Abstract
Some diseases of uncontrolled proliferation such as cancer, as well as infectious diseases, are the main cause of death in the world, and their causative agents have rapidly developed resistance to the various existing treatments, making them even more dangerous. Thereby, the discovery of new therapeutic agents is a challenge promoted by the World Health Organization (WHO). Biomacromolecules, isolated or synthesized from a natural template, have therapeutic properties which have not yet been fully studied, and represent an unexplored potential in the search for new drugs. These substances, starting from conglomerates of proteins and other substances such as animal venoms, or from minor substances such as bioactive peptides, help fight diseases or counteract harmful effects. The high effectiveness of these biomacromolecules makes them promising substances for obtaining new drugs; however, their low bioavailability or stability in biological systems is a challenge to be overcome in the coming years with the help of nanotechnology. The objective of this review article is to describe the relationship between the structure and function of biomacromolecules of animal origin that have applications already described using nanotechnology and targeted delivery.
1. Introduction
Biological diversity has proven to be a rich source of many molecules with therapeutic potential and a solution to different public health problems [1]. Some animal species possess or produce biomacromolecules. For example, some venoms can act as a defense mechanism or contribute to immobilizing, killing, and digesting the prey [2]. In recent years, the rate of registration of new drugs at the Food and Drug Administration (USFDA) has declined, despite the concern issued by the World Health Organization (WHO) due to the emergence of new resistant bacterial strains and diseases resistant to therapy drugs [3,4].
Animal venoms constitute a vast source of bioactive molecules with pharmacological properties which have evolved for millions of years to efficiently interfere with the essential physiological processes of the prey [5,6]. Due to their toxicity and general biological effects, these natural resources have long attracted scientific interest [7]. Animal venoms are complex mixtures of peptides, proteins, salts, neurotransmitters, nucleosides, and other compounds [8]. Approximately 90% of the dry weight of some venoms (like that of snakes) consists of proteins and peptides. Among the protein classes, there are phospholipases A2, proteases (serine and metallo), l-amino acid oxidase, hyaluronidases, cysteine-rich secretory proteins (CRISPs), phosphodiesterases, nerve growth factors (NGFs), etc. Among the bioactive peptide classes, there are antimicrobial, analgesic, hypotensive, cytolytic/cytotoxic, cell-penetrating, anticancer (antitumoral) peptides, neuropeptides, and others.
The advances in the search for active biomolecules based on animal venoms indicates their effectiveness, which is mainly against cancer and diseases caused by microorganisms. [9,10,11]. However, it is well known that biomacromolecules from animal venoms, mainly proteinaceous ones, have low bioavailability or stability in biological media or in vivo treatments. In this context, few studies have reported the structure-function relationship of these biomacromolecules and drug delivery strategies [12].
Nanobiotechnology is a modern tool that helps to protect these compounds and even directs the target destination of the biomacromolecules, enhancing their activity and effectiveness [13]. Here, the structure-function relationship of biomacromolecules was studied; current applications and incorporation into nanostructured systems as a potential tool in the treatment of emerging diseases.
2. Nanosystems and Nanocarriers
Nanobiotechnology combines two important branches of modern science, namely nanotechnology and biotechnology. On the one hand, nanotechnology satisfactorily meets the physicochemical characteristics of protection and drug delivery. Its particles are on a nanoscale (size around 1–1000) and have pharmacological properties of high interest worldwide [14]. This is because nanocarriers optimize bioavailability, reduce adverse effects, and increase drug activity [15]. On the other hand, biotechnology is a science that studies the application or management of a living organism to improve or obtain a beneficial product [16]. This technological combination helps to obtain new compounds with high prospects on the market and benefits for the population [17].
In recent years, the macromolecules of animal venoms have been reported to have outstanding effects, such as antivenom, anticancer, and anti-inflammatory properties, among others [18,19,20,21]. However, this potential and activity are often limited by the different factors of the biological environment to which they are exposed, and many times these macromolecules lose activity or diminish their effect. For example, some proteolytic enzymes present in the blood may denature or break peptide and protein bonds.
Snake venoms are a complex mixture of biomacromolecules, containing different components, with more than 90% of their dry weight corresponding to proteins. Among the protein content of these venoms, there is a wide range of enzymes and peptides [22]. These molecules are responsible, alone or in synergism, for the physiological effects observed after animal envenomation. Venoms have been applied as therapeutics since antiquity, and technological advances have allowed the isolation and determination of the structure and properties of their components. As a result, their potential as new medicines has been explored in many fields. Macromolecules from animal venoms are considered high-profile biotechnological products and, combined with the protection of their components conferred by nanotechnology, highly impressive results are expected in the coming years. An overview of nanotechnology with these macromolecules and their strategies is presented below. In addition, controlled drug delivery systems (CDDS) in venom-derived biomacromolecules, such as nanoliposomes, nanoemulsions, polymeric nanoparticles, microparticles, microspheres, and mesoporous silica nanoparticles (MSNs), among others, are taken into consideration (see Table 1).

Table 1.
Animal toxins and nanotechnology: biomacromolecules, sources, nanosystems, and applications.
2.1. Nanoliposomes
Nanoliposomes (NLPs) are drug delivery and carrying systems made up of phospholipidic layers that cover an aqueous fraction [60]. NLPs have many advantages, but their main importance lies in their high biocompatibility, friendliness to the environment, and non-toxicity [61]. NLPs are vesicles that can be classified and distinguished depending on the method of preparation and size. They can be unilamellar (small unilamellar vesicles—SUV, 20–80 nm; large LUV, 80 nm–1 μm; and giant GUV, >1 μm), multilamellar (MLV, >400 nm), or multivesicular (MVV, >1 μm) [62]. Vesicle preparation requires careful formulation since the size of the particle differs when performed randomly. It was previously reported that the thin film hydration method would be an adequate formulation for antimicrobial peptides (AMPs) such as bacteriocins. This technique develops heterogeneous-MLV and, with assisted energy, it is possible to obtain LUVs or SUVs [63].
NLPs encapsulated with AMPs such as nisin, a cationic peptide, could confer, through its electrostatic properties, greater affinity for anionic lipids such as dicetylphosphate, or, through a hydrophobic affinity, zwitterionic neutral lipids such as phosphatidylcholine. Therefore, a better encapsulation efficiency is achieved in relation to anionic phospholipids such as phosphatidylglycerol [64,65,66,67]. Depending on the lipid and the electrostatic effect produced by the AMP, the stability of the NLP could be altered, and consequently better stability and zeta potential higher than 30mV would be achieved. The antimicrobial activity could also be related to the net charge of the lipid system formed, since the charge of the bacterial membrane is negative, which could repel the accommodation of NLP in pathogenic bacteria; this detail must be taken into account when formulating new antimicrobial agents [68].
Another peptide, Vicrostatin (VCN), has been encapsulated with NLPs. VCN is a peptide originating from the fusion between echistatin (Echis carinatus) and contortrostatin (Agkistrodon contortrix contortrix venom) fractions [69,70]. Vicrostatin is described in the literature as a potential anti-angiogenic and pro-apoptotic tumor agent [71]. Liposomal formulation of VCN was reported in vivo in mouse breast cancer; the data demonstrated that lamellar VCN is well tolerated while exerting a significant delay in tumor growth and an increase in survival in treated animals. In addition, the results showed antitumor ability in human ovarian cancer, glioma, and prostate cancer [23,24].
The trapping of animal venoms has become relevant due to the condition of transport to specific cancer cells, and it was shown that this type of venom-NLP nanocomposite achieves better anticancer effects in various cell lines [72]. Considerable application advances are being made in venom-NLPs in the biopharmaceutical industry since NLPs are already in the preclinical and clinical phases, mainly for the treatment of cancer [25,71,72].
Specifically, regarding the encapsulation of components from snake venoms with NMPs, some peptides can be cited. Melittin is the main component of the venom of the European bee Apis mellifera. It is a 26-mer peptide, originating from a two-step enzymatic cleavage of a preproprotein. The first cleavage removes an N-terminal signal peptide, while the second cleavage removes an anionic sequence responsible for inactivating melittin through interaction with C-terminal cationic residues [73,74].
Melittin’s primary structure is amphipathic, having a more hydrophobic N-terminal half and a more hydrophilic C-terminal half. Its secondary structure varies according to its concentration in an aqueous solution: at low concentrations, it assumes a monomeric random coiled conformation, while at higher concentrations, it adopts an α-helical tetramer folding [75,76]. The adopted α-helix conformation is a helix-hinge-helix motif, with the first helix formed by residues—a hinge region formed by residues (glycine-X-proline motif), and the second helix formed by residues [77,78,79,80]. These molecular and structural characteristics are directly related to melittin binding and permeabilizing different cellular membranes [76].
Melittin has been shown to exert anti-cancer, anti-inflammatory, anti-diabetic, anti-infective, and adjuvant properties. One of the studies carried out evaluated the ability of melittin to induce apoptosis in leukemia cell lines of different origins, acute lymphoblastic leukemia (CCRF-CEM), and chronic myeloid leukemia (K-562). Cell viability methods, membrane potential, and apoptosis were confirmed using two different cytotoxicity assays with trypan blue dye, and the MTT test was performed to identify this potential. It was concluded that melittin can be incorporated into phospholipid bilayers, resulting in cell membrane disruption leading to the intensive cytotoxicity of all treated cells. It was also observed that the apoptosis process is performed by the intrinsic/mitochondrial pathway in different leukemia models [81]. The ability to prevent biofilm formation was also found [82]. A recent report indicated that lipid-based NPs loaded with melittin exhibited modulative and targeted delivery properties, triggering the activation of hepatic sinusoidal endothelial cells, causing significant changes in the production of cytokines/chemokines in the liver, and allowing melittin to prevent the formation of metastatic lesions [26]. At the same time, melittin-NLPs, combined with a surfactant (2% poloxamer 188), showed high human hepatocellular carcinoma-toxicity: LM-3 (0.94 μM; 2.13 μM), Bel-7402 (0.90 μM; 1.44 μM), SMMC-7721 (1.13 μM; 2.03 μM) and HepG2 (0.66 μM; 2.19 μM). Melittin-NLPs showed anti-hepatocellular carcinoma (HCC) potency in vitro and in vivo with decreased toxicity, which has clinical implications as a promising new drug for HCC therapy [25].
Nanotechnology and intratumoral injection greatly decrease the required dose of melittin peptide, which generates cost reductions and significantly decreases its cytotoxicity, since melittin by itself is highly toxic and an uncontrolled dose could cause irreparable damage. A study revealed that α-melittin-NPs resulted in delayed tumor growth and even in the complete regression of the distant tumor. Furthermore, α-melittin-NPs have several strengths, including simple preparation, good stability, and a lack of side effects [27].
Finally, Alyteserin-1c is a linear peptide first isolated from skin secretions of the midwife toad Alytes obstetricans [83]. Studies by Subasinghage et al. [84] and Aragon-Muriel et al. [85] showed that the β-sheet structure is predominant in this peptide in an aqueous solution, and a transition to the α-helix structure was observed in a membrane environment. One study examined the antibacterial results of Alyteserin-1c and its analog [E4K]Alyteserin-1c. The growth-inhibitory potency of [E4K]Alyteserin-1c against clinically relevant Gram-negative bacterial reference strains was between two and four times greater than that of the original peptide sequence [84]. Although it has potential antimicrobial activity, polymer-coated alyteserin-1c-NLP significantly increased the antibacterial activity of the peptide against Listeria monocytogenes at 0.06 μM, showing excellent MIC-values in comparison with conventional drugs [28].
2.2. Silica Nanoparticles (SiNPs)
SiNPs are nanosystems with high drug delivery efficiency that vary in size from 10 to 500 nm. These nanosystems are the most widely studied and scaled for drug administration. Non-porous SiNPs are a type of SiNPs, characterized mainly by their large surface area, easy functionalization, and biocompatibility. They are formulated using microemulsions, but given that this nanosystem has been scantly used for macromolecules based on animal venoms, it is not discussed in depth here. One of the systems best adapted to peptides, proteins, and DNA is MSNs, since their ordered and porous design allows a greater load of the bioactive compound for therapeutic application [86].
MSNs are nanoparticles ranging in size from 2 to 50 nm, generated mainly by the Stober method or its modifications, and they are widely used on account of their simple manufacture, low cost, and availability on an industrial scale [86,87]. They can be classified according to their nano-application as MSNs sustained drug delivery systems (MSNs-SDDSs) which exhibit deliberate drug release, responding automatically to the conditions of the medium or a concentration gradient; and as MSNs stimuli-responsive controlled drug delivery systems (MSNs-CDDSs), which instead release the drug at a controlled level in response to a physical or chemical stimulus [88]. There are various types of MSN formulations, but most of the reports used “Santa Barbara Amorphous-15” (SBA-15) systems. These systems are characterized by their particle and pore size, which in turn allows a better and controlled release of the drug [89].
SBA-15 nanoparticles have a non-toxic and adjuvant effect which modulates the immune system in mice by inducing the production of IgG2a and IgG1 isotypes. This is beneficial when it comes to anticancer, anti-inflammatory, or antimicrobial treatments, especially in people with compromised immune systems [90]. This type of biotechnological formulation using SBA-15 is generally carried out under acidic conditions and using a source of silica, like tetraethoxysilane (TEOS), in combination with an amphiphilic triblock copolymer such as Pluronic 123 composed of poly (ethylene oxide) (PEO) and poly (propylene oxide) (PPO), i.e., PEO-PPO-PEO triblock [91]. The latest studies report its effectiveness as a pH-selective, photodynamic, and thermosensitive drug administrator, which can be administered through the gastrointestinal tract or by the intravenous, intratumoral, subcutaneous, ophthalmic, nasal, and dermal routes [91,92].
There are several examples of encapsulation of molecules derived from animal sources with SiNPs. Exenatide (39-amino-acid peptide), a molecule present in the saliva of the lizard from the Gila monster (Heloderma suspectum), is very similar to the incretin hormone glucagon-like peptide-1 (GLP-1). Clinical studies and clinical experience with exenatide have shown a significant reduction in HbA1c, fasting and postprandial glucose and a marked reduction in body weight in patients with type 2 diabetes mellitus. Animal studies have shown an improvement in blood function, beta cells, and an increase in beta-cell mass after treatment with exenatide [93]. GLP-1R agonists are promising anti-obesogenic and anti-dyslipidemia drugs in the early stages of obesity, in which the integrity of the nervous system has not been affected [94]. Currently, exenatide is available commercially under the names Byetta™ and Bydureon™.
Exenatide was incorporated into PEG/PLGA-NPs modified with Fc intestinal receptors for oral delivery to improve hypoglycemic effects in mice. Fc-targeted nano-delivery systems show great promise for oral peptide/protein drug delivery [42]. Exenatide-SBA15 MSNs were administered as injectables and served as glycemic modulators, since this peptide is a glucagon-like peptide 1 receptor agonist that that caused much greater inhibition than the wild-type peptide, showing a high loading capacity (15% w/w), as well as an increase in bioavailability and life span in blood circulation [29].
Studies of BSA-15 MSNs loaded the venom, previously detoxified with Cobalt-60 irradiation, from the South American rattlesnake (Crotalus durissus terrificus) and Africanized bee (Apis mellifera). The studies showed that their application as venom-antivenom adjuvants induced the production of antibodies in sheep, making it possible to obtain serum at a low cost in comparison with the common serum made from equine [30]. In another study, the same snake venom was incorporated into non-porous SiNPs and MSNs in a stable manner with no significant differences from each other, indicating that the V proteins, as well as the venom enzymes, were joined by electrostatic or ionic forces to the nanosystem, maintaining their physicochemical properties [31]. The venom of the Micrurus ibiboboca snake also immersed within SBA-15 MSNs facilitated the modulation and stimulation of antibodies for the development of possible vaccines [32]. This type of nanosystem has been widely used, primarily to obtain antivenom serum [95].
The Walterinnesia aegyptia venom was initially reported to have antitumor activity. Subsequently, it was encapsulated in MSNs, and its potential against breast, human multiple myeloma, and prostate cancer cells was demonstrated, along with a reduction in adverse effects and improvements in chemosensitivity, bioavailability, and specificity in cancer cells [33,34,96]. The authors also confirmed that controlled doses of these MSNs increased the levels of ROS, hydroperoxides, and nitric oxide in these cell cultures, thus increasing the levels of chemokines and decreasing the surface expression of their related chemokine receptors CXCR3, CXCR4, CXCR5, and CXCR6. Furthermore, they found that MSNs potentially inhibited insulin-like growth factor 1 (mediated by breast cancer) and epidermal growth factor (EGF, mediated by prostate cancer), while they induced apoptosis by increasing the activity of caspase-3, -8, and -9 [97].
Crotoxin (CTX) (l-Arginine esterhydrolase) is a polypeptide and the main component of C. durissus terrificus snake venom. It has favorable characteristics to be used as an immunomodulatory drug [98]. In a study whose purpose was to observe anticoagulant and profibrinolytic activity in endothelial cells, Andrade et al. performed an in vitro test on HUVEC cells to determine this possible activity. CTX exerted anticoagulant and profibrinolytic action in the presence of LPS, decreasing the levels of vWF and t-PA and increasing the levels of the proteins C and PAI-1, thereby representing a potential tool against the development of thrombosis [99]. The antitumor activity has also been reported against glioma (GAMG and HCB151), and pancreatic (PSN-1 and PANC-1) cancer cells showed greater sensitivity with IC50 of <0.5, 4.1, 0.7, and <0.5 μg/mL, respectively [100].
Nanoparticles of BSA-15 MSNs loaded with CTX demonstrated that their oral administration in mice significantly reduced/increased the production of interleukin (IL) 6 and 17/10, respectively. The toxic effect of CTX was reduced and consequently the LD50 values rose, which implies a favorable option for the treatment of chronic pain and experimental autoimmune encephalomyelitis (EAE). It was also noted that this nanosystem helped to improve the efficacy of CTX to treat multiple sclerosis because it reduced the proliferation of peripheral T helper cells 17 that characterize autoimmune, inflammatory diseases and cancer [20]. Crotalphine is a 14 amino acid analgesic peptide that, like CTX, trapped in SBA-15 MSNs was capable of increasing its therapeutic action, attenuating the inflammation induced by EAE because it reduced the immunoreactivity of Egr-1 markers, microglia/macrophages and astrocytes [101]. This information indicates the nanobiotechnological potential of both macromolecules and their protection and transport system, against the treatment of neurodegenerative diseases, control of sensitivity, motor alterations, atrophy, and loss of motor function [101]. Another study revealed that CTX-SBA-15 MSNs have a long-lasting contraceptive effect, as this nanoencapsulated system releases the CTX polypeptide in a controlled manner, which could be beneficial for use as new oral or injectable contraceptives [36].
Hylin a1 is a peptide isolated from the skin of South American tree frogs, which, in addition to exhibiting antibacterial activity, showed antitumor results. This peptide encapsulated in MSNs was efficiently released in the presence of tumor cells, and the hemolytic effect of the peptide could also be contained [37]. The results were corroborated by microparticles loaded with the Ctx(Ile21)-Ha peptide, isolated from the skin of the Brazilian Cerrado frog Hypsiboas albopunctatus, which also had decreased hemolytic activity using alginate coated with hypromellose acetate/succinate and hydroxypropylmethyl cellulose. This result provides evidence of the potential of micro and nanoparticles [102]. Ctx(Ile21)-Ha is a ceratotoxin that has shown potent antimicrobial activity against resistant bacteria such as Salmonella Enteritidis [103], multi-drug resistant bacteria such as Pseudomonas aeruginosa and Acinetobacter baumannii, as well as other pathogenic bacteria and fungi of public interest [104]. Its application in alginate microparticles significantly decreased its hemolytic activity, increased its bioavailability, showed a preventive effect of systemic and intestinal infection and greater physicochemical stability during its journey in the gastrointestinal tract [105,106].
Furthermore, the biotechnological potential of snake macromolecules was extensively studied more than a decade ago, as described in this section. However, the nano-venom or nano-macromolecule relationship is still a subject that requires further study, as previous results showed a great therapeutic effect. Its application in formulations for cancer treatment was approved by the FDA and, in general, the formulations based on silica were considered “safe”. However, in this type of nanosystem, the particle size must be taken into account because it could be related to the bioavailability and the rate of excretion of the drug [88]. Likewise, controlling the negative effects of this nanosystem is a great challenge; these types of nanoparticles could induce autophagy of hippocampal neurons through oxidative stress through the AMPK/mTOR/P70S6K signaling pathway, altering the nervous system [107]. These NPs induce the expression of nitric oxide, intercellular adhesive molecule 1, and adhesive molecule 1 of vascular cells in the human umbilical vein, suggesting that SBA-15 would be toxic to vascular endothelial cells [108]. Further studies of SBA-15 MSNs must be carried out since their prolonged use could lead to a negative risk/benefit action if they are not administered carefully. The authors concluded that animal venoms for use as antidote/serum/vaccine could be excellent nanocarriers since their administration, in many cases, is carried out in a single dose and is not prolonged compared to the administration of conventional drugs.
2.3. Metallic Nanoparticles
Metallic nanoparticles (MNPs) are nanosystems that incorporate the use of metals to enhance the antimicrobial activity of different bioactive compounds, the best known of which are gold (AuNPs) and silver (AgNPs). MNPs can currently be obtained by various methods; however, it is considered that these methods may cause environmentally toxic effects because the metals are difficult to degrade. For this reason, green synthesis processes are recommended [109].
Some venom components from vipers, such as crotamine, a peptide composed of 42 amino acids, with 4.8 kDa and isoelectric point around 10.8, were first isolated from the venom of the Argentinean rattlesnake C. durissus terrificus [110]. It is a small basic myotoxin containing three disulfide bonds (Cys4-Cys36, Cys11-Cys30, and Cys18-Cys37) [111,112,113,114] which acts on sodium and potassium channels [115,116], exerting myotoxic effects. Crotamine induces microorganism death by peroxidation and lipid oxidation of target proteins, determined by substances reactive to thiobarbituric acid and sulfhydryl groups, respectively [117]. Antibacterial and antifungal activities were evaluated in ATCC strains and clinical isolates, showing promising results [10]. Likewise, antitumor treatment was confirmed after the successful treatment of a mouse model grafted with a subcutaneous melanoma tumor [118]. Other biological activities, such as anti-leishmanial, anti-helminthic, and antimalarial effects, were reported [119,120,121,122]. Crotamine-AuNPs via a PEG linker were synthesized (NP size 14.6 nm); in vitro assays confirmed the internalization of these nanoparticles into living HeLa cells. AuNPs are playing a progressively more significant role in multimodal and multifunctional molecular imaging to detect and treat diseases, such as early-stage cancer [38].
Also, viper venom-AuNPs (Daboia russellii russellii, size 30–50 nm) provided protection against venom-induced edema, hemorrhage, defibrination, organ toxicity, and inflammation in an animal model (50 µg/20 g mice, intravenous) [39]. Other NPs containing lyophilized snake venoms, such as Bothrops jararacussu, Daboia russelii and Naja kaouthia (TiO2NPs) venoms managed to manipulate the antivenom effect and were able to control bleeding, clotting, and inflammation, and even to prevent the death of the animal; likewise, bacteriological dissemination was highly controlled [40].
The list of high-priority bacteria published by the WHO indicates that studies should be carried out in Klebsiella pneumoniae (critical), as well as Staphylococcus aureus and S. typhimurium (high) [3]. For this reason, a multibacterial study was carried out using a peptide sequence (INLKAIAALVKKV) from wasp venom (Vespa orientalis) in AuNPs. The results demonstrated excellent properties: the AuNPs were found to be nontoxic, small, inexpensive, heat mediated, and environmentally friendly, and they could have extensive medical applications as well. Based on their physicochemical and morphological parameters, it was shown that AuNPs may be synthesized and that bacterial multiplication may be blocked successfully using the peptide isolated [41].
2.4. Polymeric Nanoparticles
Polymers are linear or branched conformations of covalently linked monomers [123]. The nanoparticles obtained from this material are relevant because the loading content of the bioactive is efficiently high, and in most cases, the use of surfactants is necessary since many of the drugs are usually hydrophobic [124]. The versatility of polymers allows them to adapt to the different conditions that a drug or compound needs to improve its action or bioavailability, as well as structural chemical modifications to carry out conjugations [125]. Many polymers have been used as a source of PNs; however, this review will focus on those most widely used for the administration of derivatives of animal venoms.
Chitosan nanoparticles (ChitNP) are nanosystems that have been used in formulations for treatments involving mucoadhesion. Chitosan is a biopolymer that has a positive charge, and an affinity is generated by an electrostatic bond, targeting the mucosa, which is negatively charged; in addition, it has biocompatible characteristics and the ability to permeate the intestinal barrier, which stimulates the passage of molecules through the paracellular and transcellular pathways [123]. Although this polymer has certain limitations due to its low solubility at pH > 7, it is considered a good releaser of active compounds in the duodenum for gastrointestinal formulations. In vitro and in vivo studies have shown a non-toxic response, but the results of clinical studies that fully corroborate its application are still lacking [126]. ChitNP showed promising antimicrobial as well as anti-biofilm properties and is used mainly for the development of new nanostructured products, as it can destabilize the bacterial membrane and inhibit protein synthesis and mRNA transcription [14].
Poly(lactic-co-glycolic acid) nanoparticles (PLGA-NPs) are currently highly studied nanosystems. PLGA is an FDA-approved polymer due to compound protective potential, biocompatibility, and biodegradability [127]. However, PLGA-NPs have not shown good drug delivery properties in specific cells/proteins since the release of the compound occurs in an uncontrolled manner. Generally, this type of nanoformulation is accompanied by coatings or conjugates that can help this type of targeting, such as polyethylene glycol (PEG), polydopamine (PDA), hyaluronic acid, and folic acid, among others [128,129]. In addition, the cost-benefit ratio of using this polymer does not make it attractive since PLGA is relatively expensive and the particle size is around 500 nm. However, some non-toxic chemical agents can increase encapsulation rates and the release period can be carried out for as long as one month, as with bee venom (Park, Min-Ho 2016). Hamzaoui and Laraba-Djebar [43] reported obtaining and trapping the venom of Cerastes cerastes (horned viper) and Vipera lebetina in PLGA-NPs used as a vaccine; these reptiles are native to North Africa and the Middle East, respectively, and have a high bite incidence rate in the region. These PLGA-NPs (EE 84.1%) were administered intranasally, generating antibodies and high Th2 values, which favored humoral immunity; the NPs provided venom tolerance of 6LD50 and 5LD50, respectively [43].
Orivel et al. [130] tested the antimicrobial properties of fractions from the Pachycondyla goeldii ant venom against Staphylococcus aureus 209P and Escherichia coli RL65, and the best active fraction contained 15 peptides which were named ponericins and classified into the groups G, W, and L, according to the first amino-terminal amino acid. Ponericin-G1 was one of these promising peptides, since circular dichroism results suggested that ponericins G presented a random coil in aqueous solution and adopted an α-helical structure. In addition, researchers suggested that ponericins G acts through a “carpet-like” mechanism due to its similarities with cecropins and dermaseptins. Although it was the least concentrated peptide in the crude venom, it showed a great range of action against Gram-positive and Gram-negative bacteria and yeast (Saccharomyces cerevisiae LMA 720), and insecticidal activity against the cricket Acheta domesticus [131]. Ponericin-G1 was successfully incorporated into PDA-PLGA nanofibers for treatment against Staphylococcus aureus and Escherichia coli at 250 μg mL−1 and 250 μg mL−1, respectively. PLGA nanofiber scaffolds loaded with basic fibroblast growth factor and ponericin G1 prepared by polydopamine modification developed in this study has excellent mechanical properties, hydrophilicity, and antibacterial activity. This scaffold material has appropriate morphological and surface properties that can meet the needs of skin tissue regeneration [44]. Ponericin-G1 encapsulated in PLGA/PLL also showed effectiveness against Staphylococcus aureus and E. coli at 300 μg mL−1; PLL surface modification and ponericin G1 can greatly improve the antimicrobial activity of the cell microcarrier and enhance the clinical application value of microcarriers [132].
Finally, another toxin isolated from the Leiurus quinquestriatus scorpion venom is chlorotoxin (CHTX), which is a neurotoxin composed of 36 aminoacids that was first isolated by DeBin et al. [133] and is described as a calcium channel blocker. Selective and specific binding of CHTX to glioma cells was demonstrated by immunochemical techniques, and radiolabeled CHTX was shown to bind only to cells in a glioma tumor of a mouse xenograft model [134,135]. CHTX-morusin-PLGA NPs exhibited excellent pharmacological properties against the treatment of glioblastoma or brain cancer with a mechanism directed towards tumor cells. Likewise, there is evidence that crossing the blood-brain barrier is a great challenge that NPs manage to meet [45].
On the other hand, alginate-based nanoparticles (AlgNP) have excellent properties as mucoadhesive and molecule transporters, since several formulations have been made to achieve difficult-to-treat antibacterial objectives, such as Mycobacterium tuberculosis [136]. The AlgNP is obtained by crosslinking using a positively charged solution, which is generally produced by chloride salts, giving rise to the formation of nanospheres, nanocapsules, or nanoaggregates [13]. In addition, many of the formulations use this electrostatic property to combine the alginate and chitosan (Alg/Chit) and thus achieve greater protective stability of the active compound, mainly when it comes to gastrointestinal formulations [12]. Unlike alginate, Chit-NPs are obtained using ionic crosslinking, covalent crosslinking, polyelectrolyte complexation, and self-assembly [137].
Apis melifera bee crude-lyophilized venom in Alg/Chit NPs was applied to pigs to evaluate and compare its antimicrobial effect with the respiratory syndrome virus vaccine; it was shown that these nanosystems are capable of promoting the increase of CD4 T lymphocytes, as well as reducing the viral rate in the lung and bronchial lymph node [46]. Likewise, the use of ChitNPs with bee venom was successful in eliminating cancer cells and could be an excellent option for the treatment of cervical carcinoma [48]. In addition, this venom using the same nanosystem showed induced apoptotic characteristics such as cell shrinkage, peripheral chromatin condensation, late-stage apoptotic bodies, nucleolus fragmentation, membrane blebs, a shrunken nucleus with some necrotic characteristics in the colon (Caco-2), larynx (HEp-2) and breast (MCF-7) cancer cells [47]. These promising results encouraged the search for new macromolecules such as melittin, which is currently in preclinical and clinical phase studies [138]. Melittin-based nanosystems were also highly studied as PNs, lipid NPs, metallic NPs, carbon NPs, and other nanocomposites, as described with greater emphasis and detail in Zhou et al. [139].
A fraction of the attenuated venom of the scorpion (Androctonus australis hector) was encapsulated in alginate nanoparticles. These nanosystems led to an appropriate immune response to develop an alternative vaccine. The specific IgG titer and IgG1/IgG2a isotype values showed a potentially high protective effect because the immunized rabbits did not register a mortality rate with values of 6 LD50. This result corroborates the immunomodulatory capacity of the venom, and the physicochemical stability that the polymer grants to the antigen to achieve its adjuvant effect [50].
Another macromolecule isolated from the same scorpion venom, known as Aah II toxin-ChitNPs, confirmed the modulation of the innate and systemic humoral immune system. The nanosystem favored cell viability, decreased cytotoxicity (10 LD50), and had an EE of 96.66% (values considered excellent). In addition, the toxin was released at 86% until the fifth day. Therefore, the authors concluded that this effect can maintain constant protection and help prevent the death of patients who suffer bites from this type of scorpion, mainly in the most susceptible areas [51]. Furthermore, this attenuated venom in AlgNPs was used in preclinical studies in older animals such as rabbits; an effective immunizing response and safety were observed in its administration as a vaccine, while the treatment barely caused reactogenicity [49].
In 1996, Simmaco et al. [140] isolated three peptides from the skin secretion of Rana temporaria, which showed similarity to peptides isolated from the Vespa species. One of these peptides was Temporin B (TB), a leucine-rich hydrophobic peptide [140,141]. Structural studies showed that temporin peptides tend to adopt an amphiphilic α-helical structure in hydrophobic environments. These properties are related to the membranolytic action of this peptide through a barrel-stave mechanism. Interestingly, the existence of a lysine residue in TB was proposed as the factor reducing its activity against Gram-negative bacteria [142]. Later studies revealed that this lower activity against Gram-negative bacteria was related to the formation of TB-oligomers, interfering in the diffusion to the cytoplasmic membrane [143,144]. Besides its antibacterial activity, several studies in the literature report TB’s antifungal and antiviral potential. One of these studies characterized and synthesized TB_KKG6K and d-Lys_TB_KKG6K analogs, the latter having the l-lysines replaced by the chiral counterpart d-lysines to improve their proteolytic stability. It was observed that both peptides inhibited the growth of several species of Candida spp. fungi, S. aureus and herpes simplex virus 1 (HSV-1) [145,146]. ChitNPs loaded with Temporin B (extracted from Grass frog, Rana temporaria) were optimized in such a way that their antimicrobial activity could eliminate MDR bacteria in different strains of Staphylococcus epidermidis using only 5 mg ChitNP/mL with a prolonged release of up to 15 days and a significant decrease in cytotoxicity [52]. Another single macromolecule from scorpion venom known as Tityus stigmurus Hypotensin (TistH) was encapsulated in ChitNPs, demonstrating good biocompatibility, bacterial kill, and anti-biofilm activity of the most virulent species of Candida sp. [53].
Viper venom lyophilized-ChitNPs from Bothrops jararacussu showed relatively small sizes (average size of 160 nm), EE of 70%, and antibacterial activity in Escherichia coli and Staphylococcus aureus. It was demonstrated that NPs have the ability to enhance antibacterial activity on Gram-positive strains and that the incorporation of this snake venom is promising for obtaining novel therapeutic agents [147]. Bothrops jararaca and Bothrops erythromelas are two species of snakes from South America that produce highly dangerous venom. Soares et al. [148] reported the synthesis of antivenom-ChitNPs formulations at different concentrations of the venom (size of NPs ~167 nm) and EE in the range 67.7–97.2. These results indicated that the use of ChitNPs is capable of reducing localized toxicity and inflammation and that the interaction between proteins and the nanosystem can induce an adequate immune response to counteract the harmful post-venom effect. Another study, using speckled cobra (Naja naja oxiana), reported that venom concentration directly affects EE and the carrying capacity of ChitNPs [55].
Crotalus durissus cascavella venom proteins were incorporated into ChitNPs (stable and slightly smooth spherical NPs, <160 nm, SE 90%) as antivenom. They exhibited immunoadjuvant properties and reduced the adverse effects of venom, maximizing the efficiency of conventionally used antivenoms [54]. The authors also reported that these NPs are more effective than aluminum hydroxide, which is used as a conventional immunoadjuvant, suggesting that these nanosystems can be used in immunotherapy [149]. Using synthetic PNs, other antivenoms were developed from other snakes, such as Crotalus atrox, Bitis arietans, Bitis gabonica, Echis ocellatus, and Echis carinatus. The inactivation of metalloproteinases could be achieved for all of the species studied, which is interesting for the development of new enzyme inhibitors that are effective against snake bites [56]. The authors recommended caution when trying to obtain nanoparticles of protein or peptide bioactive compounds, as some methods/time of preparation or some compounds of the preparation solutions could break or inactivate a peptide bond.
3. Nanobioconjugations
Nanosystems loaded with animal venoms tend to increase the therapeutic effect, while bioconjugation can further increase this effect [40,150]. The bioconjugation of nanoparticles is generated by embedding a molecule in another nanomolecule. For this purpose, ligand techniques can be applied, the most promising ones being click chemistry application techniques such as Diels-Alder. Some nanoconjugates that are reported to stimulate and redirect nanoparticles are addressed in this review.
The SBA-15 system loaded with melittin uses the effect known as trigger-responsive valves, and shows the opening of the silica-based porosity at pH 5.5, which is the pH of cancer cells. This system was conjugated with 3-mercaptopropyltrimethoxysilane (MPTS), then with 2,2-Bis (N-maleimidoethyloxy) propane (MK-Linker, acetal), and finally with melittin, when a Cys was added to the sequence (d-amino acids) of melittin at the N-terminus [92]. Melittin-large pore MSNs capped by electrostatic interaction with β-cyclodextrin (β-CD)-modified polyethyleneimine (PEICD) were synthesized. It was observed that the MSN/pore size ratio was 90–110/7–10 nm, offering this nanobioconjugate (with assembly and loaded nanosystem) efficacy in the controlled release of AMP and/or high and low molecular weight drugs. In addition, this product was able to reduce the formation of biofilm (between 20 and 30%) and eliminate 24 to 27% of the total bacteria. However, the authors also explained that when using melittin-MSN combined with NPs-Magnetics of MnFe2O4 loaded with ofloxacin, previously synthesized by thermal decomposition, they were able to reduce the biofilm formation of P. aeruginosa PAO1 cells by up to 97% and eliminate 100% of this bacterium [151]. This study was corroborated using an in vivo mouse model, which exhibited a high rate of serum cytokine production in the groups not treated with this combined nanosystem, which clearly shows the use of nanobioconjugates with synergistic effect (see Figure 1) [152].
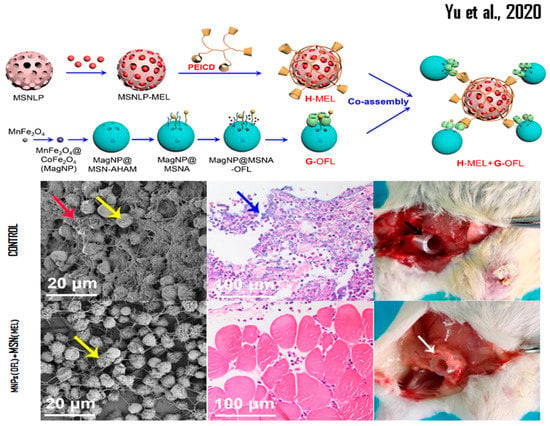
Figure 1.
Representation of bioconjugated MSN nanosystems and their effectiveness in in vivo studies. Reprinted/adapted with permission from Yu et al. [152]. Copyright 2020, ACS Chemical Society.
Some toxins, such as NN-32 (from the Indian cobra venom, Naja naja), have remarkable anticancer activity, but its high cytotoxicity does not make it an attractive macromolecule. However, when it was conjugated with AuNPs, the cytotoxicity decreased considerably without affecting its antitumor activity; this property was evaluated by means of in vitro (lymphocytes and macrophages) and in vivo (mice) assays [150]. In breast cancer cells, NN-32 incorporated into AuNPs induced apoptosis and discontinued tumor proliferation [150]. In addition, Lycosin-I from Spider (Lycosa singorensis) functionalized in AuNPs showed specific antitumor activity as an excellent nanocarrier to obtain novel drugs using these toxins [58]. Maurocalcine from Scorpio maurus palmatus functionalized with AuNPs showed selectivity for three different cell lines such as human epithelial-like HeLa, MDA-MB-231 (human breast adenocarcinoma), and A431 (epidermoid carcinoma cell), which allows nanosystems to be adapted to antitumor activity [59].
4. Prospects and Challenges
It is incontestable that peptides from animal venoms have therapeutic potential [153]. According to Van Baelen et al. [154], there are about 200,000 species of venomous animals around the world with about 40 million toxins to be exploited. However, there are only 11 peptide rugs derived from animal venom on the market, tens are in clinical development, and hundreds of patents have been filed in this field [155]. In our view, the reason for the low number of molecules currently on the market is that peptides, in general, have some limitations that need to be circumvented, such as proteolytic degradation [153].
However, to increase the half-life of peptides, many strategies involving different formulations, forms of administration, and levels of chemical modification are possible. In this context, an example of a promising strategy to increase the stability of the peptide is the introduction of d-amino acids [156], since the target of this class of peptides is the molecular membrane, which does not require interaction with specific chirality. Modifications in the N- and C-terminus of the molecule, the use of unnatural amino acid residues, cyclization, dimerization, and dimeric peptides are also used as alternative approaches to improve stability and increase biological activity [153,157,158,159,160].
Nanostructures have been utilized as vehicles intended to protect the peptides from enzymatic degradation or denaturation and prevent their release until they reach the site of action, giving larger specificity of molecules towards their targets. Furthermore, nanoscale materials have a large specific surface area, flexible surface functionalization, and some physicochemical properties that could be exploited to improve the molecule druggability [161].
The stability, toxicity, and cost of some nanoparticles should also be better studied and exploited. Large-scale synthesis will reduce the high costs associated with the synthesis and production of nano-pharmaceuticals over conventional treatments. NP stability in blood and environment depends on the materials they are synthesized with. Thus, the search for biodegradable NPs should be encouraged. Larger NPs have been shown to have a larger cytotoxic effect than smaller NPs, potentially accumulating in organs. Studies on the half-life, pharmacokinetics, and pharmacodynamics of these nanocompounds must be performed. For example, how do we remove the metal from the organism treated with MNPs? A study published in 2007 by Sadauskas et al. [162], indicates that Kupffer cells are essential in the elimination of NPs from the body. This information was confirmed and corroborated by Poon et al. [163], who added that hepatic sinusoidal endothelial cells would also be involved in this elimination through the canonical hepatobiliary pathway, as shown in Figure 2.
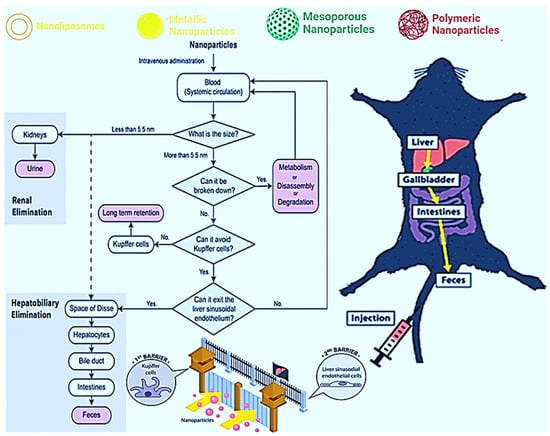
Figure 2.
Schematization of the intravenous administration of NPs and the possible mechanisms of elimination: (1) liver, (2) gallbladder, (3) intestines, and finally (4) feces. Reprinted/adapted with permission from Poon et al. [163]. Copyright 2019, ACS Chemical Society.
Regarding the complexity of therapeutic protein and peptide encapsulation, the field of nanotechnology has effectively provided several nanoscale systems that have the required encapsulating properties, as shown in the present work. As cited above, venoms are a complex mixture of compounds, produced by a tissue or organ (venom gland) and introduced mechanically into the prey by the venomous animal, with a specialized apparatus [164]. However, only a few examples of treatments using therapeutic proteins from animal venoms have been successfully translated into clinical applications, which demonstrates that the great challenge for researchers today is the translation of basic science into applied science [165]. The search for proteins and peptides to be used as therapeutic agents to treat cancer or infections has increased dramatically, mainly because of the emergence of resistance. Several biologically active toxins have been purified, characterized, and reported daily in the scientific literature. However, there is a gap between the initial drug discovery phase and its use in a clinical study [8], and this gap remains the biggest obstacle to be overcome.
5. Conclusions
Nanotechnology is a powerful tool that serves to enhance the therapeutic effect, transport, protection, and controlled release of macromolecules with biological activity. Although it is true that most studies are focused on administration as an adjuvant, current studies show that nanobiotechnology has great advantages, mainly reducing adverse effects and extending the half-life of substances derived from animal venoms. However, further studies of these biomacromolecules are necessary, as few nanosystems have been approved by the FDA for use in the pharmaceutical industry.
Author Contributions
Conceptualization, C.A.R.-B.; methodology, M.W.d.L.G. and F.H.d.F.; validation, C.A.R.-B. and N.A.S.-F.; investigation, M.W.d.L.G., F.H.d.F. and C.A.R.-B.; writing—original draft preparation, C.A.R.-B., N.A.S.-F., M.W.d.L.G. and F.H.d.F.; writing—review and editing, C.A.R.-B.; supervision, C.A.R.-B., F.R.P. and N.A.S.-F.; funding acquisition, N.A.S.-F. and F.R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by São Paulo Research Foundation (FAPESP) grant number [2020/13497-4 and 2020/16573-3]; the National Council for Scientific and Technological Development (CNPq), Research Grants: 429139/2018-7, 404181/2019-8; Productivity Research Fellows (PQ CNPq): 303603/2018-6 and The APC was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. The graphical abstract was created with BioRender.com.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Chen, N.; Xu, S.; Zhang, Y.; Wang, F. Animal protein toxins: Origins and therapeutic applications. Biophys. Rep. 2018, 4, 233–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perumal Samy, R.; Stiles, B.G.; Franco, O.L.; Sethi, G.; Lim, L.H.K. Animal venoms as antimicrobial agents. Biochem. Pharmacol. 2017, 134, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Roque-Borda, C.A.; Bento, P.; da Silva, P.B.; Rodrigues, M.C.; Azevedo, R.B.; Di Filippo, L.; Duarte, J.L.; Chorilli, M.; Festozo Vicente, E.; Pavan, F.R. Challenge in the Discovery of New Drugs: Antimicrobial Peptides against WHO-List of Critical and High-Priority Bacteria. Pharmaceutics 2021, 13, 773. [Google Scholar] [CrossRef]
- Andrei, S.; Droc, G.; Stefan, G. FDA approved antibacterial drugs: 2018–2019. Discoveries 2019, 7, e102. [Google Scholar] [CrossRef]
- Fry, B.G.; Scheib, H.; van der Weerd, L.; Young, B.; McNaughtan, J.; Ryan Ramjan, S.F.; Vidal, N.; Poelmann, R.E.; Norman, J.A. Evolution of an arsenal: Structural and functional diversification of the venom system in the advanced snakes (Caenophidia). Mol. Cell. Proteom. 2008, 7, 215–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvete, J.J.; Borges, A.; Segura, Á.; Flores-Díaz, M.; Alape-Girón, A.; Gutiérrez, J.M.; Diez, N.; De Sousa, L.; Kiriakos, D.; Sánchez, E.; et al. Snake venomics and antivenomics of Bothrops colombiensis, a medically important pitviper of the Bothrops atrox-asper complex endemic to Venezuela: Contributing to its taxonomy and snakebite management. J. Proteom. 2009, 72, 227–240. [Google Scholar] [CrossRef]
- Lomonte, B.; Angulo, Y.; Moreno, E. Synthetic Peptides Derived from the C-Terminal Region of Lys49 Phospholipase A2 Homologues from Viperidae Snake Venoms: Biomimetic Activities and Potential Applications. Curr. Pharm. Des. 2010, 16, 3224–3230. [Google Scholar] [CrossRef]
- De Bordon, K.C.F.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Júnior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cardoso, I.A.; et al. From Animal Poisons and Venoms to Medicines: Achievements, Challenges and Perspectives in Drug Discovery. Front. Pharmacol. 2020, 11, 1132. [Google Scholar] [CrossRef]
- Wang, K.R.; Zhang, B.Z.; Zhang, W.; Yan, J.X.; Li, J.; Wang, R. Antitumor effects, cell selectivity and structure–activity relationship of a novel antimicrobial peptide polybia-MPI. Peptides 2008, 29, 963–968. [Google Scholar] [CrossRef]
- Yamane, E.S.; Bizerra, F.C.; Oliveira, E.B.; Moreira, J.T.; Rajabi, M.; Nunes, G.L.C.; De Souza, A.O.; Da Silva, I.D.C.G.; Yamane, T.; Karpel, R.L.; et al. Unraveling the antifungal activity of a South American rattlesnake toxin crotamine. Biochimie 2013, 95, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Sartim, M.A.; Menaldo, D.L.; Sampaio, S.V. Immunotherapeutic potential of Crotoxin: Anti-inflammatory and immunosuppressive properties. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 39. [Google Scholar] [CrossRef] [Green Version]
- Roque-Borda, C.A.; Silva, H.R.L.; Crusca Junior, E.; Serafim, J.A.; Meneguin, A.B.; Chorilli, M.; Macedo, W.C.; Teixeira, S.R.; Guastalli, E.A.L.; Soares, N.M.; et al. Alginate-based microparticles coated with HPMCP/AS cellulose-derivatives enable the Ctx(Ile21)-Ha antimicrobial peptide application as a feed additive. Int. J. Biol. Macromol. 2021, 183, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; da Silva, C.F.; Andrade, L.N.; de Lima Oliveira, D.; Campos, J.; Souto, E.B. Alginate Nanoparticles for Drug Delivery and Targeting. Curr. Pharm. Des. 2019, 25, 1312–1334. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, L.; Su, L.; Van der Mei, H.C.; Jutte, P.C.; Ren, Y.; Busscher, H.J. Nanotechnology-based antimicrobials and delivery systems for biofilm-infection control. Chem. Soc. Rev. 2019, 48, 428–446. [Google Scholar] [CrossRef]
- Li, R.; Lyu, Y.; Luo, S.; Wang, H.; Zheng, X.; Li, L.; Ao, N.; Zha, Z. Fabrication of a multi-level drug release platform with liposomes, chitooligosaccharides, phospholipids and injectable chitosan hydrogel to enhance anti-tumor effectiveness. Carbohydr. Polym. 2021, 269, 118322. [Google Scholar] [CrossRef] [PubMed]
- Walsh, G. Pharmaceutical Biotechnology: Concepts and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Reddy, M.; Murthy, K.; Srilakshmi, A.; Rao, K.S.; Pullaiah, T. Phytosynthesis of eco-friendly silver nanoparticles and biological applications –A novel concept in nanobiotechnology. Afr. J. Biotechnol. 2015, 14, 222–247. [Google Scholar] [CrossRef]
- Tasima, L.J.; Serino-Silva, C.; Hatakeyama, D.M.; Nishiduka, E.S.; Tashima, A.K.; Sant’Anna, S.S.; Grego, K.F.; de Morais-Zani, K.; Tanaka-Azevedo, A.M. Crotamine in Crotalus durissus: Distribution according to subspecies and geographic origin, in captivity or nature. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20190053. [Google Scholar] [CrossRef]
- Misra, S.K.; Ye, M.; Kim, S.; Pan, D. Defined nanoscale chemistry influences delivery of peptido-toxins for cancer therapy. PLoS ONE 2015, 10, e0125908. [Google Scholar] [CrossRef] [Green Version]
- Sant’Anna, M.B.; Giardini, A.C.; Ribeiro, M.A.C.; Lopes, F.S.R.; Teixeira, N.B.; Kimura, L.F.; Bufalo, M.C.; Ribeiro, O.G.; Borrego, A.; Cabrera, W.H.K.; et al. The Crotoxin:SBA-15 Complex Down-Regulates the Incidence and Intensity of Experimental Autoimmune Encephalomyelitis Through Peripheral and Central Actions. Front. Immunol. 2020, 11, 591563. [Google Scholar] [CrossRef]
- Al-Rabia, M.W.; Alhakamy, N.A.; Ahmed, O.A.A.; Eljaaly, K.; Aloafi, A.L.; Mostafa, A.; Asfour, H.Z.; Aldarmahi, A.A.; Darwish, K.M.; Ibrahim, T.S.; et al. Repurposing of sitagliptin-melittin optimized nanoformula against SARS-CoV-2: Antiviral screening and molecular docking studies. Pharmaceutics 2021, 13, 307. [Google Scholar] [CrossRef]
- Santos-Filho, N.A.; Lorenzon, E.N.; Ramos, M.A.S.; Santos, C.T.; Piccoli, J.P.; Bauab, T.M.; Fusco-Almeida, A.M.; Cilli, E.M. Synthesis and characterization of an antibacterial and non-toxic dimeric peptide derived from the C-terminal region of Bothropstoxin-I. Toxicon 2015, 103, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Swenson, S.D.; Silva-Hirschberg, C.; Markland, F.S. Methods for Evaluation of a Snake Venom-Derived Disintegrin in Animal Models of Human Cancer. Methods Mol. Biol. 2020, 2068, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Minea, R.O.; Helchowski, C.M.; Zidovetzki, S.J.; Costa, F.K.; Swenson, S.D.; Markland, F.S., Jr. Vicrostatin–An Anti-Invasive Multi-Integrin Targeting Chimeric Disintegrin with Tumor Anti-Angiogenic and Pro-Apoptotic Activities. PLoS ONE 2010, 5, e10929. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Liu, S.; Ai, M.; Wang, Z.; Wang, D.; Li, X.; Hu, K.; Gao, X.; Yang, Y. A novel melittin nano-liposome exerted excellent anti-hepatocellular carcinoma efficacy with better biological safety. J. Hematol. Oncol. 2017, 10, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Chen, L.; Liu, J.; Dai, B.; Xu, G.; Shen, G.; Luo, Q.; Zhang, Z. Immune modulation of liver sinusoidal endothelial cells by melittin nanoparticles suppresses liver metastasis. Nat. Commun. 2019, 10, 574. [Google Scholar] [CrossRef]
- Yu, X.; Dai, Y.; Zhao, Y.; Qi, S.; Liu, L.; Lu, L.; Luo, Q.; Zhang, Z. Melittin-lipid nanoparticles target to lymph nodes and elicit a systemic anti-tumor immune response. Nat. Commun. 2020, 11, 1110. [Google Scholar] [CrossRef] [Green Version]
- Cantor, S.; Vargas, L.; Rojas, A.O.; Yarce, C.; Salamanca, C.; Oñate-Garzón, J. Evaluation of the Antimicrobial Activity of Cationic Peptides Loaded in Surface-Modified Nanoliposomes against Foodborne Bacteria. Int. J. Mol. Sci. 2019, 20, 680. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Zheng, H.; Xu, J.; Shi, X.; Li, F.; Wang, X. Sustained-release study on Exenatide loaded into mesoporous silica nanoparticles: In vitro characterization and in vivo evaluation. DARU J. Pharm. Sci. 2017, 25, 20. [Google Scholar] [CrossRef]
- Ferreira Junior, R.S.; Anderlini, R.P.; Pimenta, D.C.; De Oliveira Orsi, R.; Barraviera, B.; Sant’Anna, O.A. New Nanostructured Silica Adjuvant (SBA-15) Employed to Produce Antivenom in Young Sheep Using Crotalus durissus terrificus and Apis mellifera Venoms Detoxified by Cobalt-60. J. Toxicol. Environ. Health Part A 2010, 73, 926–933. [Google Scholar] [CrossRef]
- Baudou, F.G.; Fusco, L.; Giorgi, E.; Diaz, E.; Municoy, S.; Desimone, M.F.; Leiva, L.; De Marzi, M.C. Physicochemical and biological characterization of nanovenoms, a new tool formed by silica nanoparticles and Crotalus durissus terrificus venom. Colloids Surf. B Biointerfaces 2020, 193, 111128. [Google Scholar] [CrossRef]
- Mercuri, L.P.; Carvalho, L.V.; Lima, F.A.; Quayle, C.; Fantini, M.C.A.; Tanaka, G.S.; Cabrera, W.H.; Furtado, M.F.D.; Tambourgi, D.V.; do Matos, J.R.; et al. Ordered Mesoporous Silica SBA-15: A New Effective Adjuvant to Induce Antibody Response. Small 2006, 2, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Sayed, D.; Al-Sadoon, M.K.; Badr, G. Silica nanoparticles sensitize human multiple myeloma cells to snake (Walterinnesia aegyptia) venom-induced apoptosis and growth arrest. Oxid. Med. Cell. Longev. 2012, 2012, 386286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badr, G.; Al-Sadoon, M.K.; Rabah, D.M.; Sayed, D. Snake (Walterinnesia aegyptia) venom-loaded silica nanoparticles induce apoptosis and growth arrest in human prostate cancer cells. Apoptosis 2013, 18, 300–314. [Google Scholar] [CrossRef]
- Picolo, G. Crotoxin induces analgesic and immunomodulatory effects on chronic pain models that are potentiated by nanostructured silica SBA-15. Toxicon 2020, 177, S20. [Google Scholar] [CrossRef]
- Sant’Anna, M.B.; Lopes, F.S.R.; Kimura, L.F.; Giardini, A.C.; Sant’Anna, O.A.; Picolo, G. Crotoxin conjugated to SBA-15 nanostructured mesoporous silica induces long-last analgesic effect in the neuropathic pain model in mice. Toxins 2019, 11, 679. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Zhang, Y.; Shan, Y.; Wang, J.; Liu, F.; Liu, H.; Xing, G.; Lei, J.; Zhou, J. A pH-dependent Antibacterial Peptide Release Nano-system Blocks Tumor Growth in vivo without Toxicity. Sci. Rep. 2017, 7, 11242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpel, R.L.; da Silva Liberato, M.; Campeiro, J.D.; Bergeon, L.; Szychowski, B.; Butler, A.; Marino, G.; Cusic, J.F.; de Oliveira, L.C.G.; Oliveira, E.B.; et al. Design and characterization of crotamine-functionalized gold nanoparticles. Colloids Surf. B Biointerfaces 2018, 163, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Dasgupta, S.C.; Dasgupta, A.K.; Gomes, A.; Gomes, A. Gold Nanoparticles (AuNPs) Conjugated with Andrographolide Ameliorated Viper (Daboia russellii russellii) Venom-Induced Toxicities in Animal Model. J. Nanosci. Nanotechnol. 2020, 20, 3404–3414. [Google Scholar] [CrossRef]
- Oliveira, I.C.F.; de Paula, M.O.; Lastra, H.C.B.; de Alves, B.B.; Moreno, D.A.N.; Yoshida, E.H.; Amaral Filho, J.; Cogo, J.C.; Varanda, E.A.; Rai, M.; et al. Activity of silver nanoparticles on prokaryotic cells and Bothrops jararacussu snake venom. Drug Chem. Toxicol. 2019, 42, 60–64. [Google Scholar] [CrossRef]
- Jalaei, J.; Layeghi-Ghalehsoukhteh, S.; Hosseini, A.; Fazeli, M. Antibacterial effects of gold nanoparticles functionalized with the extracted peptide from Vespa orientalis wasp venom. J. Pept. Sci. 2018, 24, e3124. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, X.; Zhang, L.; Sun, K.; Li, K.; Li, Y.; Zhang, Q. Fc-modified exenatide-loaded nanoparticles for oral delivery to improve hypoglycemic effects in mice. Sci. Rep. 2018, 8, 726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamzaoui, A.; Laraba-Djebari, F. Development and evaluation of polymeric nanoparticles as a delivery system for snake envenoming prevention. Biologicals 2021, 70, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Han, F.; Zhang, W.; Yang, Y.; You, D.; Li, L. Toward improved wound dressings: Effects of polydopamine-decorated poly(lactic-co-glycolic acid) electrospinning incorporating basic fibroblast growth factor and ponericin G1. RSC Adv. 2019, 9, 33038–33051. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.; Mohamed, M.S.; Mizuki, T.; Maekawa, T.; Kumar, D.S. Chlorotoxin modified morusin–PLGA nanoparticles for targeted glioblastoma therapy. J. Mater. Chem. B 2019, 7, 5896–5919. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.M.; Kim, J.H.; Cho, C.W.; Jeon, J.W.; Park, J.K.; Lee, S.H.; Jung, B.G.; Lee, B.J. Nasal delivery of chitosan/alginate nanoparticle encapsulated bee (Apis mellifera) venom promotes antibody production and viral clearance during porcine reproductive and respiratory syndrome virus infection by modulating T cell related responses. Vet. Immunol. Immunopathol. 2018, 200, 40–51. [Google Scholar] [CrossRef]
- El-Dek, S.; Hassan, M.; Mohamed, A.; Abdel Wahab, A.W. Apoptotic and necrotic effects of chitosan nanoparticles loaded with the honeybee, Apis mellifera venom on different cancer cell lines. J. Egypt. Soc. Parasitol. 2019, 49, 115–122. [Google Scholar] [CrossRef]
- Alalawy, A.I.; El Rabey, H.A.; Almutairi, F.M.; Tayel, A.A.; Al-Duais, M.A.; Zidan, N.S.; Sakran, M.I. Effectual Anticancer Potentiality of Loaded Bee Venom onto Fungal Chitosan Nanoparticles. Int. J. Polym. Sci. 2020, 2020, 2785304. [Google Scholar] [CrossRef]
- Nait Mohamed, F.A.; Nouri, A.; Laraba-Djebari, F. Reactogenicity and safety assessment of an attenuated nanovaccine against scorpion envenomation: Preclinical study. Vaccine 2017, 35, 6657–6663. [Google Scholar] [CrossRef]
- Nait Mohamed, F.A.; Laraba-Djebari, F. Development and characterization of a new carrier for vaccine delivery based on calcium-alginate nanoparticles: Safe immunoprotective approach against scorpion envenoming. Vaccine 2016, 34, 2692–2699. [Google Scholar] [CrossRef]
- Rebbouh, F.; Martin-Eauclaire, M.F.; Laraba-Djebari, F. Chitosan nanoparticles as a delivery platform for neurotoxin II from Androctonus australis hector scorpion venom: Assessment of toxicity and immunogenicity. Acta Trop. 2020, 205, 105353. [Google Scholar] [CrossRef]
- Piras, A.M.; Maisetta, G.; Sandreschi, S.; Gazzarri, M.; Bartoli, C.; Grassi, L.; Esin, S.; Chiellini, F.; Batoni, G. Chitosan nanoparticles loaded with the antimicrobial peptide temporin B exert a long-term antibacterial activity in vitro against clinical isolates of Staphylococcus epidermidis. Front. Microbiol. 2015, 6, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Rêgo, M.; Gláucia-Silva, F.; Rocha Soares, K.S.; de Souza, L.B.F.C.; Damasceno, I.Z.; dos Santos-Silva, E.; Lacerda, A.F.; Chaves, G.M.; da Silva-Júnior, A.A.; de Fernandes-Pedrosa, M.F. Biodegradable cross-linked chitosan nanoparticles improve anti-Candida and anti-biofilm activity of TistH, a peptide identified in the venom gland of the Tityus stigmurus scorpion. Mater. Sci. Eng. C 2019, 103, 109830. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.G.; Rocha Soares, K.S.; Ribeiro Bastos De França, A.C.; Do Rêgo, M.T.; Ribeiro Bastos De França, A.C.; Da Silva, D.P.; De Sousa Ferreira, S.; Dos Santos Silva, A.M.; Da Silva Júnior, A.A.; De Freitas Fernandes Pedrosa, M. Serum production against Crotalus durissus cascavella snake venom using a biotechnological approach as immunoadjuvant. Toxicon 2019, 168, S38. [Google Scholar] [CrossRef]
- Mohammadpourdounighi, N.; Behfar, A.; Ezabadi, A.; Zolfagharian, H.; Heydari, M. Preparation of chitosan nanoparticles containing Naja naja oxiana snake venom. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 137–143. [Google Scholar] [CrossRef]
- Nakamoto, M.; Zhao, D.; Benice, O.R.; Lee, S.H.; Shea, K.J. Abiotic Mimic of Endogenous Tissue Inhibitors of Metalloproteinases: Engineering Synthetic Polymer Nanoparticles for Use as a Broad-Spectrum Metalloproteinase Inhibitor. J. Am. Chem. Soc. 2020, 142, 2338–2345. [Google Scholar] [CrossRef]
- Attarde, S.S.; Pandit, S.V. Anticancer potential of nanogold conjugated toxin GNP-NN-32 from Naja naja venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26. [Google Scholar] [CrossRef]
- Tan, H.; Huang, Y.; Xu, J.; Chen, B.; Zhang, P.; Ye, Z.; Liang, S.; Xiao, L.; Liu, Z. Spider toxin peptide lycosin-I functionalized gold nanoparticles for in vivo tumor targeting and therapy. Theranostics 2017, 7, 3168–3178. [Google Scholar] [CrossRef] [Green Version]
- Khamehchian, S.; Nikkhah, M.; Madani, R.; Hosseinkhani, S. Enhanced and selective permeability of gold nanoparticles functionalized with cell penetrating peptide derived from maurocalcine animal toxin. J. Biomed. Mater. Res. Part A 2016, 104, 2693–2700. [Google Scholar] [CrossRef]
- Di Filippo, L.D.; Duarte, J.; Fonseca-Santos, B.; Tavares Júnior, A.G.; Araújo, V.; Roque Borda, C.A.; Vicente, E.F.; Chorilli, M. Mucoadhesive Nanosystems for Nose-to-Brain Drug Delivery in the Treatment of Central Nervous System Diseases. Curr. Med. Chem. 2021, 28, 3079–3110. [Google Scholar] [CrossRef]
- Semenova, M.; Antipova, A.; Martirosova, E.; Zelikina, D.; Palmina, N.; Chebotarev, S. Essential contributions of food hydrocolloids and phospholipid liposomes to the formation of carriers for controlled delivery of biologically active substances via the gastrointestinal tract. Food Hydrocoll. 2021, 120, 106890. [Google Scholar] [CrossRef]
- da Silva Malheiros, P.; Daroit, D.J.; Brandelli, A. Food applications of liposome-encapsulated antimicrobial peptides. Trends Food Sci. Technol. 2010, 21, 284–292. [Google Scholar] [CrossRef]
- Jesorka, A.; Orwar, O. Liposomes: Technologies and analytical applications. Annu. Rev. Anal. Chem. 2008, 1, 801–832. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.L.; dos Santos, J.; Silveira, N.P.; Brandelli, A. Phospholipid nanovesicles containing a bacteriocin-like substance for control of Listeria monocytogenes. Innov. Food Sci. Emerg. Technol. 2008, 9, 49–53. [Google Scholar] [CrossRef]
- Colas, J.C.; Shi, W.; Rao, V.S.N.M.; Omri, A.; Mozafari, M.R.; Singh, H. Microscopical investigations of nisin-loaded nanoliposomes prepared by Mozafari method and their bacterial targeting. Micron 2007, 38, 841–847. [Google Scholar] [CrossRef]
- Taylor, T.M.; Bruce, B.D.; Weiss, J.; Davidson, P.M. Listeria monocytogenes and Escherichia coli O157:H7 inhibition in vitro by liposome-encapsulated nisin and ethylene diaminetetraacetic acid. J. Food Saf. 2008, 28, 183–197. [Google Scholar] [CrossRef]
- Laridi, R.; Kheadr, E.E.; Benech, R.O.; Vuillemard, J.C.; Lacroix, C.; Fliss, I. Liposome encapsulated nisin Z: Optimization, stability and release during milk fermentation. Int. Dairy J. 2003, 13, 325–336. [Google Scholar] [CrossRef]
- Were, L.M.; Bruce, B.; Davidson, P.M.; Weiss, J. Encapsulation of nisin and lysozyme in liposomes enhances efficacy against Listeria monocytogenes. J. Food Prot. 2004, 67, 922–927. [Google Scholar] [CrossRef]
- Gan, Z.R.; Gould, R.J.; Jacobs, J.W.; Friedman, P.A.; Polokoff, M.A. Echistatin. A potent platelet aggregation inhibitor from the venom of the viper, Echis carinatus. J. Biol. Chem. 1988, 263, 19827–19832. [Google Scholar] [CrossRef]
- Trikha, M.; De Clerck, Y.A.; Markland, F.S. Contortrostatin, a Snake Venom Disintegrin, Inhibits β1 Integrin-mediated Human Metastatic Melanoma Cell Adhesion and Blocks Experimental Metastasis. Cancer Res. 1994, 54, 4993–4998. [Google Scholar]
- Schönthal, A.H.; Swenson, S.D.; Chen, T.C.; Markland, F.S. Preclinical studies of a novel snake venom-derived recombinant disintegrin with antitumor activity: A review. Biochem. Pharmacol. 2020, 181, 114149. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Ullah, Z.; Al Balowi, A.; Islam, M. In vitro determination of the efficacy of scorpion venoms as anti-cancer agents against colorectal cancer cells: A nano-liposomal delivery approach. Int. J. Nanomed. 2017, 12, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, R.; Mollay, C. Import of honeybee prepromelittin into the endoplasmic reticulum. Requirements for membrane insertion, processing, and sequestration. J. Biol. Chem. 1986, 261, 12889–12895. [Google Scholar] [CrossRef]
- Müller, G.; Zimmermann, R. Import of honeybee prepromelittin into the endoplasmic reticulum: Structural basis for independence of SRP and docking protein. EMBO J. 1987, 6, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Ladokhin, A.S.; White, S.H. Folding of amphipathic α-helices on membranes: Energetics of helix formation by melittin. J. Mol. Biol. 1999, 285, 1363–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guha, S.; Ferrie, R.P.; Ghimire, J.; Ventura, C.R.; Wu, E.; Sun, L.; Kim, S.Y.; Wiedman, G.R.; Hristova, K.; Wimley, W.C. Applications and evolution of melittin, the quintessential membrane active peptide. Biochem. Pharmacol. 2021, 193, 114769. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, C.E.; Bazzo, R.; Harvey, T.S.; Syperek, I.; Boheim, G.; Campbell, I.D. Contribution of proline-14 to the structure and actions of melittin. FEBS Lett. 1991, 281, 240–244. [Google Scholar] [CrossRef] [Green Version]
- Bazzo, R.; Tappin, M.J.; Pastore, A.; Harvey, T.S.; Carver, J.A.; Campbell, I.D. The structure of melittin. A 1H-NMR study in methanol. Eur. J. Biochem. 1988, 173, 139–146. [Google Scholar] [CrossRef]
- Terwilliger, T.C.; Eisenberg, D. The structure of melittin. II. Interpretation of the structure. J. Biol. Chem. 1982, 257, 6016–6022. [Google Scholar] [CrossRef]
- Rex, S. A Pro→Ala substitution in melittin affects self-association, membrane binding and pore-formation kinetics due to changes in structural and electrostatic properties. Biophys. Chem. 2000, 85, 209–228. [Google Scholar] [CrossRef]
- Ceremuga, M.; Stela, M.; Janik, E.; Gorniak, L.; Synowiec, E.; Sliwinski, T.; Sitarek, P.; Saluk-Bijak, J.; Bijak, M. Melittin—A Natural Peptide from Bee Venom Which Induces Apoptosis in Human Leukaemia Cells. Biomolecules 2020, 10, 247. [Google Scholar] [CrossRef] [Green Version]
- Socarras, K.; Theophilus, P.; Torres, J.; Gupta, K.; Sapi, E. Antimicrobial Activity of Bee Venom and Melittin against Borrelia burgdorferi. Antibiotics 2017, 6, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conlon, J.M.; Ahmed, E.; Pal, T.; Sonnevend, A. Potent and rapid bactericidal action of alyteserin-1c and its [E4K] analog against multidrug-resistant strains of Acinetobacter baumannii. Peptides 2010, 31, 1806–1810. [Google Scholar] [CrossRef]
- Subasinghage, A.P.; O’Flynn, D.; Conlon, J.M.; Hewage, C.M. Conformational and membrane interaction studies of the antimicrobial peptide alyteserin-1c and its analogue [E4K]alyteserin-1c. Biochim. Biophys. Acta-Biomembr. 2011, 1808, 1975–1984. [Google Scholar] [CrossRef] [Green Version]
- Aragón-Muriel, A.; Ausili, A.; Sánchez, K.; Rojas A., O.E.; Mosquera, J.L.; Polo-Cerón, D.; Oñate-Garzón, J. Studies on the Interaction of Alyteserin 1c Peptide and Its Cationic Analogue with Model Membranes Imitating Mammalian and Bacterial Membranes. Biomolecules 2019, 9, 527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica Nanoparticles—A Versatile Tool for the Treatment of Bacterial Infections. Front. Chem. 2020, 8, 602. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, G.C.; Sábio, R.M.; de Cássia Ribeiro, T.; Monteiro, A.S.; Pereira, D.V.; Ribeiro, S.J.L.; Chorilli, M. Highlights in Mesoporous Silica Nanoparticles as a Multifunctional Controlled Drug Delivery Nanoplatform for Infectious Diseases Treatment. Pharm. Res. 2020, 37, 191. [Google Scholar] [CrossRef] [PubMed]
- Sábio, R.M.; Meneguin, A.B.; Ribeiro, T.C.; Silva, R.R.; Chorilli, M. New insights towards mesoporous silica nanoparticles as a technological platform for chemotherapeutic drugs delivery. Int. J. Pharm. 2019, 564, 379–409. [Google Scholar] [CrossRef]
- Schlossbauer, A.; Schaffert, D.; Kecht, J.; Wagner, E.; Bein, T. Click Chemistry for High-Density Biofunctionalization of Mesoporous Silica. J. Am. Chem. Soc. 2008, 130, 12558–12559. [Google Scholar] [CrossRef]
- Carvalho, L.V.; Ruiz, R.D.C.; Scaramuzzi, K.; Marengo, E.B.; Matos, J.R.; Tambourgi, D.V.; Fantini, M.C.A.; Anna, O.A. Immunological parameters related to the adjuvant effect of the ordered mesoporous silica SBA-15. Vaccine 2010, 28, 7829–7836. [Google Scholar] [CrossRef]
- Sábio, R.M.; Meneguin, A.B.; Martins dos Santos, A.; Monteiro, A.S.; Chorilli, M. Exploiting mesoporous silica nanoparticles as versatile drug carriers for several routes of administration. Microporous Mesoporous Mater. 2021, 312, 110774. [Google Scholar] [CrossRef]
- Schlossbauer, A.; Dohmen, C.; Schaffert, D.; Wagner, E.; Bein, T. pH-Responsive Release of Acetal-Linked Melittin from SBA-15 Mesoporous Silica. Angew. Chem. Int. Ed. 2011, 50, 6828–6830. [Google Scholar] [CrossRef] [PubMed]
- Galllwitz, B. Exenatide in type 2 diabetes: Treatment effects in clinical studies and animal study data. Int. J. Clin. Pract. 2006, 60, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.L.; Abdalla, F.M.F.; Alponti, R.F.; Silveira, P.F. Anti-obesogenic and hypolipidemic effects of a glucagon-like peptide-1 receptor agonist derived from the saliva of the Gila monster. Toxicon 2017, 135, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kuniyoshi, A.K.; Kodama, R.T.; Cajado-Carvalho, D.; Iwai, L.K.; Kitano, E.; da Silva, C.C.F.; Duzzi, B.; Dias da Silva, W.; Portaro, F.C. Experimental antivenom against serine proteases from the Bothrops jararaca venom obtained in mice, and its comparison with the antibothropic serum from the Butantan Institute. Toxicon 2019, 169, 59–67. [Google Scholar] [CrossRef]
- Badr, G.; Al-Sadoon, M.K.; Abdel-Maksoud, M.A.; Rabah, D.M.; El-Toni, A.M. Cellular and Molecular Mechanisms Underlie the Anti-Tumor Activities Exerted by Walterinnesia aegyptia Venom Combined with Silica Nanoparticles against Multiple Myeloma Cancer Cell Types. PLoS ONE 2012, 7, e51661. [Google Scholar] [CrossRef] [Green Version]
- Badr, G.; Al-Sadoon, M.K.; Rabah, D.M. Therapeutic efficacy and molecular mechanisms of snake (Walterinnesia aegyptia) venom-loaded silica nanoparticles in the treatment of breast cancer- and prostate cancer-bearing experimental mouse models. Free Radic. Biol. Med. 2013, 65, 175–189. [Google Scholar] [CrossRef]
- de Almeida, C.S.; Andrade-Oliveira, V.; Câmara, N.O.S.; Jacysyn, J.F.; Faquim-Mauro, E.L. Crotoxin from Crotalus durissus terrificus Is Able to Down-Modulate the Acute Intestinal Inflammation in Mice. PLoS ONE 2015, 10, e0121427. [Google Scholar] [CrossRef] [Green Version]
- de Andrade, C.M.; Rey, F.M.; Bianchini, F.J.; Sampaio, S.V.; Torqueti, M.R. Crotoxin, a neurotoxin from Crotalus durissus terrificus snake venom, as a potential tool against thrombosis development. Int. J. Biol. Macromol. 2019, 134, 653–659. [Google Scholar] [CrossRef]
- Muller, S.P.; Silva, V.A.O.; Silvestrini, A.V.P.; de Macedo, L.H.; Caetano, G.F.; Reis, R.M.; Mazzi, M.V. Crotoxin from Crotalus durissus terrificus venom: In vitro cytotoxic activity of a heterodimeric phospholipase A2 on human cancer-derived cell lines. Toxicon 2018, 156, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Sant’Anna, M.B.; Giardini, A.C.; Lopes, F.S.; Kimura, L.F.; Chacur, M.; Pagano, R.L.; Zambelli, V.O.; Sant’Anna, O.A.; Picolo, G. Rattlesnake venom components in the control of experimental autoimmune encephalomyelitis: Immunomodulatory properties potentiated by silica SBA-15. Toxicon 2020, 182, S22. [Google Scholar] [CrossRef]
- Vicente, E.F.; Basso, L.G.M.; Crusca Junior, E.; Roque-Borda, C.A.; Costa-Filho, A.J.; Cilli, E.M. Biophysical Studies of TOAC Analogs of the Ctx(Ile21)-Ha Antimicrobial Peptide Using Liposomes. Brazilian J. Phys. 2022, 52, 71. [Google Scholar] [CrossRef]
- Roque-Borda, C.A.; Pereira, L.P.; Guastalli, E.A.L.; Soares, N.M.; Mac-Lean, P.A.B.; Salgado, D.D.; Meneguin, A.B.; Chorilli, M.; Vicente, E.F. HPMCP-Coated Microcapsules Containing the Ctx(Ile21)-Ha Antimicrobial Peptide Reduce the Mortality Rate Caused by Resistant Salmonella Enteritidis in Laying Hens. Antibiotics 2021, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- Lorenzón, E.N.; Cespedes, G.F.; Vicente, E.F.; Nogueira, L.G.; Bauab, T.M.; Castro, M.S.; Cilli, E.M. Effects of Dimerization on the Structure and Biological Activity of Antimicrobial Peptide Ctx-Ha. Antimicrob. Agents Chemother. 2012, 56, 3004–3010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roque-Borda, C.A.; de Souza Saraiva, M.; Monte, D.F.M.; Rodrigues Alves, L.B.; de Almeida, A.M.; Ferreira, T.S.; de Lima, T.S.; Benevides, V.P.; Cabrera, J.M.; Claire, S.; et al. HPMCAS-Coated Alginate Microparticles Loaded with Ctx(Ile 21 )-Ha as a Promising Antimicrobial Agent against Salmonella Enteritidis in a Chicken Infection Model. ACS Infect. Dis. 2022, 8, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Roque-Borda, C.; de Saraiva, M.M.S.; Macedo, W.C.; Montesinos, J.M.; Meneguin, A.; de Almeida, A.; Teixeira, S.; Chorilli, M.; Pavan, F.; Junior, A.B.; et al. Development of an oral feed additive against intestinal infections based on microparticles’ systems loaded with Ctx(Ile21)-Ha peptide coated with HPMCAS/Chitosan. In Proceedings of the 7th International Electronic Conference on Medicinal Chemistry, Online, 1–30 November 2021; p. 11574. [Google Scholar] [CrossRef]
- Zhang, N.; Zhi, X.; Zhao, J.; Wei, J.; Li, J.; Yang, H. Mesoporous silica induces hippocampal neurons cell autophagy through AMPK/mTOR/P70S6K signaling pathway. Environ. Toxicol. 2020, 35, 176–187. [Google Scholar] [CrossRef]
- Zhao, J.; Bu, D.; Zhang, N.; Tian, D.; Ma, L.; Yang, H. Cytotoxicity of mesoporous silica modified by amino and carboxyl groups on vascular endothelial cells. Environ. Toxicol. 2021, 36, 1422–1433. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Goncalves, J.M.; Polson, A. The electrophoretic analysis of snake venoms. Arch. Biochem. 1947, 13, 253–259. [Google Scholar]
- Laure, C.J. The primary structure of crotamine (author’s transl). Hoppe. Seylers. Z. Physiol. Chem. 1975, 356, 213–215. [Google Scholar]
- Beltran, J.R.; Mascarenhas, Y.P.; Craievich, A.F.; Laure, C.J. SAXS study of the snake toxin α-crotamine. Eur. Biophys. J. 1990, 17, 325–329. [Google Scholar] [CrossRef]
- Nicastro, G.; Franzoni, L.; de Chiara, C.; Mancin, A.C.; Giglio, J.R.; Spisni, A. Solution structure of crotamine, a Na+ channel affecting toxin from Crotalus durissus terrificus venom. Eur. J. Biochem. 2003, 270, 1969–1979. [Google Scholar] [CrossRef] [PubMed]
- Fadel, V.; Bettendorff, P.; Herrmann, T.; De Azevedo, W.F.; Oliveira, E.B.; Yamane, T.; Wüthrich, K. Automated NMR structure determination and disulfide bond identification of the myotoxin crotamine from Crotalus durissus terrificus. Toxicon 2005, 46, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Vital Brazil, O.; Fontana, M.D. Toxins as tools in the study of sodium channel distribution in the muscle fibre membrane. Toxicon 1993, 31, 1085–1098. [Google Scholar] [CrossRef]
- Peigneur, S.; Orts, D.J.B.; Prieto Da Silva, A.R.; Oguiura, N.; Boni-Mitake, M.; De Oliveira, E.B.; Zaharenko, A.J.; De Freitas, J.C.; Tytgat, J. Crotamine pharmacology revisited: Novel insights based on the inhibition of K v channels. Mol. Pharmacol. 2012, 82, 90–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, M.A.F.; Nascimento, F.D.; Kerkis, A.; Oliveira, V.; Oliveira, E.B.; Pereira, A.; Rádis-Baptista, G.; Nader, H.B.; Yamane, T.; Kerkis, I.; et al. Cytotoxic effects of crotamine are mediated through lysosomal membrane permeabilization. Toxicon 2008, 52, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Campeiro, J.D.; Marinovic, M.P.; Carapeto, F.C.; Dal Mas, C.; Monte, G.G.; Carvalho Porta, L.; Nering, M.B.; Oliveira, E.B.; Hayashi, M.A.F. Oral treatment with a rattlesnake native polypeptide crotamine efficiently inhibits the tumor growth with no potential toxicity for the host animal and with suggestive positive effects on animal metabolic profile. Amino Acids 2018, 50, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Macedo, S.R.A.; De Barros, N.B.; Ferreira, A.S.; Moreira-Dill, L.S.; Calderon, L.A.; Soares, A.M.; Nicolete, R. Biodegradable Microparticles Containing Crotamine Isolated from Crotalus durissus terrificus Display Antileishmanial Activity in vitro. Pharmacology 2015, 95, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Passero, L.F.D.; Tomokane, T.Y.; Corbett, C.E.P.; Laurenti, M.D.; Toyama, M.H. Comparative studies of the anti-leishmanial activity of three Crotalus durissus ssp. venoms. Parasitol. Res. 2007, 101, 1365–1371. [Google Scholar] [CrossRef]
- Dal Mas, C.; Moreira, J.T.; Pinto, S.; Monte, G.G.; Nering, M.B.; Oliveira, E.B.; Gazarini, M.L.; Mori, M.A.; Hayashi, M.A.F. Anthelmintic effects of a cationic toxin from a South American rattlesnake venom. Toxicon 2016, 116, 49–55. [Google Scholar] [CrossRef]
- El Chamy Maluf, S.; Dal Mas, C.; Oliveira, E.B.; Melo, P.M.; Carmona, A.K.; Gazarini, M.L.; Hayashi, M.A.F. Inhibition of malaria parasite Plasmodium falciparum development by crotamine, a cell penetrating peptide from the snake venom. Peptides 2016, 78, 11–16. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zielinska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesk, D.N.; Durazzo, A.; Lucarin, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731–3751. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, L.M.; Calixto, G.M.F.; Chorilli, M.; Giusti, J.S.M.; Bagnato, V.S.; Soukos, N.S.; Amiji, M.M.; Fontana, C.R. Polymeric Nanoparticle-Based Photodynamic Therapy for Chronic Periodontitis in Vivo. Int. J. Mol. Sci. 2016, 17, 769. [Google Scholar] [CrossRef] [PubMed]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Lv, X.; Le, Y. Chitosan-Modified PLGA Nanoparticles for Control-Released Drug Delivery. Polymers 2019, 11, 304. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Deng, C.; Meng, F.; Zhang, J.; Sun, H.; Zhong, Z. Hyaluronic acid coated PLGA nanoparticulate docetaxel effectively targets and suppresses orthotopic human lung cancer. J. Control. Release 2017, 259, 76–82. [Google Scholar] [CrossRef]
- El-Hammadi, M.M.; Delgado, Á.V.; Melguizo, C.; Prados, J.C.; Arias, J.L. Folic acid-decorated and PEGylated PLGA nanoparticles for improving the antitumour activity of 5-fluorouracil. Int. J. Pharm. 2017, 516, 61–70. [Google Scholar] [CrossRef]
- Orivel, J.; Redeker, V.; Le Caer, J.P.; Krier, F.; Revol-Junelles, A.M.; Longeon, A.; Chaffotte, A.; Dejean, A.; Rossier, J. Ponericins, New Antibacterial and Insecticidal Peptides from the Venom of the Ant Pachycondyla goeldii. J. Biol. Chem. 2001, 276, 17823–17829. [Google Scholar] [CrossRef] [Green Version]
- Shukla, A.; Fleming, K.E.; Chuang, H.F.; Chau, T.M.; Loose, C.R.; Stephanopoulos, G.N.; Hammond, P.T. Controlling the release of peptide antimicrobial agents from surfaces. Biomaterials 2010, 31, 2348–2357. [Google Scholar] [CrossRef]
- Zhang, H.; Jiao, J.; Jin, H. Degradable poly-l-lysine-modified PLGA cell microcarriers with excellent antibacterial and osteogenic activity. Int. Mater. Rev. 2021, 66, 77–113. [Google Scholar] [CrossRef] [Green Version]
- DeBin, J.A.; Maggio, J.E.; Strichartz, G.R. Purification and characterization of chlorotoxin, a chloride channel ligand from the venom of the scorpion. Am. J. Physiol.-Cell Physiol. 1993, 264, C361–C369. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, R.E.; Yamamoto, M.; Gokaslan, Z.L.; Wang, S.W.; Mohanam, S.; Fuller, G.N.; McCutcheon, I.E.; Stetler-Stevenson, W.G.; Nicolson, G.L.; Rao, J.S. Expression and localization of 72 kDa type IV collagenase (MMP-2) in human malignant gliomas in vivo. Clin. Exp. Metastasis 1996, 14, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Deshane, J.; Garner, C.C.; Sontheimer, H. Chlorotoxin Inhibits Glioma Cell Invasion via Matrix Metalloproteinase-2. J. Biol. Chem. 2003, 278, 4135–4144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahoor, A.; Sharma, S.; Khuller, G.K. Inhalable alginate nanoparticles as antitubercular drug carriers against experimental tuberculosis. Int. J. Antimicrob. Agents 2005, 26, 298–303. [Google Scholar] [CrossRef] [PubMed]
- de Vos, P.; Faas, M.M.; Spasojevic, M.; Sikkema, J. Encapsulation for preservation of functionality and targeted delivery of bioactive food components. Int. Dairy J. 2010, 20, 292–302. [Google Scholar] [CrossRef]
- Khalil, A.; Elesawy, B.H.; Ali, T.M.; Ahmed, O.M. Bee Venom: From Venom to Drug. Molecules 2021, 26, 4941. [Google Scholar] [CrossRef]
- Zhou, J.; Wan, C.; Cheng, J.; Huang, H.; Lovell, J.F.; Jin, H. Delivery Strategies for Melittin-Based Cancer Therapy. ACS Appl. Mater. Interfaces 2021, 13, 17158–17173. [Google Scholar] [CrossRef]
- Simmaco, M.; Mignogna, G.; Canofeni, S.; Miele, R.; Mangoni, M.L.; Barra, D. Temporins, Antimicrobial Peptides from the European Red Frog Rana temporaria. Eur. J. Biochem. 1996, 242, 788–792. [Google Scholar] [CrossRef]
- Argiolas, A.; Pisano, J.J. Isolation and characterization of two new peptides, mastoparan C and crabrolin, from the venom of the European hornet, Vespa crabro. J. Biol. Chem. 1984, 259, 10106–10111. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Rinaldi, A.C.; Di Giulio, A.; Mignogna, G.; Bozzi, A.; Barra, D.; Simmaco, M. Structure–function relationships of temporins, small antimicrobialpeptides from amphibian skin. Eur. J. Biochem. 2000, 267, 1447–1454. [Google Scholar] [CrossRef] [Green Version]
- Mangoni, M.L.; Shai, Y. Temporins and their synergism against Gram-negative bacteria and in lipopolysaccharide detoxification. Biochim. Biophys. Acta-Biomembr. 2009, 1788, 1610–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahalka, A.K.; Kinnunen, P.K.J. Binding of amphipathic α-helical antimicrobial peptides to lipid membranes: Lessons from temporins B and L. Biochim. Biophys. Acta-Biomembr. 2009, 1788, 1600–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakar, A.; Holzknecht, J.; Dubrac, S.; Gelmi, M.L.; Romanelli, A.; Marx, F. New perspectives in the antimicrobial activity of the amphibian temporin b: Peptide analogs are effective inhibitors of Candida albicans growth. J. Fungi 2021, 7, 457. [Google Scholar] [CrossRef]
- Marcocci, M.E.; Amatore, D.; Villa, S.; Casciaro, B.; Aimola, P.; Franci, G.; Grieco, P.; Galdiero, M.; Palamara, A.T.; Mangoni, M.L.; et al. The amphibian antimicrobial peptide temporin b inhibits in vitro herpes simplex virus 1 infection. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro Bastos De França, A.C.; Rocha Soares, K.S.; Da Silva, F.G.; Da Silva, A.D.; Do Rêgo, M.T.; Da Silva, D.P.; De Sousa Ferreira, S.; Furtado, A.A.; Da Silva Júnior, A.A.; De Freitas Fernandes Pedrosa, M. Evaluation of self-assembled Bothrops jararacussu snake venom proteins cross-linked chitosan nanoparticles activity for use as a potential antibacterial. Toxicon 2019, 168, S32–S33. [Google Scholar] [CrossRef]
- Soares, K.; Gláucia-Silva, F.; Daniele-Silva, A.; Torres-Rêgo, M.; Araújo, N.; Menezes, Y.; Damasceno, I.; Tambourgi, D.; da Silva-Júnior, A.; Fernandes-Pedrosa, M. Antivenom Production against Bothrops jararaca and Bothrops erythromelas Snake Venoms Using Cross-Linked Chitosan Nanoparticles as an Immunoadjuvant. Toxins 2018, 10, 158. [Google Scholar] [CrossRef] [Green Version]
- Gláucia-Silva, F.; Torres-Rêgo, M.; Rocha Soares, K.S.; Damasceno, I.Z.; Tambourgi, D.V.; da Silva-Júnior, A.A.; de Freitas Fernandes-Pedrosa, M. A biotechnological approach to immunotherapy: Antivenom against Crotalus durissus cascavella snake venom produced from biodegradable nanoparticles. Int. J. Biol. Macromol. 2018, 120, 1917–1924. [Google Scholar] [CrossRef]
- Attarde, S.S.; Pandit, S.V. In Vivo Toxicity Profile of NN-32 and Nanogold Conjugated GNP-NN-32 from Indian Spectacled Cobra Venom. Curr. Pharm. Biotechnol. 2020, 21, 1479–1488. [Google Scholar] [CrossRef]
- de Pontes, J.T.C.; Toledo Borges, A.B.; Roque-Borda, C.A.; Pavan, F.R. Antimicrobial Peptides as an Alternative for the Eradication of Bacterial Biofilms of Multi-Drug Resistant Bacteria. Pharmaceutics 2022, 14, 642. [Google Scholar] [CrossRef]
- Yu, Q.; Deng, T.; Lin, F.C.; Zhang, B.; Zink, J.I. Supramolecular Assemblies of Heterogeneous Mesoporous Silica Nanoparticles to Co-deliver Antimicrobial Peptides and Antibiotics for Synergistic Eradication of Pathogenic Biofilms. ACS Nano 2020, 14, 5926–5937. [Google Scholar] [CrossRef]
- Santos-Filho, N.A.; Fernandes, R.S.; Sgardioli, B.F.; Ramos, M.A.S.; Piccoli, J.P.; Camargo, I.L.B.C.; Bauab, T.M.; Cilli, E.M. Antibacterial Activity of the Non-Cytotoxic Peptide (p-BthTX-I)2 and Its Serum Degradation Product against Multidrug-Resistant Bacteria. Mol. A J. Synth. Chem. Nat. Prod. Chem. 2017, 22, 1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Baelen, A.-C.; Robin, P.; Kessler, P.; Maïga, A.; Gilles, N.; Servent, D. Structural and Functional Diversity of Animal Toxins Interacting With GPCRs. Front. Mol. Biosci. 2022, 9, 811365. [Google Scholar] [CrossRef] [PubMed]
- Gilles, N.; Servent, D. The European FP7 Venomics Project. Future Med. Chem. 2014, 6, 1611–1612. [Google Scholar] [CrossRef] [Green Version]
- Hunter, H.N.; Jing, W.; Schibli, D.J.; Trinh, T.; Park, I.Y.; Kim, S.C.; Vogel, H.J. The interactions of antimicrobial peptides derived from lysozyme with model membrane systems. Biochim. Biophys. Acta-Biomembr. 2005, 1668, 175–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dathe, M.; Nikolenko, H.; Klose, J.; Bienert, M. Cyclization increases the antimicrobial activity and selectivity of arginine- and tryptophan-containing hexapeptides. Biochemistry 2004, 43, 9140–9150. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Schibli, D.; Vogel, H. Structural studies and model membrane interactions of two peptides derived from bovine lactoferricin. J. Pept. Sci. 2005, 11, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Chau, J.K.; Perry, N.A.; de Boer, L.; Zaat, S.A.J.; Vogel, H.J. Serum Stabilities of Short Tryptophan- and Arginine-Rich Antimicrobial Peptide Analogs. PLoS ONE 2010, 5, e12684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzon, E.N.; Piccoli, J.P.; Santos-Filho, N.A.; Cilli, E.M. Dimerization of Antimicrobial Peptides: A Promising Strategy to Enhance Antimicrobial Peptide Activity. Protein Pept. Lett. 2019, 26, 98. [Google Scholar] [CrossRef]
- Giribaldi, J.; Smith, J.J.; Schroeder, C.I. Recent developments in animal venom peptide nanotherapeutics with improved selectivity for cancer cells. Biotechnol. Adv. 2021, 50, 107769. [Google Scholar] [CrossRef]
- Sadauskas, E.; Wallin, H.; Stoltenberg, M.; Vogel, U.; Doering, P.; Larsen, A.; Danscher, G. Kupffer cells are central in the removal of nanoparticles from the organism. Part. Fibre Toxicol. 2007, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Poon, W.; Zhang, Y.-N.; Ouyang, B.; Kingston, B.R.; Wu, J.L.Y.; Wilhelm, S.; Chan, W.C.W. Elimination Pathways of Nanoparticles. ACS Nano 2019, 13, 5785–5798. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Serrano, S. Approaching the Golden Age of Natural Product Pharmaceuticals from Venom Libraries: An Overview of Toxins and Toxin-Derivatives Currently Involved in Therapeutic or Diagnostic Applications. Curr. Pharm. Des. 2007, 13, 2927–2934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smallwood, T.B.; Clark, R.J. Advances in venom peptide drug discovery: Where are we at and where are we heading? Expert Opin. Drug Discov. 2021, 16, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).