Preparation of Drug-Loaded Liposomes with Multi-Inlet Vortex Mixers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
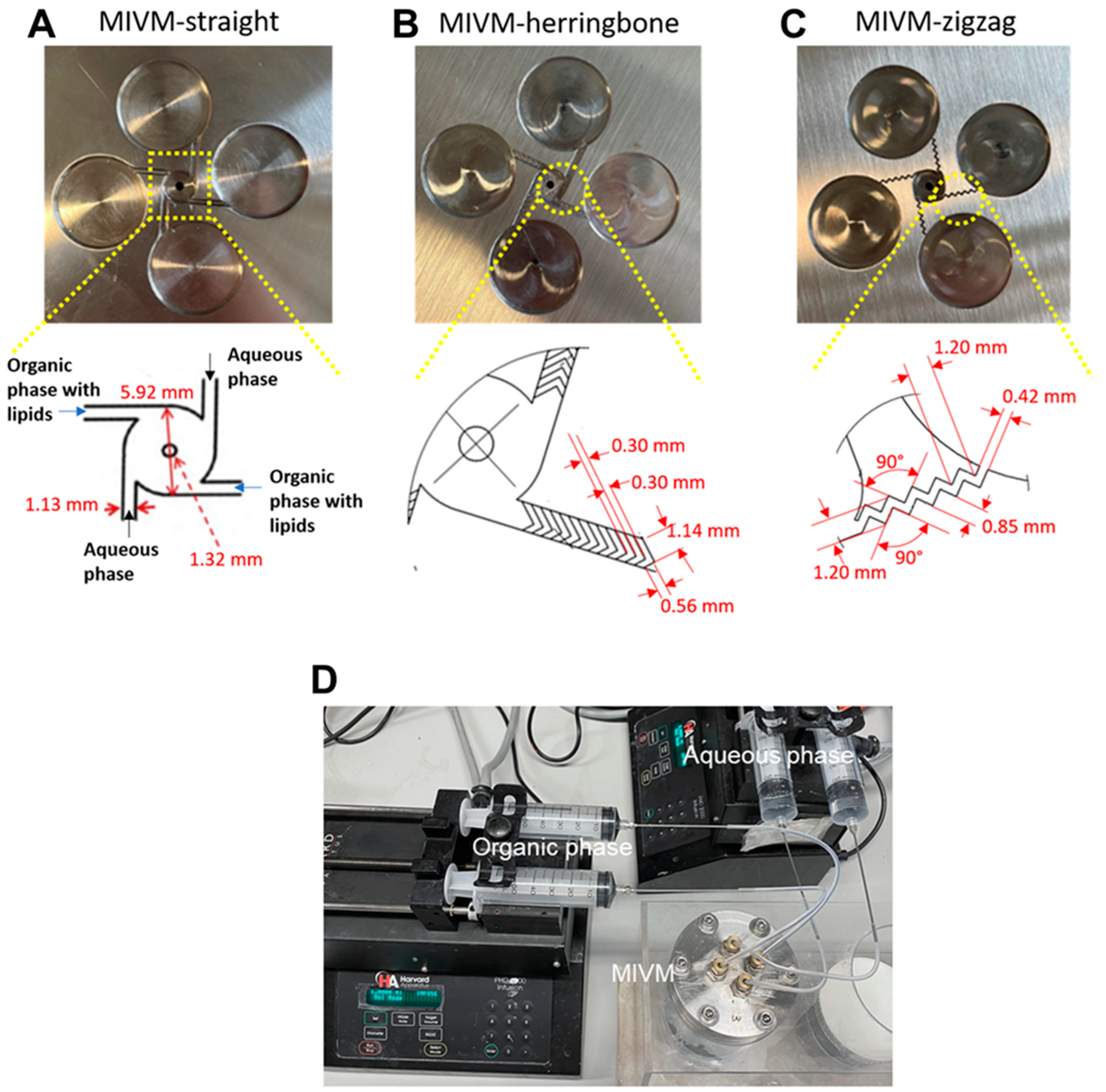
2.2. MIVM Inlet Designs
2.3. Preparation of Blank and Drug-Loaded Liposomes with the Conventional MIVM
2.4. Analysis of Particle Size and Particle Size Distribution
2.5. Transmission Electron Microscopy (TEM)
2.6. Encapsulation Efficiency Determination
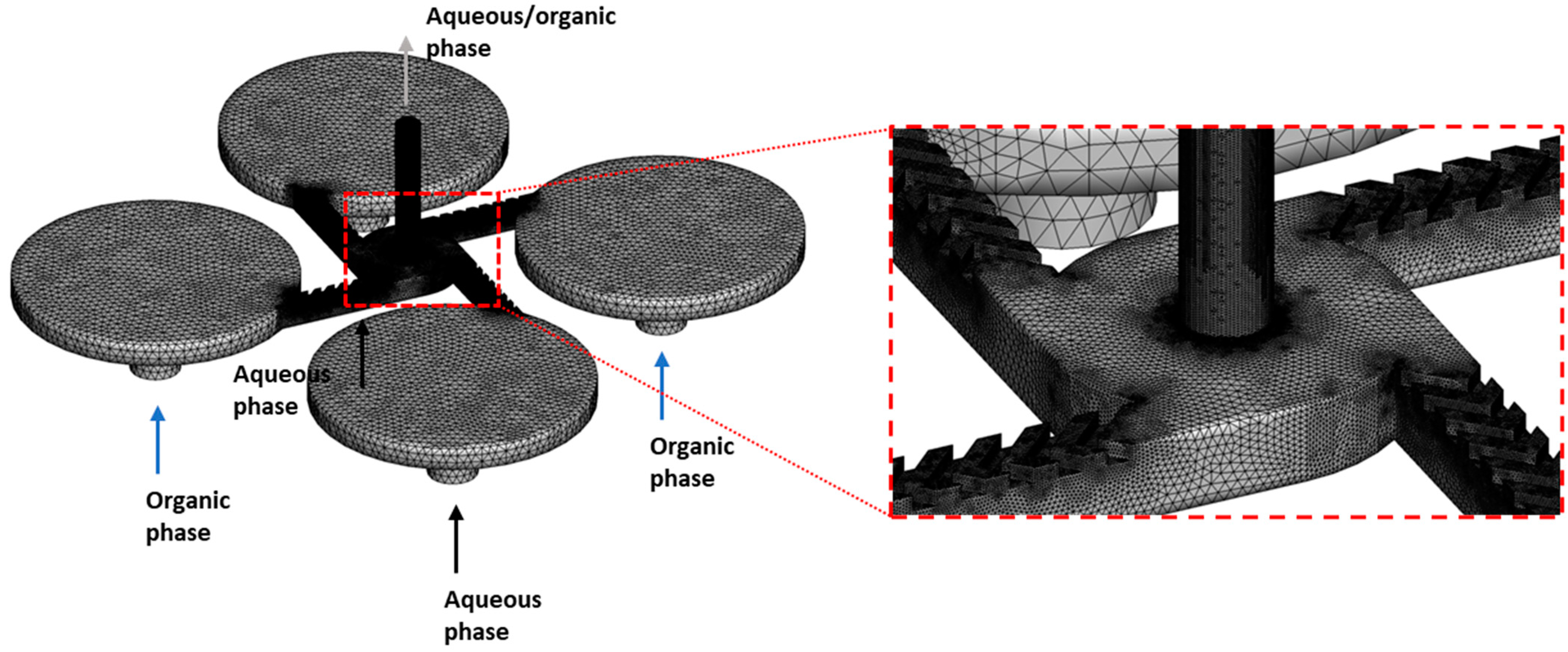
2.7. Modeling of Fluid Mixing
2.8. Statistical Analysis
3. Results and Discussion
3.1. Preparation of Blank Liposomes with Conventional MIVM
3.1.1. Effect of Lipid Concentration
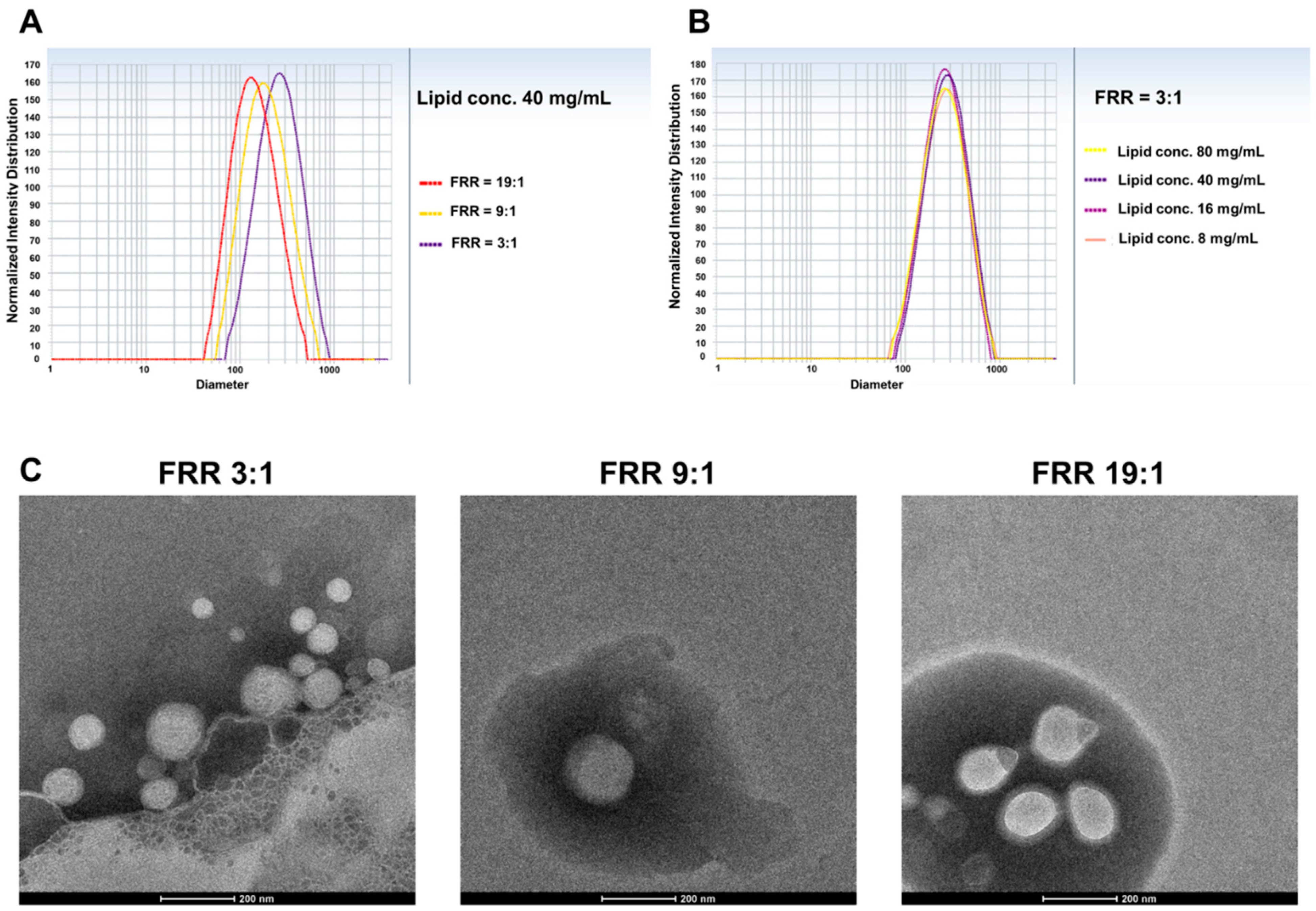
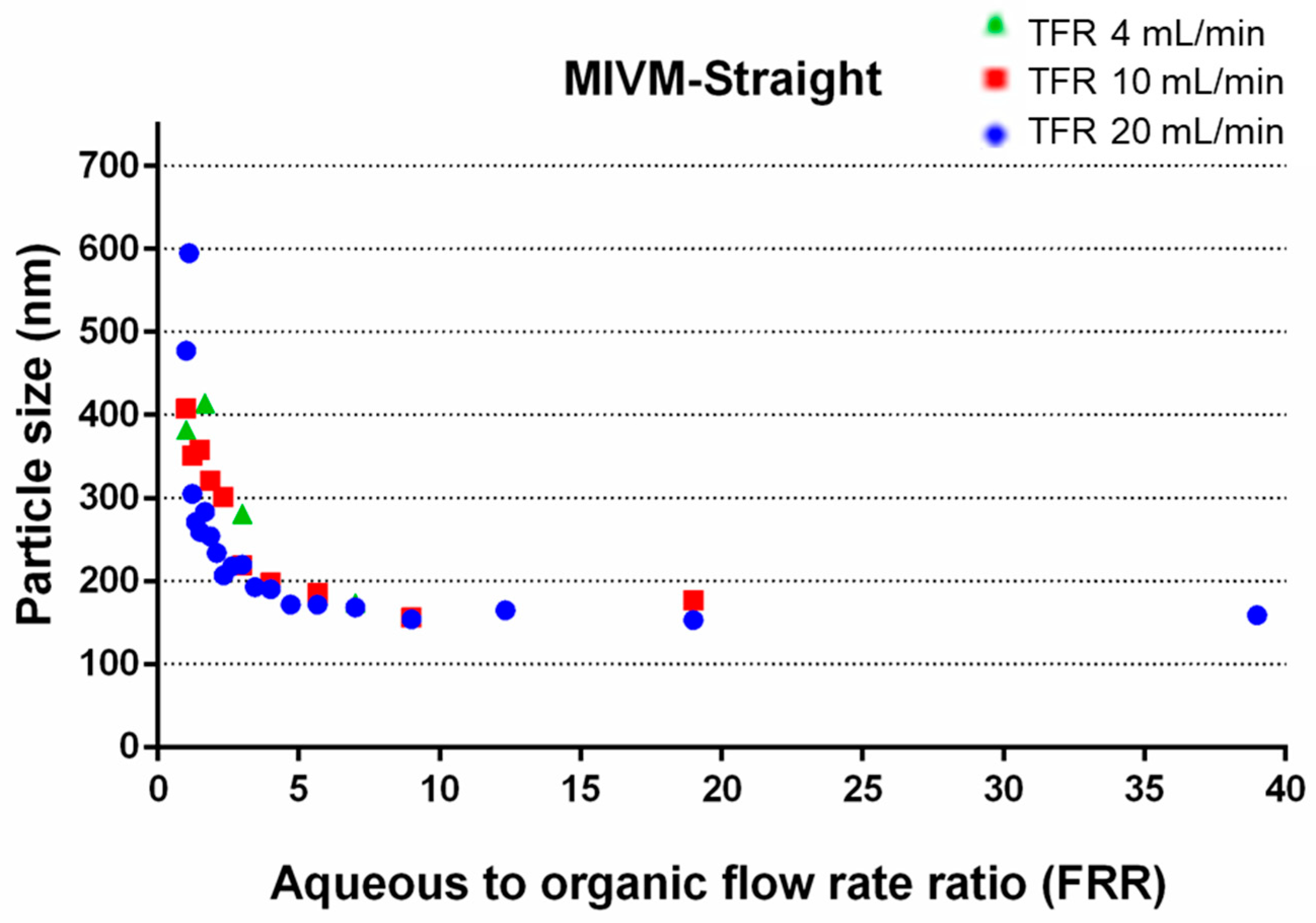
3.1.2. Effect of FRR
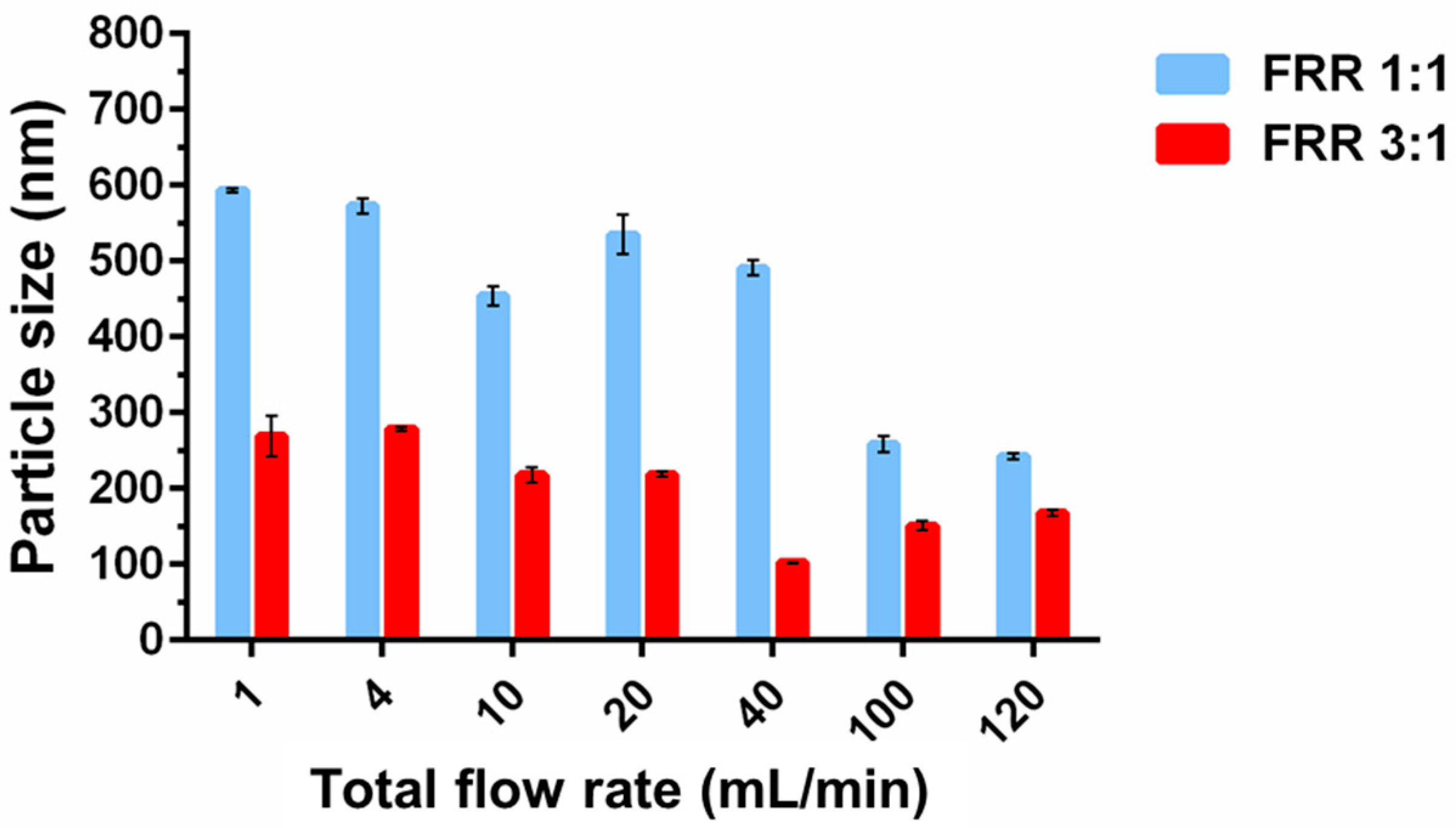
3.1.3. Effect of TFR
3.2. Preparation of Drug-Loaded Liposomes with Conventional MIVM
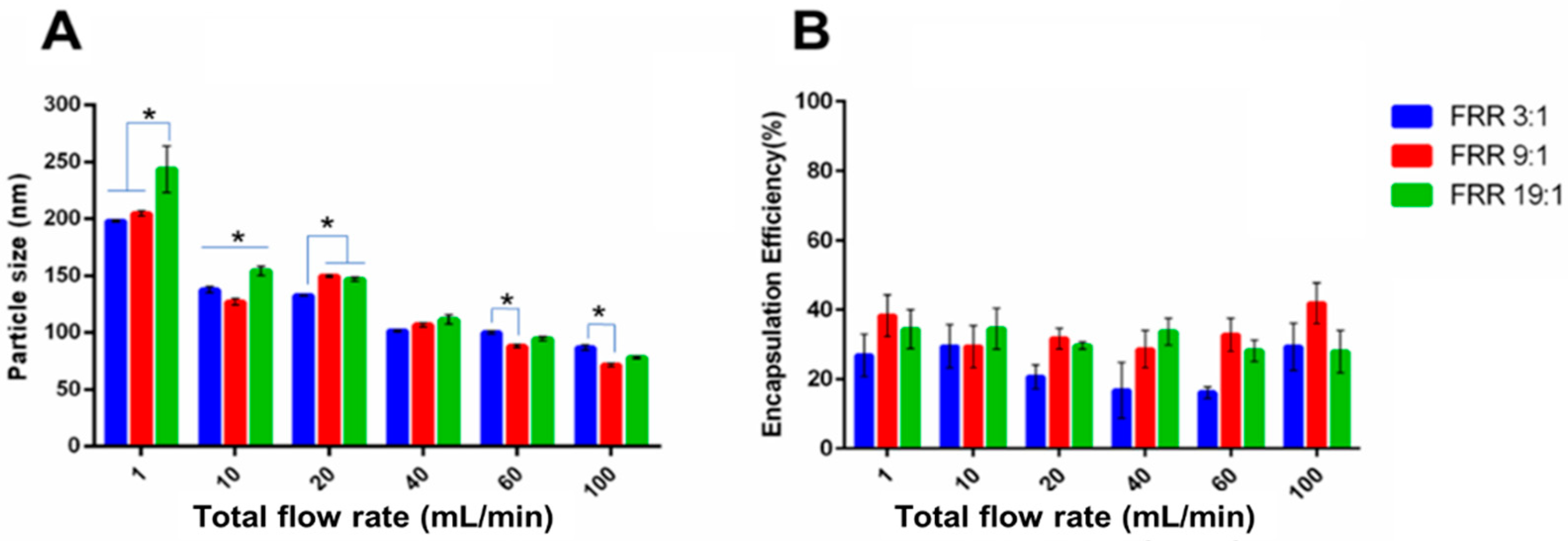
3.2.1. Lysozyme-Loaded Liposomes
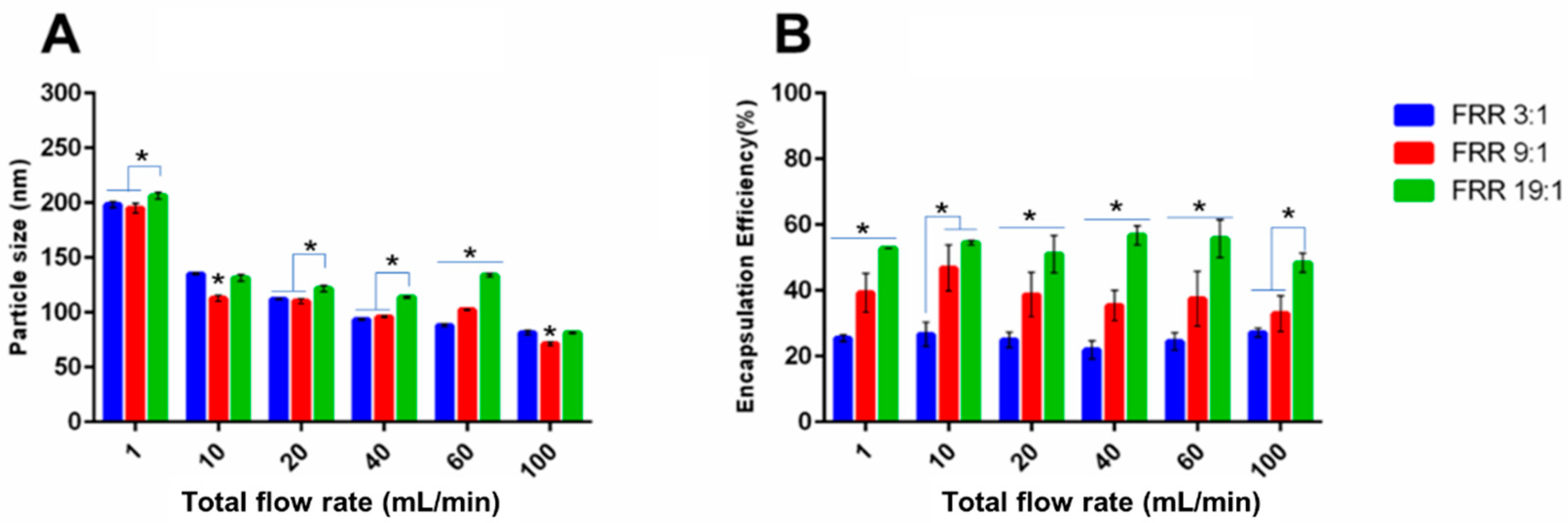
3.2.2. Erythromycin-Loaded Liposomes
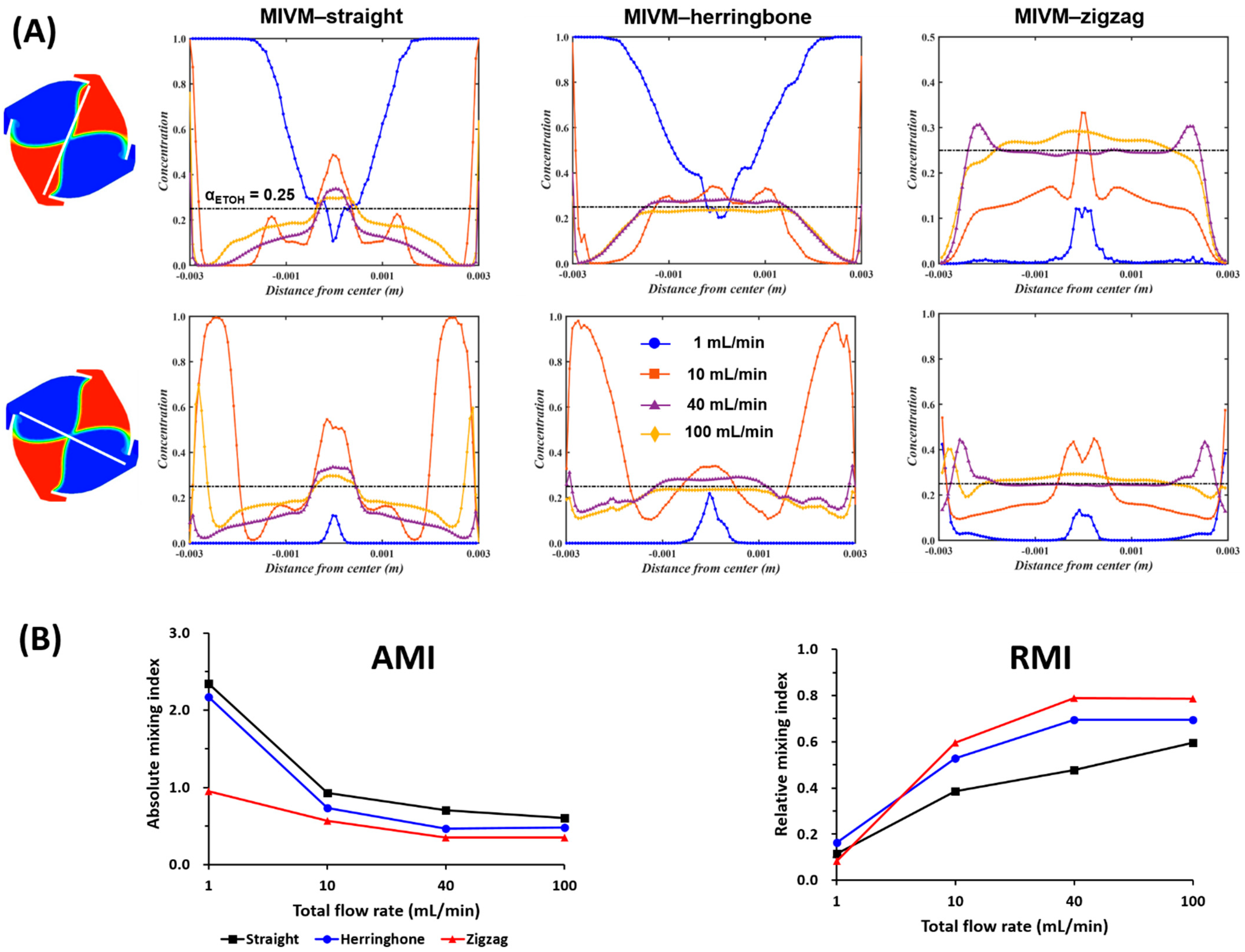
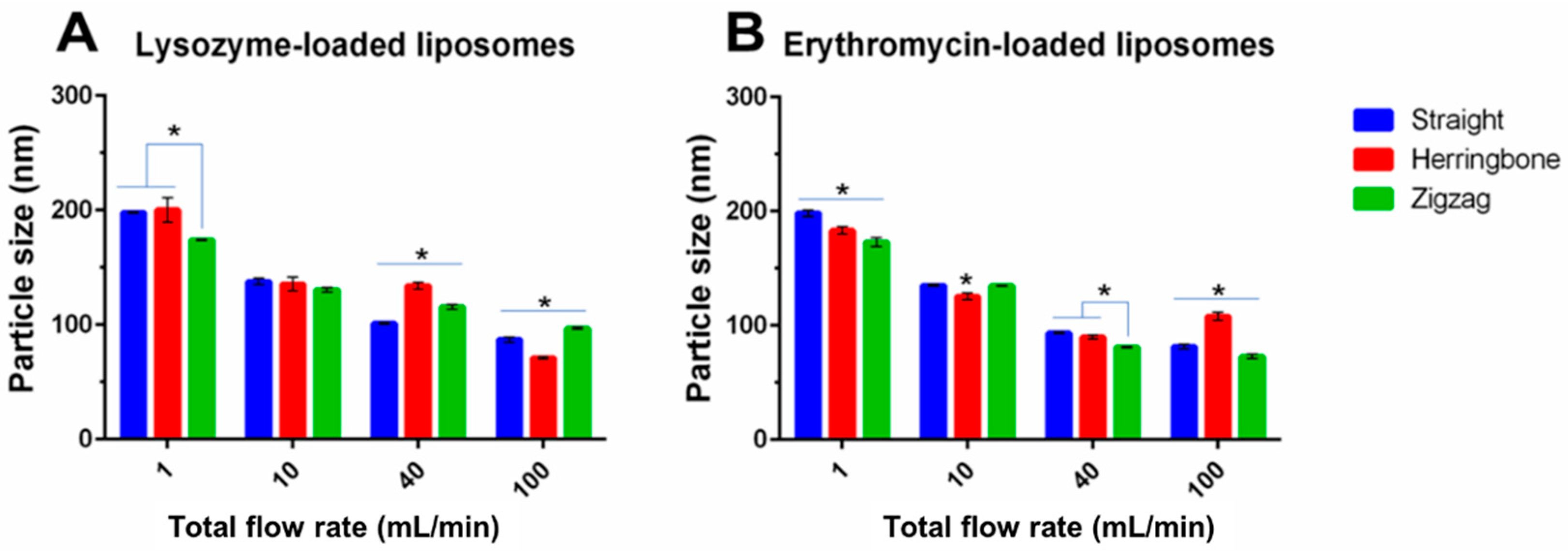
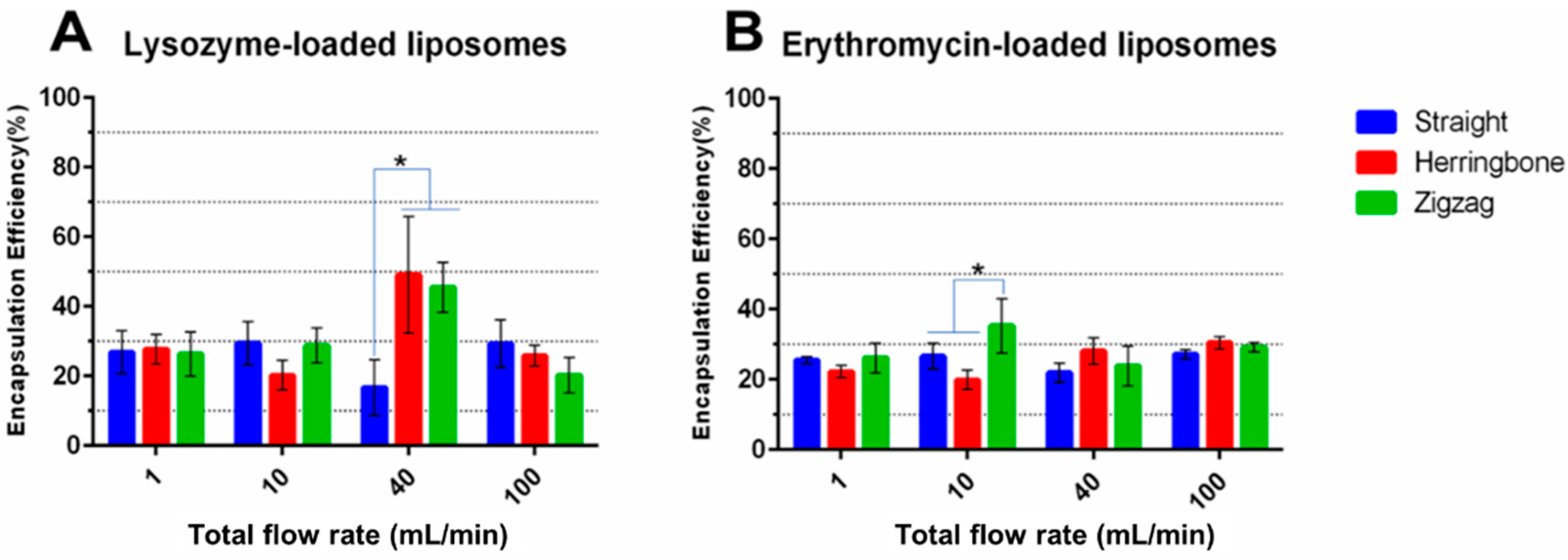
3.3. Effect of Inlet Geometries of MIVM
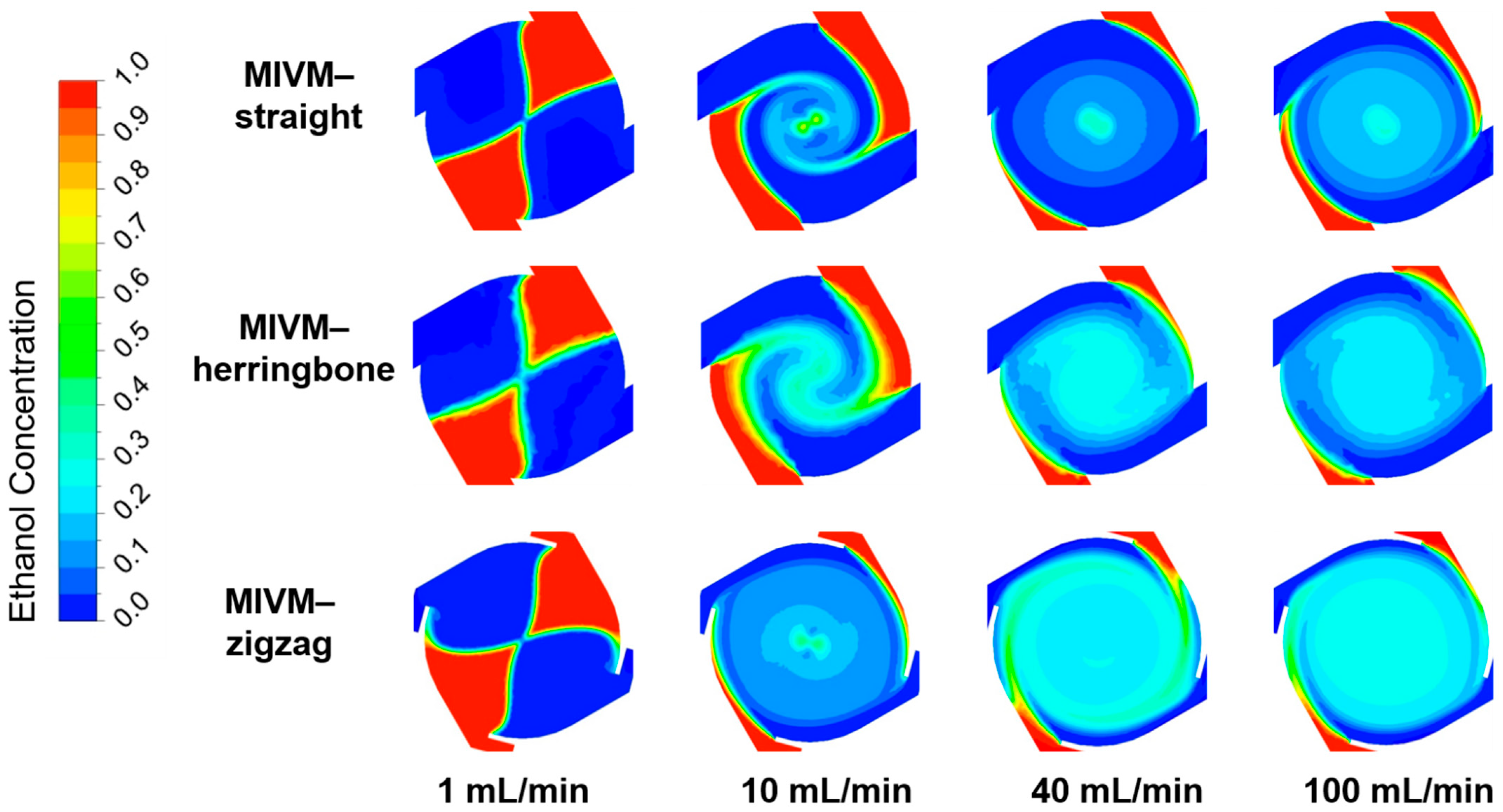
3.3.1. Computationally Determined Mixing Efficiency
3.3.2. Size of Liposomes
3.3.3. Drug Encapsulation Efficiency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.E.; Wigler, N.; Inbar, M.; Rosso, R.; Grischke, E.; Santoro, A.; Catane, R.; Kieback, D.G.; Tomczak, P.; Ackland, S.P.; et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol. 2004, 15, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Papahadjopoulos, D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc. Natl. Acad. Sci. USA 1988, 85, 6949–6953. [Google Scholar] [CrossRef] [Green Version]
- Dhoot, N.O.; Wheatley, M.A. Microencapsulated Liposomes in Controlled Drug Delivery: Strategies to Modulate Drug Release and Eliminate the Burst Effect. J. Pharm. Sci. 2003, 92, 679–689. [Google Scholar] [CrossRef]
- Huwyler, J.; Drewe, J.; Krähenbuhl, S. Tumor targeting using liposomal antineoplastic drugs. Int. J. Nanomed. 2008, 3, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Elsana, H.; Olusanya, T.O.B.; Carr-wilkinson, J.; Darby, S.; Faheem, A.; Elkordy, A.A. Evaluation of novel cationic gene based liposomes with cyclodextrin prepared by thin film hydration and microfluidic systems. Sci. Rep. 2019, 9, 15120. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, N.; Mehrnejad, F.; Vaezi, Z.; Sedghi, M.; Asghari, S.M.; Naderi-Manesh, H. Encapsulation of an endostatin peptide in liposomes: Stability, release, and cytotoxicity study. Colloids Surf. B Biointerfaces 2020, 185, 110552. [Google Scholar] [CrossRef]
- Kube, S.; Hersch, N.; Naumovska, E.; Gensch, T.; Hendriks, J.; Franzen, A.; Landvogt, L.; Siebrasse, J.-P.; Kubitscheck, U.; Hoffmann, B.; et al. Fusogenic Liposomes as Nanocarriers for the Delivery of Intracellular Proteins. Langmuir 2017, 33, 1051–1059. [Google Scholar] [CrossRef]
- Leung, S.S.Y.; Morales, S.; Britton, W.; Kutter, E.; Chan, H.-K. Microfluidic-assisted bacteriophage encapsulation into liposomes. Int. J. Pharm. 2018, 545, 176–182. [Google Scholar] [CrossRef]
- Kim, E.M.; Jeong, H.J. Liposomes: Biomedical Applications. Chonnam Med. J. 2021, 57, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carugo, D.; Bottaro, E.; Owen, J.; Stride, E.; Nastruzzi, C. Liposome production by microfluidics: Potential and limiting factors. Sci. Rep. 2016, 6, 25876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, V.M.; Nguyen, D.X.; Patel, P.; Cote, B.; Al-Fatease, A.; Pham, Y.; Huynh, M.G.; Woo, Y.; Alani, A.W. Liposomes produced by microfluidics and extrusion: A comparison for scale-up purposes. Nanomedicine 2019, 18, 146–156. [Google Scholar] [CrossRef]
- Webb, C.; Forbes, N.; Roces, C.B.; Anderluzzi, G.; Lou, G.; Abraham, S.; Ingalls, L.; Marshall, K.; Leaver, T.J.; Watts, J.A.; et al. Using microfluidics for scalable manufacturing of nanomedicines from bench to GMP: A case study using protein-loaded liposomes. Int. J. Pharm. 2020, 582, 119266. [Google Scholar] [CrossRef]
- Saad, W.S.; Prud’homme, R.K. Principles of nanoparticle formation by flash nanoprecipitation. Nano Today 2016, 11, 212–227. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, C.; Liu, Y.; Prud’homme, R.K.; Fox, R.O. Mixing in a multi-inlet vortex mixer (MIVM) for flash nano-precipitation. Chem. Eng. Sci. 2008, 63, 2829–2842. [Google Scholar] [CrossRef]
- Hu, H.; Yang, C.; Li, M.; Shao, D.; Mao, H.-Q.; Leong, K.W. Flash technology-based self-assembly in nanoformulation: Fabrication to biomedical applications. Mater. Today 2021, 42, 99–116. [Google Scholar] [CrossRef]
- Markwalter, C.E.; Prud’homme, R.K. Design of a Small-Scale Multi-Inlet Vortex Mixer for Scalable Nanoparticle Production and Application to the Encapsulation of Biologics by Inverse Flash NanoPrecipitation. J. Pharm. Sci. 2018, 107, 2465–2471. [Google Scholar] [CrossRef]
- He, Z.; Hu, Y.; Nie, T.; Tang, H.; Zhu, J.; Chen, K.; Liu, L.; Leong, K.W.; Chen, Y.; Mao, H.Q. Size-controlled lipid nanoparticle production using turbulent mixing to enhance oral DNA delivery. Acta Biomater. 2018, 81, 195–207. [Google Scholar] [CrossRef]
- Leung, S.S.; Wong, J.; Guerra, H.V.; Samnick, K.; Prud’homme, R.K.; Chan, H.K. Porous mannitol carrier for pulmonary delivery of cyclosporine A nanoparticles. Aaps J. 2017, 19, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Burke, P.A.; Gindy, M.E.; Mathre, D.J.; Kumar, V.; Prud’homme, R.K. Preparation of Lipid Nanoparticles; Sirna Therapeutics Inc.: San Francisco, CA, USA; Princeton University: Princeton, NJ, USA, 2011. [Google Scholar]
- Bokare, A.; Takami, A.; Kim, J.H.; Dong, A.; Chen, A.; Valerio, R.; Gunn, S.; Erogbogbo, F. Herringbone-Patterned 3D-Printed Devices as Alternatives to Microfluidics for Reproducible Production of Lipid Polymer Hybrid Nanoparticles. ACS Omega 2019, 4, 4650–4657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Z.; Fletcher, D.F.; Haynes, B.S. Transient laminar heat transfer simulations in periodic zigzag channels. Int. J. Heat Mass Transf. 2014, 71, 758–768. [Google Scholar] [CrossRef]
- Zheng, Z.; Fletcher, D.F.; Haynes, B.S. Laminar heat transfer simulations for periodic zigzag semicircular channels: Chaotic advection and geometric effects. Int. J. Heat Mass Transf. 2013, 62, 391–401. [Google Scholar] [CrossRef]
- Zheng, Z.; Fletcher, D.F.; Haynes, B.S. Chaotic advection in steady laminar heat transfer simulations: Periodic zigzag channels with square cross-sections. Int. J. Heat Mass Transf. 2013, 57, 274–284. [Google Scholar] [CrossRef]
- Pratt, K.C.; Wakeham, W.A.; Ubbelohde, A.R.J.P. The mutual diffusion coefficient of ethanol-water mixtures: Determination by a rapid, new method. Proc. R. Soc. Lond. A Math. Phys. Sci. 1974, 336, 393–406. [Google Scholar]
- Jesorka, A.; Orwar, O. Liposomes: Technologies and analytical applications. Annu. Rev. Anal. Chem. 2008, 1, 801–832. [Google Scholar] [CrossRef]
- Mozafari, M.R. Liposomes: An overview of manufacturing techniques. Cell. Mol. Biol. Lett. 2005, 10, 711–719. [Google Scholar]
- Yanar, F.; Mosayyebi, A.; Nastruzzi, C.; Carugo, D.; Zhang, X. Continuous-Flow Production of Liposomes with a Millireactor under Varying Fluidic Conditions. Pharmaceutics 2020, 12, 1001. [Google Scholar] [CrossRef]
- Pradhan, P.; Guan, J.; Lu, D.; Wang, P.G.; Lee, L.J.; Lee, R.J. A facile microfluidic method for production of liposomes. Anticancer Res. 2008, 28, 943–947. [Google Scholar]
- Hood, R.R.; DeVoe, D.L. High-Throughput Continuous Flow Production of Nanoscale Liposomes by Microfluidic Vertical Flow Focusing. Small 2015, 11, 5790–5799. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-Z.S.; Malmstadt, N. Liposome production and concurrent loading of drug simulants by microfluidic hydrodynamic focusing. Eur. Biophys. J. 2019, 48, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, M.G.; Jung, C.; Park, Y.-H.; Song, C.; Choi, M.C.; Park, H.G.; Park, J.-K. High-throughput nanoscale lipid vesicle synthesis in a semicircular contraction-expansion array microchannel. BioChip J. 2013, 7, 210–217. [Google Scholar] [CrossRef]
- Jahn, A.; Vreeland, W.N.; DeVoe, D.L.; Locascio, L.E.; Gaitan, M. Microfluidic directed formation of liposomes of controlled size. Langmuir 2007, 23, 6289–6293. [Google Scholar] [CrossRef]
- Colletier, J.-P.; Chaize, B.; Winterhalter, M.; Fournier, D. Protein encapsulation in liposomes: Efficiency depends on interactions between protein and phospholipid bilayer. BMC Biotechnol. 2002, 2, 9. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Guan, R.; Lyu, F.; Liu, M.; Gao, J.; Cao, G. Optimization of Preparation Conditions for Lysozyme Nanoliposomes Using Response Surface Methodology and Evaluation of Their Stability. Molecules 2016, 21, 741. [Google Scholar] [CrossRef] [Green Version]
- Matouskova, P.; Marova, I.; Bokrova, J.; Benesova, P. Effect of Encapsulation on Antimicrobial Activity of Herbal Extracts with Lysozyme. Food Technol. Biotechnol. 2016, 54, 304–316. [Google Scholar] [CrossRef]
- Shen, H.; Hong, S.; Prud’homme, R.K.; Liu, Y. Self-assembling process of flash nanoprecipitation in a multi-inlet vortex mixer to produce drug-loaded polymeric nanoparticles. J. Nanoparticle Res. 2011, 13, 4109–4120. [Google Scholar] [CrossRef]
- Eloy, J.O.; Petrilli, R.; Topan, J.F.; Antonio, H.M.R.; Barcellos, J.P.A.; Chesca, D.L.; Serafini, L.N.; Tiezzi, D.G.; Lee, R.J.; Marchetti, J.M. Co-loaded paclitaxel/rapamycin liposomes: Development, characterization and in vitro and in vivo evaluation for breast cancer therapy. Colloids Surf. B Biointerfaces 2016, 141, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Hashmi, A.; Xu, J. On the quantification of mixing in microfluidics. J. Lab. Autom. 2014, 19, 488–491. [Google Scholar] [CrossRef] [Green Version]
| Lipid Conc. (mg/mL) 1 | FRR | TFR (mL/min) | Particle Size (nm) 2 | pdi 2 |
|---|---|---|---|---|
| 8 | 3:1 | 4 | 231.5 ± 3.9 | 0.188 ± 0.016 |
| 16 | 247.7 ± 6.9 | 0.186 ± 0.029 | ||
| 40 | 227.5 ± 3.9 | 0.230 ± 0.022 | ||
| 80 | 248.9 ± 3.3 | 0.282 ± 0.019 | ||
| 8 | 9:1 | 10 | 144.7 ± 3.1 | 0.235 ± 0.037 |
| 16 | 185.8 ± 3.2 | 0.231 ± 0.018 | ||
| 40 | 167.0 ± 1.6 | 0.157 ± 0.028 | ||
| 80 | 172.3 ± 1.6 | 0.174 ± 0.020 | ||
| 8 | 19:1 | 20 | 120.9 ± 0.7 | 0.221 ± 0.009 |
| 16 | 126.5 ± 3.7 | 0.245 ± 0.013 | ||
| 40 | 124.2 ± 0.7 | 0.219 ± 0.017 | ||
| 80 | 138.3 ± 2.5 | 0.202 ± 0.023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Tao, H.; Wan, J.; Lee, K.Y.; Zheng, Z.; Leung, S.S.Y. Preparation of Drug-Loaded Liposomes with Multi-Inlet Vortex Mixers. Pharmaceutics 2022, 14, 1223. https://doi.org/10.3390/pharmaceutics14061223
Zheng H, Tao H, Wan J, Lee KY, Zheng Z, Leung SSY. Preparation of Drug-Loaded Liposomes with Multi-Inlet Vortex Mixers. Pharmaceutics. 2022; 14(6):1223. https://doi.org/10.3390/pharmaceutics14061223
Chicago/Turabian StyleZheng, Huangliang, Hai Tao, Jinzhao Wan, Kei Yan Lee, Zhanying Zheng, and Sharon Shui Yee Leung. 2022. "Preparation of Drug-Loaded Liposomes with Multi-Inlet Vortex Mixers" Pharmaceutics 14, no. 6: 1223. https://doi.org/10.3390/pharmaceutics14061223
APA StyleZheng, H., Tao, H., Wan, J., Lee, K. Y., Zheng, Z., & Leung, S. S. Y. (2022). Preparation of Drug-Loaded Liposomes with Multi-Inlet Vortex Mixers. Pharmaceutics, 14(6), 1223. https://doi.org/10.3390/pharmaceutics14061223







