Chitosan Nanoparticles in Atherosclerosis—Development to Preclinical Testing
Abstract
1. Atherosclerosis—A Brief Introduction
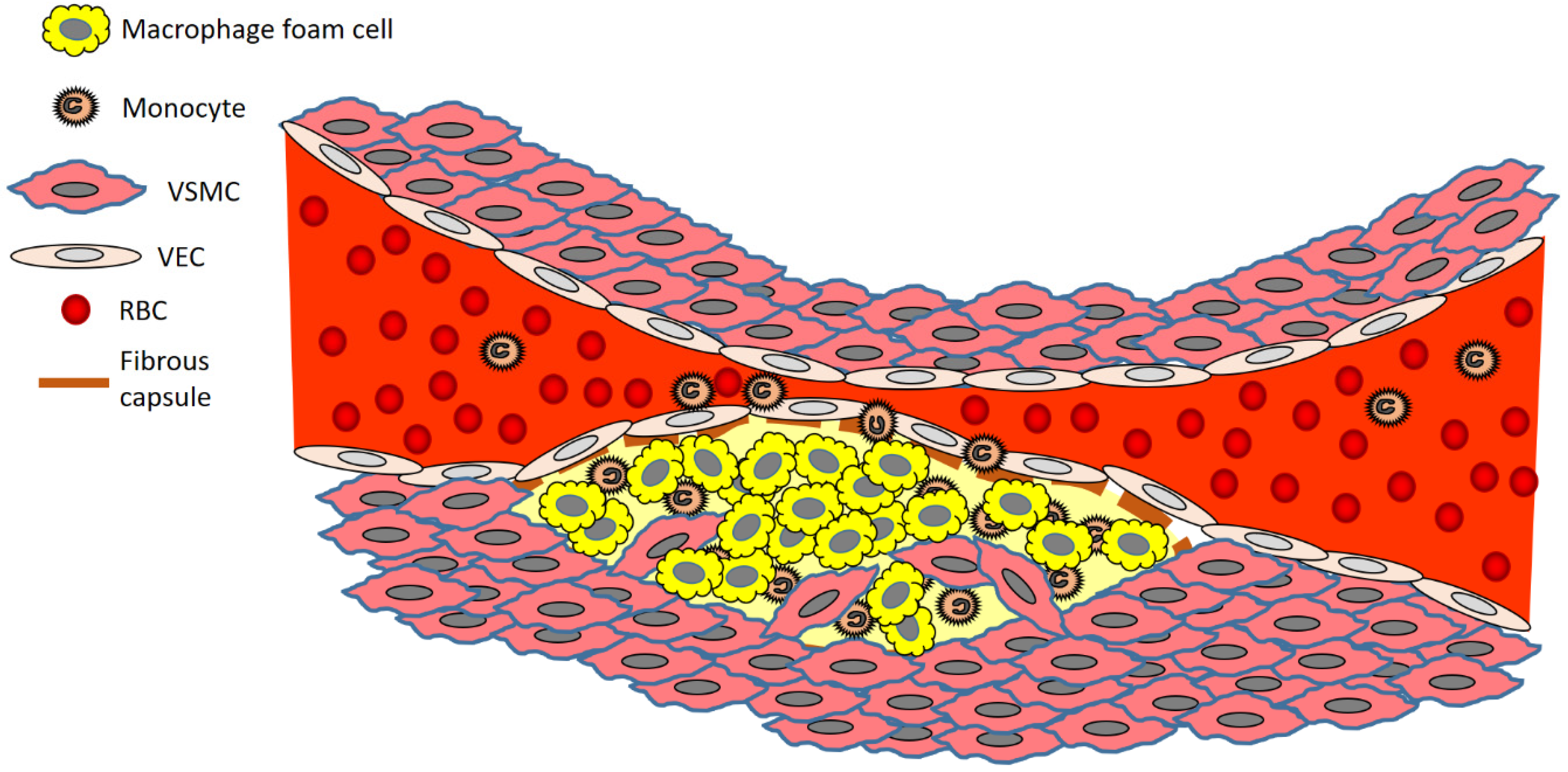
2. The Development of an Atherosclerotic Plaque—A Closer Look at the Cells Involved
3. Chitosan—A Promising Biopolymer That Has Potential in Atherosclerotic Disease Management
4. Chitosan Nanoparticles Used in Atherosclerosis Research
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Dedication
References
- Tîrziu, D.; Dobrian, A.; Tasca, C.; Simionescu, M.; Simionescu, N. Intimal thickenings of human aorta contain modified reassembled lipoproteins. Atherosclerosis 1995, 112, 101–114. [Google Scholar] [CrossRef]
- Dass, C.R.; Jessup, W. Apolipoprotein A-I, Cyclodextrins and Liposomes as Potential Drugs for the Reversal of Atherosclerosis. A Review. J. Pharm. Pharmacol. 2000, 52, 731–761. [Google Scholar] [CrossRef] [PubMed]
- Kessler, C.; Mitusch, R.; Guo, Y.; Rosengart, A.; Sheikhzadeh, A. Embolism from the aortic arch in patients with cerebral ischemia. Thromb. Res. 1996, 84, 145–155. [Google Scholar] [CrossRef]
- Tulsyan, N.; Ouriel, K.; Kashyap, V. Emerging drugs in peripheral arterial disease. Expert Opin. Emerg. Drugs 2006, 11, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.R. Myocardial infarction without obstructive coronary artery disease. Curr. Opin. Cardiol. 2012, 27, 655–660. [Google Scholar] [CrossRef]
- Wrobel, T.P.; Mateuszuk, L.; Kostogrys, R.B.; Chlopicki, S.; Baranska, M. Quantification of plaque area and characterization of plaque biochemical composition with atherosclerosis progression in ApoE/LDLR(-/-) mice by FT-IR imaging. Analyst 2013, 138, 6645–6652. [Google Scholar] [CrossRef]
- Saeedi, P.; Karuranga, S.; Hammond, L.; Kaundal, A.; Malanda, B.; Prystupiuk, M.; Matos, P. Cardiovascular diseases and risk factors knowledge and awareness in people with type 2 diabetes mellitus: A global evaluation. Diabetes Res. Clin. Pract. 2020, 165, 108194. [Google Scholar] [CrossRef]
- Lechner, K.; von Schacky, C.; McKenzie, A.L.; Worm, N.; Nixdorff, U.; Lechner, B.; Kränkel, N.; Halle, M.; Krauss, R.M.; Scherr, J. Lifestyle factors and high-risk atherosclerosis: Pathways and mechanisms beyond traditional risk factors. Eur. J. Prev. Cardiol. 2020, 27, 394–406. [Google Scholar] [CrossRef]
- Acosta, S.; Johansson, A.; Drake, I. Diet and Lifestyle Factors and Risk of Atherosclerotic Cardiovascular Disease—A Prospective Cohort Study. Nutrients 2021, 13, 3822. [Google Scholar] [CrossRef]
- Sun, F.; Zhou, X.; Li, Q.; Li, Y.; Zhang, H.; Yan, Z.; He, H.; Zhao, Z.; Ke, Z.; Gao, Y.; et al. Achieving blood pressure targets and antihypertensive effects through metabolic surgery in type 2 diabetes patients with hypertension. Diabetes/Metab. Res. Rev. 2021, 37, e3422. [Google Scholar] [CrossRef]
- Mayerl, C.; Lukasser, M.; Sedivy, R.; Niederegger, H.; Seiler, R.; Wick, G. Atherosclerosis research from past to present—On the track of two pathologists with opposing views, Carl von Rokitansky and Rudolf Virchow. Virchows Arch. 2006, 449, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Linton, M.F.; Yancey, P.G.; Davies, S.S.; Jerome, W.G.; Linton, E.F.; Song, W.L.; Doran, A.C.; Vickers, K.C. The role of lipids and lipoproteins in atherosclerosis. In Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2000; Available online: https://www.endotext.org/section/lipids (accessed on 25 December 2021).
- Boutagy, N.E.; Singh, A.K.; Sessa, W.C. Targeting the vasculature in cardiometabolic disease. J. Clin. Investig. 2022, 132, e148556. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Schilperoort, M.; Cao, Y.; Shi, J.; Tabas, I.; Tao, W. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat. Rev. Cardiol. 2022, 19, 228–249. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, N.; Giannarelli, C. How Single-Cell Technologies Have Provided New Insights into Atherosclerosis. Arter. Thromb. Vasc. Biol. 2022, 42, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Herem, J.W. Mural platelet microthrombi and major acute lesions of main epicardial arteries in sudden coronary death. Atherosclerosis 1974, 19, 529–541. [Google Scholar] [CrossRef]
- Martin, L.R. Pathophysiology of myocardial infarction. Semin. Fam. Med. 1980, 1, 3–9. [Google Scholar]
- Blekkenhorst, L.C.; Sim, M.; Bondonno, C.P.; Bondonno, N.P.; Ward, N.C.; Prince, R.L.; Devine, A.; Lewis, J.R.; Hodgson, J.M. Cardiovascular Health Benefits of Specific Vegetable Types: A Narrative Review. Nutrients 2018, 10, 595. [Google Scholar] [CrossRef]
- Hao, C.-L.; Lin, H.-L.; Ke, L.-Y.; Yen, H.-W.; Shen, K.-P. Pre-germinated brown rice extract ameliorates high-fat diet-induced metabolic syndrome. J. Food Biochem. 2019, 43, e12769. [Google Scholar] [CrossRef]
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef]
- Gylling, H.; Strandberg, T.E.; Kovanen, P.T.; Simonen, P. Lowering Low-Density Lipoprotein Cholesterol Concentration with Plant Stanol Esters to Reduce the Risk of Atherosclerotic Cardiovascular Disease Events at a Population Level: A Critical Discussion. Nutrients 2020, 12, 2346. [Google Scholar] [CrossRef]
- Zhang, Y.; Koradia, A.; Kamato, D.; Popat, A.; Little, P.J.; Ta, H.T. Treatment of atherosclerotic plaque: Perspectives on theranostics. J. Pharm. Pharmacol. 2019, 71, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Y.; Liu, S.; Sha, X.; Song, X.; Dai, Y.; Zhao, M.; Cai, L.; Xu, K.; Li, J. Theranostic nanoplatform to target macrophages enables the inhibition of atherosclerosis progression and fluorescence imaging of plaque in ApoE(−/−) mice. J. Nanobiotechnology 2021, 19, 222. [Google Scholar] [CrossRef] [PubMed]
- Winckers, K.; Cate, H.T.; Hackeng, T.M. The role of tissue factor pathway inhibitor in atherosclerosis and arterial thrombosis. Blood Rev. 2013, 27, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Finn, A.V.; Nakano, M.; Narula, J.; Kolodgie, F.D.; Virmani, R. Concept of Vulnerable/Unstable Plaque. Arter. Thromb. Vasc. Biol. 2010, 30, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- McAteer, M.A.; Choudhury, R.P. Targeted molecular imaging of vascular inflammation in cardiovascular disease using nano- and micro-sized agents. Vasc. Pharmacol. 2013, 58, 31–38. [Google Scholar] [CrossRef]
- Cybulsky, M.I.; Gimbrone, M.A., Jr. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science 1991, 251, 788791. [Google Scholar] [CrossRef]
- Hanyu, M.; Kume, N.; Ikeda, T.; Minami, M.; Kita, T.; Komeda, M. VCAM-1 expression precedes macrophage infiltration into subendothelium of vein grafts interposed into carotid arteries in hypercholesterolemic rabbits—A potential role in vein graft atherosclerosis. Atherosclerosis 2001, 158, 313–319. [Google Scholar] [CrossRef]
- Mundi, S.; Massaro, M.; Scoditti, E.; Carluccio, M.A.; Van Hinsbergh, V.W.; Iruela-Arispe, M.L.; De Caterina, R. Endothelial permeability, LDL deposition, and cardiovascular risk factors—A review. Cardiovasc. Res. 2018, 114, 35–52. [Google Scholar] [CrossRef]
- Auerbach, B.J.; Bisgaier, C.L.; Wölle, J.; Saxena, U. Oxidation of low density lipoproteins greatly enhances their association with lipoprotein lipase anchored to endothelial cell matrix. J. Biol. Chem. 1996, 271, 1329–1335. [Google Scholar] [CrossRef]
- de Freitas, F.A.; Levy, D.; Zarrouk, A.; Lizard, G.; Bydlowski, S.P. Impact of Oxysterols on Cell Death, Proliferation, and Differentiation Induction: Current Status. Cells 2021, 10, 2301. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis as an inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-W.; Ivanov, S.; Williams, J.W. Monocyte Recruitment, Specification, and Function in Atherosclerosis. Cells 2020, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Stary, H.C.; Chandler, A.B.; Glagov, S.; Guyton, J.R.; Insull, W., Jr.; Rosenfeld, M.E.; Schaffer, S.A.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis: A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis. American Heart Association. Circulation 1994, 89, 2462–2478. [Google Scholar] [CrossRef] [PubMed]
- Grebe, A.; Hoss, F.; Latz, E. NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ. Res. 2018, 122, 1722–1740. [Google Scholar] [CrossRef]
- Stary, H.C.; Chandler, A.B.; Dinsmore, R.E.; Fuster, V.; Glagov, S.; Insull, W., Jr.; Rosenfeld, M.E.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis: A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis. American Heart Association. Circulation 1995, 92, 1355–1374. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, Y.; Wu, Q.; Huang, L.; Xu, J.; Zeng, Q. Pathogenic role of microRNAs in atherosclerotic ischemic stroke: Implications for diagnosis and therapy. Genes Dis. 2022, 9, 682–696. [Google Scholar] [CrossRef]
- Tan, M.L.; Choong, P.F.; Dass, C.R. Cancer, chitosan nanoparticles and catalytic nucleic acids. J. Pharm. Pharmacol. 2009, 61, 3–12. [Google Scholar] [CrossRef]
- Suwan, J.; Zhang, Z.; Li, B.; Vongchan, P.; Meepowpan, P.; Zhang, F.; Mousa, S.A.; Mousa, S.; Premanode, B.; Kongtawelert, P.; et al. Sulfonation of papain-treated chitosan and its mechanism for anticoagulant activity. Carbohydr. Res. 2009, 344, 1190–1196. [Google Scholar] [CrossRef]
- Jo, H.-J.; Joo, S.-M.; Kim, J.Y.; Yu, K.-H.; Kim, S.W. Development of a Hybrid Chitosan- and Niacinamide-Coupled ZnO Nanoparticle Composite for Sun Protection Application. J. Nanomater. 2019, 2019, 5957606. [Google Scholar] [CrossRef]
- Ta, Q.; Ting, J.; Harwood, S.; Browning, N.; Simm, A.; Ross, K.; Olier, I.; Al-Kassas, R. Chitosan nanoparticles for enhancing drugs and cosmetic components penetration through the skin. Eur. J. Pharm. Sci. 2021, 160, 105765. [Google Scholar] [CrossRef]
- Wong, C.Y.; Martinez, J.; Al-Salami, H.; Dass, C.R. Quantification of BSA-loaded chitosan/oligonucleotide nanoparticles using reverse-phase high-performance liquid chromatography. Anal. Bioanal. Chem. 2018, 410, 6991–7006. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, H.; Bahadur, J.; Checker, R.; Ajgaonkar, P.; Vishwakarma, S.; Sen, D. Influence of molecular interactions on structure, controlled release and cytotoxicity of curcumin encapsulated chitosan—Silica nanostructured microspheres. Colloids Surf. B Biointerfaces 2021, 208, 112067. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.T.; Wijerathna, H.; Kumar, R.S.; Choi, D.; Dananjaya, S.; Attanayake, A. Preparation and characterization of succinyl chitosan and succinyl chitosan nanoparticle film: In Vitro and In Vivo evaluation of wound healing activity. Int. J. Biol. Macromol. 2021, 193, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Dutta, P.K.; Kumar, S.; Koh, J.; Lee, M.C.; Lim, J.W.; Pandey, S.; Garg, P. Synthesis, characterization and application of chitosan-N-(4-hydroxyphenyl)-methacrylamide derivative as a drug and gene carrier. Int. J. Biol. Macromol. 2022, 195, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Gallaher, C.M.; Munion, J.; Hesslink, R., Jr.; Wise, J.; Gallaher, D.D. Cholesterolreduction by glucomannan and chitosan is mediated by changes in cholesterol absorption and bile acid and fat excretion in rats. J. Nutr. 2000, 130, 2753–2759. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Tao, Y.; Guo, J.; Hu, Y.-M.; Su, Z.-Q. Hypolipidemic effects of chitosan nanoparticles in hyperlipidemia rats induced by high fat diet. Int. Immunopharmacol. 2011, 11, 457–461. [Google Scholar] [CrossRef]
- Tan, M.L.; Shao, P.; Friedhuber, A.M.; van Moorst, M.; Elahy, M.; Indumathy, S.; Dunstan, D.; Wei, Y.; Dass, C.R. The potential role of free chitosan in bone trauma and bone cancer management. Biomaterials 2014, 35, 7828–7838. [Google Scholar] [CrossRef]
- Shao, P.; Wei, Y.; Dass, C.R.; Zhang, G.; Wu, Z. Systemic Delivery of Free Chitosan Accelerates Femur Fracture Healing in Rats. Curr. Drug Targets 2018, 19, 460–466. [Google Scholar] [CrossRef]
- Dass, C.R.; Contreras, K.G.; Dunstan, D.E.; Choong, P.F. Chitosan microparticles encapsulating PEDF plasmid demonstrate efficacy in an orthotopic metastatic model of osteosarcoma. Biomaterials 2007, 28, 3026–3033. [Google Scholar] [CrossRef]
- Russo, E.; Gaglianone, N.; Baldassari, S.; Parodi, B.; Cafaggi, S.; Zibana, C.; Donalisio, M.; Cagno, V.; Lembo, D.; Caviglioli, G. Preparation, characterization and in vitro antiviral activity evaluation of foscarnet-chitosan nanoparticles. Colloids Surf. B Biointerfaces 2014, 118, 117–125. [Google Scholar] [CrossRef]
- Russo, E.; Gaglianone, N.; Baldassari, S.; Parodi, B.; Croce, I.; Bassi, A.M.; Vernazza, S.; Caviglioli, G. Chitosan-clodronate nanoparticles loaded in poloxamer gel for intra-articular administration. Colloids Surf. B Biointerfaces 2016, 143, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Ta, H.T.; Dass, C.R.; Larson, I.; Choong, P.F.; Dunstan, D.E. A chitosan–dipotassium orthophosphate hydrogel for the delivery of Doxorubicin in the treatment of osteosarcoma. Biomaterials 2009, 30, 3605–3613. [Google Scholar] [CrossRef] [PubMed]
- Ta, H.; Dass, C.R.; Larson, I.; Choong, P.F.; Dunstan, D.E. A chitosan hydrogel delivery system for osteosarcoma gene therapy with pigment epithelium-derived factor combined with chemotherapy. Biomaterials 2009, 30, 4815–4823. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.L.; Friedhuber, A.M.; Dunstan, D.E.; Choong, P.F.; Dass, C.R. The performance of doxorubicin encapsulated in chitosan–dextran sulphate microparticles in an osteosarcoma model. Biomaterials 2010, 31, 541–551. [Google Scholar] [CrossRef]
- Tan, M.L.; Dunstan, D.E.; Friedhuber, A.M.; Choong, P.F.; Dass, C.R. A nanoparticulate system that enhances the efficacy of the tumoricide Dz13 when administered proximal to the lesion site. J. Control. Release 2010, 144, 196–202. [Google Scholar] [CrossRef]
- Tan, M.L.; Friedhuber, A.M.; Dass, C.R. Co-nanoencapsulated doxorubicin and Dz13 control osteosarcoma progression in a murine model. J. Pharm. Pharmacol. 2013, 65, 35–43. [Google Scholar] [CrossRef]
- Huanbutta, K.; Sangnim, T.; Cheewatanakornkool, K.; Sutthapitaksakul, L.; Thanawuth, K.; Sriamornsak, P. Physical stability of different chitosan salts in matrix tablet formulations. Pharm. Sci. Asia 2020, 47, 347–356. [Google Scholar] [CrossRef]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. The role of chitosan on oral delivery of peptide-loaded nanoparticle formulation. J. Drug Target. 2018, 26, 551–562. [Google Scholar] [CrossRef]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Formulation and characterisation of insulin-loaded chitosan nanoparticles capable of inducing glucose uptake in skeletal muscle cells in vitro. J. Drug Deliv. Sci. Technol. 2020, 57, 101738. [Google Scholar] [CrossRef]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Lyophilisation Improves Bioactivity and Stability of Insulin-Loaded Polymeric-Oligonucleotide Nanoparticles for Diabetes Treatment. AAPS PharmSciTech 2020, 21, 108. [Google Scholar] [CrossRef]
- Wong, C.Y.; Martinez, J.; Zhao, J.; Al-Salami, H.; Dass, C.R. Development of orally administered insulin-loaded polymeric-oligonucleotide nanoparticles: Statistical optimization and physicochemical characterization. Drug Dev. Ind. Pharm. 2020, 46, 1238–1252. [Google Scholar] [CrossRef]
- Wong CY, J.; Al-Salami, H.; Dass, C.R. β-Cyclodextrin-containing chitosan-oligonucleotide nanoparticles improve insulin bioactivity, gut cellular permeation and glucose consumption. J. Pharm. Pharmacol. 2021, 73, 726–739. [Google Scholar] [CrossRef] [PubMed]
- Safarzadeh, M.; Mohammadi-Yeganeh, S.; Ghorbani-Bidkorbeh, F.; Haji Molla Hoseini, M. Chitosan based nanoformulation expressing miR-155 as a promising adjuvant to enhance Th1-biased immune responses. Life Sci. 2022, 297, 120459. [Google Scholar] [CrossRef] [PubMed]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan oligosaccharide/alginate nanoparticles as an effective carrier for astaxanthin with improving stability, in vitro oral bioaccessibility, and bioavailability. Food Hydrocoll. 2021, 124, 107246. [Google Scholar] [CrossRef]
- Liu, H.; Ojha, B.; Morris, C.; Jiang, M.; Wojcikiewicz, E.P.; Rao, P.P.N.; Du, D. Positively Charged Chitosan and N-Trimethyl Chitosan Inhibit Aβ40 Fibrillogenesis. Biomacromolecules 2015, 16, 2363–2373. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Han, L.; Qin, J.; Ru, G.; Li, R.; Wu, L.; Cui, D.; Yang, P.; He, Y.; Wang, J. N-Trimethyl chitosan chloride-coated PLGA nanoparticles overcoming multiple barriers to oral insulin absorption. ACS Appl. Mater. Interfaces 2015, 7, 15430–15441. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Cao, Z.; Xu, Y.; Lin, H.; Zhao, Y.; Wei, Y.; Qian, Z. Camptothecin nanocolloids based on N,N,N-trimethyl chitosan: Efficient suppression of growth of multiple myeloma in a murine model. Oncol. Rep. 2012, 27, 1035–1040. [Google Scholar] [CrossRef]
- Park, J.H.; Kwon, S.; Nam, J.O.; Park, R.W.; Chung, H.; Seo, S.B.; Kim, I.S.; Kwon, I.C.; Jeong, S.Y. Self-assembled nanoparticles based on glycol chitosan bearing 5 [beta]-cholanic acid for RGD peptide delivery. J. Control. Release 2004, 95, 579–588. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, Y.-S.; Kim, S.; Park, J.H.; Kim, K.; Choi, K.; Chung, H.; Jeong, S.Y.; Park, R.-W.; Kim, I.-S.; et al. Hydrophobically modified glycol chitosan nanoparticles as carriers for paclitaxel. J. Control. Release 2006, 111, 228–234. [Google Scholar] [CrossRef]
- Dass, C.R.; Choong, P.F.M. Chitosan-mediated orally delivered nucleic acids: A gutful of gene therapy. J. Drug Target. 2008, 16, 257–261. [Google Scholar] [CrossRef]
- Friedhuber, A.M.; Chandolu, V.; Manchun, S.; Donkor, O.; Sriamornsak, P.; Dass, C.R. Nucleotropic doxorubicin nanoparticles decrease cancer cell viability, destroy mitochondria, induce autophagy and enhance tumour necrosis. J. Pharm. Pharmacol. 2015, 67, 68–77. [Google Scholar] [CrossRef]
- Lee, P.X.; Martinez, J.; Dass, C.R. Stimulation of bone regeneration with pigment epithelium-derived factor microparticles: Evidence in silico, in vitro and in vivo. Die Pharm.-Int. J. Pharm. Sci. 2016, 71, 382–389. [Google Scholar] [CrossRef]
- Hecq, J.; Siepmann, F.; Siepmann, J.; Amighi, K.; Goole, J. Development and evaluation of chitosan and chitosan derivative nanoparticles containing insulin for oral administration. Drug Dev. Ind. Pharm. 2015, 41, 2037–2044. [Google Scholar] [CrossRef]
- Kumar, S.; Kaur, P.; Bernela, M.; Rani, R.; Thakur, R. Ketoconazole encapsulated in chitosan-gellan gum nanocomplexes exhibits prolonged antifungal activity. Int. J. Biol. Macromol. 2016, 93, 988–994. [Google Scholar] [CrossRef]
- Park, K.; Hong, H.-Y.; Moon, H.J.; Lee, B.-H.; Kim, I.-S.; Kwon, I.C.; Rhee, K. A new atherosclerotic lesion probe based on hydrophobically modified chitosan nanoparticles functionalized by the atherosclerotic plaque targeted peptides. J. Control. Release 2008, 128, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Xu, Y.; Yin, J.-F.; Jin, J.; Jiang, Y.; Du, Q. Improving the Effectiveness of (−)-Epigallocatechin Gallate (EGCG) against Rabbit Atherosclerosis by EGCG-Loaded Nanoparticles Prepared from Chitosan and Polyaspartic Acid. J. Agric. Food Chem. 2014, 62, 12603–12609. [Google Scholar] [CrossRef]
- Kaczyńska, A.; Guzdek, K.; Derszniak, K.; Karewicz, A.; Lewandowska-Łańcucka, J.; Mateuszuk, Ł.; Skórka, T.; Banasik, T.; Jasiński, K.; Kapusta, C.; et al. Novel nanostructural contrast for magnetic resonance imaging of endothelial inflammation: Targeting SPIONs to vascular endothelium. RSC Adv. 2016, 6, 72586–72595. [Google Scholar] [CrossRef]
- Hirpara, M.R.; Manikkath, J.; Sivakumar, K.; Managuli, R.S.; Gourishetti, K.; Krishnadas, N.; Shenoy, R.R.; Jayaprakash, B.; Rao, C.M.; Mutalik, S. Long circulating PEGylated-chitosan nanoparticles of rosuvastatin calcium: Development and in vitro and in vivo evaluations. Int. J. Biol. Macromol. 2018, 107, 2190–2200. [Google Scholar] [CrossRef]
- Ye, M.; Zhou, J.; Zhong, Y.; Xu, J.; Hou, J.; Wang, X.; Wang, Z.-G.; Guo, D. SR-A-Targeted Phase-Transition Nanoparticles for the Detection and Treatment of Atherosclerotic Vulnerable Plaques. ACS Appl. Mater. Interfaces 2019, 11, 9702–9715. [Google Scholar] [CrossRef]
- Nguyen, M.-A.; Wyatt, H.; Susser, L.; Geoffrion, M.; Rasheed, A.; Duchez, A.-C.; Cottee, M.L.; Afolayan, E.; Farah, E.; Kahiel, Z.; et al. Delivery of MicroRNAs by Chitosan Nanoparticles to Functionally Alter Macrophage Cholesterol Efflux in vitro and in vivo. ACS Nano 2019, 13, 6491–6505. [Google Scholar] [CrossRef]
- Yu, J.; Ruan, Q.; Nie, X. Synthesis and characterization of atherosclerotic target anti-CD47 functionalized by nano- polyelectrolyte complexes between chitosan and hyaluronic acid and in vivo and in vitro targeting experiments. Adv. Clin. Exp. Med. 2020, 29, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Huang, Q.; Liu, C.; Kwong, C.H.T.; Yue, L.; Wan, J.-B.; Lee, S.M.Y.; Wang, R. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat. Commun. 2020, 11, 2622. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, C.; Wu, Y. Cholesterol (Blood lipid) lowering potential of Rosuvastatin chitosan nanoparticles for atherosclerosis: Preclinical study in rabbit model. Acta Biochim. Pol. 2020, 67, 495–499. [Google Scholar] [PubMed]
- Luo, G.; Chen, W.; Luo, J.; Liu, J. Control and monitoring of lipoprotein levels in atherosclerosis induced rabbits using novel nanoparticulate medication of Lovastatin and Rosuvastatin. Micro Nano Lett. 2021, 16, 558–565. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, R.; Tran, H.D.N.; Kurniawan, N.D.; Moonshi, S.S.; Whittaker, A.K.; Ta, H.T. Chitosan Nanococktails Containing Both Ceria and Superparamagnetic Iron Oxide Nanoparticles for Reactive Oxygen Species-Related Theranostics. ACS Appl. Nano Mater. 2021, 4, 3604–3618. [Google Scholar] [CrossRef]
- Cavalcante, M.F.; Adorne, M.D.; Turato, W.M.; Kemmerer, M.; Uchiyama, M.K.; Asbahr, A.C.C.; Alves, A.D.C.S.; Farsky, S.H.P.; Drewes, C.; Spatti, M.C.; et al. scFv-Anti-LDL(-)-Metal-Complex Multi-Wall Functionalized-Nanocapsules as a Promising Tool for the Prevention of Atherosclerosis Progression. Front. Med. 2021, 8, 652137. [Google Scholar] [CrossRef]
- Yuan, X.; Yang, X.; Cai, D.; Mao, D.; Wu, J.; Zong, L.; Liu, J. Intranasal immunization with chitosan/pCETP nanoparticles inhibits atherosclerosis in a rabbit model of atherosclerosis. Vaccine 2008, 26, 3727–3734. [Google Scholar] [CrossRef]
- Lee, G.Y.; Kim, J.-H.; Oh, G.T.; Lee, B.-H.; Kwon, I.C.; Kim, I.-S. Molecular targeting of atherosclerotic plaques by a stabilin-2-specific peptide ligand. J. Control. Release 2011, 155, 211–217. [Google Scholar] [CrossRef]
- Xiao, S.; Mao, L.; Xiao, J.; Wu, Y.; Liu, H. Selenium nanoparticles inhibit the formation of atherosclerosis in apolipoprotein E deficient mice by alleviating hyperlipidemia and oxidative stress. Eur. J. Pharmacol. 2021, 902, 174120. [Google Scholar] [CrossRef]
- Hoogenboom, H.R. Designing and optimising library selection strategies for generating high-affinity antibodies. Trends Biotechnol. 1997, 15, 62–70. [Google Scholar] [CrossRef]
- Kelly, K.A.; Nahrendorf, M.; Yu, A.M.; Reynolds, F.; Weissleder, R. In Vivo Phage Display Selection Yields Atherosclerotic Plaque Targeted Peptides for Imaging. Mol. Imaging Biol. 2006, 8, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mohanraj, V.J.; Parkin, J.E. Chitosan-dextran sulfate nanoparticles for delivery of an anti-angiogenesis peptide. Lett. Pept. Sci. 2003, 10, 621–629. [Google Scholar] [CrossRef]
- Chaiyasan, W.; Srinivas, S.P.; Tiyaboonchai, W. Mucoadhesive Chitosan–Dextran Sulfate Nanoparticles for Sustained Drug Delivery to the Ocular Surface. J. Ocul. Pharmacol. Ther. 2013, 29, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Bozka, A.; Saka, O.M. Chitosan-DNA nanoparticles: Effect on DNA integrity, bacterial transformation and transfection efficiency. J. Drug Target. 2004, 12, 281–288. [Google Scholar]
- Huang, T.; Song, X.; Jing, J.; Zhao, K.; Shen, Y.; Zhang, X.; Yue, B. Chitosan-DNA nanoparticles enhanced the immunogenicity of multivalent DNA vaccination on mice against Trueperella pyogenes infection. J. Nanobiotechnol. 2018, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Dass, C.R. Biochemical and biophysical characteristics of lipoplexes pertinent to solid tumour gene therapy. Int. J. Pharm. 2002, 241, 1–25. [Google Scholar] [CrossRef]
- Nezhadi, S.H.; Choong, P.; Lotfipour, F.; Dass, C.R. Gelatin-based delivery systems for cancer gene therapy. J. Drug Target. 2009, 17, 731–738. [Google Scholar] [CrossRef]
- Carmen, I.H. A Death in the Laboratory: The Politics of the Gelsinger Aftermath. Mol. Ther. 2001, 3, 425–428. [Google Scholar] [CrossRef]
- Dass, C.R.; Walker, T.L.; Decruz, E.E.; Burton, M.A. Cationic Liposomes and Gene Therapy for Solid Tumors. Drug Deliv. 1997, 4, 151–165. [Google Scholar] [CrossRef][Green Version]
- Anderson, W.F. Human gene therapy. Nature 1998, 392, 25–30. [Google Scholar]
- Park, S.-Y.; Jung, M.-Y.; Kim, H.-J.; Lee, S.-J.; Kim, S.-Y.; Lee, B.-H.; Kwon, T.-H.; Park, R.-W.; Kim, I.-S. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 2007, 15, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Adachi, H.; Osuga, J.-I.; Ohashi, K.; Yahagi, N.; Sekiya, M.; Okazaki, H.; Tomita, S.; Iizuka, Y.; Shimano, H.; et al. FEEL-1 and FEEL-2 Are Endocytic Receptors for Advanced Glycation End Products. J. Biol. Chem. 2003, 278, 12613–12617. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Horii, Y.; Nishino, T.; Shiiki, H.; Sakaguchi, Y.; Kagoshima, T.; Dohi, K.; Makita, Z.; Vlassara, H.; Bucala, R. Immunohistochemical localization of advanced glycosylation end products in coronary atheroma and cardiac tissue in diabetes mellitus. Am. J. Pathol. 1993, 143, 1649–1656. [Google Scholar] [PubMed]
- Benlloch, M.; Cuerda-Ballester, M.; Drehmer, E.; Platero, J.L.; Carrera-Juliá, S.; López-Rodríguez, M.M.; Ceron, J.J.; Tvarijonaviciute, A.; Navarro, M.; Moreno, M.L.; et al. Possible Reduction of Cardiac Risk after Supplementation with Epigallocatechin Gallate and Increase of Ketone Bodies in the Blood in Patients with Multiple Sclerosis. A Pilot Study. Nutrients 2020, 12, 3792. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Taylor, L.S.; Ferruzzi, M.G.; Mauer, L.J. Kinetic Study of Catechin Stability: Effects of pH, Concentration, and Temperature. J. Agric. Food Chem. 2012, 60, 12531–12539. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Lambert, J.D.; Lee, M.-J.; Diamond, L.; Ju, J.; Hong, J.; Bose, M.; Newmark, H.L.; Yang, C.S. Dose-dependent levels of epigallocatechin-3-gallate in human colon cancer cells and mouse plasma and tissues. Drug Metab. Dispos. 2006, 34, 8–11. [Google Scholar] [CrossRef]
- Sang, S.; Lee, M.-J.; Hou, Z.; Ho, C.-T.; Yang, C.S. Stability of Tea Polyphenol (−)-Epigallocatechin-3-gallate and Formation of Dimers and Epimers under Common Experimental Conditions. J. Agric. Food Chem. 2005, 53, 9478–9484. [Google Scholar] [CrossRef]
- Krook, M.A.; Hagerman, A.E. Stability of polyphenols epigallocatechin gallate and pentagalloyl glucose in a simulated digestive system. Food Res. Int. 2012, 49, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 2002, 360, 7–22. [Google Scholar] [CrossRef]
- Colhoun, H.M.; Betteridge, D.J.; Durrington, P.N.; Hitman, G.A.; Neil, H.A.; Livingstone, S.J.; Thomason, M.J.; Mackness, M.I.; Charlton-Menys, V.; Fuller, J.H.; et al. Primary prevention of cardiovascular disease with atorvastatinin type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): Multicentre randomized placebo-controlled trial. Lancet 2004, 364, 685–696. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, H.; Zhou, Z.; Xia, Y.; Wang, Z.; Ran, H.; Li, P.; Ren, J. Peptide-Functionalized Phase-Transformation Nanoparticles for Low Intensity Focused Ultrasound-Assisted Tumor Imaging and Therapy. Nano Lett. 2018, 18, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-J.; Chang, Y.-C.; Yeh, C.-K. Improving Nanoparticle Penetration in Tumors by Vascular Disruption with Acoustic Droplet Vaporization. Theranostics 2015, 6, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Cybulsky, M.I.; Cheong, C.; Robbins, C.S. Macrophages and dendritic cells: Partners in atherogenesis. Circ. Res. 2016, 118, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Vent-Schmidt, J.; McGeough, M.D.; Wong, M.; Hoffman, H.M.; Steiner, T.S.; Levings, M.K. Tr1 cells, but not Foxp3þ regulatory T cells, suppress NLRP3 inflammasome activation via an IL-10-dependent mechanism. J. Immunol. 2015, 195, 488–497. [Google Scholar] [CrossRef]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nuñez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef]
- Zhong, Z.; Liang, S.; Sanchez-Lopez, E.; He, F.; Shalapour, S.; Lin, X.-J.; Wong, J.; Ding, S.; Seki, E.; Schnabl, B.; et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 2018, 560, 198–203. [Google Scholar] [CrossRef]
- Allahverdian, S.; Chehroudi, A.C.; McManus, B.M.; Abraham, T.; Francis, G.A. Contribution of Intimal Smooth Muscle Cells to Cholesterol Accumulation and Macrophage-Like Cells in Human Atherosclerosis. Circulation 2014, 129, 1551–1559. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Bardaweel, S.K.; Gul, M.; Alzweiri, M.; Ishaqat, A.; ALSalamat, H.A.; Bashatwah, R.M. Reactive Oxygen Species: The Dual Role in Physiological and Pathological Conditions of the Human Body. Eurasian J. Med. 2018, 50, 193–201. [Google Scholar] [CrossRef]
- Patetsios, P.; Song, M.; Shutze, W.P.; Pappas, C.; Rodino, W.; Ramirez, J.A.; Panetta, T.F. Identification of uric acid and xanthine oxidase in atherosclerotic plaque. Am. J. Cardiol. 2001, 88, 188–191. [Google Scholar] [CrossRef]
- Curi, R.; de Siqueira Mendes, R.; de Campos Crispin, L.A.; Norata, G.D.; Sampaio, S.C.; Newsholme, P. A past and present overview of macrophage metabolism and functional outcomes. Clin. Sci. 2017, 131, 1329–1342. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, H.; Huang, K. Selenium in the prevention of atherosclerosis and its underlying mechanisms. Metallomics 2017, 9, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Aibani, N.; Rai, R.; Patel, P.; Cuddihy, G.; Wasan, E.K. Chitosan Nanoparticles at the Biological Interface: Implications for Drug Delivery. Pharmaceutics 2021, 13, 1686. [Google Scholar] [CrossRef] [PubMed]
| Study Mode | Chemical Constituents/Type of Chitosan/Formulation Process | Size (nm)/ Shape | Agent Carried | Major Findings | Future Expectation | Reference |
|---|---|---|---|---|---|---|
| In vitro* | AP peptide HGC Stirring | 314 Spherical | NIR fluorophore Cy5.5 | In TNF-α- activated BAECs, bound more avidly than to non-activated cells, with NPs seen within cells | In vivo evaluation was performed (Table 2) | [76] |
| In vitro | PAA Chitosan Electrostatic self-assembly | 102 Spherical | EGCG | Improved stability in GI simulated fluids | In vivo evaluation was performed (Table 2) | [77] |
| In vitro | Cationic chitosan derivative Anti-VCAM-1 or anti-selectin Co-precipitation | 91 Spherical | Fe2+ Fe3+ | Specific interaction of SPIONs with aortic endothelial cells of db/db mice grown in culture | In vivo evaluation was performed (Table 2) | [78] |
| In vitro | TPP Pegylated chitosan Ionotropic gelation | <200 Spherical | RST | Sustained release of drug was observed in phosphate buffer pH 7.4 | In vivo evaluation was performed (Table 2) | [79] |
| In vitro | PFH PLGA DS Chitosan Stirring | 375 Spherical | Fe3O4 |
| In vivo evaluation was performed (Table 2) | [80] |
| In vitro | TPP Pegylated chitosan Ionic gelation | 150 Spherical | miR-33 |
| In vivo evaluation was performed (Table 2) | [81] |
| In vitro | Anti-CD47 antibody HA Chitosan Stirring | 500 Roughly spherical | Cy5.5 | Efficiently adsorbed to the surface of VECs | In vivo evaluation was performed (Table 2) | [82] |
| In vitro | Oxi-COS Extrusion | 149 Spherical | Nile red | NPs responded to presence of ROS | In vivo evaluation was performed (Table 2) | [83] |
| In vitro | TPP Tween-80 Chitosan Ionotropic gelation | ND Spherical | RST | Initial burst release of 11.89%, then gradual sustained release of drug (~88.11%) over 48 h in PBS, pH 7.4 |
| [84] |
| In vitro | TPP Poloxamer-188 Chitosan Ionic gelation | 105 Roughly spherical | LST RST | Initial burst release of 15.24% (RST) and 13.98% (LST), then sustained release of 98.95% (RST) and 99.67% (LST) respectively over 14h in PBS, pH 7.4 |
| [85] |
| In vitro | TPP Chitosan Electrostatic self-assembly | 100 Heteroge- neous | Iron oxide Cerium oxide |
| In vivo evaluation not performed | [86] |
| In vitro | Lecithin Sodium monostearate Capric caprylic triglyceride scFv-antiLDL (-) Chitosan Coated surface | 117 Spherical | Zinc |
| In vivo evaluation was performed (Table 2) | [87] |
| Study Mode | Chemical Constituents/Type of Chitosan/Formulation Process | Size (nm)/ Shape | Agent Carried | Delivery Route and Major Findings | Future Directions | Reference |
|---|---|---|---|---|---|---|
| In vivo | AP peptide HGC Stirring | 314 Spherical | NIR fluorophore Cy5.5 | Intravenous NPs bound better to atherosclerotic lesions in a low-density lipoprotein receptor-deficient (LDLr-/-) atherosclerotic mouse than to such lesions in a normal mouse |
| [76] |
| In vivo | pCETP Chitosan Complex coacervation | 340 Spherical | pCETP |
|
| [88] |
| In vivo | SP-2 HGC Self-assembly | 315 Spherical | NIR fluorophore Cy5.5 | NPs were delivered intraventricular to atherosclerotic plaques In vivo |
| [89] |
| In vivo | PAA Chitosan Electrostatic self-assembly | 102 Spherical | EGCG | Oral administration reduced lipid burden similar to simvastatin |
| [77] |
| In vivo | Cationic chitosan derivative Anti-VCAM-1 or anti-selectin Co-precipitation | 91 Spherical | Fe2+ Fe3+ | Selective delivery to the aortic arch of ApoE/LDLR-/- mice |
| [78] |
| In vivo | TPP Pegylated chitosan Ionotropic gelation | <200 Spherical | RST | Oral versus intravenous There was a greater lipid-lowering capability of RST when encapsulated |
| [79] |
| In vivo | PFH PLGA DS Chitosan Stirring | 375 Spherical | Fe3O4 |
|
| [80] |
| In vivo | TPP Pegylated chitosan Ionic gelation | 150 Spherical | miR-33 | Subcutaneous Transferred miR-33 to macrophages and reduced the expression of ABCA1 |
| [81] |
| In vivo | Anti-CD47 antibody HA Chitosan Stirring | 500 Roughly spherical | Cy5.5 | Intravenous Distributed around the atherosclerotic plaque | Deliver therapeutic agents to the plaques | [82] |
| In vivo | Oxi-COS Extrusion | 149 Spherical | AT |
| Examine whether cell membranes derived from other immune cells, including neutrophil, T cells, and B cells can also be utilised for drug delivery against atherosclerosis | [83] |
| In vivo | TPP Tween-80 Chitosan Ionotropic gelation | ND Spherical | RST |
| Assess plaque area and stability | [84] |
| In vivo | TPP Poloxamer-188 Chitosan Ionic gelation | 105 Roughly spherical | LST RST |
| Check plaques histologically for area and stability | [85] |
| In vivo | Selenium Chitosan Chemical reduction | 65.8 Spherical | AT |
| [90] | |
| In vivo | Lecithin Sodium monostearate Capric caprylic triglyceride scFv-anti-LDL (-) Chitosan Coated surface | 117 Spherical | Zinc |
| Delivery of other therapeutic, diagnostic or theranostic agents | [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sriamornsak, P.; Dass, C.R. Chitosan Nanoparticles in Atherosclerosis—Development to Preclinical Testing. Pharmaceutics 2022, 14, 935. https://doi.org/10.3390/pharmaceutics14050935
Sriamornsak P, Dass CR. Chitosan Nanoparticles in Atherosclerosis—Development to Preclinical Testing. Pharmaceutics. 2022; 14(5):935. https://doi.org/10.3390/pharmaceutics14050935
Chicago/Turabian StyleSriamornsak, Pornsak, and Crispin R. Dass. 2022. "Chitosan Nanoparticles in Atherosclerosis—Development to Preclinical Testing" Pharmaceutics 14, no. 5: 935. https://doi.org/10.3390/pharmaceutics14050935
APA StyleSriamornsak, P., & Dass, C. R. (2022). Chitosan Nanoparticles in Atherosclerosis—Development to Preclinical Testing. Pharmaceutics, 14(5), 935. https://doi.org/10.3390/pharmaceutics14050935







