Cannabinoid Receptor 2 (CB2) Inverse Agonist SMM-189 Induces Expression of Endogenous CB2 and Protein Kinase A That Differentially Modulates the Immune Response and Suppresses Experimental Colitis
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals
2.2. DSS-Induced Experimental Colitis
2.3. Pharmacological Treatments of SMM-189
2.4. Single-Cell Suspensions
2.5. Flow Cytometry Analysis
2.6. Western Blot Analysis
2.7. Histology
2.8. Statistical Analysis
3. Results
3.1. SMM-189 Modulates Body Weight and Colon Length in Mice with DSS-Induced Colitis
3.2. Activation of the CB2 Receptor by SMM-189 Inhibits Infiltration of Th17 Cells
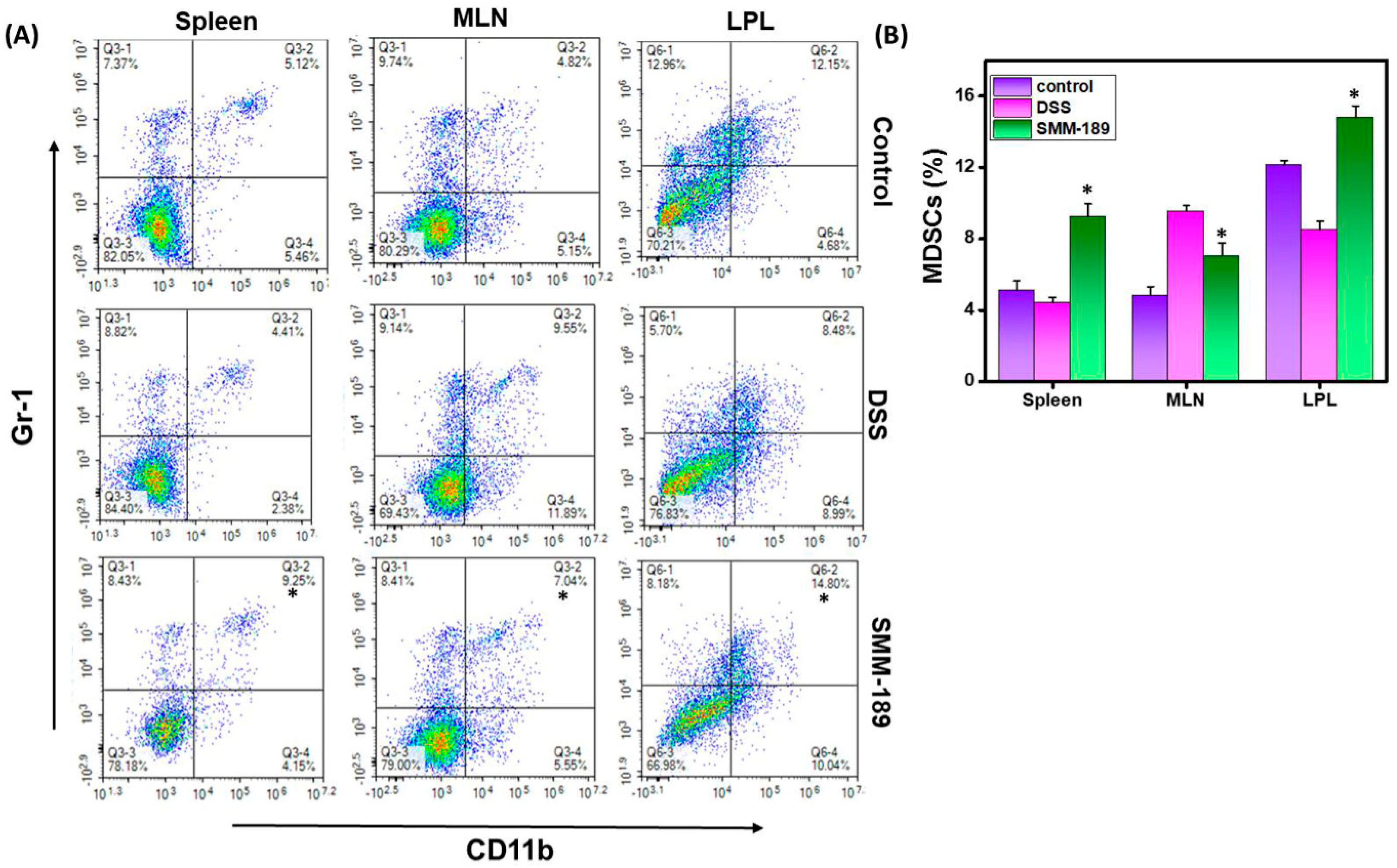
3.3. SMM-189 Increases the Frequency of Myeloid-Derived Suppressor Cells (MDSCs) in the Spleen and LPLs of Mice with DSS-Induced Colitis
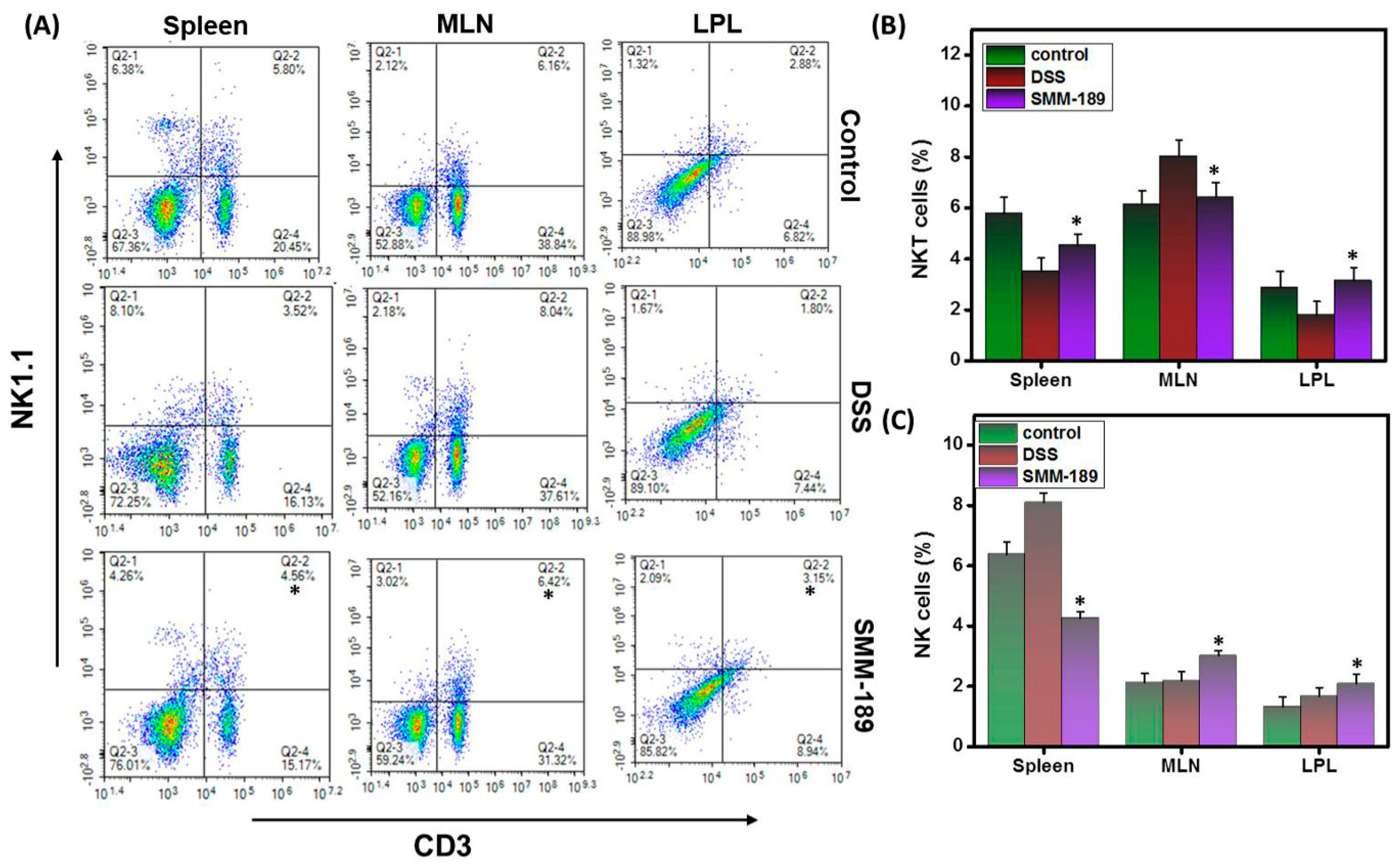
3.4. SMM-189 Differentially Alters the Percentages of NK and NKT Cells during DSS-Induced Colitis
3.5. SMM-189 Reduces Neutrophil Infiltration
3.6. SMM-189 Treatment Induces Expression of CB2 and Protein Kinase A (PKA)
3.7. SMM-189 Treatment Reduces the Severity of Colitis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Blumberg, R.S. Inflammation in the intestinal tract: Pathogenesis and treatment. Dig. Dis. 2009, 27, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, K.; Feuerstein, J.D. New developments in ulcerative colitis: Latest evidence on management, treatment, and maintenance. Drugs Context 2019, 8, 212572. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Cheifetz, A.S. The Use of Complementary and Alternative Medicine in Patients With Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2018, 14, 415–425. [Google Scholar]
- Shale, M.; Schiering, C.; Powrie, F. CD4+ T-cell subsets in intestinal inflammation. Immunol. Rev. 2013, 252, 164–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smids, C.; Horjus Talabur Horje, C.S.; Drylewicz, J.; Roosenboom, B.; Groenen, M.J.M.; van Koolwijk, E.; van Lochem, E.G.; Wahab, P.J. Intestinal T Cell Profiling in Inflammatory Bowel Disease: Linking T Cell Subsets to Disease Activity and Disease Course. J. Crohn’s Colitis 2017, 12, 465–475. [Google Scholar] [CrossRef]
- Hölttä, V.; Klemetti, P.; Sipponen, T.; Westerholm-Ormio, M.; Kociubinski, G.; Salo, H.; Räsänen, L.; Kolho, K.-L.; Färkkilä, M.; Savilahti, E.; et al. IL-23/IL-17 immunity as a hallmark of Crohn’s disease. Inflamm. Bowel Dis. 2008, 14, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Kontaki, E.; Boumpas, D.T.; Tzardi, M.; Mouzas, I.A.; Papadakis, K.A.; Verginis, P. Aberrant function of myeloid-derived suppressor cells (MDSCs) in experimental colitis and in inflammatory bowel disease (IBD) immune responses. Autoimmunity 2017, 50, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Crook, K.R.; Liu, P. Role of myeloid-derived suppressor cells in autoimmune disease. World J. Immunol. 2014, 4, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Haile, L.A.; von Wasielewski, R.; Gamrekelashvili, J.; Kruger, C.; Bachmann, O.; Westendorf, A.M.; Buer, J.; Liblau, R.; Manns, M.P.; Korangy, F.; et al. Myeloid-derived suppressor cells in inflammatory bowel disease: A new immunoregulatory pathway. Gastroenterology 2008, 135, 871–881.e5. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Ferretti, S.; Bonneau, O.; Dubois, G.R.; Jones, C.E.; Trifilieff, A. IL-17, Produced by Lymphocytes and Neutrophils, Is Necessary for Lipopolysaccharide-Induced Airway Neutrophilia: IL-15 as a Possible Trigger. J. Immunol. 2003, 170, 2106–2112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.-H.; Xia, N.; Zhou, S.-F.; Tang, T.-T.; Yan, X.-X.; Lv, B.-J.; Nie, S.-F.; Wang, J.; Iwakura, Y.; Xiao, H.; et al. Interleukin-17A contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and neutrophil infiltration. J. Am. Coll. Cardiol. 2012, 59, 420–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, V.; Iriondo De-Hond, A.; Borrelli, F.; Capasso, R.; Del Castillo, M.D.; Abalo, R. Cannabidiol and Other Non-Psychoactive Cannabinoids for Prevention and Treatment of Gastrointestinal Disorders: Useful Nutraceuticals? Int. J. Mol. Sci. 2020, 21, 3067. [Google Scholar] [CrossRef]
- Ahmed, W.; Katz, S. Therapeutic Use of Cannabis in Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2016, 12, 668–679. [Google Scholar]
- Ambrose, T.; Simmons, A. Cannabis, Cannabinoids, and the Endocannabinoid System-Is there Therapeutic Potential for Inflammatory Bowel Disease? J. Crohn’s Colitis 2019, 13, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Kienzl, M.; Storr, M.; Schicho, R. Cannabinoids and Opioids in the Treatment of Inflammatory Bowel Diseases. Clin. Transl. Gastroenterol. 2020, 11, e00120. [Google Scholar] [CrossRef] [PubMed]
- Pagano, E.; Capasso, R.; Piscitelli, F.; Romano, B.; Parisi, O.A.; Finizio, S.; Lauritano, A.; Marzo, V.D.; Izzo, A.A.; Borrelli, F. An Orally Active Cannabis Extract with High Content in Cannabidiol attenuates Chemically-induced Intestinal Inflammation and Hypermotility in the Mouse. Front. Pharm. 2016, 7, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherkasova, V.; Kovalchuk, O.; Kovalchuk, I. Cannabinoids and Endocannabinoid System Changes in Intestinal Inflammation and Colorectal Cancer. Cancers 2021, 13, 4353. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-J.; Li, Y.-Y.; Lin, X.-H.; Li, K.; Cao, M.-H. Anti-inflammatory effect of cannabinoid agonist WIN55, 212 on mouse experimental colitis is related to inhibition of p38MAPK. World J. Gastroenterol. 2016, 22, 9515–9524. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.P.; Singh, N.P.; Singh, B.; Price, R.L.; Nagarkatti, M.; Nagarkatti, P.S. Cannabinoid receptor-2 (CB2) agonist ameliorates colitis in IL-10(-/-) mice by attenuating the activation of T cells and promoting their apoptosis. Toxicol. Appl. Pharmacol. 2012, 258, 256–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storr, M.A.; Keenan, C.M.; Zhang, H.; Patel, K.D.; Makriyannis, A.; Sharkey, K.A. Activation of the cannabinoid 2 receptor (CB2) protects against experimental colitis. Inflamm. Bowel Dis. 2009, 15, 1678–1685. [Google Scholar] [CrossRef] [Green Version]
- Singh, U.P.; Singh, N.P.; Singh, B.; Hofseth, L.J.; Taub, D.D.; Price, R.L.; Nagarkatti, M.; Nagarkatti, P.S. Role of resveratrol-induced CD11b+ Gr-1+ myeloid derived suppressor cells (MDSCs) in the reduction of CXCR3+ T cells and amelioration of chronic colitis in IL-10−/− mice. Brain Behav. Immun. 2012, 26, 72–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, U.P.; Singh, S.; Taub, D.D.; Lillard, J.W. Inhibition of IFN-γ-Inducible Protein-10 Abrogates Colitis in IL-10−/− Mice. J. Immunol. 2003, 171, 1401–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breider, M.A.; Eppinger, M.; Gough, A. Intercellular adhesion molecule-1 expression in dextran sodium sulfate-induced colitis in rats. Vet. Pathol. 1997, 34, 598–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natsui, M.; Kawasaki, K.; Takizawa, H.; Hayashi, S.I.; Matsuda, Y.; Sugimura, K.; Seki, K.; Narisawa, R.; Sendo, F.; Asakura, H. Selective depletion of neutrophils by a monoclonal antibody, RP-3, suppresses dextran sulphate sodium-induced colitis in rats. J. Gastroenterol. Hepatol. 1997, 12, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Massa, F.; Marsicano, G.; Hermann, H.; Cannich, A.; Monory, K.; Cravatt, B.F.; Ferri, G.L.; Sibaev, A.; Storr, M.; Lutz, B. The endogenous cannabinoid system protects against colonic inflammation. J. Clin. Investig. 2004, 113, 1202–1209. [Google Scholar] [CrossRef] [Green Version]
- Kimball, E.S.; Schneider, C.R.; Wallace, N.H.; Hornby, P.J. Agonists of cannabinoid receptor 1 and 2 inhibit experimental colitis induced by oil of mustard and by dextran sulfate sodium. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G364–G371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storr, M.A.; Keenan, C.M.; Emmerdinger, D.; Zhang, H.; Yuce, B.; Sibaev, A.; Massa, F.; Buckley, N.E.; Lutz, B.; Goke, B.; et al. Targeting endocannabinoid degradation protects against experimental colitis in mice: Involvement of CB1 and CB2 receptors. J. Mol. Med. 2008, 86, 925–936. [Google Scholar] [CrossRef]
- Leinwand, K.L.; Jones, A.A.; Huang, R.H.; Jedlicka, P.; Kao, D.J.; de Zoeten, E.F.; Ghosh, S.; Moaddel, R.; Wehkamp, J.; Ostaff, M.J.; et al. Cannabinoid Receptor-2 Ameliorates Inflammation in Murine Model of Crohn’s Disease. J Crohn’s Colitis 2017, 11, 1369–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grinspoon, L.; Bakalar, J.B. Marihuana as medicine. A plea for reconsideration. JAMA 1995, 273, 1875–1876. [Google Scholar] [CrossRef] [PubMed]
- Jamontt, J.M.; Molleman, A.; Pertwee, R.G.; Parsons, M.E. The effects of Delta-tetrahydrocannabinol and cannabidiol alone and in combination on damage, inflammation and in vitro motility disturbances in rat colitis. Br. J. Pharm. 2010, 160, 712–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, M.A.; Kellermann, C.A.; Burnat, G.; Hahn, E.G.; Rau, T.; Konturek, P.C. Mice lacking cannabinoid CB1-, CB2-receptors or both receptors show increased susceptibility to trinitrobenzene sulfonic acid (TNBS)-induced colitis. J. Physiol. Pharm. 2010, 61, 89–97. [Google Scholar]
- D’Argenio, G.; Valenti, M.; Scaglione, G.; Cosenza, V.; Sorrentini, I.; Di Marzo, V. Up-regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J. 2006, 20, 568–570. [Google Scholar] [CrossRef] [PubMed]
- Cluny, N.L.; Chambers, A.P.; Vemuri, V.K.; Wood, J.T.; Eller, L.K.; Freni, C.; Reimer, R.A.; Makriyannis, A.; Sharkey, K.A. The neutral cannabinoid CB receptor antagonist AM4113 regulates body weight through changes in energy intake in the rat. Pharm. Biochem. Behav. 2011, 97, 537–543. [Google Scholar] [CrossRef] [Green Version]
- Ignatowska-Jankowska, B.; Jankowski, M.M.; Swiergiel, A.H. Cannabidiol decreases body weight gain in rats: Involvement of CB2 receptors. Neurosci. Lett. 2011, 490, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Qin, H.; Wang, L.; Benveniste, E.N.; Elson, C.O.; Cong, Y. Th17 cells induce colitis and promote Th1 cell responses through IL-17 induction of innate IL-12 and IL-23 production. J. Immunol. 2011, 186, 6313–6318. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zheng, M.; Bindas, J.; Schwarzenberger, P.; Kolls, J.K. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm. Bowel Dis. 2006, 12, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Guillot, A.; Hamdaoui, N.; Bizy, A.; Zoltani, K.; Souktani, R.; Zafrani, E.S.; Mallat, A.; Lotersztajn, S.; Lafdil, F. Cannabinoid receptor 2 counteracts interleukin-17-induced immune and fibrogenic responses in mouse liver. Hepatology 2014, 59, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Li, H.; Tuma, R.F.; Ganea, D. Selective CB2 receptor activation ameliorates EAE by reducing Th17 differentiation and immune cell accumulation in the CNS. Cell Immunol. 2014, 287, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Carrillo-Salinas, F.J.; Navarrete, C.; Mecha, M.; Feliu, A.; Collado, J.A.; Cantarero, I.; Bellido, M.L.; Munoz, E.; Guaza, C. A cannabigerol derivative suppresses immune responses and protects mice from experimental autoimmune encephalomyelitis. PLoS ONE 2014, 9, e94733. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Hegde, V.L.; Nagarkatti, M.; Nagarkatti, P.S. Cannabinoid receptor activation leads to massive mobilization of myeloid-derived suppressor cells with potent immunosuppressive properties. Eur. J. Immunol. 2010, 40, 3358–3371. [Google Scholar] [CrossRef]
- Palmen, M.J.; Dijkstra, C.D.; van der Ende, M.B.; Pena, A.S.; van Rees, E.P. Anti-CD11b/CD18 antibodies reduce inflammation in acute colitis in rats. Clin. Exp. Immunol. 1995, 101, 351–356. [Google Scholar] [CrossRef]
- Larmonier, C.B.; Midura-Kiela, M.T.; Ramalingam, R.; Laubitz, D.; Janikashvili, N.; Larmonier, N.; Ghishan, F.K.; Kiela, P.R. Modulation of neutrophil motility by curcumin: Implications for inflammatory bowel disease. Inflamm. Bowel Dis. 2011, 17, 503–515. [Google Scholar] [CrossRef] [Green Version]
- Saubermann, L.J.; Beck, P.; De Jong, Y.P.; Pitman, R.S.; Ryan, M.S.; Kim, H.S.; Exley, M.; Snapper, S.; Balk, S.P.; Hagen, S.J.; et al. Activation of natural killer T cells by alpha-galactosylceramide in the presence of CD1d provides protection against colitis in mice. Gastroenterology 2000, 119, 119–128. [Google Scholar] [CrossRef]
- Heller, F.; Fuss, I.J.; Nieuwenhuis, E.E.; Blumberg, R.S.; Strober, W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity 2002, 17, 629–638. [Google Scholar] [CrossRef] [Green Version]
- Murikinati, S.; Juttler, E.; Keinert, T.; Ridder, D.A.; Muhammad, S.; Waibler, Z.; Ledent, C.; Zimmer, A.; Kalinke, U.; Schwaninger, M. Activation of cannabinoid 2 receptors protects against cerebral ischemia by inhibiting neutrophil recruitment. FASEB J. 2010, 24, 788–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Presley, C.; Abidi, A.; Suryawanshi, S.; Mustafa, S.; Meibohm, B.; Moore, B.M. Preclinical evaluation of SMM-189, a cannabinoid receptor 2-specific inverse agonist. Pharmacol. Res. Perspect. 2015, 3, e00159. [Google Scholar] [CrossRef]
- Pressly, J.D.; Soni, H.; Jiang, S.; Wei, J.; Liu, R.; Moore, B.; Adebiyi, A.; Park, F. Activation of the cannabinoid receptor 2 increases renal perfusion. Physiol. Genom. 2019, 51, 90–96. [Google Scholar] [CrossRef]
- Hall, L.J.; Murphy, C.T.; Quinlan, A.; Hurley, G.; Shanahan, F.; Nally, K.; Melgar, S. Natural killer cells protect mice from DSS-induced colitis by regulating neutrophil function via the NKG2A receptor. Mucosal Immunol. 2013, 6, 1016–1026. [Google Scholar] [CrossRef]
- Wright, K.; Rooney, N.; Feeney, M.; Tate, J.; Robertson, D.; Welham, M.; Ward, S. Differential expression of cannabinoid receptors in the human colon: Cannabinoids promote epithelial wound healing. Gastroenterology 2005, 129, 437–453. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Li, L.; Jiang, B.; Feng, Z.; Yang, L.; Tang, J.; Chen, Q.; Zhang, J.; Tan, Q.; Feng, H. Cannabinoid receptor-2 stimulation suppresses neuroinflammation by regulating microglial M1/M2 polarization through the cAMP/PKA pathway in an experimental GMH rat model. Brain Behav. Immun. 2016, 58, 118–129. [Google Scholar] [CrossRef]
- Duff, G.; Argaw, A.; Cecyre, B.; Cherif, H.; Tea, N.; Zabouri, N.; Casanova, C.; Ptito, M.; Bouchard, J.-F. Cannabinoid receptor CB2 modulates axon guidance. PLoS ONE 2013, 8, e70849. [Google Scholar]
 ), or DSS plus 100 µL of SMM-189 (
), or DSS plus 100 µL of SMM-189 (  ) every day, starting at the beginning of day 0. The body weight of the mice was recorded every day, and the change from initial body weight was expressed as a percentage change in body weight Panel (A). On day 9, the colon length was recorded after euthanization Panel (B). Panel (C) Represent the picture of colon length of mice taken from individual groups. The statistical significance between values of each group was assessed using Student’s t-test. Data represent the mean of three independent experiments involving six mice per group (n = 18). Asterisks (*) (**) indicate a statistically significant difference at p < 0.01 and p < 0.001, respectively, between the group of mice treated with vehicle (DSS) and SMM-189.
) every day, starting at the beginning of day 0. The body weight of the mice was recorded every day, and the change from initial body weight was expressed as a percentage change in body weight Panel (A). On day 9, the colon length was recorded after euthanization Panel (B). Panel (C) Represent the picture of colon length of mice taken from individual groups. The statistical significance between values of each group was assessed using Student’s t-test. Data represent the mean of three independent experiments involving six mice per group (n = 18). Asterisks (*) (**) indicate a statistically significant difference at p < 0.01 and p < 0.001, respectively, between the group of mice treated with vehicle (DSS) and SMM-189.
 ), or DSS plus 100 µL of SMM-189 (
), or DSS plus 100 µL of SMM-189 (  ) every day, starting at the beginning of day 0. The body weight of the mice was recorded every day, and the change from initial body weight was expressed as a percentage change in body weight Panel (A). On day 9, the colon length was recorded after euthanization Panel (B). Panel (C) Represent the picture of colon length of mice taken from individual groups. The statistical significance between values of each group was assessed using Student’s t-test. Data represent the mean of three independent experiments involving six mice per group (n = 18). Asterisks (*) (**) indicate a statistically significant difference at p < 0.01 and p < 0.001, respectively, between the group of mice treated with vehicle (DSS) and SMM-189.
) every day, starting at the beginning of day 0. The body weight of the mice was recorded every day, and the change from initial body weight was expressed as a percentage change in body weight Panel (A). On day 9, the colon length was recorded after euthanization Panel (B). Panel (C) Represent the picture of colon length of mice taken from individual groups. The statistical significance between values of each group was assessed using Student’s t-test. Data represent the mean of three independent experiments involving six mice per group (n = 18). Asterisks (*) (**) indicate a statistically significant difference at p < 0.01 and p < 0.001, respectively, between the group of mice treated with vehicle (DSS) and SMM-189.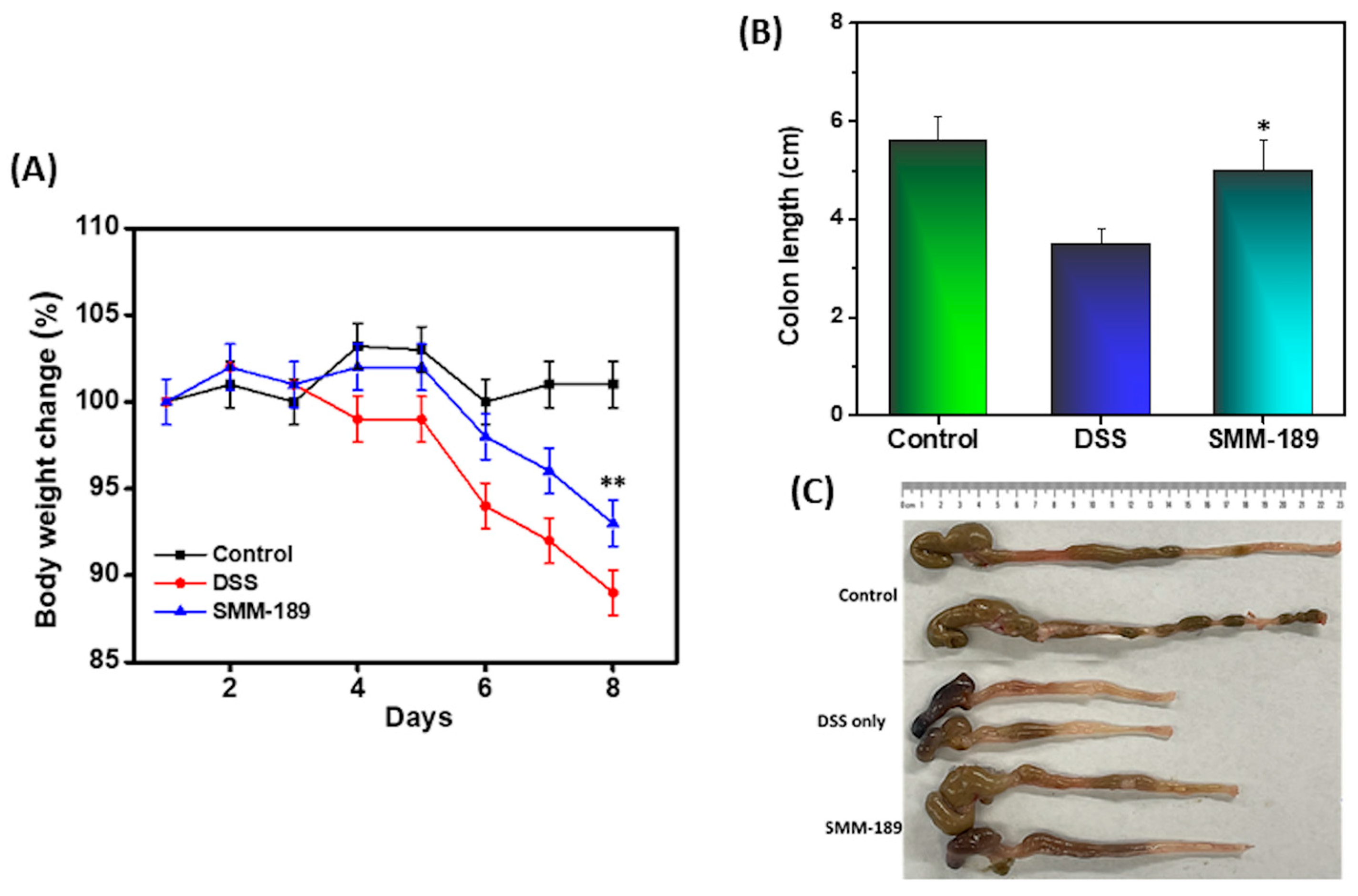



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiran, S.; Rakib, A.; Moore, B.M.; Singh, U.P. Cannabinoid Receptor 2 (CB2) Inverse Agonist SMM-189 Induces Expression of Endogenous CB2 and Protein Kinase A That Differentially Modulates the Immune Response and Suppresses Experimental Colitis. Pharmaceutics 2022, 14, 936. https://doi.org/10.3390/pharmaceutics14050936
Kiran S, Rakib A, Moore BM, Singh UP. Cannabinoid Receptor 2 (CB2) Inverse Agonist SMM-189 Induces Expression of Endogenous CB2 and Protein Kinase A That Differentially Modulates the Immune Response and Suppresses Experimental Colitis. Pharmaceutics. 2022; 14(5):936. https://doi.org/10.3390/pharmaceutics14050936
Chicago/Turabian StyleKiran, Sonia, Ahmed Rakib, Bob M. Moore, and Udai P. Singh. 2022. "Cannabinoid Receptor 2 (CB2) Inverse Agonist SMM-189 Induces Expression of Endogenous CB2 and Protein Kinase A That Differentially Modulates the Immune Response and Suppresses Experimental Colitis" Pharmaceutics 14, no. 5: 936. https://doi.org/10.3390/pharmaceutics14050936
APA StyleKiran, S., Rakib, A., Moore, B. M., & Singh, U. P. (2022). Cannabinoid Receptor 2 (CB2) Inverse Agonist SMM-189 Induces Expression of Endogenous CB2 and Protein Kinase A That Differentially Modulates the Immune Response and Suppresses Experimental Colitis. Pharmaceutics, 14(5), 936. https://doi.org/10.3390/pharmaceutics14050936






