Beneath the Skin: A Review of Current Trends and Future Prospects of Transdermal Drug Delivery Systems
Abstract
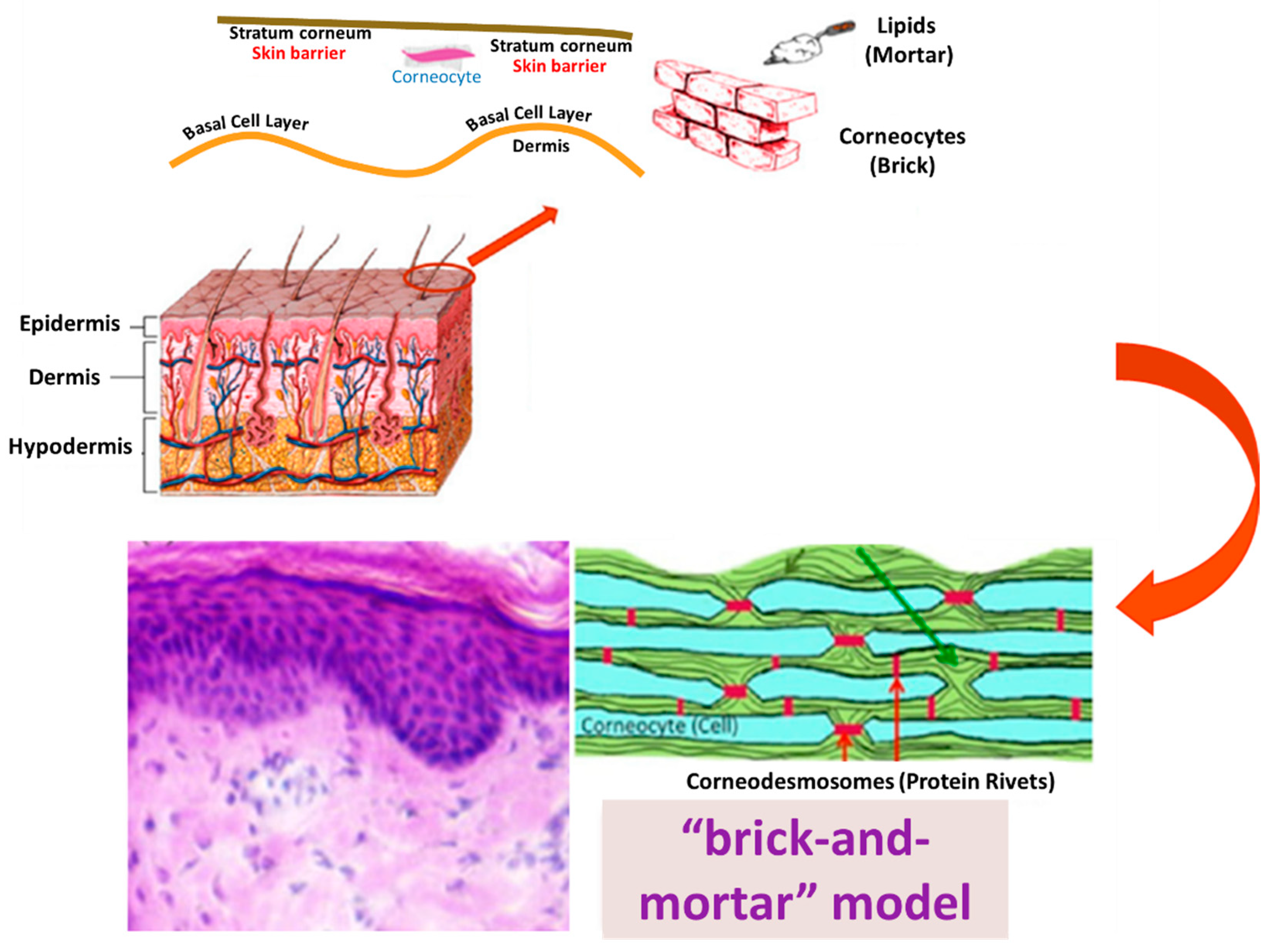
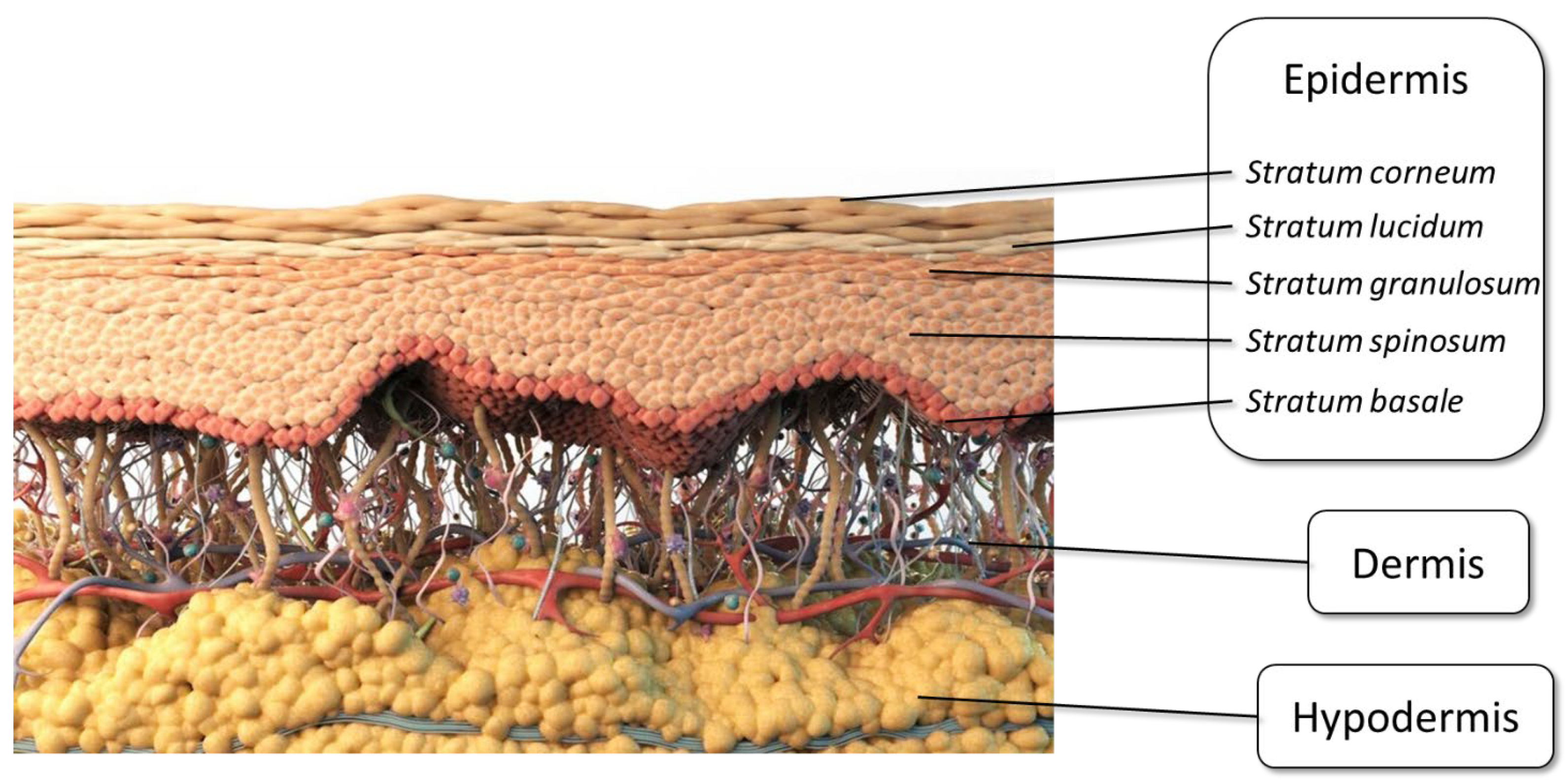
:1. Introduction
1.1. An Overview of Transdermal Drug Delivery
1.2. Currently Approved Transdermally Delivered Drugs
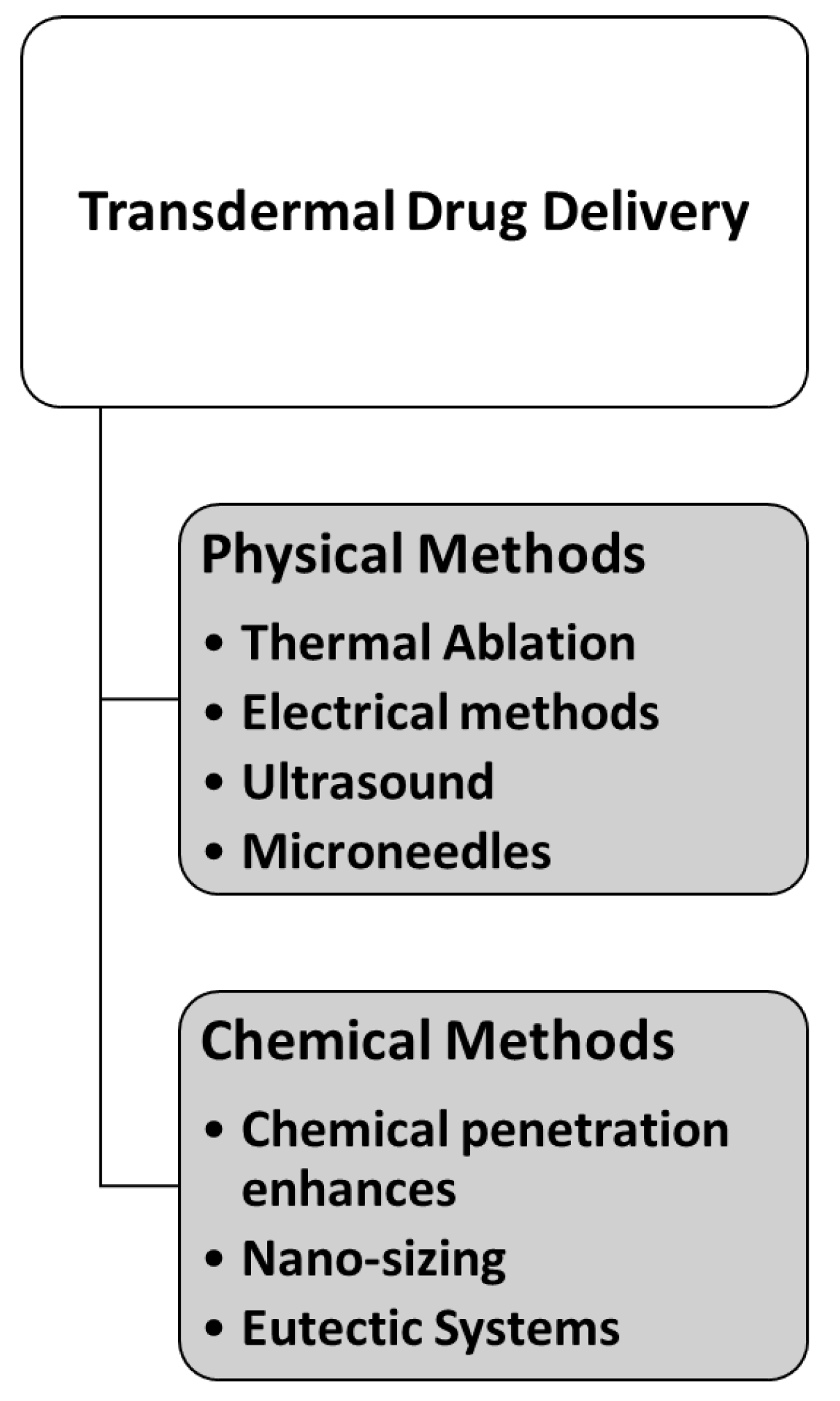
2. Techniques for Enhancement of Skin Permeabilisation
2.1. Chemical Methods for Transdermal Drug Delivery
2.1.1. Chemical Penetration Enhancers (CPEs)
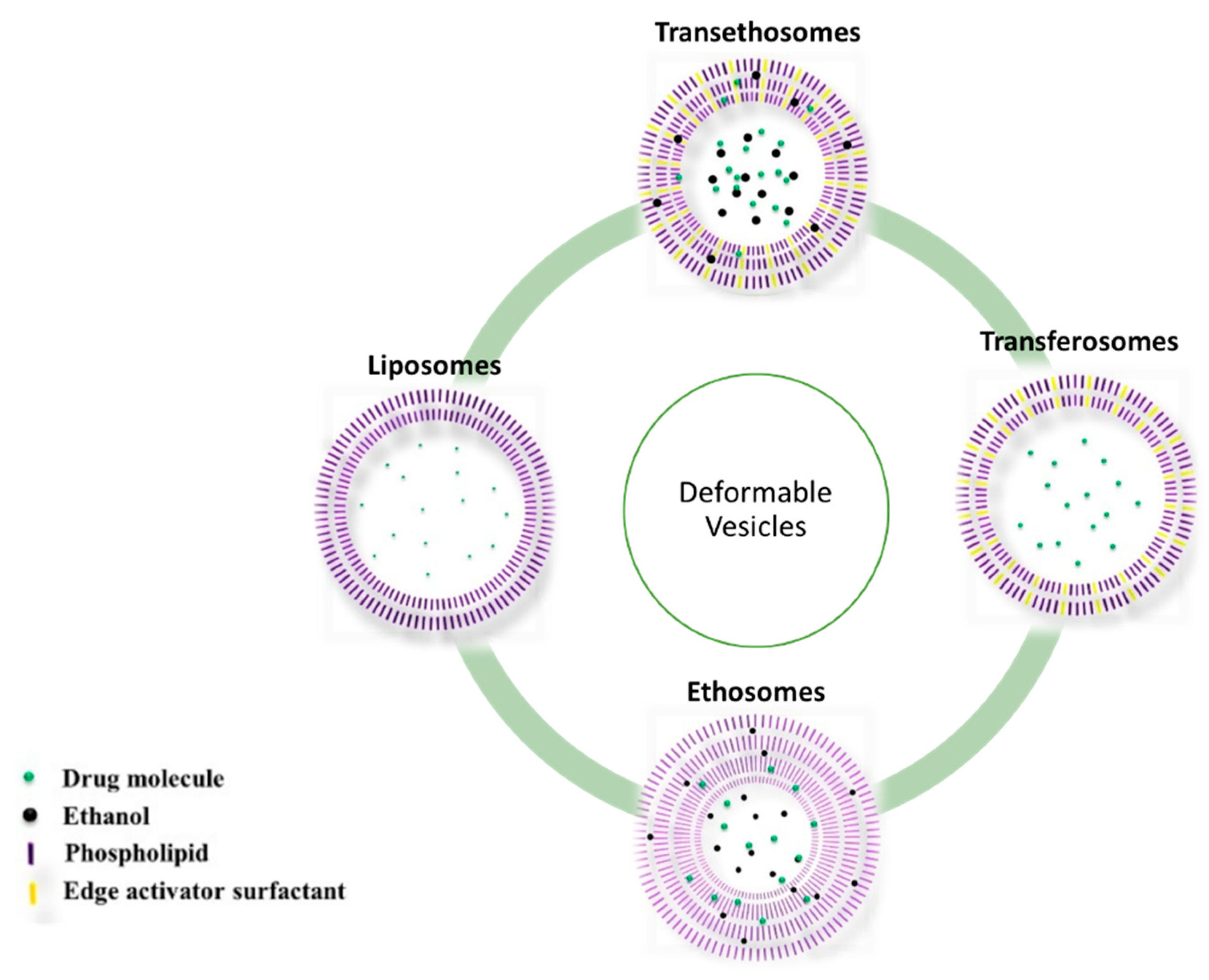
2.1.2. Vesicles
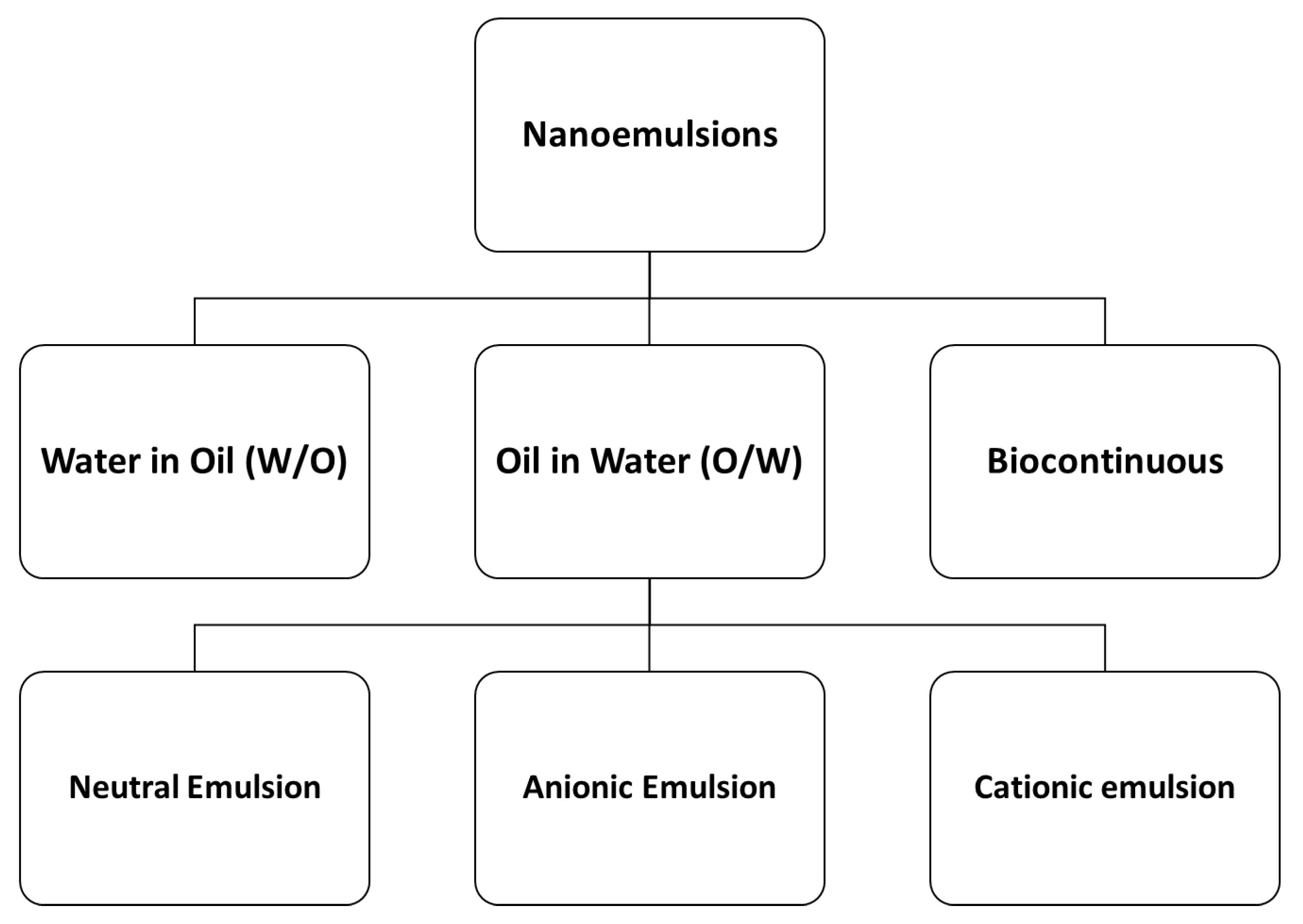
2.1.3. Nanoemulsions (NEs)
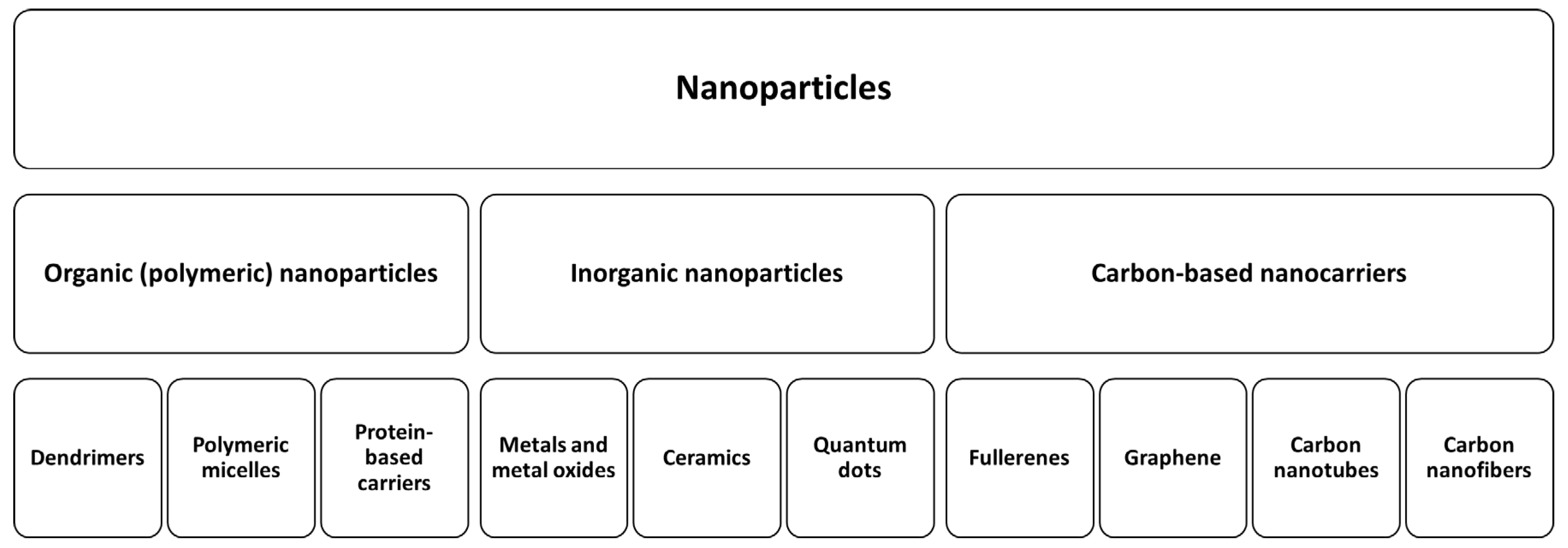
2.1.4. Nanoparticles
2.1.5. Nanocrystals
2.1.6. Solid Dispersions
2.2. Physical Methods for Transdermal Drug Delivery
2.2.1. Electrical Techniques
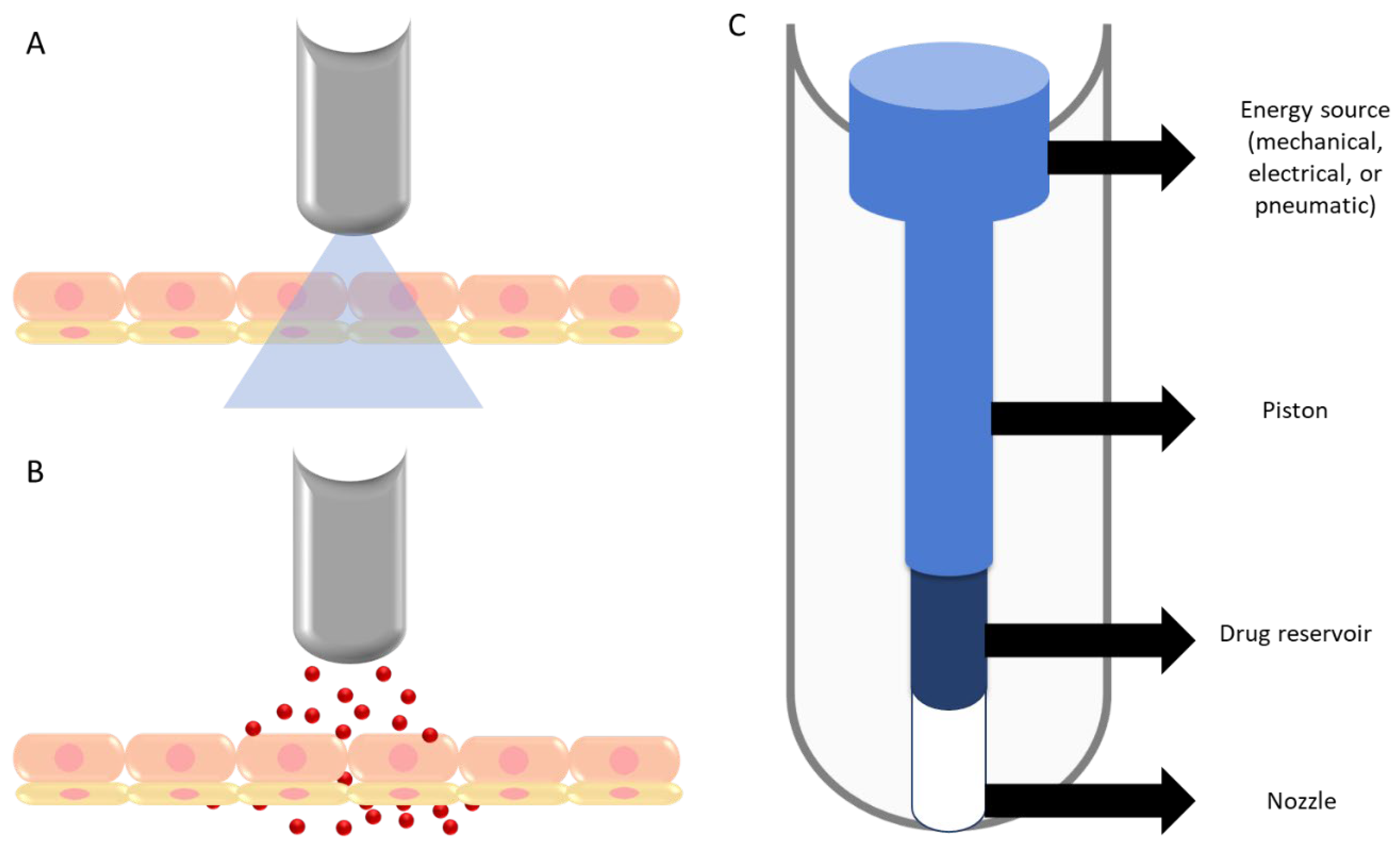
2.2.2. High Pressure-Based Devices
2.2.3. Mechanical Approaches
2.3. Integrating Chemical and Physical Technologies
3. Challenges and Future Prospects
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahoo, D.; Bandaru, R.; Samal, S.K.; Naik, R.; Kumar, P.; Kesharwani, P.; Dandela, R. Chapter 9—Oral drug delivery of nanomedicine. In Theory and Applications of Nonparenteral Nanomedicines; Kesharwani, P., Taurin, S., Greish, K., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 181–207. [Google Scholar]
- He, M.; Zhu, L.; Yang, N.; Li, H.; Yang, Q. Recent advances of oral film as platform for drug delivery. Int. J. Pharm. 2021, 604, 120759. [Google Scholar] [CrossRef]
- Kaur, G.; Arora, M.; Ravi Kumar, M.N.V. Oral Drug Delivery Technologies-A Decade of Developments. J. Pharmacol. Exp. Ther. 2019, 370, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Hayat, U.; Bilal, M.; Iqbal, H.M.N.; Wang, J.-Y. Zein-based micro- and nano-constructs and biologically therapeutic cues with multi-functionalities for oral drug delivery systems. J. Drug Deliv. Sci. Technol. 2020, 58, 101818. [Google Scholar] [CrossRef]
- Gulati, N.; Gupta, H. Parenteral drug delivery: A review. Recent Pat. Drug Deliv. Formul. 2011, 5, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Nagarsenkar, M.S.; Dhawan, V.V. Chapter 29—Parenteral preparations. In Remington (Twenty-third Edition); Adejare, A., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 577–603. [Google Scholar]
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal patches: History, development and pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209. [Google Scholar] [CrossRef] [Green Version]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Sharma, G.; Alle, M.; Chakraborty, C.; Kim, J.C. Strategies for transdermal drug delivery against bone disorders: A preclinical and clinical update. J. Control. Release 2021, 336, 375–395. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kwon, M.; Choi, H.E.; Kim, K.S. Recent advances in transdermal drug delivery systems: A review. Biomater. Res. 2021, 25, 24. [Google Scholar] [CrossRef]
- Jung, J.H.; Jin, S.G. Microneedle for transdermal drug delivery: Current trends and fabrication. J. Pharm. Investig. 2021, 51, 503–517. [Google Scholar] [CrossRef]
- Khoury, R.; Rajamanickam, J.; Grossberg, G.T. An update on the safety of current therapies for Alzheimer’s disease: Focus on rivastigmine. Ther. Adv. Drug Saf. 2018, 9, 171–178. [Google Scholar] [CrossRef]
- Tsuno, N.; Mori, T.; Ishikawa, I.; Bando, N.; Park, H.; Matsumoto, Y.; Mori, I.; Tanaka, M.; Hirano, T.; Nakamura, Y. Efficacy of rivastigmine transdermal therapy on low food intake in patients with Alzheimer’s disease: The Attitude Towards Food Consumption in Alzheimer’s Disease Patients Revive with Rivastigmine Effects study. Geriatr. Gerontol. Int. 2019, 19, 571–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkilani, A.Z.; McCrudden, M.T.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajaj, S.; Whiteman, A.; Brandner, B. Transdermal drug delivery in pain management. Contin. Educ. Anaesth. Crit. Care Pain 2011, 11, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Abrams, L.S.; Skee, D.M.; Natarajan, J.; Wong, F.A.; Anderson, G.D. Pharmacokinetics of a contraceptive patch (Evra/Ortho Evra) containing norelgestromin and ethinyloestradiol at four application sites. Br. J. Clin. Pharmacol. 2002, 53, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Frampton, J.E. Rotigotine Transdermal Patch: A Review in Parkinson’s Disease. CNS Drugs 2019, 33, 707–718. [Google Scholar] [CrossRef]
- Schaer, D.H.; Buff, L.A.; Katz, R.J. Sustained antianginal efficacy of transdermal nitroglycerin patches using an overnight 10-hour nitrate-free interval. Am. J. Cardiol. 1988, 61, 46–50. [Google Scholar] [CrossRef]
- Vartan, C.M. Buprenorphine Transdermal Patch: An Overview for Use in Chronic Pain. US Pharm 2014, 10, 16. [Google Scholar]
- Tanida, N.; Akiyama, K.; Terahara, T. [Pharmacological profile and clinical efficacy of transdermal patch containing emedastine difumarate (ALLESAGA(®) TAPE)]. Nihon Yakurigaku Zasshi. Folia Pharmacol. Jpn. 2018, 152, 246–255. [Google Scholar] [CrossRef]
- Birks, J.S.; Grimley Evans, J. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2015, 4, Cd001191. [Google Scholar] [CrossRef]
- Westhoff, C.L.; Reinecke, I.; Bangerter, K.; Merz, M. Impact of body mass index on suppression of follicular development and ovulation using a transdermal patch containing 0.55-mg ethinyl estradiol/2.1-mg gestodene: A multicenter, open-label, uncontrolled study over three treatment cycles. Contraception 2014, 90, 272–279. [Google Scholar] [CrossRef]
- Yousef, H.; Alhajj, M.; Sharma, S. Anatomy, Skin (Integument), Epidermis; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kahraman, E.; Kaykın, M.; Şahin Bektay, H.; Güngör, S. Recent Advances on Topical Application of Ceramides to Restore Barrier Function of Skin. Cosmetics 2019, 6, 52. [Google Scholar] [CrossRef] [Green Version]
- Bouwstra, J.A.; Groenink, H.W.W.; Kempenaar, J.A.; Romeijn, S.G.; Ponec, M. Water Distribution and Natural Moisturizer Factor Content in Human Skin Equivalents Are Regulated by Environmental Relative Humidity. J. Investig. Dermatol. 2008, 128, 378–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai-Cheong, J.E.; McGrath, J.A. Structure and function of skin, hair and nails. Medicine 2021, 49, 337–342. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Adwan, S.; Khanfar, M.; Idkaidek, N.; Al-Remawi, M. A Novel Eutectic-Based Transdermal Delivery System for Risperidone. AAPS PharmSciTech 2020, 22, 4. [Google Scholar] [CrossRef]
- Richard, C.; Cassel, S.; Blanzat, M. Vesicular systems for dermal and transdermal drug delivery. RSC Adv. 2021, 11, 442–451. [Google Scholar] [CrossRef]
- Moronkeji, K.; Todd, S.; Dawidowska, I.; Barrett, S.D.; Akhtar, R. The role of subcutaneous tissue stiffness on microneedle performance in a representative in vitro model of skin. J. Control. Release 2017, 265, 102–112. [Google Scholar] [CrossRef] [Green Version]
- Karande, P.; Mitragotri, S. Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochim. et Biophys. Acta (BBA) Biomembr. 2009, 1788, 2362–2373. [Google Scholar] [CrossRef] [Green Version]
- Alkilani, A.Z.; Alkalbani, R.; Jaber, D.; Hamed, R.; Hamad, I.; Abumansour, H.; Assab, M.A. Knowledge, attitude, practice and satisfaction of patients using analgesic patches in Jordan. Trop. J. Pharm. Res. 2019, 18, 1745–1753. [Google Scholar]
- Berner, B.; John, V.A. Pharmacokinetic Characterisation of Transdermal Delivery Systems. Clin. Pharmacokinet. 1994, 26, 121–134. [Google Scholar] [CrossRef]
- Parhi, R.; Mandru, A. Enhancement of skin permeability with thermal ablation techniques: Concept to commercial products. Drug Deliv. Transl. Res. 2021, 11, 817–841. [Google Scholar] [CrossRef]
- Kurz, A.; Farlow, M.; Lefèvre, G. Pharmacokinetics of a novel transdermal rivastigmine patch for the treatment of Alzheimer’s disease: A review. Int. J. Clin. Pract. 2009, 63, 799–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, M.E. Skin penetration enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Hamed, R.; Al Baraghthi, T.; Alkilani, A.Z.; Abu-Huwaij, R. Correlation between rheological properties and in vitro drug release from penetration enhancer-loaded Carbopol® gels. J. Pharm. Innov. 2016, 11, 339–351. [Google Scholar] [CrossRef]
- Hao, Y.; Li, W.; Zhou, X.; Yang, F.; Qian, Z. Microneedles-based transdermal drug delivery systems: A review. J. Biomed. Nanotechnol. 2017, 13, 1581–1597. [Google Scholar] [CrossRef]
- Ramadon, D.; McCrudden, M.T.; Courtenay, A.J.; Donnelly, R.F. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv. Transl. Res. 2022, 4, 758–791. [Google Scholar] [CrossRef]
- Akhtar, N.; Singh, V.; Yusuf, M.; Khan, R.A. Non-invasive drug delivery technology: Development and current status of transdermal drug delivery devices, techniques and biomedical applications. Biomed. Eng./Biomed. Tech. 2020, 65, 243–272. [Google Scholar] [CrossRef] [Green Version]
- Subedi, R.K.; Oh, S.Y.; Chun, M.-K.; Choi, H.-K. Recent advances in transdermal drug delivery. Arch. Pharmacal Res. 2010, 33, 339–351. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, J.-H.; Prausnitz, M.R. Dissolving microneedles for transdermal drug delivery. Biomaterials 2008, 29, 2113–2124. [Google Scholar] [CrossRef] [Green Version]
- Finnin, B.C.; Morgan, T.M. Transdermal penetration enhancers: Applications, limitations, and potential. J. Pharm. Sci. 1999, 88, 955–958. [Google Scholar] [CrossRef]
- Arora, A.; Prausnitz, M.R.; Mitragotri, S. Micro-scale devices for transdermal drug delivery. Int. J. Pharm. 2008, 364, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Zorec, B.; Préat, V.; Miklavčič, D.; Pavšelj, N. Active enhancement methods for intra-and transdermal drug delivery: A review. Slov. Med. J. 2013, 82, 5. [Google Scholar]
- Kling, J.; DeFrancesco, L. The paper trail to commercialization. Nat. Biotechnol. 2007, 25, 1217. [Google Scholar] [CrossRef] [PubMed]
- Karande, P.; Jain, A.; Mitragotri, S. Discovery of transdermal penetration enhancers by high-throughput screening. Nat. Biotechnol. 2004, 22, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Bozdaganyan, M.E.; Orekhov, P.S. Synergistic Effect of Chemical Penetration Enhancers on Lidocaine Permeability Revealed by Coarse-Grained Molecular Dynamics Simulations. Membranes 2021, 11, 410. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.W.; Shin, S.C. Enhanced transdermal delivery of atenolol from the ethylene-vinyl acetate matrix. Int. J. Pharm. 2004, 287, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic, N.; Maibach, H.I. Percutaneous Penetration Enhancers Physical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Kanikkannan, N.; Singh, M. Skin permeation enhancement effect and skin irritation of saturated fatty alcohols. Int. J. Pharm. 2002, 248, 219–228. [Google Scholar] [CrossRef]
- Maibach, H.I.; Feldmann, R.J. The effect of DMSO on percutaneous penetration of hydrocortisone and testosterone in man. Ann. N. Y. Acad. Sci. 1967, 141, 423–427. [Google Scholar] [CrossRef]
- Hadgraft, J.; Lane, M.E. Transdermal delivery of testosterone. Eur. J. Pharm. Biopharm. 2015, 92, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, J.; Poduri, R.; Panchagnula, R. Transdermal delivery of naloxone: Ex vivo permeation studies. Int. J. Pharm. 1999, 179, 129–134. [Google Scholar] [CrossRef]
- Liu, C.; Guan, Y.; Tian, Q.; Shi, X.; Fang, L. Transdermal enhancement strategy of ketoprofen and teriflunomide: The effect of enhanced drug-drug intermolecular interaction by permeation enhancer on drug release of compound transdermal patch. Int. J. Pharm. 2019, 572, 118800. [Google Scholar] [CrossRef]
- Ameen, D.; Michniak-Kohn, B. Transdermal delivery of dimethyl fumarate for Alzheimer’s disease: Effect of penetration enhancers. Int. J. Pharm. 2017, 529, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Singh, R.B.; Singh, J. Effects of ionization and penetration enhancers on the transdermal delivery of 5-fluorouracil through excised human stratum corneum. Int. J. Pharm. 2005, 298, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.J.; Langer, R.; Shastri, V.P. Role of n-methyl pyrrolidone in the enhancement of aqueous phase transdermal transport. J. Pharm. Sci. 2005, 94, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Ogiso, T.; Hata, T.; Iwaki, M.; TANINO, T. Transdermal absorption of bupranolol in rabbit skin in vitro and in vivo. Biol. Pharm. Bull. 2001, 24, 588–591. [Google Scholar] [CrossRef] [Green Version]
- Van Zyl, L.; Du Preez, J.; Gerber, M.; Du Plessis, J.; Viljoen, J. Essential fatty acids as transdermal penetration enhancers. J. Pharm. Sci. 2016, 105, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Stott, P.W.; Williams, A.C.; Barry, B.W. Mechanistic study into the enhanced transdermal permeation of a model β-blocker, propranolol, by fatty acids: A melting point depression effect. Int. J. Pharm. 2001, 219, 161–176. [Google Scholar] [CrossRef]
- Klimentová, J.; Kosák, P.; Vávrová, K.; Holas, T.; Hrabálek, A. Influence of terminal branching on the transdermal permeation-enhancing activity in fatty alcohols and acids. Bioorganic Med. Chem. 2006, 14, 7681–7687. [Google Scholar] [CrossRef]
- Melero, A.; Garrigues, T.; Almudever, P.; Martı, A.; Lehr, C.; Schäfer, U. Nortriptyline hydrochloride skin absorption: Development of a transdermal patch. Eur. J. Pharm. Biopharm. 2008, 69, 588–596. [Google Scholar] [CrossRef]
- Haq, A.; Michniak-Kohn, B. Effects of solvents and penetration enhancers on transdermal delivery of thymoquinone: Permeability and skin deposition study. Drug Deliv. 2018, 25, 1943–1949. [Google Scholar] [CrossRef] [Green Version]
- Stahl, J.; Kietzmann, M. The effects of chemical and physical penetration enhancers on the percutaneous permeation of lidocaine through equine skin. BMC Vet. Res. 2014, 10, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ogiso, T.; Iwaki, M.; Paku, T. Effect of various enhancers on transdermal penetration of indomethacin and urea, and relationship between penetration parameters and enhancement factors. J. Pharm. Sci. 1995, 84, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Vijaya, C.; Bingi, M.; Vigneshwaran, L. Transdermal delivery of venlafaxine hydrochloride: The effects of enhancers on permeation across pig ear skin. Indian J. Pharm. Sci. 2011, 73, 456. [Google Scholar] [PubMed]
- Björklund, S.; Engblom, J.; Thuresson, K.; Sparr, E. Glycerol and urea can be used to increase skin permeability in reduced hydration conditions. Eur. J. Pharm. Sci. 2013, 50, 638–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narishetty, S.T.K.; Panchagnula, R. Transdermal delivery of zidovudine: Effect of terpenes and their mechanism of action. J. Control. Release 2004, 95, 367–379. [Google Scholar] [CrossRef]
- Jain, A.K.; Thomas, N.S.; Panchagnula, R. Transdermal drug delivery of imipramine hydrochloride.: I. Effect of terpenes. J. Control. Release 2002, 79, 93–101. [Google Scholar] [CrossRef]
- Nokhodchi, A.; Shokri, J.; Dashbolaghi, A.; Hassan-Zadeh, D.; Ghafourian, T.; Barzegar-Jalali, M. The enhancement effect of surfactants on the penetration of lorazepam through rat skin. Int. J. Pharm. 2003, 250, 359–369. [Google Scholar] [CrossRef]
- Piret, J.; Désormeaux, A.; Cormier, H.; Lamontagne, J.; Gourde, P.; Juhász, J.; Bergeron, M.G. Sodium lauryl sulfate increases the efficacy of a topical formulation of foscarnet against herpes simplex virus type 1 cutaneous lesions in mice. Antimicrob. Agents Chemother. 2000, 44, 2263–2270. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, N.; Rehman, M.; Khan, H.; Rasool, F.; Saeed, T.; Murtaz, G. Penetration enhancing effect of polysorbate 20 and 80 on the in vitro percutaneous absorption of lascorbic acid. Trop. J. Pharm. Res. 2011, 10, 3. [Google Scholar] [CrossRef]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.; Assi, R.A.; Khan, A.A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279–2304. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, P.; Tripathi, P.; Gupta, R.; Pandey, S. Niosomes: A review on niosomal research in the last decade. J. Drug Deliv. Sci. Technol. 2020, 56, 101581. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chacko, I.A.; Ghate, V.M.; Dsouza, L.; Lewis, S.A. Lipid vesicles: A versatile drug delivery platform for dermal and transdermal applications. Colloids Surf. B Biointerfaces 2020, 195, 111262. [Google Scholar] [CrossRef] [PubMed]
- Babaie, S.; Bakhshayesh, A.R.D.; Ha, J.W.; Hamishehkar, H.; Kim, K.H. Invasome: A Novel Nanocarrier for Transdermal Drug Delivery. Nanomaterials 2020, 10, 341. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Jain, V.; Mahajan, S.C. Lipid Based Vesicular Drug Delivery Systems. Adv. Pharm. 2014, 2014, 574673. [Google Scholar] [CrossRef] [Green Version]
- Pandita, A.; Sharma, P. Pharmacosomes: An emerging novel vesicular drug delivery system for poorly soluble synthetic and herbal drugs. ISRN Pharm. 2013, 2013, 348186. [Google Scholar] [CrossRef] [Green Version]
- Witika, B.A.; Mweetwa, L.L.; Tshiamo, K.O.; Edler, K.; Matafwali, S.K.; Ntemi, P.V.; Chikukwa, M.T.R.; Makoni, P.A. Vesicular drug delivery for the treatment of topical disorders: Current and future perspectives. J. Pharm. Pharmacol. 2021, 73, 1427–1441. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.L.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Nakhaei, P.; Margiana, R.; Bokov, D.O.; Abdelbasset, W.K.; Jadidi Kouhbanani, M.A.; Varma, R.S.; Marofi, F.; Jarahian, M.; Beheshtkhoo, N. Liposomes: Structure, Biomedical Applications, and Stability Parameters With Emphasis on Cholesterol. Front. Bioeng. Biotechnol. 2021, 9, 748. [Google Scholar] [CrossRef]
- Nayak, D.; Tippavajhala, V.K. A Comprehensive Review on Preparation, Evaluation and Applications of Deformable Liposomes. Iran. J. Pharm. Res. 2021, 20, 186–205. [Google Scholar]
- Alavi, M.; Karimi, N.; Safaei, M. Application of Various Types of Liposomes in Drug Delivery Systems. Adv. Pharm. Bull. 2017, 7, 3–9. [Google Scholar] [CrossRef]
- Hussain, A.; Singh, S.; Sharma, D.; Webster, T.J.; Shafaat, K.; Faruk, A. Elastic liposomes as novel carriers: Recent advances in drug delivery. Int. J. Nanomed. 2017, 12, 5087–5108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ntimenou, V.; Fahr, A.; Antimisiaris, S.G. Elastic vesicles for transdermal drug delivery of hydrophilic drugs: A comparison of important physicochemical characteristics of different vesicle types. J. Biomed. Nanotechnol. 2012, 8, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Romero, E.L.; Morilla, M.J. Ultradeformable phospholipid vesicles as a drug delivery system: A review. Res. Rep. Transdermal Drug Deliv. 2015, 4, 55–69. [Google Scholar] [CrossRef] [Green Version]
- Sudhakar, K.; Fuloria, S.; Subramaniyan, V.; Sathasivam, K.V.; Azad, A.K.; Swain, S.S.; Sekar, M.; Karupiah, S.; Porwal, O.; Sahoo, A.; et al. Ultraflexible Liposome Nanocargo as a Dermal and Transdermal Drug Delivery System. Nanomaterials 2021, 11, 2557. [Google Scholar] [CrossRef]
- Rai, S.; Pandey, V. Transfersomes as versatile and flexible nano-vesicular carriers in skin cancer therapy: The state of the art. Nano Rev. Exp. 2017, 8, 1325708. [Google Scholar] [CrossRef]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A Promising Nanoencapsulation Technique for Transdermal Drug Delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef]
- Akram, M.W.; Jamshaid, H.; Rehman, F.U.; Zaeem, M.; Khan, J.Z.; Zeb, A. Transfersomes: A Revolutionary Nanosystem for Efficient Transdermal Drug Delivery. AAPS PharmSciTech 2021, 23, 7. [Google Scholar] [CrossRef]
- Duangjit, S.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. Characterization and In Vitro Skin Permeation of Meloxicam-Loaded Liposomes versus Transfersomes. J. Drug Deliv. 2011, 2011, 418316. [Google Scholar] [CrossRef] [Green Version]
- Sardana, V.; Burzynski, J.; Zalzal, P. Safety and efficacy of topical ketoprofen in transfersome gel in knee osteoarthritis: A systematic review. Musculoskelet. Care 2017, 15, 114–121. [Google Scholar] [CrossRef]
- Bnyan, R.; Khan, I.; Ehtezazi, T.; Saleem, I.; Gordon, S.; O’Neill, F.; Roberts, M. Formulation and optimisation of novel transfersomes for sustained release of local anaesthetic. J. Pharm. Pharmacol. 2019, 71, 1508–1519. [Google Scholar] [CrossRef]
- Cevc, G. Transdermal drug delivery of insulin with ultradeformable carriers. Clin. Pharmacokinet. 2003, 42, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Cevc, G.; Blume, G.; Schätzlein, A. Transfersomes-mediated transepidermal delivery improves the regio-specificity and biological activity of corticosteroids in vivo. J. Control. Release 1997, 45, 211–226. [Google Scholar] [CrossRef]
- Chen, S.; Hanning, S.; Falconer, J.; Locke, M.; Wen, J. Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications. Eur. J. Pharm. Biopharm. 2019, 144, 18–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoee, S.; Yaghoobian, M. Chapter 6—Niosomes: A novel approach in modern drug delivery systems. In Nanostructures for Drug Delivery; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 207–237. [Google Scholar]
- Masjedi, M.; Montahaei, T. An illustrated review on nonionic surfactant vesicles (niosomes) as an approach in modern drug delivery: Fabrication, characterization, pharmaceutical, and cosmetic applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102234. [Google Scholar] [CrossRef]
- Durak, S.; Esmaeili Rad, M.; Alp Yetisgin, A. Niosomal Drug Delivery Systems for Ocular Disease-Recent Advances and Future Prospects. Nanomaterials 2020, 10, 1191. [Google Scholar] [CrossRef]
- Ge, X.; Wei, M.; He, S.; Yuan, W.E. Advances of Non-Ionic Surfactant Vesicles (Niosomes) and Their Application in Drug Delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.; Irchhaiya, R. Niosomes: A potential tool for novel drug delivery. J. Pharm. Investig. 2016, 46, 195–204. [Google Scholar] [CrossRef]
- El-Ridy, M.S.; Yehia, S.A.; Mohsen, A.M.; El-Awdan, S.A.; Darwish, A.B. Formulation of Niosomal Gel for Enhanced Transdermal Lornoxicam Delivery: In-Vitro and In-Vivo Evaluation. Curr. Drug Deliv. 2018, 15, 122–133. [Google Scholar] [CrossRef]
- Patel, K.K.; Kumar, P.; Thakkar, H.P. Formulation of niosomal gel for enhanced transdermal lopinavir delivery and its comparative evaluation with ethosomal gel. AAPS PharmSciTech 2012, 13, 1502–1510. [Google Scholar] [CrossRef] [Green Version]
- Honeywell-Nguyen, P.L.; Bouwstra, J.A. The in vitro transport of pergolide from surfactant-based elastic vesicles through human skin: A suggested mechanism of action. J. Control. Release 2003, 86, 145–156. [Google Scholar] [CrossRef]
- El Maghraby, G.M.; Barry, B.W.; Williams, A.C. Liposomes and skin: From drug delivery to model membranes. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2008, 34, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Benson, H.A.E.; Grice, J.E.; Mohammed, Y.; Namjoshi, S.; Roberts, M.S. Topical and Transdermal Drug Delivery: From Simple Potions to Smart Technologies. Curr. Drug Deliv. 2019, 16, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Halnor, V.; Pande, V.; Borawake, D.; Nagare, H. Nanoemulsion: A novel platform for drug delivery system. J. Mat. Sci. Nanotechol. 2018, 6, 104. [Google Scholar]
- Hamed, R.; Basil, M.; AlBaraghthi, T.; Sunoqrot, S.; Tarawneh, O. Nanoemulsion-based gel formulation of diclofenac diethylamine: Design, optimization, rheological behavior and in vitro diffusion studies. Pharm. Dev. Technol. 2016, 21, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Abu-Huwaij, R.; Al-Assaf, S.F.; Hamed, R. Recent exploration of nanoemulsions for drugs and cosmeceuticals delivery. J. Cosmet. Dermatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Hamed, R.; Al-Adhami, Y.; Abu-Huwaij, R. Concentration of a microemulsion influences the mechanical properties of ibuprofen in situ microgels. Int. J. Pharm. 2019, 570, 118684. [Google Scholar] [CrossRef]
- Hamed, R.; Farhan, A.; Abu-Huwaij, R.; Mahmoud, N.N.; Kamal, A. Lidocaine microemulsion-laden organogels as lipid-based systems for topical delivery. J. Pharm. Innov. 2019, 15, 1–14. [Google Scholar] [CrossRef]
- Ganesan, P.; Karthivashan, G.; Park, S.Y.; Kim, J.; Choi, D.-K. Microfluidization trends in the development of nanodelivery systems and applications in chronic disease treatments. Int. J. Nanomed. 2018, 13, 6109. [Google Scholar] [CrossRef] [Green Version]
- Qian, C.; McClements, D.J. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: Factors affecting particle size. Food Hydrocoll. 2011, 25, 1000–1008. [Google Scholar] [CrossRef]
- Hashtjin, A.M.; Abbasi, S. Nano-emulsification of orange peel essential oil using sonication and native gums. Food Hydrocoll. 2015, 44, 40–48. [Google Scholar] [CrossRef]
- Liu, W.; Sun, D.; Li, C.; Liu, Q.; Xu, J. Formation and stability of paraffin oil-in-water nano-emulsions prepared by the emulsion inversion point method. J. Colloid Interface Sci. 2006, 303, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, P.; Esquena, J.; Tadros, T.F.; Dederen, C.; Garcia, M.; Azemar, N.; Solans, C. Formation and stability of nano-emulsions prepared using the phase inversion temperature method. Langmuir 2002, 18, 26–30. [Google Scholar] [CrossRef]
- Azmi, N.A.N.; Elgharbawy, A.A.; Motlagh, S.R.; Samsudin, N.; Salleh, H.M. Nanoemulsions: Factory for Food, Pharmaceutical and Cosmetics. Processes 2019, 7, 617. [Google Scholar] [CrossRef] [Green Version]
- Devarajan, V.; Ravichandran, V. Nanoemulsions: As modified drug delivery tool. Int. J. Compr. Pharm. 2011, 2, 1–6. [Google Scholar]
- Chavda, V.P.; Shah, D. A review on novel emulsification technique: A nanoemulsion. J. Pharmacol. Toxicol. Stud. 2017, 5, 32–33. [Google Scholar]
- Sutradhar, K.B.; Amin, M.L. Nanoemulsions: Increasing possibilities in drug delivery. Eur. J. Nanomed. 2013, 5, 97–110. [Google Scholar] [CrossRef]
- Hamed, R.; Mahmoud, N.N.; Alnadi, S.H.; Alkilani, A.Z.; Hussein, G. Diclofenac diethylamine nanosystems-loaded bigels for topical delivery: Development, rheological characterization, and release studies. Drug Dev. Ind. Pharm. 2020, 46, 1705–1715. [Google Scholar] [CrossRef]
- Koroleva, M.; Nagovitsina, T.; Yurtov, E. Nanoemulsions stabilized by non-ionic surfactants: Stability and degradation mechanisms. Phys. Chem. Chem. Phys. 2018, 20, 10369–10377. [Google Scholar] [CrossRef]
- Fernandes, A.R.; Sanchez-Lopez, E.; Santos, T.d.; Garcia, M.L.; Silva, A.M.; Souto, E.B. Development and Characterization of Nanoemulsions for Ophthalmic Applications: Role of Cationic Surfactants. Materials 2021, 14, 7541. [Google Scholar] [CrossRef]
- Fraga, M.; de Carvalho, T.G.; da Silva Diel, D.; Bruxel, F.; Teixeira, H.F.; Matte, U. Cationic nanoemulsions as a gene delivery system: Proof of concept in the mucopolysaccharidosis I murine model. J. Nanosci. Nanotechnol. 2015, 15, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.; Agrawal, A.; Mateen, H.; Mishra, I.M. Stability of oil-in-water macro-emulsion with anionic surfactant: Effect of electrolytes and temperature. Chem. Eng. Sci. 2013, 102, 176–185. [Google Scholar] [CrossRef]
- Ribeiro, R.C.d.A.; Barreto, S.M.A.G.; Ostrosky, E.A.; Rocha-Filho, P.A.d.; Veríssimo, L.M.; Ferrari, M. Production and characterization of cosmetic nanoemulsions containing Opuntia ficus-indica (L.) Mill extract as moisturizing agent. Molecules 2015, 20, 2492–2509. [Google Scholar] [CrossRef] [Green Version]
- Hamed, R.; Seder, B.Y.; Bardaweel, S.K.; Qawass, H. Lipid-based formulations of microemulsion-loaded oleogels for the oral delivery of carvedilol. J. Dispers. Sci. Technol. 2021, 1–11. [Google Scholar] [CrossRef]
- Praveen Kumar, G.; Divya, A. Nanoemulsion based targeting in cancer therapeutics. Med. Chem. 2015, 5, 272–284. [Google Scholar] [CrossRef]
- Lovelyn, C.; Attama, A.A. Current state of nanoemulsions in drug delivery. J. Biomater. Nanobiotechnol. 2011, 2, 626. [Google Scholar] [CrossRef] [Green Version]
- Shaker, D.S.; Ishak, R.A.; Ghoneim, A.; Elhuoni, M.A. Nanoemulsion: A review on mechanisms for the transdermal delivery of hydrophobic and hydrophilic drugs. Sci. Pharm. 2019, 87, 17. [Google Scholar] [CrossRef] [Green Version]
- Zaid Alkilani, A.; Hamed, R.; Hussein, G.; Alnadi, S. Nanoemulsion-based patch for the dermal delivery of ascorbic acid. J. Dispers. Sci. Technol. 2021, 1–11. [Google Scholar] [CrossRef]
- Zhengguang, L.; Jie, H.; Yong, Z.; Jiaojiao, C.; Xingqi, W.; Xiaoqin, C. Study on the transdermal penetration mechanism of ibuprofen nanoemulsions. Drug Dev. Ind. Pharm. 2019, 45, 465–473. [Google Scholar] [CrossRef]
- Shakeel, F.; Baboota, S.; Ahuja, A.; Ali, J.; Aqil, M.; Shafiq, S. Nanoemulsions as vehicles for transdermal delivery of aceclofenac. AAPS PharmSciTech 2007, 8, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Pathan, I.; Mangle, M.; Bairagi, S. Design and characterization of nanoemulsion for transdermal delivery of meloxicam. Anal. Chem. Lett. 2016, 6, 286–295. [Google Scholar] [CrossRef]
- Khurana, S.; Jain, N.; Bedi, P. Nanoemulsion based gel for transdermal delivery of meloxicam: Physico-chemical, mechanistic investigation. Life Sci. 2013, 92, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, F.; Baboota, S.; Ahuja, A.; Ali, J.; Shafiq, S. Skin permeation mechanism and bioavailability enhancement of celecoxib from transdermally applied nanoemulsion. J. Nanobiotechnol. 2008, 6, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakeel, F.; Baboota, S.; Ahuja, A.; Ali, J.; Shafiq, S. Celecoxib nanoemulsion for transdermal drug delivery: Characterization and in vitro evaluation. J. Dispers. Sci. Technol. 2009, 30, 834–842. [Google Scholar] [CrossRef]
- Kim, B.S.; Won, M.; Yang; Lee, K.M.; Kim, C.S. In vitro permeation studies of nanoemulsions containing ketoprofen as a model drug. Drug Deliv. 2008, 15, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Arora, R.; Aggarwal, G.; Harikumar, S.; Kaur, K. Nanoemulsion based hydrogel for enhanced transdermal delivery of ketoprofen. Adv. Pharm. 2014, 2014. [Google Scholar] [CrossRef]
- Barakat, N.; Fouad, E.; Elmedany, A. Formulation design of indomethacin-loaded nanoemulsion for transdermal delivery. Pharm. Anal. Acta 2011, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- El-Leithy, E.S.; Ibrahim, H.K.; Sorour, R.M. In vitro and in vivo evaluation of indomethacin nanoemulsion as a transdermal delivery system. Drug Deliv. 2015, 22, 1010–1017. [Google Scholar] [CrossRef]
- Shakeel, F.; Ramadan, W.; Ahmed, M.A. Investigation of true nanoemulsions for transdermal potential of indomethacin: Characterization, rheological characteristics, and ex vivo skin permeation studies. J. Drug Target. 2009, 17, 435–441. [Google Scholar] [CrossRef]
- Dhawan, B.; Aggarwal, G.; Harikumar, S. Enhanced transdermal permeability of piroxicam through novel nanoemulgel formulation. Int. J. Pharm. Investig. 2014, 4, 65. [Google Scholar]
- Kumar, D.; Ali, J.; Baboota, S. Omega 3 fatty acid-enriched nanoemulsion of thiocolchicoside for transdermal delivery: Formulation, characterization and absorption studies. Drug Deliv. 2016, 23, 591–600. [Google Scholar] [CrossRef]
- Pratap, S.B.; Brajesh, K.; Jain, S.; Kausar, S. Development and characterization of a nanoemulsion gel formulation for transdermal delivery of carvedilol. Int. J. Drug Dev. Res. 2012, 4, 151–161. [Google Scholar]
- Alkilani, A.Z.; Hamed, R.; Al-Marabeh, S.; Kamal, A.; Abu-Huwaij, R.; Hamad, I. Nanoemulsion-based film formulation for transdermal delivery of carvedilol. J. Drug Deliv. Sci. Tec. 2018, 46, 122–128. [Google Scholar] [CrossRef]
- Aqil, M.; Kamran, M.; Ahad, A.; Imam, S.S. Development of clove oil based nanoemulsion of olmesartan for transdermal delivery: Box–Behnken design optimization and pharmacokinetic evaluation. J. Mol. Liq. 2016, 214, 238–248. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, A.; Harikumar, S. Development and optimization of nanoemulsion based gel for enhanced transdermal delivery of nitrendipine using box-behnken statistical design. Drug Dev. Ind. Pharm. 2020, 46, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, F.; Ramadan, W. Transdermal delivery of anticancer drug caffeine from water-in-oil nanoemulsions. Colloids Surf. B Biointerfaces 2010, 75, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-J.; Fu, Y.-S.; Lin, Y.-H.; Huang, Y.-B.; Wu, P.-C. The effect of nanoemulsion as a carrier of hydrophilic compound for transdermal delivery. PLoS ONE 2014, 9, e102850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Ramachandran, C.; Weiner, N.D.; Roessler, B.J. Topical transport of hydrophilic compounds using water-in-oil nanoemulsions. Int. J. Pharm. 2001, 220, 63–75. [Google Scholar] [CrossRef]
- Harwansh, R.K.; Patra, K.C.; Pareta, S.K.; Singh, J.; Rahman, M.A. Nanoemulsions as vehicles for transdermal delivery of glycyrrhizin. Braz. J. Pharm. Sci. 2011, 47, 769–778. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.S.; Alam, M.S.; Alam, N.; Siddiqui, M.R. Preparation, characterization and stability study of dutasteride loaded nanoemulsion for treatment of benign prostatic hypertrophy. Iran. J. Pharm. Res. 2014, 13, 1125. [Google Scholar]
- Pathan, I.B.; Mallikarjuna Setty, C. Nanoemulsion system for transdermal delivery of tamoxifen citrate: design, characterization, effect of penetration enhancers and in vivo studies. Dig. J. Nanomater. Biostructures (DJNB) 2012, 7, 1373–1387. [Google Scholar]
- Zheng, W.-W.; Zhao, L.; Wei, Y.-M.; Ye, Y.; Xiao, S.-H. Preparation and the in vitro evaluation of nanoemulsion system for the transdermal delivery of granisetron hydrochloride. Chem. Pharm. Bull. 2010, 58, 1015–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Ouyang, W.-Q.; Wei, Y.-P.; Syed, S.F.; Hao, C.-S.; Wang, B.-Z.; Shang, Y.-H. Effects of Carbopol® 934 proportion on nanoemulsion gel for topical and transdermal drug delivery: A skin permeation study. Int. J. Nanomed. 2016, 11, 5971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wais, M.; Samad, A.; Nazish, I.; Khale, A.; Aqil, M.; Khan, M. Formulation Development Ex-Vivo and in-Vivo Evaluation of Nanoemulsion for transdermal delivery of glibenclamide. Int. J. Pharm. Pharm. Sci. 2013, 5, 747–754. [Google Scholar]
- Sandig, A.G.; Campmany, A.C.; Campos, F.F.; Villena, M.M.; Naveros, B.C. Transdermal delivery of imipramine and doxepin from newly oil-in-water nanoemulsions for an analgesic and anti-allodynic activity: Development, characterization and in vivo evaluation. Colloids Surf. B Biointerfaces 2013, 103, 558–565. [Google Scholar] [CrossRef]
- Altamimi, M.; Haq, N.; Alshehri, S.; Qamar, W.; Shakeel, F. Enhanced skin permeation of hydrocortisone using nanoemulsion as potential vehicle. ChemistrySelect 2019, 4, 10084–10091. [Google Scholar] [CrossRef]
- Shaker, D.S.; Ishak, R.A.; Elhuoni, M.A.; Ghoneim, A.M. Boosting transdermal delivery of atorvastatin calcium via o/w nanoemulsifying system: Two-step optimization, ex vivo and in vivo evaluation. Int. J. Pharm. 2020, 578, 119073. [Google Scholar] [CrossRef]
- Abdulbaqi, M.R.; Rajab, N. Apixaban Ultrafine O/W Nano Emulsion Transdermal Drug Delivery System: Formulation, In Vitro and Ex Vivo Characterization. Syst. Rev. Pharm. 2020, 11, 82–94. [Google Scholar]
- Campano-Cuevas, E.; Mora-Boza, A.; Castillo-Dalí, G.; RodríGuez-Gonzalez-Elipe, A.; Serrera-Figallo, M.-A.; Angel, B.; Torres-Lagares, D. Nanotechnology in Medicine: Drug Delivery Systems. In Drug Delivery Approaches and Nanosystems, Volume 1; Apple Academic Press: Cambridge, MA, USA, 2017; pp. 55–93. [Google Scholar]
- Sunoqrot, S.; Hamed, R.; Abdel-Halim, H.; Tarawneh, O. Synergistic Interplay of Medicinal Chemistry and Formulation Strategies in Nanotechnology—From Drug Discovery to Nanocarrier Design and Development. Curr. Top. Med. Chem. 2017, 17, 1451–1468. [Google Scholar] [CrossRef]
- Ealias, A.M.; Saravanakumar, M. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, K.; Makino, K. Chapter 7—Nanoparticles for transdermal drug delivery system (TDDS). In Colloid and Interface Science in Pharmaceutical Research and Development; Ohshima, H., Makino, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 131–147. [Google Scholar]
- Palmer, B.C.; DeLouise, L.A. Nanoparticle-Enabled Transdermal Drug Delivery Systems for Enhanced Dose Control and Tissue Targeting. Molecules 2016, 21, 1719. [Google Scholar] [CrossRef] [PubMed]
- Lohcharoenkal, W.; Wang, L.; Chen, Y.C.; Rojanasakul, Y. Protein nanoparticles as drug delivery carriers for cancer therapy. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdivia-Olivares, R.Y.; Rodriguez-Fernandez, M. The Importance of Nanocarrier Design and Composition for an Efficient Nanoparticle-Mediated Transdermal Vaccination. Vaccines 2021, 9, 1420. [Google Scholar] [CrossRef]
- Zhang, K.; Zhuang, Y.; Li, J.; Liu, X.; He, S. Poly(Acrylic Acid)-Modified MoS2 Nanoparticle-Based Transdermal Delivery of Atenolol. Int. J. Nanomed. 2020, 15, 5517–5526. [Google Scholar] [CrossRef]
- Zhao, Q.H.; Zhang, Y.; Liu, Y.; Wang, H.L.; Shen, Y.Y.; Yang, W.J.; Wen, L.P. Anticancer effect of realgar nanoparticles on mouse melanoma skin cancer in vivo via transdermal drug delivery. Med. Oncol. 2010, 27, 203–212. [Google Scholar] [CrossRef]
- Sunoqrot, S.; Mahmoud, N.N.; Ibrahim, L.H.; Al-Dabash, S.; Raschke, H.; Hergenröder, R. Tuning the Surface Chemistry of Melanin-Mimetic Polydopamine Nanoparticles Drastically Enhances Their Accumulation into Excised Human Skin. ACS Biomater. Sci. Eng. 2020, 6, 4424–4432. [Google Scholar] [CrossRef]
- Al-Nemrawi, N.; Nimrawi, S. A novel formulation of chitosan nanoparticles functionalized with titanium dioxide nanoparticles. J. Adv. Pharm. Technol. Res. 2021, 12, 402–407. [Google Scholar] [CrossRef]
- Nabipour, Z.; Nourbakhsh, M.S.; Baniasadi, M. Evaluation of ibuprofen release from gelatin/hydroxyapatite/polylactic acid nanocomposites. Iran. J. Pharm. Sci. 2018, 14, 75–84. [Google Scholar]
- Rasouli, R.; Barhoum, A.; Bechelany, M.; Dufresne, A. Nanofibers for biomedical and healthcare applications. Macromol. Biosci. 2019, 19, 1800256. [Google Scholar] [CrossRef]
- Astete, C.E.; Sabliov, C.M. Synthesis and characterization of PLGA nanoparticles. J. Biomater. Sci. Polym. Ed. 2006, 17, 247–289. [Google Scholar] [CrossRef] [PubMed]
- Rancan, F.; Papakostas, D.; Hadam, S.; Hackbarth, S.; Delair, T.; Primard, C.; Verrier, B.; Sterry, W.; Blume-Peytavi, U.; Vogt, A. Investigation of Polylactic Acid (PLA) Nanoparticles as Drug Delivery Systems for Local Dermatotherapy. Pharm. Res. 2009, 26, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-C.; Wu, Q.; Song, K.; Lee, S.; Qing, Y.; Wu, Y. Cellulose Nanoparticles: Structure–Morphology–Rheology Relationships. ACS Sustain. Chem. Eng. 2015, 3, 821–832. [Google Scholar] [CrossRef]
- Vauthier, C.; Bouchemal, K. Methods for the Preparation and Manufacture of Polymeric Nanoparticles. Pharm. Res. 2009, 26, 1025–1058. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.N.; Le, T.G.; Dao, T.T.T.; Le, T.H.; Dinh, T.T.H.; Nguyen, D.H.; Tran, T.C.; Nguyen, C.N. Development of Itraconazole-Loaded Polymeric Nanoparticle Dermal Gel for Enhanced Antifungal Efficacy. J. Nanomater. 2020, 2020. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kim, S.; Lee, S.Y.; Lee, H.; Han, D.W.; Yang, S.Y.; Kim, K.S. Transdermal delivery of Minoxidil using HA-PLGA nanoparticles for the treatment in alopecia. Biomater. Res. 2019, 23, 16. [Google Scholar] [CrossRef]
- Wang, M.; Marepally, S.; Vemula, P.; Xu, C. Inorganic nanoparticles for transdermal drug delivery and topical application. In Nanoscience in Dermatology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 57–72. [Google Scholar]
- Abu-Huwaij, R.; Abbas, M.M.; Al-Shalabi, R.; Almasri, F.N. Synthesis of transdermal patches loaded with greenly synthesized zinc oxide nanoparticles and their cytotoxic activity against triple negative breast cancer. Appl. Nanosci. 2022, 12, 69–78. [Google Scholar] [CrossRef]
- Naser, R.; Abu-Huwaij, R.; Al-khateeb, I.; Abbas, M.M.; Atoom, A.M. Green synthesis of zinc oxide nanoparticles using the root hair extract of Phoenix dactylifera: Antimicrobial and anticancer activity. Appl. Nanosci. 2021, 11, 1747–1757. [Google Scholar] [CrossRef]
- Abu-Huwaij, R.; Al-Assaf, S.F.; Mousli, F.; Kutkut, M.S.; Al-Bashtawi, A. Perceptive review on properties of iron oxide nanoparticles and their antimicrobial and anticancer activity. Sys. Rev. Pharm. 2020, 11, 418–431. [Google Scholar]
- Manivasagan, P.; Bharathiraja, S.; Moorthy, M.S.; Oh, Y.-O.; Seo, H.; Oh, J. Marine biopolymer-based nanomaterials as a novel platform for theranostic applications. Polym. Rev. 2017, 57, 631–667. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Hamed, R.; Khalil, E.A. Colloidal stability and rheological properties of gold nanoparticle–loaded polymeric hydrogels: Impact of nanoparticle’s shape, surface modification, and concentration. Colloid Polym. Sci. 2020, 298, 989–999. [Google Scholar] [CrossRef]
- Wang, M.; Marepally, S.K.; Vemula, P.K.; Xu, C. Chapter 5—Inorganic Nanoparticles for Transdermal Drug Delivery and Topical Application. In Nanoscience in Dermatology; Hamblin, M.R., Avci, P., Prow, T.W., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 57–72. [Google Scholar]
- Labouta, H.I.; Schneider, M. Interaction of inorganic nanoparticles with the skin barrier: Current status and critical review. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.L.; Elmotasem, H.; Salama, A.A.A. Colchicine mesoporous silica nanoparticles/hydrogel composite loaded cotton patches as a new encapsulator system for transdermal osteoarthritis management. Int. J. Biol. Macromol. 2020, 164, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.K.; Srivastava, R. Drug Delivery With Carbon-Based Nanomaterials as Versatile Nanocarriers: Progress and Prospects. Front. Nanotechnol. 2021, 3, 644564. [Google Scholar] [CrossRef]
- Strasinger, C.; Paudel, K.S.; Wu, J.; Hammell, D.; Pinninti, R.R.; Hinds, B.J.; Stinchcomb, A. Programmable Transdermal Clonidine Delivery Through Voltage-Gated Carbon Nanotube Membranes. J. Pharm. Sci. 2014, 103, 1829–1838. [Google Scholar] [CrossRef] [Green Version]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Vehring, R. Pharmaceutical particle engineering via spray drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef] [Green Version]
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J.H. Strategies to address low drug solubility in discovery and development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef]
- Kolter, K.; Karl, M.; Gryczke, A. Introduction to Solid Dispersions. In Hot-Melt Extrusion with BASF Pharma Polymers. 2012, pp. 9–18. Available online: https://pharma.basf.com/solutions/solubilization/solid-dispersions/hot-melt-extrusion (accessed on 15 March 2022).
- Gigliobianco, M.R.; Casadidio, C.; Censi, R.; Martino, P.D. Nanocrystals of Poorly Soluble Drugs: Drug Bioavailability and Physicochemical Stability. Pharmaceutics 2018, 10, 134. [Google Scholar] [CrossRef] [Green Version]
- Junghanns, J.-U.A.H.; Müller, R.H. Nanocrystal technology, drug delivery and clinical applications. Int. J. Nanomed. 2008, 3, 295. [Google Scholar]
- Chen, L.; Wang, Y.; Zhang, J.; Hao, L.; Guo, H.; Lou, H.; Zhang, D. Bexarotene nanocrystal-Oral and parenteral formulation development, characterization and pharmacokinetic evaluation. Eur. J. Pharm. Biopharm. 2014, 87, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Staufenbiel, S.; Rühl, E.; Bodmeier, R. In situ determination of the saturation solubility of nanocrystals of poorly soluble drugs for dermal application. Int. J. Pharm. 2017, 521, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Ige, P.P.; Baria, R.K.; Gattani, S.G. Fabrication of fenofibrate nanocrystals by probe sonication method for enhancement of dissolution rate and oral bioavailability. Colloids Surf. B Biointerfaces 2013, 108, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hu, J.; Sui, H.; Zhao, Q.; Zhang, X.; Wang, W. Enhanced skin permeation of glabridin using eutectic mixture-based nanoemulsion. Drug Deliv. Transl. Res. 2017, 7, 325–332. [Google Scholar] [CrossRef]
- Fu, Q.; Sun, J.; Ai, X.; Zhang, P.; Li, M.; Wang, Y.; Liu, X.; Sun, Y.; Sui, X.; Sun, L.; et al. Nimodipine nanocrystals for oral bioavailability improvement: Role of mesenteric lymph transport in the oral absorption. Int. J. Pharm. 2013, 448, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Mauludin, R.; Müller, R.H.; Keck, C.M. Development of an oral rutin nanocrystal formulation. Int. J. Pharm. 2009, 370, 202–209. [Google Scholar] [CrossRef]
- Yu, L. Amorphous pharmaceutical solids: Preparation, characterization and stabilization. Adv. Drug Deliv. Rev. 2001, 48, 27–42. [Google Scholar] [CrossRef]
- Cerdeira, A.M.; Mazzotti, M.; Gander, B. Miconazole nanosuspensions: Influence of formulation variables on particle size reduction and physical stability. Int. J. Pharm. 2010, 396, 210–218. [Google Scholar] [CrossRef]
- Mitri, K.; Shegokar, R.; Gohla, S.; Anselmi, C.; Müller, R.H. Lutein nanocrystals as antioxidant formulation for oral and dermal delivery. Int. J. Pharm. 2011, 420, 141–146. [Google Scholar] [CrossRef]
- Müller, R.H.; Zhai, X.; Romero, G.B.; Keck, C.M. Nanocrystals for Passive Dermal Penetration Enhancement. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Nanocarriers; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 283–295. [Google Scholar]
- Parmar, P.K.; Wadhawan, J.; Bansal, A.K. Pharmaceutical nanocrystals: A promising approach for improved topical drug delivery. Drug Discov. Today 2021, 26, 2329–2349. [Google Scholar] [CrossRef]
- Zhou, H.; Luo, D.; Chen, D.; Tan, X.; Bai, X.; Liu, Z.; Yang, X.; Liu, W. Current Advances of Nanocarrier Technology-Based Active Cosmetic Ingredients for Beauty Applications. Clin. Cosmet. Investig. Dermatol. 2021, 14, 867–887. [Google Scholar] [CrossRef] [PubMed]
- Eckert, R.W.; Wiemann, S.; Keck, C.M. Improved dermal and transdermal delivery of curcumin with smartfilms and nanocrystals. Molecules 2021, 26, 1633. [Google Scholar] [CrossRef] [PubMed]
- Wadhawan, J.; Parmar, P.K.; Bansal, A.K. Nanocrystals for improved topical delivery of medium soluble drug: A case study of acyclovir. J. Drug Deliv. Sci. Technol. 2021, 65, 102662. [Google Scholar] [CrossRef]
- Khan, B.A.; Rashid, F.; Khan, M.K.; Alqahtani, S.S.; Sultan, M.H.; Almoshari, Y. Fabrication of capsaicin loaded nanocrystals: Physical characterizations and in vivo evaluation. Pharmaceutics 2021, 13, 841. [Google Scholar] [CrossRef]
- Tekko, I.A.; Permana, A.D.; Vora, L.; Hatahet, T.; McCarthy, H.O.; Donnelly, R.F. Localised and sustained intradermal delivery of methotrexate using nanocrystal-loaded microneedle arrays: Potential for enhanced treatment of psoriasis. Eur. J. Pharm. Sci. 2020, 152, 105469. [Google Scholar] [CrossRef] [PubMed]
- Avasatthi, V.; Pawar, H.; Dora, C.P.; Bansod, P.; Gill, M.S.; Suresh, S. A novel nanogel formulation of methotrexate for topical treatment of psoriasis: Optimization, in vitro and in vivo evaluation. Pharm. Dev. Technol. 2016, 21, 554–562. [Google Scholar] [CrossRef]
- Parmar, P.K.; Bansal, A.K. Novel nanocrystal-based formulations of apremilast for improved topical delivery. Drug Deliv. Transl. Res. 2021, 11, 966–983. [Google Scholar] [CrossRef]
- Kumar, M.; Shanthi, N.; Mahato, A.K.; Soni, S.; Rajnikanth, P.S. Preparation of luliconazole nanocrystals loaded hydrogel for improvement of dissolution and antifungal activity. Heliyon 2019, 5, e01688. [Google Scholar] [CrossRef] [Green Version]
- Döge, N.; Hönzke, S.; Schumacher, F.; Balzus, B.; Colombo, M.; Hadam, S.; Rancan, F.; Blume-Peytavi, U.; Schäfer-Korting, M.; Schindler, A.; et al. Ethyl cellulose nanocarriers and nanocrystals differentially deliver dexamethasone into intact, tape-stripped or sodium lauryl sulfate-exposed ex vivo human skin—Assessment by intradermal microdialysis and extraction from the different skin layers. J. Control. Release 2016, 242, 25–34. [Google Scholar] [CrossRef]
- Wang, W.P.; Hul, J.; Sui, H.; Zhao, Y.S.; Feng, J.; Liu, C. Glabridin nanosuspension for enhanced skin penetration: Formulation optimization, in vitro and in vivo evaluation. Pharmazie 2016, 71, 252–257. [Google Scholar]
- Assem, M.; Khowessah, O.M.; Ghorab, D. Nano-crystallization as a tool for the enhancement of beclomethasone dipropionate dermal deposition: Formulation, in vitro characterization and ex vivo study. J. Drug Deliv. Sci. Technol. 2019, 54, 101318. [Google Scholar] [CrossRef]
- Oktay, A.N.; Ilbasmis-Tamer, S.; Han, S.; Uludag, O.; Celebi, N. Preparation and in vitro/in vivo evaluation of flurbiprofen nanosuspension-based gel for dermal application. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2020, 155, 105548. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, S.; Tekko, I.A.; Vora, L.; Larrañeta, E.; Permana, A.D.; Donnelly, R.F. Nanosuspension-based dissolving microneedle arrays for intradermal delivery of curcumin. Pharmaceutics 2019, 11, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamed, R.; Mohamed, E.M.; Sediri, K.; Khan, M.A.; Rahman, Z. Development of stable amorphous solid dispersion and quantification of crystalline fraction of lopinavir by spectroscopic-chemometric methods. Int. J. Pharm. 2021, 602, 120657. [Google Scholar] [CrossRef]
- Chiou, W.L.; Riegelman, S. Pharmaceutical applications of solid dispersion systems. J. Pharm. Sci. 1971, 60, 1281–1302. [Google Scholar] [CrossRef]
- Zhao, M.; Barker, S.A.; Belton, P.S.; McGregor, C.; Craig, D.Q.M. Development of fully amorphous dispersions of a low Tg drug via co-spray drying with hydrophilic polymers. Eur. J. Pharm. Biopharm. 2012, 82, 572–579. [Google Scholar] [CrossRef]
- Alhijjaj, M.; Belton, P.; Qi, S. An investigation into the use of polymer blends to improve the printability of and regulate drug release from pharmaceutical solid dispersions prepared via fused deposition modeling (FDM) 3D printing. Eur. J. Pharm. Biopharm. 2016, 108, 111–125. [Google Scholar] [CrossRef] [Green Version]
- Brough, C.; Williams, R.O. Amorphous solid dispersions and nano-crystal technologies for poorly water-soluble drug delivery. Int. J. Pharm. 2013, 453, 157–166. [Google Scholar] [CrossRef]
- Friesen, D.T.; Shanker, R.; Crew, M.; Smithey, D.T.; Curatolo, W.J.; Nightingale, J.A.S. Hydroxypropyl methylcellulose acetate succinate-based spray-dried dispersions: An overview. Mol. Pharm. 2008, 5, 1003–1019. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Dai, W.-G. Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharm. Sin. B 2014, 4, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Sarode, A.L.; Sandhu, H.; Shah, N.; Malick, W.; Zia, H. Hot melt extrusion (HME) for amorphous solid dispersions: Predictive tools for processing and impact of drug-polymer interactions on supersaturation. Eur. J. Pharm. Sci. 2013, 48, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Obaidat, R.M.; Khanfar, M.; Ghanma, R. A Comparative Solubility Enhancement Study of Cefixime Trihydrate Using Different Dispersion Techniques. AAPS PharmSciTech 2019, 20, 194. [Google Scholar] [CrossRef] [PubMed]
- Gato, K.; Fujii, M.Y.; Hisada, H.; Carriere, J.; Koide, T.; Fukami, T. Molecular state evaluation of active pharmaceutical ingredients in adhesive patches for transdermal drug delivery. J. Drug Deliv. Sci. Technol. 2020, 58, 101800. [Google Scholar] [CrossRef]
- Jermain, S.V.; Brough, C.; Williams, R.O. Amorphous solid dispersions and nanocrystal technologies for poorly water-soluble drug delivery—An update. Int. J. Pharm. 2018, 535, 379–392. [Google Scholar] [CrossRef]
- Peltonen, L.; Strachan, C.J. Degrees of order: A comparison of nanocrystal and amorphous solids for poorly soluble drugs. Int. J. Pharm. 2020, 586, 119492. [Google Scholar] [CrossRef]
- Yani, F.; Arianto, A.; Noersal, R. Formulation of Ketoprofen Transdermal Solid Dispersion Patch as an Analgesic and Anti-Inflammatory. Asian J. Pharm. Res. Dev. 2020, 8, 51–58. [Google Scholar] [CrossRef]
- Marreto, R.N.; Cardoso, G.; dos Santos Souza, B.; Martin-Pastor, M.; Cunha-Filho, M.; Taveira, S.F.; Concheiro, A.; Alvarez-Lorenzo, C. Hot melt-extrusion improves the properties of cyclodextrin-based poly(pseudo)rotaxanes for transdermal formulation. Int. J. Pharm. 2020, 586, 119510. [Google Scholar] [CrossRef] [PubMed]
- Azizoğlu, E.; Özer, Ö. Fabrication of Montelukast sodium loaded filaments and 3D printing transdermal patches onto packaging material. Int. J. Pharm. 2020, 587, 119588. [Google Scholar] [CrossRef]
- Chaudhari, V.S.; Malakar, T.K.; Murty, U.S.; Banerjee, S. Extruded filaments derived 3D printed medicated skin patch to mitigate destructive pulmonary tuberculosis: Design to delivery. Expert Opin. Drug Deliv. 2021, 18, 301–313. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Bhatia, S.; Singh, S. Transdermal Delivery of Ondansetron Hydrochloride in the management of Hyperemesis Gravidarum. Int. J. Pharm. Sci. Res. 2020, 11, 206–214. [Google Scholar]
- Soral, M.; Nanjappa, S.H.; Alayadan, P. Formulation and evaluation of transdermal patch of rabeprazole sodium. J. Rep. Pharm. Sci. 2021, 10, 240. [Google Scholar] [CrossRef]
- Lakshmi, S.S.; Rao, Y.S.; Asha, D.; Kumari, P.K.; Mallikarjun, P. Formulation and evaluation of rosuvastatin-calcium drug transdermal patch. Res. J. Pharm. Technol. 2020, 13, 4784–4790. [Google Scholar] [CrossRef]
- Parhi, R.; Suresh, P.; Patnaik, S. Physical means of stratum corneum barrier manipulation to enhance transdermal drug delivery. Curr. Drug Deliv. 2015, 12, 122–138. [Google Scholar] [CrossRef]
- Abd-Elghany, A.A.; Mohamad, E.A. Ex-vivo transdermal delivery of Annona squamosa entrapped in niosomes by electroporation. J. Radiat. Res. Appl. Sci. 2020, 13, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Anantrao, J.H.; Nath, P.A.; Nivrutti, P.R. Drug Penetration Enhancement Techniques in Transdermal Drug Delivery System: A Review. J. Pharm. Res. Int. 2021, 46–61. [Google Scholar] [CrossRef]
- Ita, K. Perspectives on transdermal electroporation. Pharmaceutics 2016, 8, 9. [Google Scholar] [CrossRef]
- Demiryurek, Y.; Nickaeen, M.; Zheng, M.; Yu, M.; Zahn, J.D.; Shreiber, D.I.; Lin, H.; Shan, J.W. Transport, resealing, and re-poration dynamics of two-pulse electroporation-mediated molecular delivery. Acta (BBA)-Biomembr. 2015, 1848, 1706–1714. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zeng, L.; Song, W.; Liu, J. Influencing factors and drug application of iontophoresis in transdermal drug delivery: An overview of recent progress. Drug Deliv. Transl. Res. 2021, 12, 15–26. [Google Scholar] [CrossRef]
- Ita, K. Percutaneous transport of psychotropic agents. J. Drug Deliv. Sci. Technol. 2017, 39, 247–259. [Google Scholar] [CrossRef]
- Petrilli, R.; Lopez, R.F.V. Physical methods for topical skin drug delivery: Concepts and applications. Braz. J. Pharm. Sci. 2018, 54. [Google Scholar] [CrossRef]
- Dixit, N.; Bali, V.; Baboota, S.; Ahuja, A.; Ali, J. Iontophoresis-an approach for controlled drug delivery: A review. Curr. Drug Deliv. 2007, 4, 1–10. [Google Scholar] [PubMed]
- Pikal, M.J. The role of electroosmotic flow in transdermal iontophoresis. Adv. Drug Deliv. Rev. 2001, 46, 281–305. [Google Scholar] [CrossRef]
- Dhote, V.; Bhatnagar, P.; Mishra, P.K.; Mahajan, S.C.; Mishra, D.K. Iontophoresis: A potential emergence of a transdermal drug delivery system. Sci. Pharm. 2012, 80, 1–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saepang, K.; Li, S.K.; Chantasart, D. Effect of pH on iontophoretic transport of pramipexole dihydrochloride across human epidermal membrane. Pharm. Res. 2021, 38, 657–668. [Google Scholar] [CrossRef]
- Monti, D.; Egiziano, E.; Burgalassi, S.; Tampucci, S.; Terreni, E.; Tivegna, S.; Chetoni, P. Influence of a combination of chemical enhancers and iontophoresis on in vitro transungual permeation of nystatin. AAPS PharmSciTech 2018, 19, 1574–1581. [Google Scholar] [CrossRef]
- Giri, T.K.; Chakrabarty, S.; Ghosh, B.J.C.P.A. Non-Invasive Extraction of Gabapentin for Therapeutic Drug Monitoring by Reverse Iontophoresis: Effect of pH, Ionic Strength, and Polyethylene Glycol 400 in the Receiving Medium. Curr. Pharm. Anal. 2019, 15, 632–639. [Google Scholar] [CrossRef]
- Azad Khan, M.Y.; Asif, M.; Chauhan, I.; Singh, A.P.; Sharma, R.; Singh, P.; Rai, S.J. Iontophoretic drug delivery: History and applications. J. Ocul. Pharmacol. Ther. 2011, 1, 11–24. [Google Scholar]
- Chen, K.; Puri, V.; Michniak-Kohn, B. Iontophoresis to Overcome the Challenge of Nail Permeation: Considerations and Optimizations for Successful Ungual Drug Delivery. AAPS J. 2021, 23, 1–15. [Google Scholar] [CrossRef]
- Banga, A.K. Electrically Assisted Transdermal and Topical Drug Delivery; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Michov, B. 4 Iontophoresis. In Electrophoresis; De Gruyter: Berlin, Germany, 2020; pp. 739–752. [Google Scholar]
- Perez, V.L.; Wirostko, B.; Korenfeld, M.; From, S.; Raizman, M. Ophthalmic drug delivery using iontophoresis: Recent clinical applications. J. Ocul. Pharmacol. 2020, 36, 75–87. [Google Scholar] [CrossRef]
- Schoellhammer, C.M.; Blankschtein, D.; Langer, R. Skin permeabilization for transdermal drug delivery: Recent advances and future prospects. Expert Opin. Drug Deliv. 2014, 11, 393–407. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, G.; Roberts, M.S.; Grice, J.; Anissimov, Y.G.; Moghimi, H.R.; Benson, H.A. Iontophoretic skin permeation of peptides: An investigation into the influence of molecular properties, iontophoretic conditions and formulation parameters. Drug Deliv. Transl. Res. 2014, 4, 222–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpiński, T.M. Selected medicines used in iontophoresis. Pharmaceutics 2018, 10, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatima, T.; Ajjarapu, S.; Shankar, V.K.; Rangappa, S.; Shivakumar, H.N.; Biswas, S.K.; Hoque, M.; Murthy, S. Topical pilocarpine formulation for diagnosis of cystic fibrosis. J. Pharm. Sci. 2020, 109, 1747–1751. [Google Scholar] [CrossRef] [PubMed]
- Yengin, C.; Kilinc, E.; Der, F.G.; Sezgin, M.C.; Alcin, I. Optimization of Extraction Parameters of Reverse Iontophoretic Determination of Blood Glucose in an Artificial Skin Model. Curr. Anal. Chem. 2020, 16, 722–737. [Google Scholar] [CrossRef]
- Lemke, J.; Sardariani, E.; Phipps, J.B.; Patel, N.; Itri, L.M.; Caravelli, J.; Viscusi, E.R. Fentanyl Iontophoretic Transdermal System (IONSYS®) can be Safely used in the Hospital Environment with X-Rays, Computerized Tomography and Radiofrequency Identification Devices. Adv. Ther. 2016, 33, 1649–1659. [Google Scholar] [CrossRef] [Green Version]
- Vikelis, M.; Mitsikostas, D.D.; Rapoport, A.M. Sumatriptan iontophoretic transdermal system for the acute treatment of migraine. Pain Manag. 2014, 4, 123–128. [Google Scholar] [CrossRef]
- Scott, J.A.; Banga, A.K. Cosmetic devices based on active transdermal technologies. Ther. Deliv. 2015, 6, 1089–1099. [Google Scholar] [CrossRef]
- Roustit, M.; Blaise, S.; Cracowski, J.L. Trials and tribulations of skin iontophoresis in therapeutics. Br. J. Clin. Pharmacol. 2014, 77, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Bian, Q.; Xu, Y.; Xu, D.; Gao, J. Recent advances in mechanical force-assisted transdermal delivery of macromolecular drugs. Int. J. Pharm. 2021, 602, 120598. [Google Scholar] [CrossRef]
- Marwah, H.; Garg, T.; Goyal, A.K.; Rath, G. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 2016, 23, 564–578. [Google Scholar] [CrossRef]
- Miyazaki, H.; Atobe, S.; Suzuki, T.; Iga, H.; Terai, K. Development of pyro-drive jet injector with controllable jet pressure. J. Pharm. Sci. 2019, 108, 2415–2420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, A. Liquid and powder jet injectors in drug delivery: Mechanisms, designs, and applications. In Percutaneous Penetration Enhancers Physical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2017; pp. 221–230. [Google Scholar]
- Mitragotri, S. Current status and future prospects of needle-free liquid jet injectors. Nat. Rev. Drug Discov. 2006, 5, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Kale, T.R.; Momin, M. Needle free injection technology-An overview. Inov. Pharm. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Trimzi, M.A.; Ham, Y.-B. A Needle-Free Jet Injection System for Controlled Release and Repeated Biopharmaceutical Delivery. Pharmaceutics 2021, 13, 1770. [Google Scholar] [CrossRef]
- Kendall, M.; Mitchell, T.; Wrighton-Smith, P. Intradermal ballistic delivery of micro-particles into excised human skin for pharmaceutical applications. J. Biomech. 2004, 37, 1733–1741. [Google Scholar] [CrossRef]
- Mulholland, W.J.; Kendall, M.A.; White, N.; Bellhouse, B.J. Characterization of powdered epidermal vaccine delivery with multiphoton microscopy. Phys. Med. Biol. 2004, 49, 5043. [Google Scholar] [CrossRef]
- Mathur, V.; Satrawala, Y.; Rajput, M.S. Physical and chemical penetration enhancers in transdermal drug delivery system. Asian J. Pharm. (AJP) 2014, 4. [Google Scholar] [CrossRef]
- Khan, S.; Hasan, A.; Attar, F.; Babadaei, M.M.N.; Zeinabad, H.A.; Salehi, M.; Alizadeh, M.; Hassan, M.; Derakhshankhah, H.; Hamblin, M.R.; et al. Diagnostic and drug release systems based on microneedle arrays in breast cancer therapy. J. Control. Release 2021, 338, 341–357. [Google Scholar] [CrossRef]
- Nagarkar, R.; Singh, M.; Nguyen, H.X.; Jonnalagadda, S. A review of recent advances in microneedle technology for transdermal drug delivery. J. Drug Deliv. Sci. Technol. 2020, 59, 101923. [Google Scholar] [CrossRef]
- Obaidat, R.; BaniAmer, F.; Assaf, S.M.; Yassin, A. Fabrication and Evaluation of Transdermal Delivery of Carbamazepine Dissolving Microneedles. AAPS PharmSciTech 2021, 22, 253. [Google Scholar] [CrossRef]
- Chevala, N.T.; Jitta, S.R.; Marques, S.M.; Vaz, V.M.; Kumar, L. Polymeric microneedles for transdermal delivery of nanoparticles: Frontiers of formulation, sterility and stability aspects. J. Drug Deliv. Sci. Technol. 2021, 65, 102711. [Google Scholar]
- Yang, D.; Chen, M.; Sun, Y.; Jin, Y.; Lu, C.; Pan, X.; Quan, G.; Wu, C. Microneedle-mediated transdermal drug delivery for treating diverse skin diseases. Acta Biomater. 2020, 121, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.R.R.; McMillan, H.; Mooney, K.; Alkilani, A.Z.; Donnelly, R.F. Fabrication of Microneedles. In Percutaneous Penetration Enhancers Physical Methods in Penetration Enhancement; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 305–323. [Google Scholar]
- Ingrole, R.S.J.; Azizoglu, E.; Dul, M.; Birchall, J.C.; Gill, H.S.; Prausnitz, M.R. Trends of microneedle technology in the scientific literature, patents, clinical trials and internet activity. Biomaterials 2021, 267, 120491. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, H.; Hu, T.; Xu, C.; Jiang, L.; Shrike Zhang, Y.; Xie, M. Recent advances of microneedles used towards stimuli-responsive drug delivery, disease theranostics, and bioinspired applications. Chem. Eng. J. 2021, 426, 130561. [Google Scholar] [CrossRef]
- McCrudden, M.T.C.; Alkilani, A.Z.; McCrudden, C.M.; McAlister, E.; McCarthy, H.O.; Woolfson, A.D.; Donnelly, R.F. Design and physicochemical characterisation of novel dissolving polymeric microneedle arrays for transdermal delivery of high dose, low molecular weight drugs. J. Control. Release 2014, 180, 71–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kearney, M.-C.; Caffarel-Salvador, E.; Fallows, S.J.; McCarthy, H.O.; Donnelly, R.F. Microneedle-mediated delivery of donepezil: Potential for improved treatment options in Alzheimer’s disease. Eur. J. Pharm. Biopharm. 2016, 103, 43–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.Q.; Zhang, X.P.; Hao, Y.Y.; Zhang, B.L.; Guo, X.D. Codelivery of hydrophilic and hydrophobic drugs in a microneedle patch for the treatment of skin pigmentation. J. Ind. Eng. Chem. 2020, 88, 241–250. [Google Scholar] [CrossRef]
- Oh, J.-H.; Park, H.-H.; Do, K.-Y.; Han, M.; Hyun, D.-H.; Kim, C.-G.; Kim, C.-H.; Lee, S.S.; Hwang, S.-J.; Shin, S.-C.; et al. Influence of the delivery systems using a microneedle array on the permeation of a hydrophilic molecule, calcein. Eur. J. Pharm. Biopharm. 2008, 69, 1040–1045. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Yu, H.; Li, C.; Feng, J.; Haq, F.; Khan, A.; Khan, R.U. Preparation, properties and challenges of the microneedles-based insulin delivery system. J. Control. Release 2018, 288, 173–188. [Google Scholar] [CrossRef]
- Yu, W.; Jiang, G.; Liu, D.; Li, L.; Tong, Z.; Yao, J.; Kong, X. Transdermal delivery of insulin with bioceramic composite microneedles fabricated by gelatin and hydroxyapatite. Mater. Sci. Eng. C 2017, 73, 425–428. [Google Scholar] [CrossRef]
- Stinson, J.A.; Boopathy, A.V.; Cieslewicz, B.M.; Zhang, Y.; Hartman, N.W.; Miller, D.P.; Dirckx, M.; Hurst, B.L.; Tarbet, E.B.; Kluge, J.A.; et al. Enhancing influenza vaccine immunogenicity and efficacy through infection mimicry using silk microneedles. Vaccine 2021, 39, 5410–5421. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, M.; Fu, J.; Sun, Y.; Lu, C.; Quan, G.; Pan, X.; Wu, C. Recent advances in microneedles-mediated transdermal delivery of protein and peptide drugs. Acta Pharm. Sin. B 2021, 11, 2326–2343. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Kwon, H.J. Hyaluronic acid microneedle patch for the improvement of crow’s feet wrinkles. Dermatol. Ther. 2017, 30, e12546. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, Y.H.; Yamada, M.; Lin, L.L.; Grice, J.E.; Roberts, M.S.; Raphael, A.P.; Benson, H.A.; Prow, T.W. Microneedle enhanced delivery of cosmeceutically relevant peptides in human skin. PLoS ONE 2014, 9, e101956. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Park, J.; Chu, G.S.; Kim, K.S.; Sung, J.H.; Kim, B. Transdermal delivery of cosmetic ingredients using dissolving polymer microneedle arrays. Biotechnol. Bioprocess Eng. 2015, 20, 543–549. [Google Scholar] [CrossRef]
- Peng, K.; Vora, L.K.; Tekko, I.A.; Permana, A.D.; Domínguez-Robles, J.; Ramadon, D.; Chambers, P.; McCarthy, H.O.; Larrañeta, E.; Donnelly, R.F. Dissolving microneedle patches loaded with amphotericin B microparticles for localised and sustained intradermal delivery: Potential for enhanced treatment of cutaneous fungal infections. J. Control. Release 2021, 339, 361–380. [Google Scholar] [CrossRef]
- Zhang, D.; Das, D.B.; Rielly, C.D. An Experimental Study of Microneedle-Assisted Microparticle Delivery. J. Pharm. Sci. 2013, 102, 3632–3644. [Google Scholar] [CrossRef] [Green Version]
- Ilić, T.; Savić, S.; Batinić, B.; Marković, B.; Schmidberger, M.; Lunter, D.; Savić, M.; Savić, S. Combined use of biocompatible nanoemulsions and solid microneedles to improve transport of a model NSAID across the skin: In vitro and in vivo studies. Eur. J. Pharm. Sci. 2018, 125, 110–119. [Google Scholar] [CrossRef]
- Yin, Y.; Su, W.; Zhang, J.; Huang, W.; Li, X.; Ma, H.; Tan, M.; Song, H.; Cao, G.; Yu, S.; et al. Separable Microneedle Patch to Protect and Deliver DNA Nanovaccines Against COVID-19. ACS Nano 2021, 15, 14347–14359. [Google Scholar] [CrossRef]
- Niu, L.; Chu, L.Y.; Burton, S.A.; Hansen, K.J.; Panyam, J. Intradermal delivery of vaccine nanoparticles using hollow microneedle array generates enhanced and balanced immune response. J. Control. Release 2019, 294, 268–278. [Google Scholar] [CrossRef]
- Larrañeta, E.; Lutton, R.E.M.; Woolfson, A.D.; Donnelly, R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Haq, M.I.; Smith, E.; John, D.N.; Kalavala, M.; Edwards, C.; Anstey, A.; Morrissey, A.; Birchall, J.C. Clinical administration of microneedles: Skin puncture, pain and sensation. Biomed. Microdevices 2009, 11, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.T.M.; Gavitt, T.D.; Farrell, N.J.; Curry, E.J.; Mara, A.B.; Patel, A.; Brown, L.; Kilpatrick, S.; Piotrowska, R.; Mishra, N.; et al. Transdermal microneedles for the programmable burst release of multiple vaccine payloads. Nat. Biomed. Eng. 2021, 5, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Kathuria, H.; Lim, D.; Cai, J.; Chung, B.G. Microneedles with Tunable Dissolution Rate. ACS Biomater. Sci. Eng. 2020, 6, 5061–5068. [Google Scholar] [CrossRef] [PubMed]
- Alhnan, M.A.; Okwuosa, T.C.; Sadia, M.; Wan, K.W.; Ahmed, W.; Arafat, B. Emergence of 3D Printed Dosage Forms: Opportunities and Challenges. Pharm. Res. 2016, 33, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh, S.R.; Sarabi, M.R.; Rahbarghazi, R.; Sokullu, E.; Yetisen, A.K.; Tasoglu, S. 3D-printed microneedles in biomedical applications. iScience 2020, 24, 102012. [Google Scholar] [CrossRef] [PubMed]
- Pere, C.P.P.; Economidou, S.N.; Lall, G.; Ziraud, C.; Boateng, J.S.; Alexander, B.D.; Lamprou, D.A.; Douroumis, D. 3D printed microneedles for insulin skin delivery. Int. J. Pharm. 2018, 544, 425–432. [Google Scholar] [CrossRef] [Green Version]
- Economidou, S.N.; Uddin, M.J.; Marques, M.J.; Douroumis, D.; Sow, W.T.; Li, H.; Reid, A.; Windmill, J.F.C.; Podoleanu, A. A novel 3D printed hollow microneedle microelectromechanical system for controlled, personalized transdermal drug delivery. Addit. Manuf. 2021, 38, 101815. [Google Scholar] [CrossRef]
- Caudill, C.; Perry, J.L. Transdermal vaccination via 3D-printed microneedles induces potent humoral and cellular immunity. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Economidou, S.N.; Pissinato Pere, C.P.; Okereke, M.; Douroumis, D. Optimisation of Design and Manufacturing Parameters of 3D Printed Solid Microneedles for Improved Strength, Sharpness, and Drug Delivery. Micromachines 2021, 12, 117. [Google Scholar] [CrossRef]
- Elahpour, N.; Pahlevanzadeh, F.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Ramakrishna, S.; Berto, F. 3D printed microneedles for transdermal drug delivery: A brief review of two decades. Int. J. Pharm. 2021, 597, 120301. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Luo, G.; Xing, M. Biomedical applications of polymeric microneedles for transdermal therapeutic delivery and diagnosis: Current status and future perspectives. Adv. Ther. 2020, 3, 1900140. [Google Scholar] [CrossRef]
- Szeto, B.; Aksit, A.; Valentini, C.; Yu, M.; Werth, E.G.; Goeta, S.; Tang, C.; Brown, L.M.; Olson, E.S.; Kysar, J.W.; et al. Novel 3D-printed hollow microneedles facilitate safe, reliable, and informative sampling of perilymph from guinea pigs. Hear. Res. 2021, 400, 108141. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhong, W.; Xu, L.; Li, H.; Yan, Q.; She, Y.; Yang, G. Recent progress of 3D-printed microneedles for transdermal drug delivery. Int. J. Pharm. 2021, 593, 120106. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef]
- Biospace. Available online: https://www.biospace.com/article/releases/pharmather-enters-into-process-development-agreement-with-lts-lohmann-for-ketamine-microneedle-patch/ (accessed on 13 April 2022).
- Jeong, H.R.; Jun, H.; Cha, H.R.; Lee, J.M.; Park, J.H. Safe coated microneedles with reduced puncture occurrence after administration. Micromachines 2020, 11, 710. [Google Scholar] [CrossRef]
- Kapoor, Y.; Milewski, M.; Dick, L.; Zhang, J.; Bothe, J.R.; Gehrt, M.; Manser, K.; Nissley, B.; Petrescu, I.; Johnson, P.; et al. Coated microneedles for transdermal delivery of a potent pharmaceutical peptide. Biomed. Microdevices 2020, 22, 7. [Google Scholar] [CrossRef]
- Liang, L.; Chen, Y.; Zhang, B.L.; Zhang, X.P.; Liu, J.L.; Shen, C.B.; Cui, Y.; Guo, X.D. Optimization of dip-coating methods for the fabrication of coated microneedles for drug delivery. J. Drug Deliv. Sci. Technol. 2020, 55, 101464. [Google Scholar] [CrossRef]
- Chang, H.; Zheng, M.; Chew, S.W.T.; Xu, C. Advances in the Formulations of Microneedles for Manifold Biomedical Applications. In Advanced Materials Technologies; Wiley-Blackwell: Hoboken, NJ, USA, 2020; Volume 5. [Google Scholar]
- Moussi, K.; Bukhamsin, A.; Hidalgo, T.; Kosel, J. Biocompatible 3D printed microneedles for transdermal, intradermal, and percutaneous applications. Adv. Eng. Mater. 2020, 22, 1901358. [Google Scholar] [CrossRef] [Green Version]
- Sachan, A.; Sachan, R.J.; Lu, J.; Sun, H.; Jin, Y.J.; Erdmann, D.; Zhang, J.Y.; Narayan, R.J. Injection molding for manufacturing of solid poly(l-lactide-co-glycolide) microneedles. MRS Adv. 2021, 6, 61–65. [Google Scholar] [CrossRef]
- Turner, J.G.; White, L.R.; Estrela, P.; Leese, H.S. Hydrogel-Forming Microneedles: Current Advancements and Future Trends. In Macromolecular Bioscience; Wiley-VCH: Hoboken, NJ, USA, 2021; Volume 21. [Google Scholar]
- Donnelly, R.F.; McCrudden, M.T.; Zaid Alkilani, A.; Larrañeta, E.; McAlister, E.; Courtenay, A.J.; Kearney, M.C.; Singh, T.R.; McCarthy, H.O.; Kett, V.L.; et al. Hydrogel-forming microneedles prepared from "super swelling" polymers combined with lyophilised wafers for transdermal drug delivery. PLoS ONE 2014, 9, e111547. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. ClinicalTrials.gov Is a Resource Provided by the U.S. National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=transdermal+drug+delivery&cntry=&state=&city=&dist= (accessed on 18 April 2022).
- Roep, B.O.; Wheeler, D.C.S.; Peakman, M. Antigen-based immune modulation therapy for type 1 diabetes: The era of precision medicine. Lancet. Diabetes Endocrinol. 2019, 7, 65–74. [Google Scholar] [CrossRef]
- Alimardani, V.; Abolmaali, S.S.; Yousefi, G.; Rahiminezhad, Z.; Abedi, M. Microneedle Arrays Combined with Nanomedicine Approaches for Transdermal Delivery of Therapeutics. J. Clin. Med. 2021, 10, 181. [Google Scholar] [CrossRef] [PubMed]
| CPEs | Drugs Used | Mechanism of Action |
|---|---|---|
| Dimethyl sulphoxide | Hydrocortisone [51] Testosterone [52] Naloxone [53] |
|
| Azone | Ketoprofen [54] Dimethyl fumarate [55] 5-Fluorouracil [56] |
|
| Pyrrolidone | Ketoprofen [54] Lidocaine hydrochloride [57] Bupranolol [58] |
|
| Fatty acids | Flurbiprofen [59] Propranolol [60] Theophylline [61] |
|
| Alcohols | Nortriptyline hydrochloride [62] Thymoquinone [63] Lidocaine [64] |
|
| Urea | Indometacin [65] Venlafaxine hydrochloride [66] Metronidazole [67] |
|
| Terpenes | Zidovudine [68] Dimethyl fumarate [55] Imipramine hydrochloride [69] |
|
| Surfactants | Lorazepam [70] Foscarnet [71] L-Ascorbic acid [72] Dimethyl fumarate [55] |
|
| Cosolvents | Diclofenac sodium [36] |
|
| Drug | Type of NE | Method of Preparation | Drug Class | TDD |
|---|---|---|---|---|
| Ibuprofen | O/W | Spontaneous emulsification | NSAID | NE [134] |
| Aceclofenac | O/W | Spontaneous emulsification | NSAID | NE [135] |
| Meloxicam | O/W | Spontaneous emulsification | NSAID | NE [136] NE-loaded gel [137] |
| Celecoxib | O/W | Spontaneous emulsification | NSAID | NE [138,139] |
| Ketoprofen | W/O | Spontaneous emulsification | NSAID | NE [140] NE-loaded gel [141] |
| Indomethacin | O/W | Spontaneous emulsification | NSAID | NE [142,143] NE-loaded gel [144] |
| Piroxicam | O/W | Spontaneous emulsification | NSAID | NE-loaded gel [145] |
| Thiocolchicoside | W/O | Spontaneous emulsification | Muscle relaxant with anti-inflammatory and analgesic effects | NE [146] |
| Carvedilol | O/W | Spontaneous emulsification | Congestive heart failure | NE-loaded gel [147] NE-loaded film [148] |
| Olmesartan | O/W | Spontaneous emulsification | Antihypertensive | NE [149] |
| Nitrendipine | O/W | Spontaneous emulsification | Antihypertensive | NE-loaded gel [150] |
| Caffeine | W/O | Oil phase titration method | Anticancer drug | NE [151] |
| Ropinirole hydrochloride | W/O | Spontaneous emulsification | Parkinson’s disease | NE [152] |
| Inulin | W/O | Not mentioned | Model drug | NE [153] |
| Glycyrrhizin | W/O | Spontaneous emulsification | Gastric ulcer | NE [154] |
| Dutasteride | O/W | Spontaneous emulsification | Prostate cancer | NE-loaded patch [155] |
| Tamoxifen citrate | O/W | Spontaneous emulsification | Anticancer | NE [156] |
| Granisetron hydrochloride | O/W | Spontaneous emulsification | Antiemetic | NE [157] |
| Terbinafine and citral | O/W | Spontaneous emulsification | Model drugs | NE-loaded gel [158] |
| Glibenclamide | O/W | Not mentioned | Antidiabetic | NE-loaded gel [159] |
| Imipramine and doxepin | O/W | Not mentioned | Local anesthetics | NE [160] |
| Hydrocortisone | O/W | Spontaneous emulsification | Corticosteroid | NE [161] |
| Atorvastatin | O/W | Spontaneous emulsification | Lower cholesterol | NE [162] |
| Apixaban | O/W | Spontaneous emulsification | Anticoagulant | NE [163] |
| Drug | Dosage Form | Reference |
|---|---|---|
| Apremilast | Gel | [218] |
| Luliconazole | Hydrogel patch | [219] |
| Dexamethasone | Nanosuspension | [220] |
| Glabridin | Nanosuspension | [204,221] |
| Beclomethasone | Nanosuspension | [222] |
| Ibuprofen | Gel | [202] |
| Flurbiprofen | Gel | [223] |
| Methotrexate | Gel | [217] |
| Methotrexate | MNs | [216] |
| Curcumin | Adhesive film | [213] |
| Curcumin | Nanosuspension | [224] |
| Rank | Title | Conditions | Interventions | Trial Code 1 |
|---|---|---|---|---|
| 1 | Gabapentin Versus Transdermal Fentanyl Matrix for Chronic Neuropathic Pain | Neuropathic pain| Spinal stenosis | Drug: transdermal fentanyl matrix, gabapentin | NCT01127100 |
| 2 | Transdermal Basal Insulin Patch Study in Type 1 Diabetes | Type 1 diabetes | Other: PassPort(R) Transdermal Insulin Delivery System | NCT00519623 |
| 3 | Disease-modifying Potential of Transdermal nicotine in Early Parkinson’s Disease | Parkinson’s disease | Drug: nicotine transdermal patch | NCT01560754 |
| 4 | Effect of Transdermal Magnesium Chloride on Quality of Life in Patients with Fibromyalgia | Fibromyalgia|fibromyalgia syndrome | Other: Transdermal Magnesium Chloride | NCT01968772 |
| 5 | Granisetron Transdermal Patch for Prophylaxis of Delayed CINV | Chemotherapy-induced nausea and vomiting (CINV) | Drug: Granisetron transdermal patch|Drug: Palonosetron|Drug: Aprepitant|Drug: Fosaprepitant|Drug: Dexamethasone | NCT04912271 |
| 6 | Granisetron Transdermal Patch for Prophylaxis of Nausea and Vomiting in Patients Receiving Oral Anticancer Agents | Chemotherapy-induced nausea and vomiting (CINV) | Drug: Granisetron Transdermal Delivery System | NCT04472143 |
| 7 | Totally Transdermal Sedation in the Weaning from Remifentanil Infusion | Respiratory insufficiency|ventilator weaning|analgesics, opioid | Drug: Fentanyl Transdermal System|Drug: Remifentanil | NCT04204967 |
| 8 | Granisetron Transdermal Patch System for Prevention of CINV by CapeOX | Chemotherapy-induced nausea and vomiting | Drug: Granisetron Transdermal Patch System | NCT05325190 |
| 9 | Opioid Titration With 12.5 ug/h Fentanyl Transdermal Patch vs Orally Morphine for Opioid-Naive Patients with Moderate Cancer Pain | Opioid, moderate cancer pain, transdermal fentanyl, 12.5 ug/h, opioid-naive | Drug: 2.5 ug/h transdermal fentanyl|Drug: Oral immediate-released morphine | NCT04533243 |
| 10 | Comparison of Blood Pressure Measurements Between Transdermal Optical Imaging and Standard of Care | Blood pressure | Device: Transdermal Optical Imaging | NCT04539860 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkilani, A.Z.; Nasereddin, J.; Hamed, R.; Nimrawi, S.; Hussein, G.; Abo-Zour, H.; Donnelly, R.F. Beneath the Skin: A Review of Current Trends and Future Prospects of Transdermal Drug Delivery Systems. Pharmaceutics 2022, 14, 1152. https://doi.org/10.3390/pharmaceutics14061152
Alkilani AZ, Nasereddin J, Hamed R, Nimrawi S, Hussein G, Abo-Zour H, Donnelly RF. Beneath the Skin: A Review of Current Trends and Future Prospects of Transdermal Drug Delivery Systems. Pharmaceutics. 2022; 14(6):1152. https://doi.org/10.3390/pharmaceutics14061152
Chicago/Turabian StyleAlkilani, Ahlam Zaid, Jehad Nasereddin, Rania Hamed, Sukaina Nimrawi, Ghaid Hussein, Hadeel Abo-Zour, and Ryan F. Donnelly. 2022. "Beneath the Skin: A Review of Current Trends and Future Prospects of Transdermal Drug Delivery Systems" Pharmaceutics 14, no. 6: 1152. https://doi.org/10.3390/pharmaceutics14061152
APA StyleAlkilani, A. Z., Nasereddin, J., Hamed, R., Nimrawi, S., Hussein, G., Abo-Zour, H., & Donnelly, R. F. (2022). Beneath the Skin: A Review of Current Trends and Future Prospects of Transdermal Drug Delivery Systems. Pharmaceutics, 14(6), 1152. https://doi.org/10.3390/pharmaceutics14061152