Nanocarrier-Based Targeted Therapies for Myocardial Infarction
Abstract
1. Introduction
1.1. Myocardial Infarction and Treatment Challenges
1.2. Treatment Challenges
2. Nanomedicines and Nanocarriers
3. Nanomedicines for Cardiac Therapy
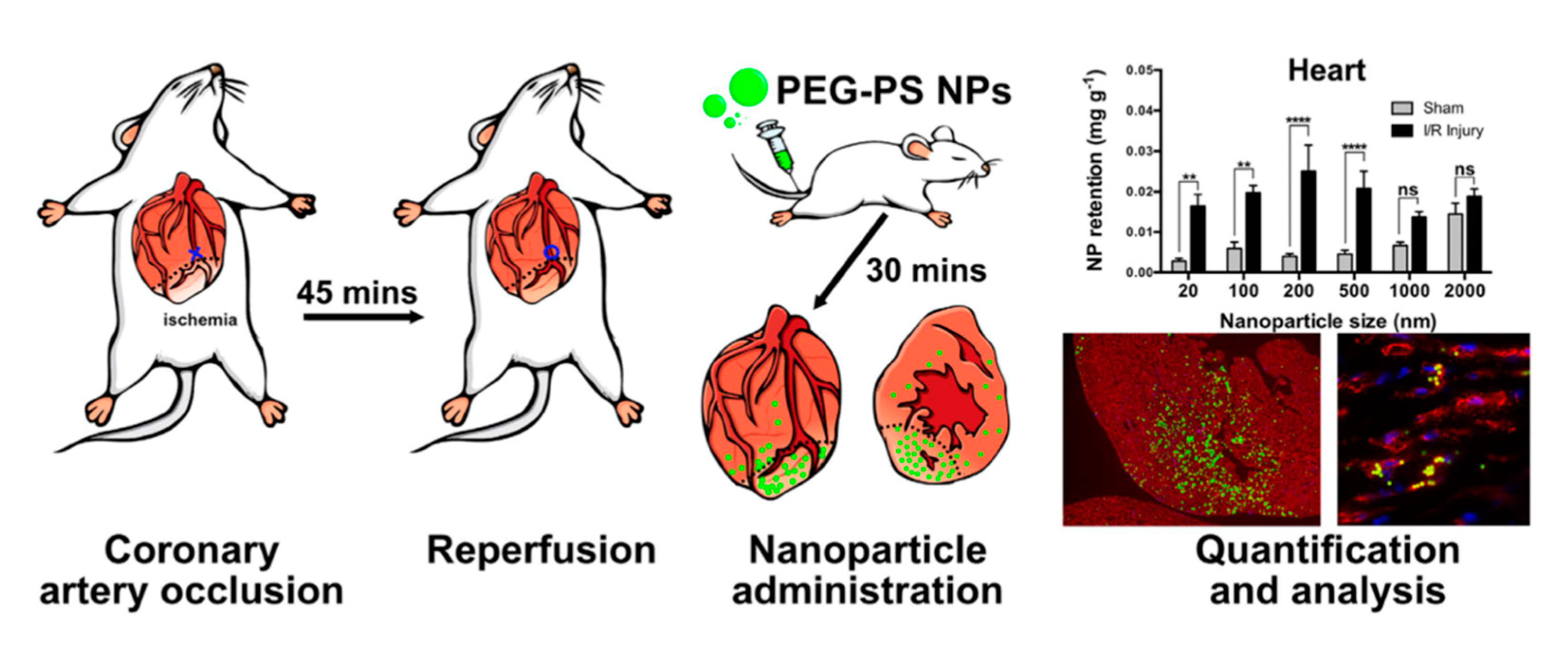
3.1. Passive Targeting
3.2. Active Targeting
3.2.1. Polymer-Based Nanocarriers
3.2.2. Lipid-Based Nanocarriers
3.2.3. Inorganic Nanocarriers
3.3. Cell-Based and Biomimicry-Based Targeting
3.4. Exosomes for Cardiac Therapy
4. Limitations and Future Challenges
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Global Health Estimates: Leading Causes of Death. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 23 February 2022).
- Aparicio, H.J.; Benjamin, E.J.; Callaway, C.W.; Carson, A.P.; Cheng, S.; Elkind, M.S.; Evenson, K.R.; Ferguson, J.F.; Knutson, K.L.; Lee, C.D.; et al. Heart Disease and Stroke Statistics-2021 Update A Report from the American Heart Association. Circulation 2021, 143, E254–E743. [Google Scholar] [CrossRef]
- Dani, S.S.; Lone, A.N.; Javed, Z.; Khan, M.S.; Zia Khan, M.; Kaluski, E.; Virani, S.S.; Shapiro, M.D.; Cainzos-Achirica, M.; Nasir, K.; et al. Trends in Premature Mortality from Acute Myocardial Infarction in the United States, 1999 to 2019. J. Am. Heart Assoc. 2021, 11, e021682. [Google Scholar] [CrossRef] [PubMed]
- McGill, H.C.; McMahan, C.A.; Gidding, S.S. Preventing Heart Disease in the 21st Century: Implications of the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Study. Circulation 2008, 117, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The Changing Landscape of Atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Scarabelli, T.; Stephanou, A.; Rayment, N.; Pasini, E.; Comini, L.; Curello, S.; Ferrari, R.; Knight, R.; Latchman, D. Apoptosis of Endothelial Cells Precedes Myocyte Cell Apoptosis in Ischemia/Reperfusion Injury. Circulation 2001, 104, 253–256. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Pathophysiology of Myocardial Infarction. In Comprehensive Physiology; Wiley for American Physiological Society: Bethesda, MD, USA, 2015; pp. 1841–1875. [Google Scholar]
- Bergmann, O.; Zdunek, S.; Felker, A.; Salehpour, M.; Alkass, K.; Bernard, S.; Sjostrom, S.L.; Szewczykowska, M.; Jackowska, T.; dos Remedios, C.; et al. Dynamics of Cell Generation and Turnover in the Human Heart. Cell 2015, 161, 1566–1575. [Google Scholar] [CrossRef]
- Vilahur, G.; Juan-Babot, O.; Pena, E.; Onate, B.; Casani, L.; Badimon, L. Molecular and Cellular Mechanisms Involved in Cardiac Remodeling after Acute Myocardial Infarction. J. Mol. Cell. Cardiol. 2011, 50, 522–533. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Awada, H.K.; Hwang, M.P.; Wang, Y. Towards Comprehensive Cardiac Repair and Regeneration after Myocardial Infarction: Aspects to Consider and Proteins to Deliver. Biomaterials 2016, 82, 94–112. [Google Scholar] [CrossRef]
- Broughton, K.M.; Wang, B.J.; Firouzi, F.; Khalafalla, F.; Dimmeler, S.; Fernandez-Aviles, F.; Sussman, M.A. Mechanisms of Cardiac Repair and Regeneration. Circ. Res. 2018, 122, 1151–1163. [Google Scholar] [CrossRef]
- Kukielka, G.L.; Smith, C.W.; LaRosa, G.J.; Manning, A.M.; Mendoza, L.H.; Daly, T.J.; Hughes, B.J.; Youker, K.A.; Hawkins, H.K.; Michael, L.H.; et al. Interleukin-8 Gene Induction in the Myocardium after Ischemia and Reperfusion in Vivo. J. Clin. Investig. 1995, 95, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.D.; Annex, B.H.; McKendall, G.R.; Azrin, M.A.; Lopez, J.J.; Giordano, F.J.; Shah, P.K.; Willerson, J.T.; Benza, R.L.; Berman, D.S.; et al. The VIVA Trial: Vascular Endothelial Growth Factor in Ischemia for Vascular Angiogenesis. Circulation 2003, 107, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.B.; Hernández-Reséndiz, S.; Crespo-Avilan, G.E.; Mukhametshina, R.T.; Kwek, X.Y.; Cabrera-Fuentes, H.A.; Hausenloy, D.J. Inflammation Following Acute Myocardial Infarction: Multiple Players, Dynamic Roles, and Novel Therapeutic Opportunities. Pharmacol. Ther. 2018, 186, 73–87. [Google Scholar] [CrossRef]
- Eckhouse, S.R. Local Hydrogel Release of Recombinant TIMP-3 Attenuates Adverse Left Ventricular Remodeling after Experimental Myocardial Infarction. Sci. Transl. Med. 2014, 6, 223ra21. [Google Scholar] [CrossRef] [PubMed]
- Bejerano, T.; Etzion, S.; Elyagon, S.; Etzion, Y.; Cohen, S. Nanoparticle Delivery of MiRNA-21 Mimic to Cardiac Macrophages Improves Myocardial Remodeling after Myocardial Infarction. Nano Lett. 2018, 18, 5885–5891. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth Factor Delivery-Based Tissue Engineering: General Approaches and a Review of Recent Developments. J. R. Soc. Interface/R. Soc. 2011, 8, 153–170. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Singh, I.; Khademhosseini, A. Engineered Biomaterials for in Situ Tissue Regeneration. Nat. Rev. Mater. 2020, 5, 686–705. [Google Scholar] [CrossRef]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef]
- Oduk, Y.; Zhu, W.; Kannappan, R.; Zhao, M.; Borovjagin, A.; Oparil, S.; Zhang, J. (Jay) VEGF Nanoparticles Repair the Heart after Myocardial Infarction. Am. J. Physiol.-Heart Circ. Physiol. 2018, 314, H278–H284. [Google Scholar] [CrossRef]
- Fan, C.; Joshi, J.; Li, F.; Xu, B.; Khan, M.; Yang, J.; Zhu, W. Nanoparticle-Mediated Drug Delivery for Treatment of Ischemic Heart Disease. Front. Bioeng. Biotechnol. 2020, 8, 687. [Google Scholar] [CrossRef]
- Prajnamitra, R.P.; Chen, H.-C.; Lin, C.-J.; Chen, L.-L.; Hsieh, P.C.-H. Nanotechnology Approaches in Tackling Cardiovascular Diseases. Molecules 2019, 24, 2017. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.; Batist, G.; Belt, R.; Rovira, D.; Navari, R.; Azarnia, N.; Welles, L.; Winer, E.; Garrett, T.; Blayney, D.; et al. Liposome-Encapsulated Doxorubicin Compared with Conventional Doxorubicin in a Randomized Multicenter Trial as First-Line Therapy of Metastatic Breast Carcinoma. Cancer 2002, 94, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.Y.; Yang, Y.J.; Chang, C.H.; Tang, A.C.L.; Liao, W.Y.; Cheng, F.Y.; Yeh, C.S.; Lai, J.J.; Stayton, P.S.; Hsieh, P.C.H. Functionalized Nanoparticles Provide Early Cardioprotection after Acute Myocardial Infarction. J. Control. Release 2013, 170, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Kugeratski, F.G.; Hodge, K.; Lilla, S.; McAndrews, K.M.; Zhou, X.; Hwang, R.F.; Zanivan, S.; Kalluri, R. Quantitative proteomics identifies the core proteome of exosomes with syntenin-1 as the highest abundant protein and a putative universal biomarker. Nat. Cell Biol. 2021, 23, 631–641. [Google Scholar] [CrossRef]
- Tieu, A.; Lalu, M.M.; Slobodian, M.; Gnyra, C.; Fergusson, D.A.; Montroy, J.; Burger, D.; Stewart, D.J.; Allan, D.S. An Analysis of Mesenchymal Stem Cell-Derived Extracellular Vesicles for Preclinical Use. ACS Nano 2020, 14, 9728–9743. [Google Scholar] [CrossRef]
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics 2018, 10, 218. [Google Scholar] [CrossRef]
- Lai, R.C.; Yeo, R.W.Y.; Tan, K.H.; Lim, S.K. Exosomes for Drug Delivery—A Novel Application for the Mesenchymal Stem Cell. Biotechnol. Adv. 2013, 31, 543–551. [Google Scholar] [CrossRef]
- Morad, G.; Carman, C.V.; Hagedorn, E.J.; Perlin, J.R.; Zon, L.I.; Mustafaoglu, N.; Park, T.; Ingber, D.E.; Daisy, C.C.; Moses, M.A. Tumor-Derived Extracellular Vesicles Breach the Intact Blood–Brain Barrier via Transcytosis. ACS Nano 2019, 13, 13853–13865. [Google Scholar] [CrossRef]
- Ge, X.; Meng, Q.; Wei, L.; Liu, J.; Li, M.; Liang, X.; Lin, F.; Zhang, Y.; Li, Y.; Liu, Z.; et al. Myocardial Ischemia-Reperfusion Induced Cardiac Extracellular Vesicles Harbour Proinflammatory Features and Aggravate Heart Injury. J. Extracell. Vesicles 2021, 10, e12072. [Google Scholar] [CrossRef]
- Bei, Y.; Das, S.; Rodosthenous, R.S.; Holvoet, P.; Vanhaverbeke, M.; Monteiro, M.C.; Monteiro, V.V.S.; Radosinska, J.; Bartekova, M.; Jansen, F.; et al. Extracellular Vesicles in Cardiovascular Theranostics. Theranostics 2017, 7, 4168–4182. [Google Scholar] [CrossRef] [PubMed]
- Huda, M.N.; Nafiujjaman, M.; Deaguero, I.G.; Okonkwo, J.; Hill, M.L.; Kim, T.; Nurunnabi, M. Potential Use of Exosomes as Diagnostic Biomarkers and in Targeted Drug Delivery: Progress in Clinical and Preclinical Applications. ACS Biomater. Sci. Eng. 2021, 7, 2106–2149. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Agrahari, V.; Burnouf, P.A.; Chew, C.H.; Burnouf, T. Extracellular Microvesicles as New Industrial Therapeutic Frontiers. Trends Biotechnol. 2019, 37, 707–729. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, T.; Agrahari, V.; Agrahari, V. Extracellular Vesicles as Nanomedicine: Hopes and Hurdles in Clinical Translation. Int. J. Nanomed. 2019, 14, 8847–8859. [Google Scholar] [CrossRef]
- Wu, Y.W.; Huang, C.C.; Changou, C.A.; Lu, L.S.; Goubran, H.; Burnouf, T. Clinical-Grade Cryopreserved Doxorubicin-Loaded Platelets: Role of Cancer Cells and Platelet Extracellular Vesicles Activation Loop. J. Biomed. Sci. 2020, 27, 45. [Google Scholar] [CrossRef]
- Armstrong, J.P.K.; Holme, M.N.; Stevens, M.M. Re-Engineering Extracellular Vesicles as Smart Nanoscale Therapeutics. ACS Nano 2017, 11, 69–83. [Google Scholar] [CrossRef]
- Price, P.M.; Mahmoud, W.E.; Al-Ghamdi, A.A.; Bronstein, L.M. Magnetic Drug Delivery: Where the Field Is Going. Front. Chem. 2018, 6, 619. [Google Scholar] [CrossRef]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the Dynamics of the EPR Effect and Strategies to Improve the Therapeutic Effects of Nanomedicines by Using EPR Effect Enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef]
- Wu, Y.; Yin, X.; Wijaya, C.; Huang, M.-H.; McConnell, B.K. Acute Myocardial Infarction in Rats. J. Vis. Exp. 2011, 2464. [Google Scholar] [CrossRef]
- Mao, S.; Wang, L.; Chen, P.; Lan, Y.; Guo, R.; Zhang, M. Nanoparticle-Mediated Delivery of Tanshinone IIA Reduces Adverse Cardiac Remodeling Following Myocardial Infarctions in a Mice Model: Role of NF-ΚB Pathway. Artif. Cells Nanomed. Biotechnol. 2018, 46, S707–S716. [Google Scholar] [CrossRef]
- Asanuma, H.; Sanada, S.; Yoshitomi, T.; Sasaki, H. Novel Synthesized Radical-Containing Nanoparticles Limit Infarct Size Following Ischemia and Reperfusion in Canine Hearts. Cardiovasc. Drugs Ther. 2017, 31, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Allijn, I.E.; Czarny, B.M.S.; Wang, X.; Chong, S.Y.; Weiler, M.; da Silva, A.E.; Metselaar, J.M.; Lam, C.S.P.; Pastorin, G.; de Kleijn, D.P.V.; et al. Liposome Encapsulated Berberine Treatment Attenuates Cardiac Dysfunction after Myocardial Infarction. J. Control. Release 2017, 247, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Evers, M.J.W.; Du, W.; Yang, Q.; Kooijmans, S.A.A.; Vink, A.; van Steenbergen, M.; Vader, P.; de Jager, S.C.A.; Fuchs, S.A.; Mastrobattista, E.; et al. Delivery of Modified MRNA to Damaged Myocardium by Systemic Administration of Lipid Nanoparticles. J. Control. Release 2022, 343, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Lundy, D.J.; Chen, K.-H.; Toh, E.K.-W.; Hsieh, P.C.-H. Distribution of Systemically Administered Nanoparticles Reveals a Size-Dependent Effect Immediately Following Cardiac Ischaemia-Reperfusion Injury. Sci. Rep. 2016, 6, 25613. [Google Scholar] [CrossRef]
- Kannan, L.; Kis-Toth, K.; Yoshiya, K.; Thai, T.H.; Sehrawat, S.; Mayadas, T.N.; Dalle Lucca, J.J.; Tsokos, G.C. R-Spondin3 Prevents Mesenteric Ischemia/Reperfusioninduced Tissue Damage by Tightening Endothelium and Preventing Vascular Leakage. Proc. Natl. Acad. Sci. USA 2013, 110, 14348–14353. [Google Scholar] [CrossRef]
- Paulis, L.E.; Geelen, T.; Kuhlmann, M.T.; Coolen, B.F.; Schäfers, M.; Nicolay, K.; Strijkers, G.J. Distribution of Lipid-Based Nanoparticles to Infarcted Myocardium with Potential Application for MRI-Monitored Drug Delivery. J. Control. Release 2012, 162, 276–285. [Google Scholar] [CrossRef]
- Cheng, C.J.; Tietjen, G.T.; Saucier-Sawyer, J.K.; Saltzman, W.M. A Holistic Approach to Targeting Disease with Polymeric Nanoparticles. Nat. Rev. Drug Discov. 2015, 14, 239–247. [Google Scholar] [CrossRef]
- Emami, F.; Mostafavi Yazdi, S.J.; Na, D.H. Poly(Lactic Acid)/Poly(Lactic-Co-Glycolic Acid) Particulate Carriers for Pulmonary Drug Delivery. J. Pharm. Investig. 2019, 49, 427–442. [Google Scholar] [CrossRef]
- Boltnarova, B.; Kubackova, J.; Skoda, J.; Stefela, A.; Smekalova, M.; Svacinova, P.; Pavkova, I.; Dittrich, M.; Scherman, D.; Zbytovska, J.; et al. PLGA Based Nanospheres as a Potent Macrophage-Specific Drug Delivery System. Nanomaterials 2021, 11, 749. [Google Scholar] [CrossRef]
- Nakano, Y.; Matoba, T.; Tokutome, M.; Funamoto, D.; Katsuki, S.; Ikeda, G.; Nagaoka, K.; Ishikita, A.; Nakano, K.; Koga, J.I.; et al. Nanoparticle-Mediated Delivery of Irbesartan Induces Cardioprotection from Myocardial Ischemia-Reperfusion Injury by Antagonizing Monocyte-Mediated Inflammation. Sci. Rep. 2016, 6, 29601. [Google Scholar] [CrossRef]
- Sun, B.; Liu, S.; Hao, R.; Dong, X.; Fu, L.; Han, B. RGD-PEG-PLA Delivers MiR-133 to Infarct Lesions of Acute Myocardial Infarction Model Rats for Cardiac Protection. Pharmaceutics 2020, 12, 575. [Google Scholar] [CrossRef]
- Schanze, N.; Bode, C.; Duerschmied, D. Platelet Contributions to Myocardial Ischemia/Reperfusion Injury. Front. Immunol. 2019, 10, 1260. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.M.; Carlini, A.S.; Chien, M.P.; Sonnenberg, S.; Luo, C.; Braden, R.L.; Osborn, K.G.; Li, Y.; Gianneschi, N.C.; Christman, K.L. Enzyme-Responsive Nanoparticles for Targeted Accumulation and Prolonged Retention in Heart Tissue after Myocardial Infarction. Adv. Mater. 2015, 27, 5547–5552. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.P.A.; Ranjan, S.; Kinnunen, S.; Correia, A.; Talman, V.; Mäkilä, E.; Barrios-Lopez, B.; Kemell, M.; Balasubramanian, V.; Salonen, J.; et al. Drug-Loaded Multifunctional Nanoparticles Targeted to the Endocardial Layer of the Injured Heart Modulate Hypertrophic Signaling. Small 2017, 13, 1701276. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Shi, X.; Dong, H.; You, S.; Cao, H.; Wang, K.; Wen, Y.; Shi, D.; He, B.; Li, Y. Delivery of MicroRNA-1 Inhibitor by Dendrimer-Based Nanovector: An Early Targeting Therapy for Myocardial Infarction in Mice. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Seo, M.J.; Deci, M.B.; Weil, B.R.; Canty, J.M.; Nguyen, J. Effect of CCR2 Inhibitor-Loaded Lipid Micelles on Inflammatory Cell Migration and Cardiac Function after Myocardial Infarction. Int. J. Nanomed. 2018, 13, 6441–6451. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, M.; Sun, S.; Li, B.; Du, D.; Sun, J.; Cao, F.; Li, H.; Jia, F.; Wang, T.; et al. The Use of Antibody Modified Liposomes Loaded with AMO-1 to Deliver Oligonucleotides to Ischemic Myocardium for Arrhythmia Therapy. Biomaterials 2014, 35, 3697–3707. [Google Scholar] [CrossRef]
- Dong, Z.; Guo, J.; Xing, X.; Zhang, X.; Du, Y.; Lu, Q. RGD Modified and PEGylated Lipid Nanoparticles Loaded with Puerarin: Formulation, Characterization and Protective Effects on Acute Myocardial Ischemia Model. Biomed. Pharmacother. 2017, 89, 297–304. [Google Scholar] [CrossRef]
- Dasa, S.S.K.; Suzuki, R.; Gutknecht, M.; Brinton, L.T.; Tian, Y.; Michaelsson, E.; Lindfors, L.; Klibanov, A.L.; French, B.A.; Kelly, K.A. Development of Target-Specific Liposomes for Delivering Small Molecule Drugs after Reperfused Myocardial Infarction. J. Control. Release 2015, 220, 556–567. [Google Scholar] [CrossRef]
- Nguyen, J.; Sievers, R.; Motion, J.P.M.; Kivimäe, S.; Fang, Q.; Lee, R.J. Delivery of Lipid Micelles into Infarcted Myocardium Using a Lipid-Linked Matrix Metalloproteinase Targeting Peptide. Mol. Pharm. 2015, 12, 1150–1157. [Google Scholar] [CrossRef]
- Li, M.; Tang, X.; Liu, X.; Cui, X.; Lian, M.; Zhao, M.; Peng, H.; Han, X. Targeted MiR-21 Loaded Liposomes for Acute Myocardial Infarction. J. Mater. Chem. B 2020, 8, 10384–10391. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Yang, W.; Han, G. Protective Effects on Myocardial Infarction Model: Delivery of Schisandrin B Using Matrix Metalloproteinase-Sensitive Peptide-Modified, Pegylated Lipid Nanoparticles. Int. J. Nanomed. 2017, 12, 7121–7130. [Google Scholar] [CrossRef] [PubMed]
- Ben-Mordechai, T.; Kain, D.; Holbova, R.; Landa, N.; Levin, L.P.; Elron-Gross, I.; Glucksam-Galnoy, Y.; Feinberg, M.S.; Margalit, R.; Leor, J. Targeting and Modulating Infarct Macrophages with Hemin Formulated in Designed Lipid-Based Particles Improves Cardiac Remodeling and Function. J. Control. Release 2017, 257, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Lee, K.J.; Chen, H.C.; Prajnamitra, R.P.; Hsu, C.H.; Jian, C.B.; Yu, X.E.; Chueh, D.Y.; Kuo, C.W.; Chiang, T.C.; et al. Immune Cell Shuttle for Precise Delivery of Nanotherapeutics for Heart Disease and Cancer. Sci. Adv. 2021, 7, eabf2400. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, Y.; Liu, D.; Liu, M.; Cui, H.; Zhang, B.; Mei, Q.; Zhou, S. Mitochondria-Targeted Cyclosporin A Delivery System to Treat Myocardial Ischemia Reperfusion Injury of Rats. J. Nanobiotechnology 2019, 17, 18. [Google Scholar] [CrossRef]
- Yu, J.; Li, W.; Yu, D. Atrial Natriuretic Peptide Modified Oleate Adenosine Prodrug Lipid Nanocarriers for the Treatment of Myocardial Infarction: In Vitro and in Vivo Evaluation. Drug Des. Dev. Ther. 2018, 12, 1697–1706. [Google Scholar] [CrossRef]
- Cung, T.-T.; Morel, O.; Cayla, G.; Rioufol, G.; Garcia-Dorado, D.; Angoulvant, D.; Bonnefoy-Cudraz, E.; Guérin, P.; Elbaz, M.; Delarche, N.; et al. Cyclosporine before PCI in Patients with Acute Myocardial Infarction. N. Engl. J. Med. 2015, 373, 1021–1031. [Google Scholar] [CrossRef]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as Nanocarrier for Drug Delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Díaz-Herráez, P.; Saludas, L.; Pascual-Gil, S.; Simón-Yarza, T.; Abizanda, G.; Prósper, F.; Garbayo, E.; Blanco-Prieto, M.J. Transplantation of Adipose-Derived Stem Cells Combined with Neuregulin-Microparticles Promotes Efficient Cardiac Repair in a Rat Myocardial Infarction Model. J. Control. Release 2017, 249, 23–31. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric Micelles in Drug Delivery: An Insight of the Techniques for Their Characterization and Assessment in Biorelevant Conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Zielinska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Nagasamy Venkatesh, D.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- George, T.A.; Chen, M.M.; Czosseck, A.; Chen, H.-P.; Huang, H.-S.; Lundy, D.J. Liposome-Encapsulated Anthraquinone Improves Efficacy and Safety in Triple Negative Breast Cancer. J. Control. Release 2022, 342, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Castro Bravo, K.M.; Liu, J. Targeted Liposomal Drug Delivery: A Nanoscience and Biophysical Perspective. Nanoscale Horiz. 2021, 6, 78–94. [Google Scholar] [CrossRef]
- Won, Y.W.; Bull, D.A.; Kim, S.W. Functional Polymers of Gene Delivery for Treatment of Myocardial Infarct. J. Control. Release 2014, 195, 110–119. [Google Scholar] [CrossRef]
- Wang, X.; Huang, H.; Zhang, L.; Bai, Y.; Chen, H. PCM and TAT Co-Modified Liposome with Improved Myocardium Delivery: In Vitro and in Vivo Evaluations. Drug Deliv. 2017, 24, 339–345. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Di, L. Acute Myocardial Infarction Therapy: In Vitro and in Vivo Evaluation of Atrial Natriuretic Peptide and Triphenylphosphonium Dual Ligands Modified, Baicalin-Loaded Nanoparticulate System. Drug Deliv. 2021, 28, 2198–2204. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Carbone, C.; Souto, E.B. Beyond Liposomes: Recent Advances on Lipid Based Nanostructures for Poorly Soluble/Poorly Permeable Drug Delivery. Prog. Lipid Res. 2017, 68, 1–11. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Oliveira, C.; Granja, P.L.; Sarmento, B. Antibodies and Associates: Partners in Targeted Drug Delivery. Pharmacol. Ther. 2017, 177, 129–145. [Google Scholar] [CrossRef]
- Sato, Y.; Fujiwara, H.; Takatsu, Y. Biochemical Markers in Heart Failure. J. Cardiol. 2012, 59, 1–7. [Google Scholar] [CrossRef][Green Version]
- Liu, B.; Wang, B.; Zhang, X.; Lock, R.; Nash, T.; Vunjak-Novakovic, G. Cell Type–Specific MicroRNA Therapies for Myocardial Infarction. Sci. Transl. Med. 2021, 13, eabd0914. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Rossi, J. Aptamers as Targeted Therapeutics: Current Potential and Challenges. Nat. Rev. Drug Discov. 2016, 16, 181–202. [Google Scholar] [CrossRef]
- Isa, E.D.M.; Ahmad, H.; Rahman, M.B.A.; Gill, M.R. Progress in Mesoporous Silica Nanoparticles as Drug Delivery Agents for Cancer Treatment. Pharmaceutics 2021, 13, 152. [Google Scholar] [CrossRef]
- Rusinkevich, V.; Huang, Y.; Chen, Z.Y.; Qiang, W.; Wang, Y.G.; Shi, Y.F.; Yang, H. tian Temporal Dynamics of Immune Response Following Prolonged Myocardial Ischemia/Reperfusion with and without Cyclosporine A. Acta Pharmacol. Sin. 2019, 40, 1168–1183. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Pittet, M.J.; Swirski, F.K. Monocytes: Protagonists of Infarct Inflammation and Repair after Myocardial Infarction. Circulation 2010, 121, 2437–2445. [Google Scholar] [CrossRef]
- Wojakowski, W.; Tendera, M.; Michałowska, A.; Majka, M.; Kucia, M.; Maślankiewicz, K.; Wyderka, R.; Ochała, A.; Ratajczak, M.Z. Mobilization of CD34/CXCR4+, CD34/CD117+, c-Met + Stem Cells, and Mononuclear Cells Expressing Early Cardiac, Muscle, and Endothelial Markers into Peripheral Blood in Patients with Acute Myocardial Infarction. Circulation 2004, 110, 3213–3220. [Google Scholar] [CrossRef] [PubMed]
- Mathiyalagan, P.; Liang, Y.; Kim, D.; Misener, S.; Thorne, T.; Kamide, C.E.; Klyachko, E.; Losordo, D.W.; Hajjar, R.J.; Sahoo, S. Angiogenic Mechanisms of Human CD34 + Stem Cell Exosomes in the Repair of Ischemic Hindlimb. Circ. Res. 2017, 120, 1466–1476. [Google Scholar] [CrossRef]
- Stellos, K.; Bigalke, B.; Borst, O.; Pfaff, F.; Elskamp, A.; Sachsenmaier, S.; Zachmann, R.; Stamatelopoulos, K.; Schönberger, T.; Geisler, T.; et al. Circulating Platelet-Progenitor Cell Coaggregate Formation Is Increased in Patients with Acute Coronary Syndromes and Augments Recruitment of Cd341 Cells in the Ischaemic Microcirculation. Eur. Heart J. 2013, 34, 2548–2556. [Google Scholar] [CrossRef]
- Cheng, B.; Toh, E.K.W.; Chen, K.-H.; Chang, Y.-C.; Hu, C.-M.J.; Wu, H.-C.; Chau, L.-Y.; Chen, P.; Hsieh, P.C.H. Biomimicking Platelet-Monocyte Interactions as a Novel Targeting Strategy for Heart Healing. Adv. Healthc. Mater. 2016, 5, 2686–2697. [Google Scholar] [CrossRef]
- Tang, J.; Su, T.; Huang, K.; Dinh, P.U.; Wang, Z.; Vandergriff, A.; Hensley, M.T.; Cores, J.; Allen, T.; Li, T.; et al. Targeted Repair of Heart Injury by Stem Cells Fused with Platelet Nanovesicles. Nat. Biomed. Eng. 2018, 2, 17–26. [Google Scholar] [CrossRef]
- Moebiust, J.; Zahedi, R.P.; Lewandrowski, U.; Berger, C.; Walter, U.; Sickmann, A. The Human Platelet Membrane Proteome Reveals Several New Potential Membrane Proteins. Mol. Cell. Proteom. 2005, 4, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.J.O.; Floriano, J.F.; Nicastro, L.; Emanueli, C.; Catapano, F. Novel Applications of Mesenchymal Stem Cell-Derived Exosomes for Myocardial Infarction Therapeutics. Biomolecules 2020, 10, 707. [Google Scholar] [CrossRef]
- Xiao, C.; Wang, K.; Xu, Y.; Hu, H.; Zhang, N.; Wang, Y.; Zhong, Z.; Zhao, J.; Li, Q.; Zhu, D.; et al. Transplanted Mesenchymal Stem Cells Reduce Autophagic Flux in Infarcted Hearts via the Exosomal Transfer of MiR-125b. Circ. Res. 2018, 123, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alonso, S.; Alcaraz-Serna, A.; Sánchez-Madrid, F.; Alfranca, A. Extracellular Vesicle-Mediated Immune Regulation of Tissue Remodeling and Angiogenesis after Myocardial Infarction. Front. Immunol. 2018, 9, 2799. [Google Scholar] [CrossRef] [PubMed]
- Vandergriff, A.; Huang, K.; Shen, D.; Hu, S.; Hensley, M.T.; Caranasos, T.G.; Qian, L.; Cheng, K. Targeting Regenerative Exosomes to Myocardial Infarction Using Cardiac Homing Peptide. Theranostics 2018, 8, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Kanki, S.; Jaalouk, D.E.; Lee, S.; Yu, A.Y.C.; Gannon, J.; Lee, R.T. Identification of Targeting Peptides for Ischemic Myocardium by in Vivo Phage Display. J. Mol. Cell. Cardiol. 2011, 50, 841–848. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Zhao, Z.; Meng, Q.; Yu, Y.; Sun, J.; Yang, Z.; Chen, Y.; Li, J.; Ma, T.; et al. Engineered Exosomes with Ischemic Myocardium-Targeting Peptide for Targeted Therapy in Myocardial Infarction. J. Am. Heart Assoc. 2018, 7, e008737. [Google Scholar] [CrossRef]
- Gallet, R.; Dawkins, J.; Valle, J.; Simsolo, E.; De Couto, G.; Middleton, R.; Tseliou, E.; Luthringer, D.; Kreke, M.; Smith, R.R.; et al. Exosomes Secreted by Cardiosphere-Derived Cells Reduce Scarring, Attenuate Adverse Remodelling, and Improve Function in Acute and Chronic Porcine Myocardial Infarction. Eur. Heart J. 2017, 38, 201–211. [Google Scholar] [CrossRef]
- Malliaras, K.; Makkar, R.R.; Smith, R.R.; Cheng, K.; Wu, E.; Bonow, R.O.; Marbán, L.; Mendizabal, A.; Cingolani, E.; Johnston, P.V.; et al. Intracoronary Cardiosphere-Derived Cells after Myocardial Infarction. J. Am. Coll. Cardiol. 2014, 63, 110–122. [Google Scholar] [CrossRef]
- Tseliou, E.; Fouad, J.; Reich, H.; Slipczuk, L.; De Couto, G.; Aminzadeh, M.A.; Middleton, R.C.; Valle, J.; Weixin, L.; Marbán, E. Exosomes from Cardiac Stem Cells Amplify Their Own Bioactivity by Converting Fibroblasts to Therapeutic Cells. J. Am. Coll. Cardiol. 2015, 66, 599–611. [Google Scholar] [CrossRef]
- Cambier, L.; de Couto, G.; Ibrahim, A.; Marbán, E. Abstract 16009: Y RNA Fragments Enriched in Exosomes from Cardiosphere-Derived Cells Mediate Cardioprotection and Macrophage Polarization. Circulation 2015, 132, A16009. [Google Scholar] [CrossRef]
- Qiao, L.; Hu, S.; Liu, S.; Zhang, H.; Ma, H.; Huang, K.; Li, Z.; Su, T.; Vandergriff, A.; Tang, J.; et al. MicroRNA-21-5p Dysregulation in Exosomes Derived from Heart Failure Patients Impairs Regenerative Potential. J. Clin. Investig. 2019, 129, 2237–2250. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.K.; Khan, M.A.; Zubair, H.; Srivastava, S.K.; Khushman, M.; Singh, S.; Singh, A.P. Comparative Analysis of Exosome Isolation Methods Using Culture Supernatant for Optimum Yield, Purity and Downstream Applications. Sci. Rep. 2019, 9, 5335. [Google Scholar] [CrossRef] [PubMed]
- Willms, E.; Johansson, H.J.; Mäger, I.; Lee, Y.; Blomberg, K.E.M.; Sadik, M.; Alaarg, A.; Smith, C.I.E.; Lehtiö, J.; El Andaloussi, S.; et al. Cells Release Subpopulations of Exosomes with Distinct Molecular and Biological Properties. Sci. Rep. 2016, 6, 22519. [Google Scholar] [CrossRef] [PubMed]
- Butreddy, A.; Kommineni, N.; Dudhipala, N. Exosomes as Naturally Occurring Vehicles for Delivery of Biopharmaceuticals: Insights from Drug Delivery to Clinical Perspectives. Nanomaterials 2021, 11, 1481. [Google Scholar] [CrossRef]
- Boengler, K.; Schulz, R.; Heusch, G. Loss of Cardioprotection with Ageing. Cardiovasc. Res. 2009, 83, 247–261. [Google Scholar] [CrossRef]
- Boyle, A.J.; Hwang, J.; Ye, J.; Shih, H.; Jun, K.; Zhang, Y.; Fang, Q.; Sievers, R.; Yeghiazarians, Y.; Lee, R.J. The Effects of Aging on Apoptosis Following Myocardial Infarction. Cardiovasc. Ther. 2013, 31, e102–e110. [Google Scholar] [CrossRef]
- Schloss, M.J.; Horckmans, M.; Nitz, K.; Duchene, J.; Drechsler, M.; Bidzhekov, K.; Scheiermann, C.; Weber, C.; Soehnlein, O.; Steffens, S. The Time-of-day of Myocardial Infarction Onset Affects Healing through Oscillations in Cardiac Neutrophil Recruitment. EMBO Mol. Med. 2016, 8, 937–948. [Google Scholar] [CrossRef]
- Lindsey, M.L.; Brunt, K.R.; Kirk, J.A.; Kleinbongard, P.; Calvert, J.W.; de Castro Brás, L.E.; DeLeon-Pennell, K.Y.; Del Re, D.P.; Frangogiannis, N.G.; Frantz, S.; et al. Guidelines for in Vivo Mouse Models of Myocardial Infarction. Am. J. Physiol.—Heart Circ. Physiol. 2021, 321, H1056–H1073. [Google Scholar] [CrossRef]
- Carlsson, L.; Clarke, J.C.; Yen, C.; Gregoire, F.; Albery, T.; Billger, M.; Egnell, A.C.; Gan, L.M.; Jennbacken, K.; Johansson, E.; et al. Biocompatible, Purified VEGF-A MRNA Improves Cardiac Function after Intracardiac Injection 1 Week Post-Myocardial Infarction in Swine. Mol. Ther.-Methods Clin. Dev. 2018, 9, 330–346. [Google Scholar] [CrossRef]
- Chris, Y.; Patrick, H. Pathology of Permanent, LAD-Ligation Induced Myocardial Infarction Differs across Small (Mice, Rat) and Large (Pig) Animal Models. Front. Bioeng. Biotechnol. 2016, 4. [Google Scholar] [CrossRef]
- Garbern, J.C.; Mummery, C.L.; Lee, R.T. Model Systems for Cardiovascular Regenerative Biology. Cold Spring Harb. Perspect. Med. 2013, 3, a014019. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cicha, I.; Chauvierre, C.; Texier, I.; Cabella, C.; Metselaar, J.M.; Szebeni, J.; Dézsi, L.; Alexiou, C.; Rouzet, F.; Storm, G.; et al. From Design to the Clinic: Practical Guidelines for Translating Cardiovascular Nanomedicine. Cardiovasc. Res. 2018, 114, 1714. [Google Scholar] [CrossRef] [PubMed]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]

| Targeting | Nanocarrier/Payload | Findings | Ref. |
|---|---|---|---|
| Passive/EPR Intravenous | PLGA NPs/irbesartan | Reduced infarct size vs. free drug (mouse) | [52] |
| Passive/EPR Direct injection | PLGA NPs/VEGF | Improved cardiac performance and vascular density (mouse) | [21] |
| Passive/EPR Intravenous | Copolymer micelles/TEMPO 1 | Reduced infarct size, apoptosis, (canine I/R model) | [43] |
| Passive/EPR | Liposome/berberine | Preserved cardiac function (mouse) compared to free Berberine | [44] |
| Passive/EPR | Liposome/tanshinone and puerarin | Improved drug delivery to heart compared to free drug (rat) | [42] |
| Passive/EPR | Liposome/modRNA | Delivery and expression of functional protein at infarct site following intravenous injection(mouse) | [45] |
| Responsive/EPR | MMP-responsive NPs | Improved retention in infarcted myocardium (rat) | [55] |
| Ligand-based (ANP) | Porous silica NPs | Improved retention in ischemic left ventricle (rat) | [56] |
| Ligand-based (AT1) | Dendrimer/AMO 2 | Improved delivery vs. non-targeted. Reduced apoptosis and infarct size (mouse) | [57] |
| Ligand-based (anti-CCR2 antibody) | PEG-DSPE micelle/ CCR2 antagonist | Reduced inflammatory cell recruitment and infarct size | [58] |
| Ligand-based (anti-Troponin antibody) | Liposome/AMO 2 | Increased delivery to infarct area compared to non-targeted liposomes (rat) | [59] |
| Ligand-based (RGD) | Liposome/Peurarin | Increased delivery to heart, reduced infarct size (rat) | [60] |
| Ligand-based (multiple targeting peptides) | Liposome/ PARP inhibitor | Ninefold higher delivery to cardiomyocytes than non-targeted peptides (mouse) | [61] |
| Ligand-based (MMP targeting peptide) | Micelle/ MMP-targeting peptide | Increased micelle delivery to infarct area compared to non-targeted micelles (mouse) | [62] |
| Ligand-based (anti-troponin antibody) | Liposome/miR-21 | Increased binding and retention in heart (rat) | [63] |
| Ligand-based (MMP-targeting peptide) | Lipid NPs/ schisandrin B | Slightly improved drug delivery and reduced infarct size (rat) | [64] |
| Cell/Ligand-based (hyaluronan) | Liposome/hemin | Targeting macrophages to deliver to infarcted heart. Improved cardiac function, angiogenesis and reduced scar vs. free drug (mouse) | [65] |
| Ligand-based (RGD) | PEG + PLA NPs/ miR-133 | Slightly improved vs. free miRNA or non-targeted liposomes (mouse) | [53] |
| Ligand-based (aptamer) | Liposome/IOX2 | Delivered to ischemic heart via macrophages. Improved cardiac function (mouse) | [66] |
| Ligand-based (mitochondria-targeting peptide) | PLGA/cyclosporine A | Passive targeting combined with active targeting of the mitochondria. Increased accumulation in ischemia compared to normal (rat) | [67] |
| Ligand-based (ANP) | Lipid NP/adenosine | Improved delivery and reduced infarct size (rat) | [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
George, T.A.; Hsu, C.-C.; Meeson, A.; Lundy, D.J. Nanocarrier-Based Targeted Therapies for Myocardial Infarction. Pharmaceutics 2022, 14, 930. https://doi.org/10.3390/pharmaceutics14050930
George TA, Hsu C-C, Meeson A, Lundy DJ. Nanocarrier-Based Targeted Therapies for Myocardial Infarction. Pharmaceutics. 2022; 14(5):930. https://doi.org/10.3390/pharmaceutics14050930
Chicago/Turabian StyleGeorge, Thomashire A., Chuan-Chih Hsu, Annette Meeson, and David J. Lundy. 2022. "Nanocarrier-Based Targeted Therapies for Myocardial Infarction" Pharmaceutics 14, no. 5: 930. https://doi.org/10.3390/pharmaceutics14050930
APA StyleGeorge, T. A., Hsu, C.-C., Meeson, A., & Lundy, D. J. (2022). Nanocarrier-Based Targeted Therapies for Myocardial Infarction. Pharmaceutics, 14(5), 930. https://doi.org/10.3390/pharmaceutics14050930






