Enalapril and Enalaprilat Pharmacokinetics in Children with Heart Failure Due to Dilated Cardiomyopathy and Congestive Heart Failure after Administration of an Orodispersible Enalapril Minitablet (LENA-Studies)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
Study Outcomes
2.2. Patient Selection
2.3. Treatment
2.3.1. Administration and Food Restrictions
2.3.2. Method of Assigning Patients to Treatment Groups
2.3.3. Selection of Doses in the Study
2.3.4. Selection and Timing of Dose for Each Patient
2.3.5. Treatment Adherence
2.3.6. Prior and Concomitant Therapy
2.4. Pharmacokinetic Sampling and Pharmacokinetic Assessment
2.4.1. Blood Sampling, Sample Preparation, Storage, and Transport of Samples
2.4.2. Determination of Enalapril and Enalaprilat
2.4.3. Data Analysis
2.4.4. Noncompartmental Analysis (NCA)
2.5. Glomerular Filtration Rate
2.6. Statistics
2.7. Safety Data
3. Results
3.1. Patient Characteristics
3.1.1. Demographic Characteristics
3.1.2. Medical History of Patients with DCM and CHD
3.1.3. Enalapril Dosing Distribution
3.1.4. Pretreatment and Concomitant Medication of Patients with DCM
3.1.5. Pretreatment and Concomitant Medication of Patients with CHD
3.1.6. Renal Status
3.2. Pharmacokinetics of Enalapril and Enalaprilat
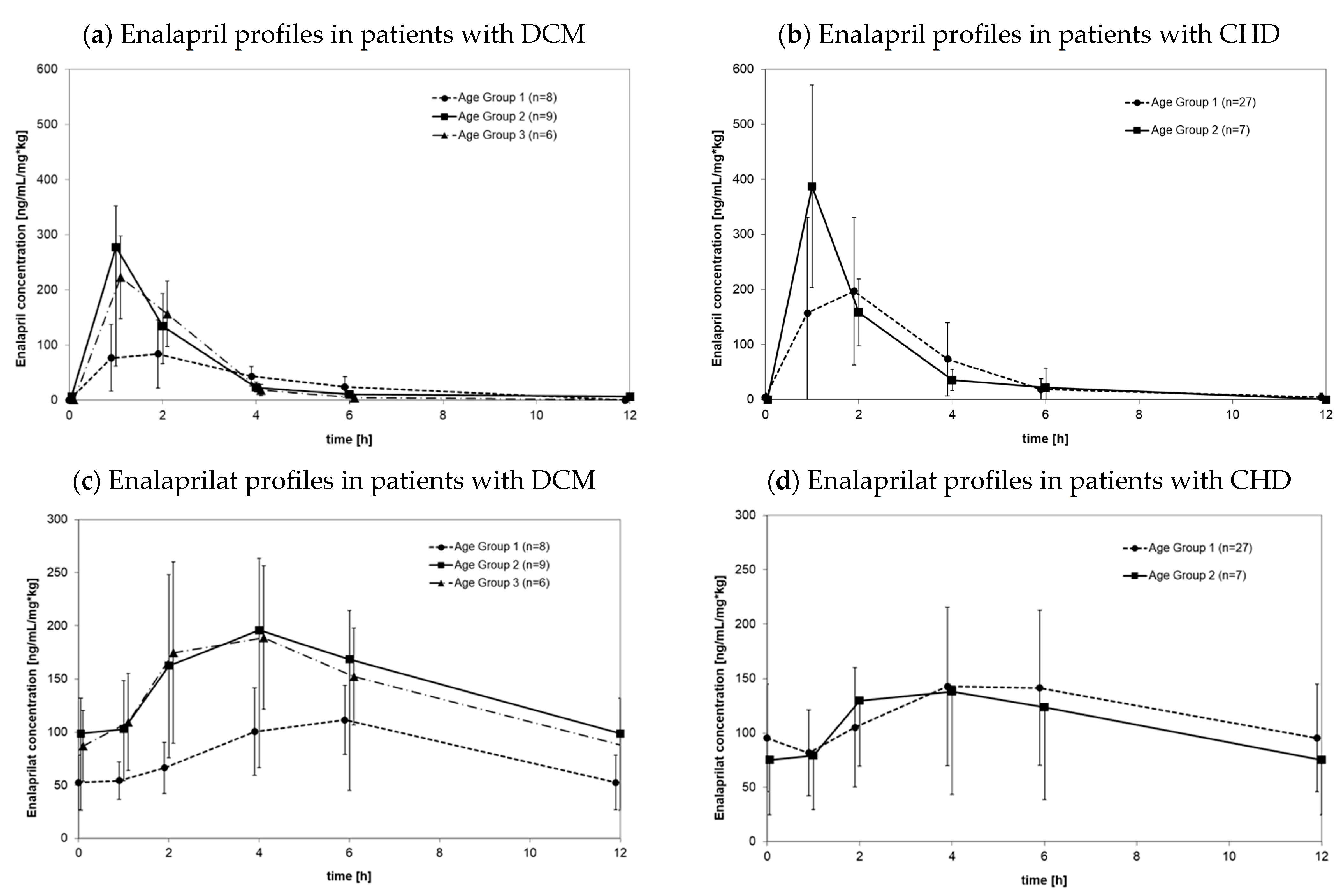
3.2.1. Pharmacokinetics in Patients with DCM
3.2.2. Pharmacokinetics in Patients with CHD
3.2.3. Pharmacokinetic Differences between DCM Patients and CHD Patients
3.2.4. Body-Weight-Normalized Clearance of Enalapril and Enalaprilat as Functions of Age
3.3. Safety Evaluation
3.3.1. Cohort of Patients with DCM
3.3.2. Cohort of Patients with CHD
4. Discussion
4.1. Pharmacokinetic Data in Children with Heart Failure Due to Congenital Heart Disease and Dilated Cardiomyopathy
4.2. Pharmacokinetics in Patients with Heart Failure and Age as Influencing Factors
4.3. Dosing Schedule of Enalapril Orodispersible Minitablets
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EMA. Committee for Proprietary Medicinal Products (CPMP) Summary Information of Referral Opinion Pursuant to Article 30 of Council Directive 2001/83/EC for Renitec and associated names (See Annex I), London 4 December 2003. Available online: https://www.ema.europa.eu/documents/referral/summary-information-referral-opinion-pursuant-article-30-council-directive-2001/83/ec-renitec-associated-names-see-annex-i-international-non-proprietary-name-inn-enalapril-background_en.pdf (accessed on 31 March 2022).
- Cleland, J.G.; Dargie, H.J.; Ball, S.G.; Gillen, G.; Hodsman, G.P.; Morton, J.J.; East, B.W.; Robertson, I.; Ford, I.; Robertson, J.I. Effects of enalapril in heart failure: A double blind study of effects on exercise performance, renal function, hormones, and metabolic state. Heart 1985, 54, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Hunt, S.A. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J. Am. Coll. Cardiol. 2005, 46, e1–e82. [Google Scholar] [PubMed] [Green Version]
- EMA. Report on the Expert Group Meeting of Paediatric Heart Failure, London 29 November 2010. Available online: https://www.ema.europa.eu/documents/other/report-expert-group-meeting-paediatric-heart-failure-london-29-november-2010_en.pdf (accessed on 31 March 2022).
- Lloyd, T.R.; Mahoney, L.T.; Knoedel, D.; Marvin Jr, W.J.; Robillard, J.E.; Lauer, R.M. Orally administered enalapril for infants with congestive heart failure: A dose-finding study. J. Pediatrics 1989, 114, 650–654. [Google Scholar] [CrossRef]
- Nakamura, H.; Ishii, M.; Sugimura, T.; Chiba, K.; Kato, H.; Ishizaki, T. The kinetic profiles of enalapril and enalaprilat and their possible developmental changes in pediatric patients with congestive heart failure. Clin. Pharmacol. Ther. 1994, 56, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Wells, T.; Rippley, R.; Hogg, R.; Sakarcan, A.; Blowey, D.; Walson, P.; Vogt, B.; Delucchi, A.; Lo, M.-W.; Hand, E.; et al. The pharmacokinetics of enalapril in children and infants with hypertension. J. Clin. Pharmacol. 2001, 41, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Bajcetic, M.; de Wildt, S.N.; Dalinghaus, M.; Breitkreutz, J.; Klingmann, I.; Lagler, F.B.; Keatley-Clarke, A.; Breur, J.M.; Male, C.; Jovanovic, I.; et al. Orodispersible minitablets of enalapril for use in children with heart failure (LENA): Rationale and protocol for a multicentre pharmacokinetic bridging study and follow-up safety study. Contemp. Clin. Trials Commun. 2019, 15, 100393. [Google Scholar] [CrossRef]
- Vinarov, Z.; Abdallah, M.; Agundez, J.A.; Allegaert, K.; Basit, A.W.; Braeckmans, M.; Ceulemans, J.; Corsetti, M.; Griffin, B.T.; Grimm, M.; et al. Impact of gastrointestinal tract variability on oral drug absorption and pharmacokinetics: An UNGAP review. Eur. J. Pharm. Sci. 2021, 162, 105812. [Google Scholar] [CrossRef]
- Sethi, P.K.; White, C.A.; Cummings, B.S.; Hines, R.N.; Muralidhara, S.; Bruckner, J.V. Ontogeny of plasma proteins, albumin and binding of diazepam, cyclosporine, and deltamethrin. Pediatr. Res. 2016, 79, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Van den Anker, J.; Reed, M.D.; Allegaert, K.; Kearns, G.L. Developmental Changes in Pharmacokinetics and Pharmacodynamics. J. Clin. Pharmacol. 2018, 58, S10–S25. [Google Scholar] [CrossRef] [Green Version]
- Boberg, M.; Vrana, M.; Mehrotra, A.; Pearce, R.E.; Gaedigk, A.; Bhatt, D.K.; Leeder, J.S.; Prasad, B. Age-Dependent Absolute Abundance of Hepatic Carboxylesterases (CES1 and CES2) by LC-MS/MS Proteomics: Application to PBPK Modeling of Oseltamivir In Vivo Pharmacokinetics in Infants. Drug Metab. Dispos. 2017, 45, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Ulm, E.H.; Hichens, M.; Gomez, H.J.; Till, A.E.; Hand, E.; Vassil, T.C.; Biollaz, J.; Brunner, H.R.; Schelling, J.L. Enalapril maleate and a lysine analog (MK-521): Disposition in man. Br. J. Clin. Pharmacol. 1982, 14, 357–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodieux, F.; Wilbaux, M.; Anker, J.N.; Van, D.; Pfister, M. Effect of Kidney Function on Drug Kinetics and Dosing in Neonates, Infants, and Children. Clin. Pharmacokinet. 2015, 54, 1183–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamper, A.L.; Thomsen, H.S.; Nielsen, S.L.; Strandgaard, S. Initial effect of enalapril on kidney function in patients with moderate to severe chronic nephropathy. Scand. J. Urol. Nephrol. 1990, 24, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, A.A.; Jarmuzewska, E.A. The influence of heart failure on the pharmacokinetics of cardiovascular and non-cardiovascular drugs: A critical appraisal of the evidence. Br. J. Clin. Pharmacol. 2019, 85, 36. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Meister, W. Pharmacokinetics in patients with cardiac failure. Clin. Pharmacokinet. 1976, 1, 389–405. [Google Scholar] [CrossRef]
- Ogawa, R.; Stachnik, J.M.; Echizen, H. Clinical Pharmacokinetics of Drugs in Patients with Heart Failure. Clin. Pharmacokinet. 2013, 52, 169–185. [Google Scholar] [CrossRef]
- Dickstein, K.; Till, A.E.; Aarsland, T.; Tjelta, K.; Abrahamsen, A.M.; Kristianson, K.; Gomez, H.J.; Gregg, H.; Hichens, M. The pharmacokinetics of enalapril in hospitalized patients with congestive heart failure. Br. J. Clin. Pharmacol. 1987, 23, 403–410. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, J.B.; Taylor, A.; Abernethy, D.; O’Meara, M.; Farmer, J.; Young, J.; Nelson, E.; Pool, J.; Mitchell, J.R. Pharmacokinetics and pharmacodynamics of enalapril in patients with congestive heart failure and patients with hypertension. J. Cardiovasc. Pharmacol. 1985, 7, 767–776. [Google Scholar] [CrossRef]
- Hsu, D.T.; Pearson, G.D. Heart failure in children, part I: History, etiology, and pathophysiology. Circ. Heart Fail. 2009, 2, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Läer, S.; Mir, T.S.; Behn, F.; Eiselt, M.; Scholz, H.; Venzke, A.; Meibohm, B.; Weil, J. Carvedilol therapy in pediatric patients with congestive heart failure: A study investigating clinical and pharmacokinetic parameters. Am. Heart J. 2002, 143, 916–922. [Google Scholar] [CrossRef]
- Stoltenberg, I.; Breitkreutz, J. Orally disintegrating mini-tablets (ODMTs)–A novel solid dosage form for paediatric use. Eur. J. Pharm. Biopharm. 2011, 78, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Van Hecken, A.; Burckhardt, B.B.; Khalil, F.; de Hoon, J.; Klingmann, I.; Herbots, M.; Laeer, S.; Lagler, F.B.; Breitkreutz, J. Relative Bioavailability of Enalapril Administered as Orodispersible Minitablets in Healthy Adults. Clin. Pharmacol. Drug Dev. 2020, 9, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Cawello, W.; Burckhardt, B.B.; Läer, S.; on behalf of the LENA consortium. Model-dependent pharmacokinetic analysis of enalapril administered to healthy adult volunteers using orodispersible mini-tablets for use in paediatrics. Drug Des. Dev. Ther. 2019, 13, 481–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramusovic, S.; Laeer, S. An integrated physiology-based model for the interaction of RAA system biomarkers with drugs. J. Cardiovasc. Pharmacol. 2012, 60, 417–428. [Google Scholar] [CrossRef]
- Burckhardt, B.B.; Tins, J.; Ramusovic, S.; Läer, S. Tailored Assays for Pharmacokinetic and Pharmacodynamic Investigations of Aliskiren and Enalapril in Children: An Application in Serum, Urine, and Saliva. J. Pediatric Pharmacol. Ther. 2015, 20, 431–452. [Google Scholar] [CrossRef] [Green Version]
- Faisal, M.; Cawello, W.; Burckhardt, B.B.; de Hoon, J.; Laer, S.; LENA Consortium. Simultaneous Semi-Mechanistic Population Pharmacokinetic Modeling Analysis of Enalapril and Enalaprilat Serum and Urine Concentrations From Child Appropriate Orodispersible Minitablets. Front. Pediatrics 2019, 7, 281. [Google Scholar] [CrossRef]
- Thabet, Y.; Walsh, J.; Breitkreutz, J. Flexible and precise dosing of enalapril maleate for all paediatric age groups utilizing orodispersible minitablets. Int. J. Pharm. 2018, 541, 136–142. [Google Scholar] [CrossRef]
- Khalil, F.; Laer, S. Physiologically based pharmacokinetic modeling: Methodology, applications, and limitations with a focus on its role in pediatric drug development. J. Biomed. Biotechnol. 2011, 2011, 907461. [Google Scholar] [CrossRef]
- Khalil, F.; Laer, S. Physiologically based pharmacokinetic models in the prediction of oral drug exposure over the entire pediatric age range—Sotalol as a model drug. AAPS J. 2014, 16, 226–239. [Google Scholar] [CrossRef] [Green Version]
- Takagi, T.; Ramachandran, C.; Bermejo, M.; Yamashita, S.; Yu, L.X.; Amidon, G.L. A provisional biopharmaceutical classification of the top 200 oral drug products in the United States, Great Britain, Spain, and Japan. Mol. Pharm. 2006, 3, 631–643. [Google Scholar] [CrossRef]
- Remko, M. Acidity, lipophilicity, solubility, absorption, and polar surface area of some ACE inhibitors. Chem. Pap. 2007, 61, 133–141. [Google Scholar] [CrossRef]
- Kasim, N.A.; Whitehouse, M.; Ramachandran, C.; Bermejo, M.; Lennernäs, H.; Hussain, A.S.; Junginger, H.E.; Stavchansky, S.A.; Midha, K.K.; Shah, V.P.; et al. Molecular properties of WHO essential drugs and provisional biopharmaceutical classification. Mol. Pharm. 2004, 1, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Sirianni, G.L.; Pang, K.S. Intracellular and not intraluminal esterolysis of enalapril in kidney. Studies with the single pass perfused nonfiltering rat kidney. Drug Metab. Dispos. 1998, 26, 324–331. [Google Scholar] [PubMed]
- Hockings, N.; Ajayi, A.A.; Reid, I.L. Age and the pharmacokinetics of angiotensin converting enzyme inhibitors enalapril and enalaprilat. Br. J. Clin. Pharmacol. 1986, 21, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.; Miyashita, A.; Iwatsubo, T.; Usui, T. Simultaneous absolute protein quantification of carboxylesterases 1 and 2 in human liver tissue fractions using liquid chromatography-tandem mass spectrometry. Drug Metab. Dispos. 2012, 40, 1389–1396. [Google Scholar] [CrossRef] [Green Version]
- Avdeef, A.; Berger, C.M. pH-metric solubility. 3. Dissolution titration template method for solubility determination. Eur. J. Pharm. Sci. 2001, 14, 281–291. [Google Scholar] [CrossRef]
- Rodgers, T.; Leahy, D.; Rowland, M. Physiologically based pharmacokinetic modeling 1: Predicting the tissue distribution of moderate-to-strong bases. J. Pharm. Sci. 2005, 94, 1259–1276. [Google Scholar] [CrossRef]
- Rodgers, T.; Rowland, M. Physiologically based pharmacokinetic modelling 2: Predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J. Pharm. Sci. 2006, 95, 1238–1257. [Google Scholar] [CrossRef]
- Rodgers, T.; Rowland, M. Mechanistic approaches to volume of distribution predictions: Understanding the processes. Pharm. Res. 2007, 24, 918–933. [Google Scholar] [CrossRef]
- Zhu, H.-J.; Appel, D.I.; Jiang, Y.; Markowitz, J.S. Age- and sex-related expression and activity of carboxylesterase 1 and 2 in mouse and human liver. Drug Metab. Dispos. 2009, 37, 1819–1853. [Google Scholar] [CrossRef] [Green Version]
- Shi, D.; Yang, D.; Prinssen, E.P.; Davies, B.E.; Yan, B. Surge in expression of carboxylesterase 1 during the post-neonatal stage enables a rapid gain of the capacity to activate the anti-influenza prodrug oseltamivir. J. Infect. Dis. 2011, 203, 937–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, F.; Läer, S. Development of a physiologically based model to support the choice of paediatric enalapril dosing regimen for orodispersible minitablets. In Proceedings of the 50th Annual Meeting of the AEPC, Rome, Italy, 1–4 June 2016; pp. P2–P132. [Google Scholar]
- Frenneaux, M.; Stewart, R.A.; Newman, C.M.; Hallidie-Smith, K.A. Enalapril for severe heart failure in infancy. Arch. Dis. Child. 1989, 64, 219–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leversha, A.M.; Wilson, N.J.; Clarkson, P.M.; Calder, A.L.; Ramage, M.C.; Neutze, J.M. Efficacy and dosage of enalapril in congenital and acquired heart disease. Arch. Dis. Child. 1994, 70, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Schilder, J.L.; Van den Anker, J.N. Use of enalapril in neonatal hypertension. Acta Paediatr. 1995, 84, 1426–1428. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Narang, A. Enalapril-induced acute renal failure in a newborn infant. Pediatric Nephrol. 2003, 18, 570–572. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, B.B.; Ciplea, A.M.; Laven, A.; Ablonczy, L.; Klingmann, I.; Läer, S.; Kleine, K.; Dalinghaus, M.; Đukić, M.; Breur, J.M.P.J.; et al. Simulation Training to Improve Informed Consent and Pharmacokinetic/Pharmacodynamic Sampling in Pediatric Trials. Front. Pharmacol. 2020, 11, 603042. [Google Scholar] [CrossRef] [PubMed]
- Ciplea, A.M.; Laeer, S.; Burckhardt, B.B. A feasibility study prior to an international multicentre paediatric study to assess pharmacokinetic/pharmacodynamic sampling and sample preparation procedures, logistics and bioanalysis. Contemp. Clin. Trials Commun. 2018, 12, 32–39. [Google Scholar] [CrossRef]
- Ali, M.; Tins, J.; Burckhardt, B.B.; LENA Consortium. Fit-for-Purpose Quality Control System in Continuous Bioanalysis During Long-Term Pediatric Studies. AAPS J. 2019, 21, 104. [Google Scholar] [CrossRef]
- Brett, M. Parameters for compartment-free pharmacokinetics. In Standardisation of Study Design, Data Analysis and Reporting; Cawello, W., Ed.; Shaker Verlag: Aachen, Germany, 2003; ISBN 3-8265-4767-5. [Google Scholar]
- Schwartz, G.J.; Feld, L.G.; Langford, D.J. A simple estimate of glomerular filtration rate in full-term infants during the first year of life. J. Pediatrics 1984, 104, 849–854. [Google Scholar] [CrossRef]
- Schwartz, G.J.; Gauthier, B. A simple estimate of glomerular filtration rate in adolescent boys. J. Pediatrics 1985, 106, 522–526. [Google Scholar] [CrossRef]
- Faisal, M.; Cawello, W.; Laeer, S. LENA Consortium. Clinical Pharmacokinetics of Enalapril and Enalaprilat in Pediatric Patients-A Systematic Review. Front. Pediatrics 2021, 9, 611322. [Google Scholar] [CrossRef] [PubMed]
- Shaddy, R.E.; Boucek, M.M.; Hsu, D.T.; Boucek, R.J.; Canter, C.E.; Mahony, L.; Ross, R.D.; Pahl, E.; Blume, E.D.; Dodd, D.A.; et al. Carvedilol for children and adolescents with heart failure: A randomized controlled trial: Pediatric Carvedilol Study Group. JAMA 2007, 298, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.J.; Vajjah, P.; Abduljalil, K.; Jamei, M.; Rostami-Hodjegan, A.; Tucker, G.T.; Johnson, T.N. Does age affect gastric emptying time? A model-based meta-analysis of data from premature neonates through to adults. Biopharm. Drug Dispos. 2015, 36, 245–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maharaj, A.A.; Edginton, A.N. Examining Small Intestinal Transit Time as a Function of Age: Is There Evidence to Support Age-Dependent Differences among Children? Drug Metab. Dispos. 2016, 44, 1080–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Patient Group | n | Gender (m) | Age (Years) | Body Weight (kg) | Dose (mg/kg) | Height (cm) | GFR * (mL/min) | BSA * (m2) |
|---|---|---|---|---|---|---|---|---|
| Total (ALL) | 89 | 49 (55%) | 0.422 (0.064–11.0) | 5.93 (2.52–49.1) | 0.0718 (0.0167–0.290) | 65 (49.8–168.4) | 118.8 (49.6–233.1) | 0.327 (0.184–1.42) |
| 1 day to <1 year | 66 | 38 (58%) | 0.363 (0.064–0.997) | 4.93 (2.52–9.2) | 0.06906 (0.0347–0.133) | 61 (49.8–77.7) | 115.8 (49.6–233.1) | 0.285 (0.187–0.441) |
| 1 year to <6 years | 17 | 10 (59%) | 1.75 (1.036–5.25) | 11.9 (6.87–21) | 0.09617 (0.0167–0.148) | 85.6 (67.6–115.9) | 125 (78.4–168.5 | 0.532 (0.370–0.795) |
| 6 years to <12 years | 6 | 4 (67%) | 10.5 (6–11.02) | 32.3 (17.8–49.1) | 0.15658 (0.0396–0.290) | 142.1 (111.9–168.4) | 109.95 (94.2–151.1) | 1.11 (0.74–1.41) |
| Dilated Cardiomyopathy | 26 | 17 (65%) | 1.54 0.296–11.02 | 9.83 5.48–49.1 | 0.107 0.027–0.290 | 81.5 62–168.4 | 112.7 71.8–151.1 | 0.463 0.311–1.41 |
| 1 months to <1 year | 9 | 6 (67%) | 0.844 (0.296–0.997) | 8.9 (5.48–9.2) | 0.107 (0.04–0.133) | 73.5 (62–76.5) | 100 (71.8–171.5) | 0.429 (0.311–0.441) |
| 1 year to <6 years | 11 | 7 (64%) | 1.75 (1.25–5.25) | 10.3 (6.87–19.6) | 0.1 (0.027–0.143) | 84.4 (74.0–115.9) | 114.5 (78.4–148.6) | 0.491 (0.386–0.795) |
| 6 years to <12 years | 6 | 4 (67%) | 10.5 (6–11.0) | 32.3 (17.8–49.1) | 0.157 (0.0396–0.290) | 142.1 (111.9–168.4) | 109.95 (94.2–151.1) | 1.11 (0.74–1.41) |
| Congenital Heart Disease | 63 | 32 (51%) | 0.362 0.064–5 | 4.84 2.52–21.06 | 0.068 0.017–0.148 | 61.0 49.8–115 | 125.7 49.6–233.1 | 0.284 0.187–0.768 |
| 1 day to <1 year | 56 | 29 (52%) | 0.322 (0.064–0.751) | 4.535 (2.52–8.78) | 0.0651 (0.0347–0.133) | 58.6 (49.8–77.7) | 122.2 (49.6–233.1) | 0.273 (0.187–0.435) |
| 1 year to <6 years | 7 | 3 (43%) | 3.67 (1.04–5) | 13.6 (7.3–21.0) | 0.08 (0.017–0.148) | 96.7 (67.5–115) | 156.3 (108.2–168.5) | 0.606 (0.370–0.768) |
| Patient Group | Enalapril | Enalaprilat | Enalapril | Enalaprilat | Enalapril | Enalaprilat | ||
|---|---|---|---|---|---|---|---|---|
| n | AUCtau, ss, norm (ng/mL·h/mg·kg) | Cmax, ss, norm (ng/mL/mg·kg) | tmax or tmax, ss (h) | |||||
| Total (All) | 89 | 637.5 | 1166.4 | 231.6 | 138.5 | 1.98 | 5.95 | |
| (0–3144.8) | (0–5451.9) | (0–760.8) | (0–719.5) | (0–5.95) | (0–12.2) | |||
| 1 day to <1 year (Ia) | 65 | 721.9 | 1111.7 | 219.4 | 128.8 | 2 | 6 | |
| (0–3144.8) | (0–5451.9) | (0–603.5) | (0–719.5) | (0–5.95) | (0–12.2) | |||
| 1 year to <6 years (Ib) | 18 | 580.2 | 1272.2 | 279.2 | 139.7 | 1.03 | 4 | |
| (313.0–1338.2) | (0–4468.2) | (78.9–279.2) | (0–516.3) | (1–4.08) | (0–12.02) | |||
| 6 years to <12 years (Ic) | 6 | 514.6 | 1618.7 | 240.95 | 187.8 | 1 | 3.92 | |
| (373.1–597.8) | (872.6–1968.6) | (155.5–273.2) | (103.3–268) | (1–2) | (2–4) | |||
| p, Ia vs. Ib | 0.849 | 0.4039 | 0.1038 | 0.3613 | 0.011 # | 0.003 # | ||
| p, Ia vs. Ic | 0.0809 | 0.0333 | 0.1851 | 0.1721 | 0.022 | <0.0001 # | ||
| p, Ib vs. Ic | 0.1476 | 0.3257 | 0.4547 | 0.5413 | 0.8683 | 0.5149 | ||
| Dilated Cardiomyopathy | 26 | 455.4 | 1194.4 | 148.0 | 129.2 | 1.51 | 4.56 | |
| (235.5–1338.2) | (0–4468.2) | (44–760.8) | (0–516.3) | (0.93–4.17) | (0–12.0) | |||
| 1 months to <1 year (IIa) | 9 | 387.7 | 929.5 | 97.1 | 109.6 | 2.03 | 6 | |
| (235.5–626.1) | (390.7–1569) | (44–219.4) | (66.8–187.7) | (0.93–4.17) | (4.03–6.08) | |||
| 1 year to <6 years (IIb) | 11 | 470.7 | 1285.4 | 225.3 | 148.8 | 1.03 | 4 | |
| (313–1338.2) | (0–4468.2) | (78.9–760.8) | (0–516.3) | (1–4.08) | (0–12.0) | |||
| 6 years to <12 years (IIc) | 6 | 514.6 | 1618.7 | 240.95 | 187.8 | 1 | 3.92 | |
| (373.1–597.8) | (872.6–1968.6) | (155.5–273.2) | (103.3–268) | (1–2) | (2–4) | |||
| p, IIa vs. IIb | 0.1005 | 0.0212 | 0.0039 # | 0.0213 | 0.0646 | 0.128 | ||
| p, IIa vs. IIc | 0.1018 | 0.0062 # | 0.0005 # | 0.015 # | 0.0229 | 0.0003 # | ||
| p, IIb vs. IIc | 0.7078 | 0.7708 | 0.8555 | 0.8426 | 0.6496 | 0.6443 | ||
| Congenital Heart Disease | 63 | 778.2 | 1166.1 | 260.3 | 142.2 | 1.98 | 6.0 | |
| (0–3144.8) | (0–5451.9) | (0–603.5) | (0–719.5) | (0–5.95) | (0–12.2) | |||
| 1 day to <1 year (IIIa) | 56 | 783.7 | 1156.6 | 257.1 | 142.7 | 2 | 6 | |
| (0–3144.8) | (0–5451.9) | (0–603.5) | (0–719.5) | (0–5.95) | (0–12.2) | |||
| 1 year to <6 years (IIIb) | 7 | 778.2 | 1258.9 | 471.9 | 130.5 | 1.03 | 4 | |
| (448.8–1334.8) | (166.5–2706.9) | (105.1–564) | (81.8–289.3) | (1–2.1) | (2.07–6) | |||
| p, IIIa vs. IIIb | 0.5217 | 0.8282 | 0.1021 | 0.9591 | 0.0026 # | 0.0006 # | ||
| Age | Body Weight | Enalapril | Enalaprilat | Enalapril | Enalaprilat | Enalapril | Enalaprilat |
|---|---|---|---|---|---|---|---|
| (days) | (kg) | AUCtau, ss, norm (ng/mL·h/mg·kg) | Cmax, ss, norm (ng/mL/mg·kg) | tmax or tmax, ss (h) | |||
| 17 | 4.35 | 775.3 | 259.5 | 1344.6 | 174.9 | 2.05 | 5.983 |
| 24 | 3.28 | 996 | 274.8 | 504.9 | 101.8 | 3.983 | 11.9 |
| 25 | 3.50 | 318.6 | 103.8 | 1111.7 | 152.5 | 1 | 5.983 |
| Enalapril | Enalaprilat | Enalapril | Enalaprilat | Enalapril | Enalaprilat | ||
|---|---|---|---|---|---|---|---|
| n | AUCtau, ss, norm (ng/mL·h/mg·kg) | Cmax, ss, norm (ng/mL/mg·kg) | t-max or tmax, ss (h) | ||||
| Dilated Cardiomyopathy 1 months to <6 year | 20 | 428.3 | 1040.1 | 136.4 | 120.4 | 1.99 | 5.37 |
| (235.5–1338.2) | (0–4468.2) | (44–760.8) | (0–516.3) | (0.93–4.17) | (0–12.02) | ||
| Congenital Heart Disease 1 months to <6 years | 60 | 785.1 | 1166.3 | 261.0 | 142.1 | 1.98 | 6.0 |
| (0–3144.8) | (0–5451.9) | (0–603.5) | (0–719.5) | (0–5.95) | (0–12.2) | ||
| p DCM versus CHD | 0.0025 # | 0.4517 | 0.051 | 0.9543 | 0.7632 | 0.0095 # | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laeer, S.; Cawello, W.; Burckhardt, B.B.; Ablonczy, L.; Bajcetic, M.; Breur, J.M.P.J.; Dalinghaus, M.; Male, C.; de Wildt, S.N.; Breitkreutz, J.; et al. Enalapril and Enalaprilat Pharmacokinetics in Children with Heart Failure Due to Dilated Cardiomyopathy and Congestive Heart Failure after Administration of an Orodispersible Enalapril Minitablet (LENA-Studies). Pharmaceutics 2022, 14, 1163. https://doi.org/10.3390/pharmaceutics14061163
Laeer S, Cawello W, Burckhardt BB, Ablonczy L, Bajcetic M, Breur JMPJ, Dalinghaus M, Male C, de Wildt SN, Breitkreutz J, et al. Enalapril and Enalaprilat Pharmacokinetics in Children with Heart Failure Due to Dilated Cardiomyopathy and Congestive Heart Failure after Administration of an Orodispersible Enalapril Minitablet (LENA-Studies). Pharmaceutics. 2022; 14(6):1163. https://doi.org/10.3390/pharmaceutics14061163
Chicago/Turabian StyleLaeer, Stephanie, Willi Cawello, Bjoern B. Burckhardt, László Ablonczy, Milica Bajcetic, Johannes M. P. J. Breur, Michiel Dalinghaus, Christoph Male, Saskia N. de Wildt, Jörg Breitkreutz, and et al. 2022. "Enalapril and Enalaprilat Pharmacokinetics in Children with Heart Failure Due to Dilated Cardiomyopathy and Congestive Heart Failure after Administration of an Orodispersible Enalapril Minitablet (LENA-Studies)" Pharmaceutics 14, no. 6: 1163. https://doi.org/10.3390/pharmaceutics14061163
APA StyleLaeer, S., Cawello, W., Burckhardt, B. B., Ablonczy, L., Bajcetic, M., Breur, J. M. P. J., Dalinghaus, M., Male, C., de Wildt, S. N., Breitkreutz, J., Faisal, M., Keatley-Clarke, A., Klingmann, I., & Lagler, F. B. (2022). Enalapril and Enalaprilat Pharmacokinetics in Children with Heart Failure Due to Dilated Cardiomyopathy and Congestive Heart Failure after Administration of an Orodispersible Enalapril Minitablet (LENA-Studies). Pharmaceutics, 14(6), 1163. https://doi.org/10.3390/pharmaceutics14061163







