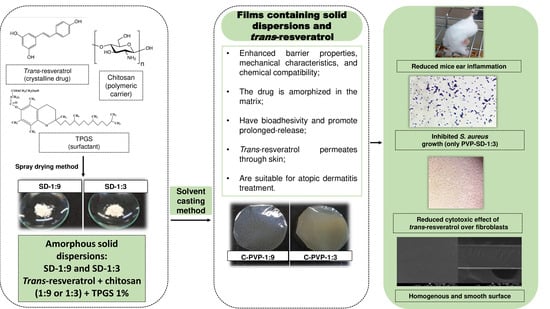
Solid Dispersions Incorporated into PVP Films for the Controlled Release of Trans-Resveratrol: Development, Physicochemical and In Vitro Characterizations and In Vivo Cutaneous Anti-Inflammatory Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Solid Dispersions’ Manufacture
2.2.2. PVP Films Preparation
2.3. Liquid Uptake Ability
2.4. Water Vapor Permeability (WVP)
2.5. Mechanical Properties
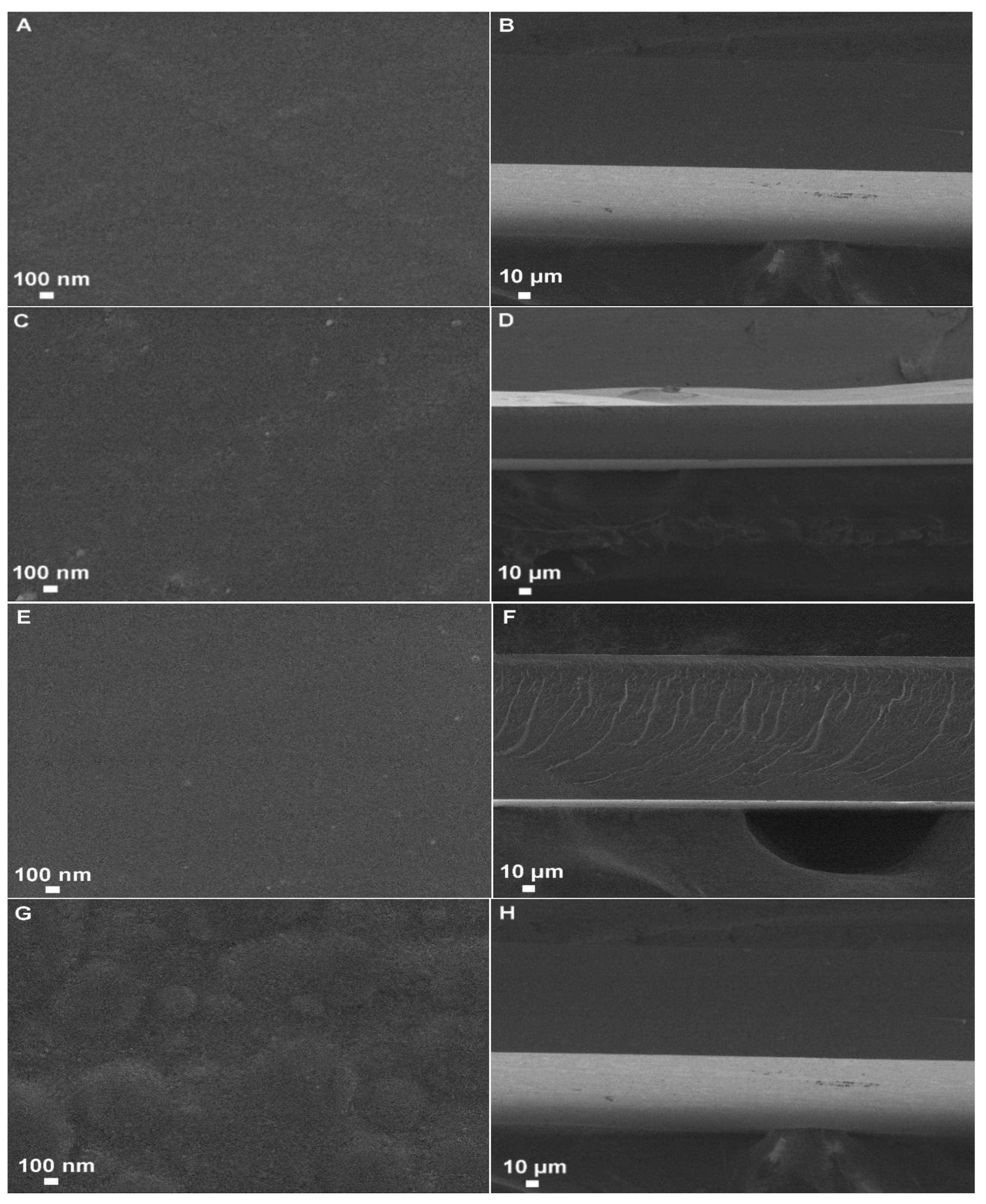
2.6. Scanning Electron Microscopy (SEM)
2.7. X-ray Diffraction (DRX)
2.8. Fourier Transformed Infrared Spectroscopy (FT-IR)
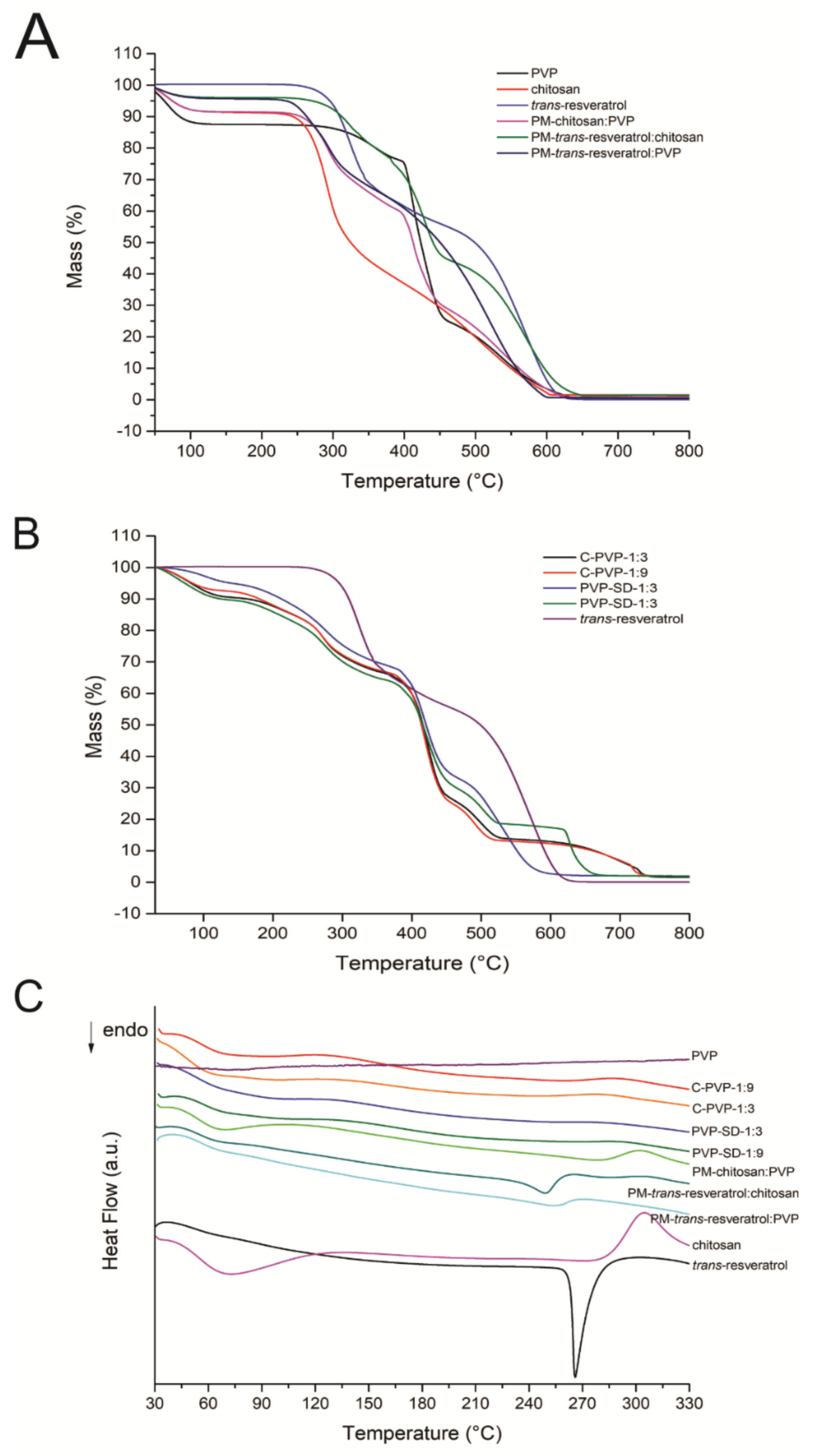
2.9. Thermogravimetry and Differential Scanning Calorimetry (TG-DSC)
2.10. In Vitro Bioadhesion
2.11. In Vitro Trans-Resveratrol Release
2.12. In Vitro Permeation
2.13. In Vitro Cytotoxicity
2.14. In Vivo Anti-Inflammatory Activity
2.15. In Vitro Antimicrobial Activity Evaluation against Staphylococcus aureus
2.15.1. Bacterial Strain
2.15.2. Culture Media and Bacterial Strain Reactivation
2.15.3. Disk Diffusion Assay
2.16. Statistical Analysis
3. Results
3.1. PVP Films Preparation
3.2. Liquid Uptake Ability
3.3. Water Vapor Permeability (WVP)
3.4. Mechanical Properties
3.5. Scanning Electron Microscope (SEM)
3.6. Fourier-Transformed Infrared Spectroscopy (FT-IR)
3.7. X-ray Diffraction (XRD)
3.8. Thermal Analysis (TG and DSC)
3.9. In Vitro Bioadhesion
3.10. In Vitro Release of Trans-Resveratrol
3.11. In Vitro Cutaneous Permeation
3.12. In Vitro Cytotoxicity
3.13. In Vivo Anti-Inflammatory Activity
3.14. In Vitro Antimicrobial Effect against Staphylococcus aureus—Disk Diffusion Assay
4. Discussion
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miyashiro, C.A.; Bernegossi, J.; Bonifácio, B.V.; de Toledo, L.G.; Ramos, M.A.S.; Bauab, T.M.; Chorilli, M. Development and characterization of a novel liquid crystalline system containing sodium alginate for incorporation of trans-resveratrol intended for treatment of buccal candidiasis. Scopus 2020, 75, 179–185. [Google Scholar] [CrossRef]
- Riccio, B.V.F.; Spósito, L.; Carvalho, G.C.; Chorilli, M. Resveratrol isoforms and conjugates: A review from biosynthesis in plants to elimination from the human body. Arch. Pharm. 2020, 353, 2000146. [Google Scholar] [CrossRef] [PubMed]
- Fachinetti, N.; Rigon, R.B.; Eloy, J.O.; Sato, M.R.; dos Santos, K.C.; Chorilli, M. Comparative Study of Glyceryl Behenate or Polyoxyethylene 40 Stearate-Based Lipid Carriers for Trans-Resveratrol Delivery: Development, Characterization and Evaluation of the In Vitro Tyrosinase Inhibition. AAPS PharmSciTech 2018, 19, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, A.T.; Martinez, R.M.; Pinho-Ribeiro, F.A.; Lopes Dias Da Silva, A.M.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A.; Chorilli, M.; Casagrande, R. Resveratrol-Loaded Liquid-Crystalline System Inhibits UVB-Induced Skin Inflammation and Oxidative Stress in Mice. J. Nat. Prod. 2016, 79, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Ding, Q.; Gao, C.; Sun, X. Resveratrol attenuates neuropathic pain through balancing pro-in flammatory and anti-in flammatory cytokines release in mice. Int. Immunopharmacol. 2016, 34, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Fiod Riccio, B.V.; Fonseca-Santos, B.; Colerato Ferrari, P.; Chorilli, M. Characteristics, Biological Properties and Analytical Methods of Trans-Resveratrol: A Review. Crit. Rev. Anal. Chem. 2020, 50, 339–358. [Google Scholar] [CrossRef]
- Sharma, D.K.; Joshi, S.B. Solubility enhancement strategies for poorly water soluble drugs in solid dispersion: A review. Asian J. Pharm. 2007, 1, 9–19. [Google Scholar]
- Baghel, S.; Cathcart, H.; Reilly, N.J.O. Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef] [Green Version]
- Tambosi, G.; Coelho, P.F.; Soares, L.; Cristina, I.; Lenschow, S.; Zétola, M.; Stulzer, H.K.; Pezzini, B.R.; Pós-graduação, P.; De Ambiente, M.; et al. Challenges to improve the biopharmaceutical properties of poorly water-soluble drugs and the application of the solid dispersion technology. Matéria 2018, 23. [Google Scholar] [CrossRef]
- Chaudhari, S.P.; Dugar, R.P. Application of surfactants in solid dispersion technology for improving solubility of poorly water soluble drugs. J. Drug Deliv. Sci. Technol. 2017, 41, 68–77. [Google Scholar] [CrossRef]
- Wilson, V.R.; Lou, X.; Osterling, D.J.; Stolarik, D.F.; Jenkins, G.J.; Nichols, B.L.B.; Dong, Y.; Edgar, K.J.; Zhang, G.G.Z.; Taylor, L.S. Amorphous solid dispersions of enzalutamide and novel polysaccharide derivatives: Investigation of relationships between polymer structure and performance. Sci. Rep. 2020, 10, 18535. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.; Kim, H.; Na, S.J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef] [Green Version]
- Prezotti, F.G.; Siedle, I.; Boni, F.I.; Chorilli, M.; Müller, I.; Cury, B.S.F. Mucoadhesive films based on gellan gum/pectin blends as potential platform for buccal drug delivery. Pharm. Dev. Technol. 2019, 25, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Bathe, R.; Kapoor, R.; Bathe, R.S. Transdermal drug delivery system: Formulation, development and evaluation—An overview. Int. J. Biomed. Adv. Res. 2015, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bouthillette, M.; Beccati, D.; Akthakul, A.; Sakamoto, F.H. A crosslinked polymer skin barrier film for moderate to severe atopic dermatitis: A pilot study in adults. J. Am. Acad. Dermatol. 2019, 82, 895–901. [Google Scholar] [CrossRef]
- Pünnel, L.C.; Lunter, D.J. Film-Forming Systems for Dermal Drug Delivery. Pharmaceutics 2021, 13, 932. [Google Scholar] [CrossRef]
- Meneguin, A.B.; Cury, B.S.F.; Evangelista, R.C. Films from resistant starch-pectin dispersions intended for colonic drug delivery. Carbohydr. Polym. 2014, 99, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, M.J. Functionalization and Characterization of Bioactive Films Based on HPMC: Influence of Antioxidants Inclusion on Films Properties and Food Preservation. Doctoral Thesis, University of Lorraine, Thionville, France, 2012. [Google Scholar]
- Haddad, A.; Hugo, V.; Araújo, S.; Eloy, J.O.; Fonseca-santos, B.; Pereira-da-silva, M.A.; Peccinini, R.G.; Chorilli, M. Synthesis and Characterization of Nanostructured Lipid Nanocarriers for Enhanced Sun Protection Factor of Octyl p-methoxycinnamate. AAPS PharmSciTech 2020, 21, 125. [Google Scholar] [CrossRef]
- Sethia, S.; Squillante, E. Solid dispersion of carbamazepine in PVP K30 by conventional solvent evaporation and supercritical methods. Int. J. Pharm. 2004, 272, 1–10. [Google Scholar] [CrossRef]
- Grégoire, B.; Dazas, B.; Hubert, F.; Tertre, E.; Ferrage, E.; Grasset, L.; Petit, S. Journal of Colloid and Interface Science Orientation measurements of clay minerals by polarized attenuated total reflection infrared spectroscopy. J. Colloid Interface Sci. 2020, 567, 274–284. [Google Scholar] [CrossRef]
- Schroeder, I.Z.; Franke, P.; Schaefer, U.F.; Lehr, C. Development and characterization of film forming polymeric solutions for skin drug delivery. Eur. J. Pharm. Biopharm. 2007, 65, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Nanoemulsion, F.; Applicable, N.E.; Primo, F.L.; Bentley, M.V.L.B.; Tedesco, A.C. Photophysical Studies and In Vitro Skin Permeation/Retention of Photophysical Studies and In Vitro Skin Permeation/Retention of Foscan®/Nanoemulsion (NE) Applicable to Photodynamic Therapy Skin Cancer Treatment. J. Nanosci. Nanotechnol. 2008, 8, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Rodero, C.F.; Fioramonti Calixto, G.M.; Cristina Dos Santos, K.; Sato, M.R.; Aparecido Dos Santos Ramos, M.; Miró, M.S.; Rodríguez, E.; Vigezzi, C.; Bauab, T.M.; Sotomayor, C.E.; et al. Curcumin-Loaded Liquid Crystalline Systems for Controlled Drug Release and Improved Treatment of Vulvovaginal Candidiasis. Mol. Pharm. 2018, 15, 4491–4504. [Google Scholar] [CrossRef] [PubMed]
- Mas, D.; Press, D. Nanoemulsion as a carrier to improve the topical anti-inflammatory activity of stem bark extract of Rapanea ferruginea. Int. J. Nanomed. 2016, 11, 4495–4507. [Google Scholar] [CrossRef] [Green Version]
- Baricevic, D.; Sosa, S.; Loggia, R.D.; Tubaro, A.; Simonovska, B. Topical anti-inflammatory activity of Sal 6 ia officinalis L. leaves: The relevance of ursolic acid. J. Ethnopharmacol. 2001, 75, 125–132. [Google Scholar] [CrossRef]
- Sampaio, L.S.; de Annunzio, S.R.; de Freitas, L.M.; Dantas, L.O.; de Boni, L.; Donatoni, M.C.; de Oliveira, K.T.; Fontana, C.R. Influence of light intensity and irradiation mode on methylene blue, chlorin-e6 and curcumin-mediated photodynamic therapy against Enterococcus faecalis. Photodiagn. Photodyn. Ther. 2020, 31, 101925. [Google Scholar] [CrossRef]
- Limbago, B. M100-S11, Performance standards for antimicrobial susceptibility testing. Clin. Microbiol. Newsl. 2001, 23, 49. [Google Scholar] [CrossRef]
- Kroustalli, A.; Zisimopoulou, A.E.; Koch, S.; Rongen, L.; Deligianni, D.; Diamantouros, S.; Athanassiou, G.; Kokozidou, M.; Mavrilas, D.; Jockenhoevel, S. Carbon nanotubes reinforced chitosan films: Mechanical properties and cell response of a novel biomaterial for cardiovascular tissue engineering. J. Mater. Sci. Mater. Med. 2013, 24, 2889–2896. [Google Scholar] [CrossRef]
- Brazilian’s Health Ministery. RDC n° 31 de 11 de Agosto de 2010; Brazilian’s Health Ministery: Rio de Janeiro, Brazil, 2010. (in Portuguese)
- Arora, P.; Mukherjee, B. Design, Development, Physicochemical and In Vitro and In Vivo Evaluation of Transdermal Patches Containing Diclofenac Diethylammonium Salt. J. Pharm. Sci. 2002, 91, 2076–2089. [Google Scholar] [CrossRef]
- Wagh, M.P.; Dalvi, H.; Bagal, M. Formulation and evaluation of transdermal drug delivery system for simvastatin. Indian Drugs 2009, 46, 221–225. [Google Scholar] [CrossRef]
- Muralter, F.; Perrotta, A.; Coclite, A.M. Thickness-Dependent Swelling Behavior of Vapor-Deposited Smart Polymer Thin Films. Macromolecules 2018, 51, 9692–9699. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Bharath, S. Design and Characterization of Double Layered Mucoadhesive System Containing Bisphosphonate Derivative. ISRN Pharm. 2013, 2013, 604690. [Google Scholar] [CrossRef] [PubMed]
- Turan, D. Water Vapor Transport Properties of Polyurethane Films for Packaging of Respiring Foods. Food Eng. Rev. 2021, 13, 54–65. [Google Scholar] [CrossRef] [Green Version]
- Pamela, V.Y.; Meindrawan, B.; Syarief, R.; Iriani, E.S.; Suyatma, N.E. Barrier and antimicrobial properties of PVA films incorporated with ZnO nanoparticles and stearic acid. IOP Conf. Ser. Earth Environ. Sci. 2018, 195, 012060. [Google Scholar] [CrossRef]
- Othman, S.H.; Amirah, S.; Edwal, M.; Risyon, N.P.; Basha, R.K.; Talib, R.A. Water sorption and water permeability properties of edible film made from potato peel waste. Food Sci. Technol. 2017, 37, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Anatoly, C.; Pavel, Z.; Tatiana, C.; Alexei, R.; Svetlana, Z. Water vapor permeability through porous polymeric membranes with various hydrophilicity as synthetic and natural barriers. Polymers 2020, 12, 282. [Google Scholar] [CrossRef] [Green Version]
- Felton, L.A. Mechanisms of polymeric film formation. Int. J. Pharm. 2013, 457, 423–427. [Google Scholar] [CrossRef]
- Meneguin, A.B.; Ferreira Cury, B.S.; dos Santos, A.M.; Franco, D.F.; Barud, H.S.; da Silva Filho, E.C. Resistant Starch/Pectin Free-Standing Films Reinforced with Nanocellulose Intended for Colonic Methotrexate Release. Carbohydrate Pol. 2017, 157. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Bryaskova, R.; Pencheva, D.; Nikolov, S.; Kantardjiev, T. Synthesis and comparative study on the antimicrobial activity of hybrid materials based on silver nanoparticles (AgNps) stabilized by polyvinylpyrrolidone (PVP). J. Chem. Biol. 2011, 4, 185. [Google Scholar] [CrossRef] [Green Version]
- Karavas, E.; Georgarakis, E.; Bikiaris, D. Adjusting drug release by using miscible polymer blends as effective drug carries. J. Therm. Anal. Calorim. 2006, 84, 125–133. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Hu, S.C.-S.; Huang, P.-H.; Lin, T.-C.; Yen, F.-L. Electrospun Resveratrol-Loaded Polyvinylpyrrolidone/Cyclodextrin Nanofibers and Their Biomedical Applications. Pharmaceutics 2020, 12, 552. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, C.; Thangam, R.; Mary, S.A.; Kannan, P.R.; Arun, G.; Madhan, B. Targeted delivery and apoptosis induction of trans-resveratrol-ferulic acid loaded chitosan coated folic acid conjugate solid lipid nanoparticles in colon cancer cells. Carbohydr. Polym. 2020, 231, 115682. [Google Scholar] [CrossRef] [PubMed]
- Porto, I.C.C.M.; Nascimento, T.G.; Oliveira, J.M.S.; Freitas, P.H.; Haimeur, A.; França, R. Use of polyphenols as a strategy to prevent bond degradation in the dentin-resin interface. Eur. J. Oral Sci. 2018, 126, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Dai, Y.; Cai, J.; Zhong, N.; Xiao, H.; McClements, D.J.; Hu, K. Resveratrol encapsulation in core-shell biopolymer nanoparticles: Impact on antioxidant and anticancer activities. Food Hydrocoll. 2017, 64, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Koh, J.; Kim, H.; Gupta, M.K.; Dutta, P.K. A new chitosan-thymine conjugate: Synthesis, characterization and biological activity. Int. J. Biol. Macromol. 2012, 50, 493–502. [Google Scholar] [CrossRef]
- El Hotaby, W.; Sherif, H.H.A.; Hemdan, B.A.; Khalil, W.A.; Khalil, S.K.H. Assessment of in situ-prepared polyvinylpyrrolidone-silver nanocomposite for antimicrobial applications. Acta Phys. Pol. A 2017, 131, 1554–1560. [Google Scholar] [CrossRef]
- da Silva, R.C.; Teixeira, J.A.; Nunes, W.D.G.; Zangaro, G.A.C.; Pivatto, M.; Caires, F.J.; Ionashiro, M. Resveratrol: A thermoanalytical study. Food Chem. 2017, 237, 561–565. [Google Scholar] [CrossRef]
- Chen, X.; Taguchi, T. Enhanced Skin Adhesive Property of Hydrophobically Modi fi ed Poly(vinyl alcohol) Films. ACS Omega 2020, 5, 1519–1527. [Google Scholar] [CrossRef]
- Maswadeh, H.M.; Kanaan, R.A.; Aljarbou, A.N. Effect of Different Biological Membranes on In Vitro Bioadhesion Property Effect of Different Biological Membranes on In Vitro Bioadhesion Property. Drug Invent. Today 2010, 2, 155–159. [Google Scholar]
- Lee, J. Intrinsic Adhesion Properties of Poly(vinyl pyrrolidone) to Pharmaceutical Materials: Humidity Effect. Macromol. Biosci. 2005, 5, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Pagano, C.; Rachele, M.; Calarco, P.; Scuota, S.; Conte, C.; Primavilla, S.; Ricci, M.; Perioli, L. Colloids and Surfaces B: Biointerfaces Bioadhesive polymeric films based on usnic acid for burn wound treatment: Antibacterial and cytotoxicity studies. Colloids Surf. B Biointerfaces 2019, 178, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Carreras, J.J.; Canales, P.; Melero, A. Mucoadhesion of Polymeric Drug Delivery Systems: Polymeric Nanoparticles and its Interactions with the Intestinal Barrier. JSM Nanotechnol. Nanomed. 2016, 4, 1–5. [Google Scholar]
- Franco, P.; De Marco, I. The use of poly(N-vinyl pyrrolidone) in the delivery of drugs: A review. Polymers 2020, 12, 1114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, L.; Zhang, P.; Liu, T.; Yang, G.; Lin, R.; Zhou, J. Extraction of polydatin and resveratrol from Polygonum cuspidatum root: Kinetics and modeling. Food Bioprod. Process. 2014, 94, 518–524. [Google Scholar] [CrossRef]
- Ferrari, P.C.; Souza, F.M.; Giorgetti, L.; Oliveira, G.F.; Chaud, M.V.; Ferraz, H.G.; Evangelista, R.C. In vitro drug permeation from chitosan pellets. Carbohydr. Polym. 2012, 87, 2526–2531. [Google Scholar] [CrossRef]
- Ahmadi, F.; Vatanara, A.; Moazeni, E.; Hoseini, F.; Darabi, M. Preparation and physicochemical evaluation of transdermal aerosols containing ketoprofen. Trop. J. Pharm. Res. 2017, 16, 1813–1818. [Google Scholar] [CrossRef] [Green Version]
- Tavares Luiz, M.; Delello Di Filippo, L.; Carolina Alves, R.; Sousa Araújo, V.H.; Lobato Duarte, J.; Maldonado Marchetti, J.; Chorilli, M. The use of TPGS in drug delivery systems to overcome biological barriers. Eur. Polym. J. 2021, 142, 110129. [Google Scholar] [CrossRef]
- Maccario, C.; Savio, M.; Ferraro, D.; Bianchi, L.; Pizzala, R.; Pretali, L.; Forti, L.; Stivala, L.A. The resveratrol analog 4,4′-dihydroxy-trans-stilbene suppresses transformation in normal mouse fibroblasts and inhibits proliferation and invasion of human breast cancer cells. Carcinogenesis 2012, 33, 2172–2180. [Google Scholar] [CrossRef] [Green Version]
- Anlar, H.G.; Bacanlı, M.; Kutluk, B.; Başaran, A.A.; Başaran, N. Cytotoxıc Actıvıty of Resveratrol in Dıfferent Cell Lınes Evaluated by MTT and NRU Assays. Turkish J. Pharm. Sci. 2016, 13, 109–118. [Google Scholar] [CrossRef]
- Libio, I.C.; Demori, R.; Ferrão, M.F.; Lionzo, M.I.Z.; da Silveira, N.P. Films based on neutralized chitosan citrate as innovative composition for cosmetic application. Mater. Sci. Eng. C 2016, 67, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Marino-Marmolejo, E.N.; Flores-Hernández, F.Y.; Flores-Valdez, M.A.; García-Morales, L.F.; González-Villegas, A.C.; Bravo-Madrigal, J. A quantitative model for dermal infection and oedema in BALB/c mice pinna. BMC Microbiol. 2016, 16, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- dos Santos, D.M.; Chagas, P.A.M.; Leite, I.S.; Inada, N.M.; de Annunzio, S.R.; Fontana, C.R.; Campana-Filho, S.P.; Correa, D.S. Core-sheath nanostructured chitosan-based nonwovens as a potential drug delivery system for periodontitis treatment. Int. J. Biol. Macromol. 2020, 142, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, H.; Wróbel, J.; Jenerowicz, D.; Sadowska-Przytocka, A.; Wachal, M.; Adamski, Z.; Czarnecka-Operacz, M.M. The role of Staphylococcus aureus in atopic dermatitis: Microbiological and immunological implications. Postepy Derm. Alergol. 2019, 36, 485–491. [Google Scholar] [CrossRef]
- Vivero-Lopez, M.; Muras, A.; Silva, D.; Serro, A.P.; Otero, A.; Concheiro, A.; Alvarez-Lorenzo, C. Resveratrol-loaded hydrogel contact lenses with antioxidant and antibiofilm performance. Pharmaceutics 2021, 13, 532. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef]
- Nøhr-Meldgaard, K.; Ovsepian, A.; Ingmer, H.; Vestergaard, M. Resveratrol enhances the efficacy of aminoglycosides against Staphylococcus aureus. Int. J. Antimicrob. Agents 2018, 52, 390–396. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Meng, X.M.; Li, B.; Wang, C.Q.; Wang, S.Q.; Wang, T.L.; Tian, Y.M. Inhibition of alpha-hemolysin expression by resveratrol attenuates Staphylococcus aureus virulence. Microb. Pathog. 2019, 127, 85–90. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Moskovicz, V.; Gross, A.; Mizrahi, B. Extrinsic factors shaping the skin microbiome. Microorganisms 2020, 8, 1023. [Google Scholar] [CrossRef]
- Abedini, E.; Khodadadi, E.; Zeinalzadeh, E.; Moaddab, S.R.; Asgharzadeh, M.; Mehramouz, B.; Dao, S.; Samadi Kafil, H. A Comprehensive Study on the Antimicrobial Properties of Resveratrol as an Alternative Therapy. Evid.-Based Complement. Altern. Med. 2021, 2021, 8866311. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Bolster, M.B. Neurorheumatology; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]


| Formulation | Trans-Resveratrol:Chitosan Ratio | Chitosan (g) | TPGS (%) |
|---|---|---|---|
| SD-1:9 | 1:9 | - | 1 |
| SD-1:3 | 1:3 | - | 1 |
| PVP-SD-1:9 | 1:9 | - | 1 |
| PVP-SD-1:3 | 1:3 | - | 1 |
| C-PVP-1:9 | - | 0.90 | 1 |
| C-PVP-1:3 | - | 0.75 | 1 |
| Grade (Cytotoxicity) | Cytotoxicity Zone |
|---|---|
| 0 (absent) | No signal of discoloration under the sample area |
| 1 (light) | Discoloration zone only under the sample area |
| 2 (mild) | Discoloration up to 0.5 cm beyond the sample area |
| 3 (moderated) | Discoloration between 0.5 and 1.0 cm beyond the sample area |
| 4 (severe) | Discoloration greater than 1.0 cm beyond the sample area |
| Samples | Thickness (μm) | Liquid Uptake (%) | WVP (×10−5 g mm m−2 h−1 Pa−1) | PS (Mpa) | Eb (%) | PE (kJ m−3) |
|---|---|---|---|---|---|---|
| C-PVP-1:9 | 157 ± 5.39 a | 1579.59 ± 31.16 a | 2.07 | 30.36 ± 2.05 a | 13.41 ± 2.45 a | 3.22 ± 0.647 a |
| C-PVP-1:3 | 163 ± 8.35 a | 760.78 ± 52.64 b | 2.10 | 22.99 ± 5.37 a,c | 12.70 ± 3.78 a | 1.69 ± 0.790 b |
| PVP-SD-1:9 | 123 ± 5.18 b | 983.94 ± 49.90 c | 1.38 | 32.20 ± 3.88 b | 15.43 ± 6.93 a | 4.24 ± 1.11 a,c |
| PVP-SD-1:3 | 122 ± 4.74 b | 606.69 ± 27.10 b,c | 1.43 | 39.25 ±2.67 a,b | 14.72 ± 3.18 a | 5.28 ± 1.77 a,b,c |
| Sample | Step 1 | Step 2 | Step 3 | Step 4 | Step 5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TRange (°C) | Tpeak (°C) | ∆m (%) | TRange (°C) | Tpeak (°C) | ∆m (%) | TRange (°C) | Tpeak (°C) | ∆m (%) | TRange (°C) | Tpeak (°C) | ∆m (%) | TRange (°C) | Tpeak (°C) | |
| C-PVP-1:9 | 30–105 | ↓75 | 6.66 | 105–335 | ↓206 ↑278 | 26.44 | 360–450 | ↓415 | 39.95 | 450–520 | ↑495 | 16.66 | 520–745 | ↑720 |
| C-PVP-1:3 | 30–120 | ↓82 | 8.97 | 120–370 | ↑274 | 25.32 | 370–445 | ↓415 ↑442 | 37.30 | 445–530 | ↑505 | 14.96 | 530–750 | ↑730 |
| PVP-SD-1:9 | 30–140 | ↓75 | 10.35 | 140–370 | ↑280 | 25.94 | 370–460 | ↓415 ↑440 | 33.33 | 460–535 | ↑505 | 12.01 | 535–700 | ↑730 |
| PVP-SD-1:3 | 30–120 | ↓100 | 4.17 | 120–380 | ↑290 | 28.25 | 380–460 | ↑345 | 33.74 | 460–640 | ↑535 ↑548 | 31.87 | ---- | ---- |
| Chitosan | 30–126 | ↓73 | 8.47 | 149–310 | ↑300 | 36.40 | 310–607 | ↑325 ↑485 | 53.62 | ---- | ---- | ---- | ---- | ---- |
| PVP | 30–135 | ↓75 | 12.45 | 240–400 | ↓400 | 11.98 | 400–455 | ↓440 | 49.22 | 455–650 | ↑480 | 26.23 | ---- | ---- |
| Trans- resveratrol | 242–350 | ↓266 | 31.11 | 350–650 | ↑565 | 68.84 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| Chitosan: PVP | 30–129 | ↓70 | 8.43 | 215–393 | ↑380 | 32.35 | 393–450 | ↓398 ↓435 | 28.75 | 450–625 | ↑485 | 29.88 | ---- | ---- |
| Trans-resveratrol: PVP | 30–120 | ↓80 | 3.90 | 240–380 | ↓265 | 19.03 | 380–450 | ↓430 | 30.44 | 450–650 | ↑560 | 45.23 | ---- | ---- |
| Trans-resveratrol: chitosan | 30–120 | ↓65 | 4.20 | 195–330 | ↑255 ↑265 ↑310 | 24.94 | 330–600 | ↑525 | 70.46 | ---- | ---- | ---- | ---- | ---- |
| Formulations | Mean ± SD (N) |
|---|---|
| C-PVP-1:9 | 0.1373 ± 0.2240 a |
| C-PVP-1:3 | 0.0629 ± 0.0155 b |
| PVP-SD-1:9 | 0.1142 ± 0.0171 a,c |
| PVP-SD-1:3 | 0.0999 ± 0.0115 d |
| PVP-SD-1:9 | PVP-SD-1:3 | ||
|---|---|---|---|
| Stratum corneum | Accumulative concentration (μg/cm2) | 47.14 ± 53.41 | 189.56 ± 65.10 |
| Retained drug (%) | 35.10 ± 4.87 | 27.29 ± 4.48 | |
| Dermis and epidermis layers (below the stratum corneum) | Accumulative concentration (μg/cm2) | 59.06 ± 25.82 | 117.26 ± 14.53 |
| Retained drug (%) | 18.18 ± 10.28 | 15.59 ± 1.42 | |
| Films | Halo (cm) | Cytotoxicity Degree |
|---|---|---|
| Control (+) | 0.9 | Severe |
| Control (−) | 0 | Absent |
| Trans-resveratrol | 0.5 | Moderate |
| C-PVP-1:9 | 0 | Absent |
| C-PVP-1:3 | 0 | Absent |
| PVP-SD-1:9 | 0.4 | Light |
| PVP-SD-1:3 | 0.1 | Light |
| Groups | Mean Inflammation (%) | Reduction in Inflammation (%) |
|---|---|---|
| Trans-resveratrol 3 mg/mL | 30.85 ± 4.29 | 69.15 a |
| PVP-SD-1:9 | 56.89 ± 7.19 | 43.11 a |
| PVP-SD-1:3 | 33.98 ± 7.09 | 66.02 a |
| C-PVP-1:9 | 80.37 ± 8.04 | 19.63 b,e |
| C-PVP-1:3 | 83.58 ± 21.54 | 15.42 c,e |
| Control + (dexamethasone 1 mg/g) | 24.82 ± 6.96 | 75.18 d |
| Control - (no treatment) | 98.45 ± 7.21 | 1.55 e |
| Experimental Group | Mean ± SD |
|---|---|
| C-PVP-1:3 | NI |
| C-PVP-1:9 | NI |
| PVP-SD-1:3 | 4.11 ± 1.53 |
| PVP-SD-1:9 | NI |
| ETH-1:3 | NI |
| ETH-1:9 | NI |
| ETH | NI |
| NC | NI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riccio, B.V.F.; Nascimento, A.L.C.S.d.; Meneguin, A.B.; Rodero, C.F.; Santos, K.P.; Sábio, R.M.; Annunzio, S.R.d.; Fontana, C.R.; Barud, H.d.S.; Ferrari, P.C.; et al. Solid Dispersions Incorporated into PVP Films for the Controlled Release of Trans-Resveratrol: Development, Physicochemical and In Vitro Characterizations and In Vivo Cutaneous Anti-Inflammatory Evaluation. Pharmaceutics 2022, 14, 1149. https://doi.org/10.3390/pharmaceutics14061149
Riccio BVF, Nascimento ALCSd, Meneguin AB, Rodero CF, Santos KP, Sábio RM, Annunzio SRd, Fontana CR, Barud HdS, Ferrari PC, et al. Solid Dispersions Incorporated into PVP Films for the Controlled Release of Trans-Resveratrol: Development, Physicochemical and In Vitro Characterizations and In Vivo Cutaneous Anti-Inflammatory Evaluation. Pharmaceutics. 2022; 14(6):1149. https://doi.org/10.3390/pharmaceutics14061149
Chicago/Turabian StyleRiccio, Bruno Vincenzo Fiod, André Luiz Carneiro Soares do Nascimento, Andréia Bagliotti Meneguin, Camila Fernanda Rodero, Kaio Pini Santos, Rafael Miguel Sábio, Sarah Raquel de Annunzio, Carla Raquel Fontana, Hernane da Silva Barud, Priscileila Colerato Ferrari, and et al. 2022. "Solid Dispersions Incorporated into PVP Films for the Controlled Release of Trans-Resveratrol: Development, Physicochemical and In Vitro Characterizations and In Vivo Cutaneous Anti-Inflammatory Evaluation" Pharmaceutics 14, no. 6: 1149. https://doi.org/10.3390/pharmaceutics14061149
APA StyleRiccio, B. V. F., Nascimento, A. L. C. S. d., Meneguin, A. B., Rodero, C. F., Santos, K. P., Sábio, R. M., Annunzio, S. R. d., Fontana, C. R., Barud, H. d. S., Ferrari, P. C., & Chorilli, M. (2022). Solid Dispersions Incorporated into PVP Films for the Controlled Release of Trans-Resveratrol: Development, Physicochemical and In Vitro Characterizations and In Vivo Cutaneous Anti-Inflammatory Evaluation. Pharmaceutics, 14(6), 1149. https://doi.org/10.3390/pharmaceutics14061149