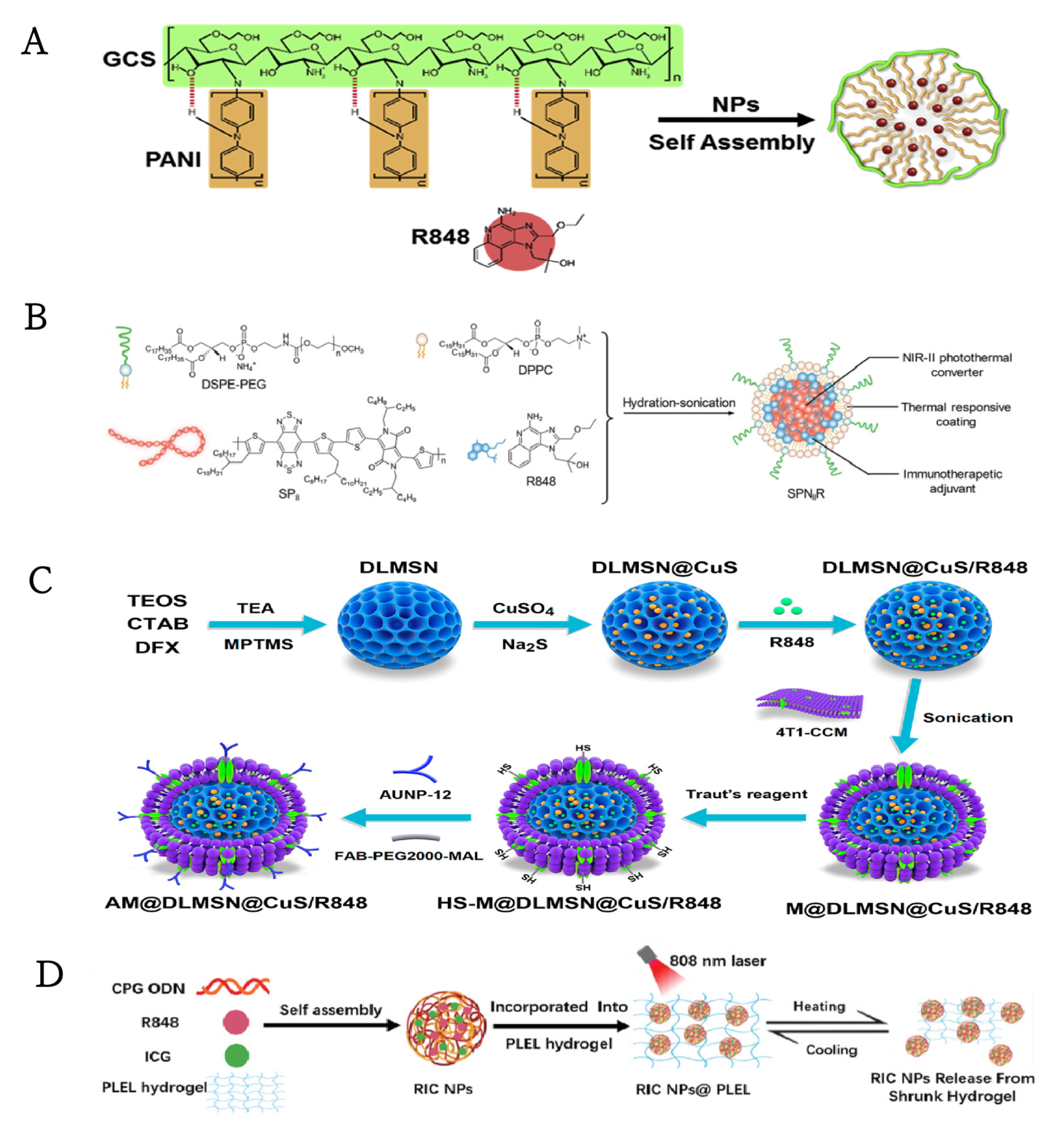
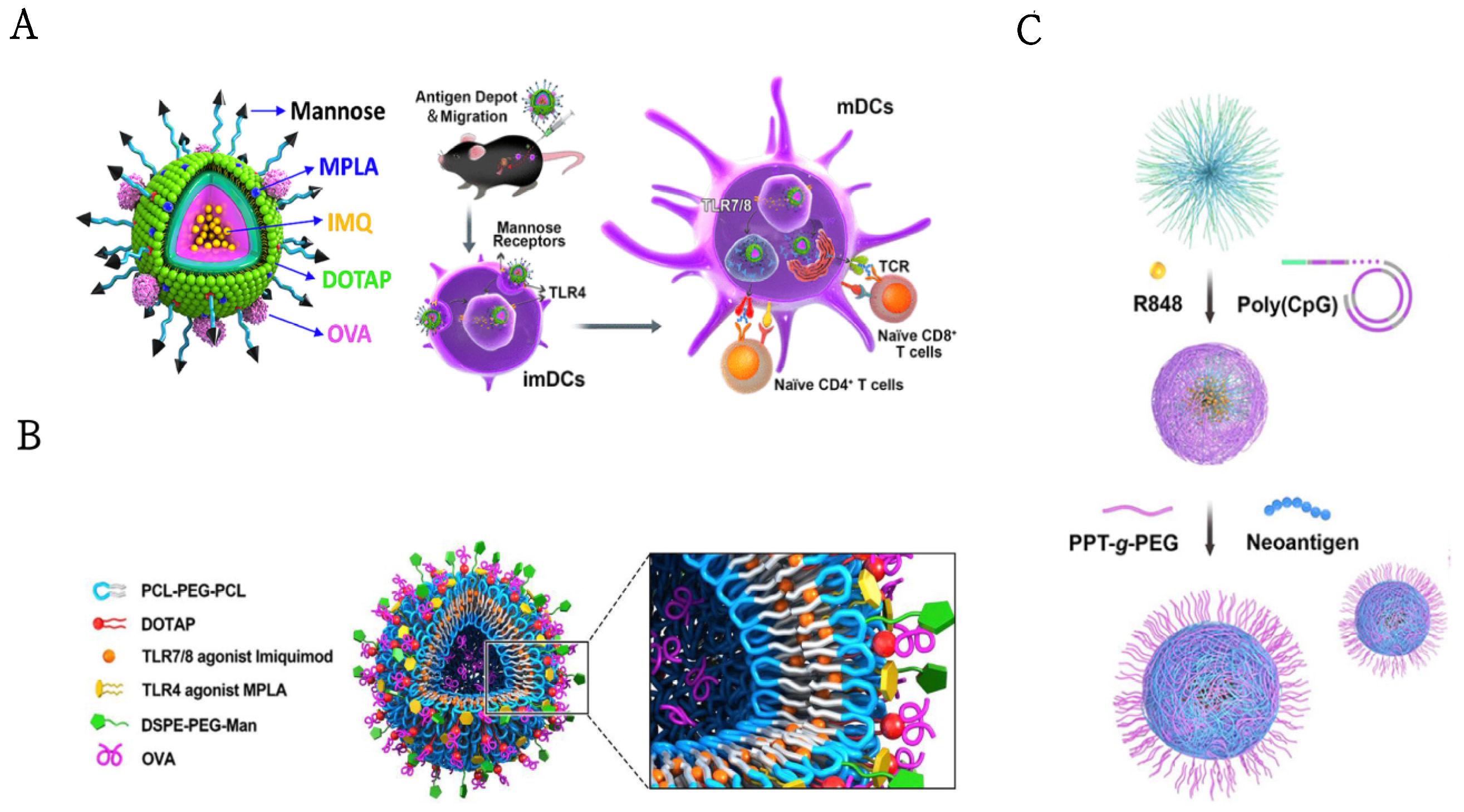
| Nanoparticles | Sub Classses | TLRa | Immunoadjuvants | Administration Route | Tumor Model | References |
|---|---|---|---|---|---|---|
| nanoplexed formulation complexed with PEI | TLR3 | poly(I:C) | anti-CD137, anti-PD-L1 | intratumoral | MC38, 4T1, B16-F10 | [38] |
| PLGA-based nanoparticles | TLR4 | LPS | N/A | peritumoral | C26, GL261 | [78] |
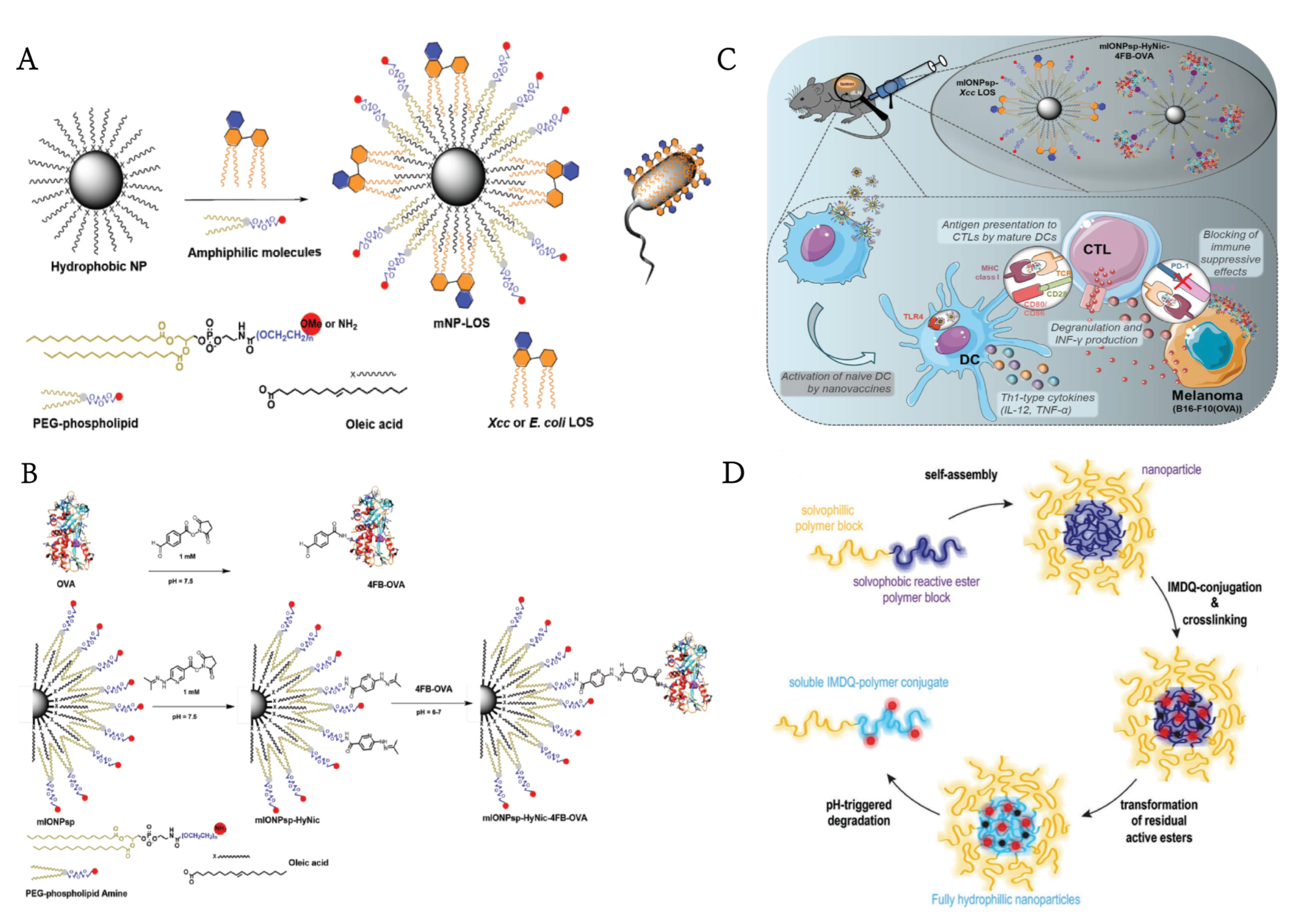
| mIONPsp | TLR4 | LOS | ovalbumin | subcutaneous | B16-F10 | [84] |
| Hf-DBP nMOF | TLR7 | R837 | anti-PD-L1 | intratumoral | CT26 | [83] |
| poly(L-glutamic acid)-graft-methoxy poly(ethylene glycol)/combretastatin A4 (CA4-NPs) | TLR7 | hypoxia-sensitive imiquimod (hs-IMQ) | N/A | intraperitoneal | 4T1 | [85] |
| upconversion nanoparticles (UCNPs) | TLR7 | R837 | chlorin e6 (Ce6), anti-CTLA-4 | subcutaneous | CT26 | [86] |
| NP-based cancer vaccine (nanovaccine) | TLR7/8 | 522 | tyrosine kinase inhibitor (TKI) sunitinib, anti-PD-L1 | subcutaneous | MB49, B16F10 | [87] |
| PEG-histamine-modified alginate (PHA) nano-complex | TLR7 | let-7b | cationic Bletilla Striata polysaccharide (cBSP) | intravenous | 4T1 | [88] |
| poly(2-oxazoline) (POx)–based nanomicellar | TLR7/8 | R848 | anti-PD-1 | intravenous | 344SQ lung adenocarcinoma (LUAD) | [89] |
| acidic pH-responsive PLGA nanoparticles | TLR7/8 | 522 | N/A | subcutaneous | B16F10 | [79] |
| PLGA nanoparticles | TLR7/8 | 522 | N/A | subcutaneous | B16F10, MB49, Renca-GL | [80] |
| PLGA nanoparticles | TLR7/8 | Gardiquimod | 5,6-Dimethylxanthenone-4-acetic acid (DMXAA) | intratumoral | B16-F10 | [81] |
| mTEGMA&PFPMA in DMSO into NPs | TLR7/8 | IMDQ | anti-PDL1, Fms-related tyrosine kinase 3 ligand (Flt3L) | peritumoral | CT26, B16 | [76] |
| platelet membrane-coated nanoparticles | TLR7/8 | R848 | N/A | intratumoral | MC38, HT-29, 4T1, and MDA-MB-231 | [90] |
| β-cyclodextrin nanoparticles(CDNP-R848) | TLR7/8 | R848 | anti-PD-1 | intravenous | MC38, B16.F10 | [91] |
| adamantane-modified cyclodextrin nanoparticles (CDNPs) | TLR7/8 | R848 | N/A | intravenous | MC38 | [92] |
| PANI-GCS NPs | TLR7/8 | R848 | N/A | intratumoral | CT26 | [93] |
| HA-Toco nano-suspension | TLR7/8 | R848 | N/A | intratumoral | AT84 | [94] |
| PLGA-PLA NPs | TLR7/8 TLR9 TLR3 | R848 or CpG ODNs or poly(I:C) | HPV-16 E7/E6 fusion protein, anti-PD-L1, cisplatin | subcutaneous | TC-1 | [95] |
| human ferritin heavy chain (rHF) nanocages | TLR9 | CpG ODNs | M2 macrophage-targeting peptide (M2pep) | intravenous | 4T1 | [96] |
| CHP nanogel | TLR9 | CpG ODN | long peptide antigen (LPA) | intravenous | CMS5a | [97] |
| PEI nanocomplex | TLR9 | CpG | N/A | intratumoral | B16F10 | [98] |
| PEG-PE & PSA polymeric hybrid micelles (HMs) | TLR9 | CpG ODN | Tyrosinase-related protein 2 (Trp2) peptide | subcutaneous | B16F10 | [99] |
| Nanoparticles | Sub Classses | TLRa | Immunoadjuvants | Administration Route | Tumor Model | References |
|---|---|---|---|---|---|---|
| phospholipid micelles loaded with zinc-doped iron oxide magnetic nanoparticles (MNPs) | TLR3, TLR7 | Poly(I:C), R837 | ovalbumin | subcutaneous | B16-F10 | [114] |
| liposomes | TLR3, TLR9 | Poly(I:C), CpG-ODN | ovalbumin | intraperitoneal | EL4 | [115] |
| PCL−PEG-PCL & DOTAP(IMNPs) & DSPE-PEG-mannose (MAN-IMNPS)lipid-polymer hybrid nanoparticles | TLR4, TLR7/8 | MPLA, R837 | ovalbumin, anti-PD-1 | subcutaneous | E.G7-OVA | [43] |
| mannose-functionalized lipid-hybrid polymersomes (MAN-IMO-PS) | TLR4, TLR7/8 | MPLA, R837 | ovalbumin | intramuscular | E.G7-OVA | [117] |
| the lipids shell POPC/DMPG, PLGA of PEGylated-coated nanoparticles (NPs) | TLR4, TLR9 | MPLA, CpG | α-galactosylceramide (GalCer), melanoma antigens | subcutaneous | B16-F10 | [118] |
| mesoporous silicon vector (MSV) microparticles | TLR4, TLR9 | MPLA, CpG | tyrosinase related protein 2 (TRP2) peptide | intravenous | B16 | [119] |
| sHDL nanodiscs(ND) | TLR4, TLR9 | MPLA, CpG | ovalbumin, E7 peptide antigen | subcutaneous | B16F10-OVA, TC-1 | [120] |
| PLGA/PEI NPs | TLR9 + TLR4 or TLR7/8 | CpG ODN, MPLA or R848 | N/A | subcutaneous | J774 | [116] |
| BanNVs | TLR7/8, TLR9 | R848, CpG-ODNs | Adpgk, aPD-1 | subcutaneous | MC38 | [121] |
| Sub Classses | TLRa | Immunoadjuvants | Nanoparticles | Administration Route | Phototherapy | Tumor Model | References |
|---|---|---|---|---|---|---|---|
| TLR4 | LPS | Quercetin (inhibitor of HSP70) | Quercetin-loaded liposomes | N/A | AG-IR820 | TT | [146] |
| TLR7 | R837 | anti-CTLA4 | PLGA nanoparticles | intratumoral | ICG | 4T1,CT26 | [147] |
| TLR7/8 | IMDQ | N/A | calcium crosslinked polyaspartic acid nanocomplex(denoted as NanopIR/mpIM) | intratumoral | IR780 | CT26 | [148] |
| TLR7/8 | IMDQ | N/A | (folic acid (FA)-Poly(N,N-dimethylacrylamide)(PDMA)& CuS@OXA& Mannose-Poly(acetone oxime acrylamide) (PAA) | intratumoral | copper sulfide (CuS) | CT-26 | [149] |
| TLR7/8 | R848 | N/A | semiconducting polymer nanoparticle (SPN) | intravenous | SPII Photothermal | 4T1 | [106] |
| TLR7/8 | R848 | anti-PD-1 | dendritic large-pore mesoporous silica nanoparticles(DLMSNs) | intravenous | copper sulfide (CuS) | MDA-MB-231,4T1 | [108] |
| TLR7/8 | R848 | αPD-1 | thermosensitive liposomes (TSLs) | intravenous | FeSO4 | Neu deletion (NDL) | [150] |
| TLR7/8 | R848 | anti-PD-L1 | polyethylene glycol-modified (PDA)&PEG NPs | intravenous | carbon dots (CDs) | 4T1 | [151] |
| TLR7/8 | R848 | N/A | PLGA nanoparticles | intratumoral | ICG | RM9 | [152] |
| TLR7/8, TLR9 | R848, CpG ODN | N/A | RIC NPs&PLEL hydrogel | intratumoral | ICG | 4T1 | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Ge, X.; Liu, Y.; Li, H.; Zhang, Z. The Role of Toll-like Receptor Agonists and Their Nanomedicines for Tumor Immunotherapy. Pharmaceutics 2022, 14, 1228. https://doi.org/10.3390/pharmaceutics14061228
Huang L, Ge X, Liu Y, Li H, Zhang Z. The Role of Toll-like Receptor Agonists and Their Nanomedicines for Tumor Immunotherapy. Pharmaceutics. 2022; 14(6):1228. https://doi.org/10.3390/pharmaceutics14061228
Chicago/Turabian StyleHuang, Lingling, Xiaoyan Ge, Yang Liu, Hui Li, and Zhiyue Zhang. 2022. "The Role of Toll-like Receptor Agonists and Their Nanomedicines for Tumor Immunotherapy" Pharmaceutics 14, no. 6: 1228. https://doi.org/10.3390/pharmaceutics14061228
APA StyleHuang, L., Ge, X., Liu, Y., Li, H., & Zhang, Z. (2022). The Role of Toll-like Receptor Agonists and Their Nanomedicines for Tumor Immunotherapy. Pharmaceutics, 14(6), 1228. https://doi.org/10.3390/pharmaceutics14061228







