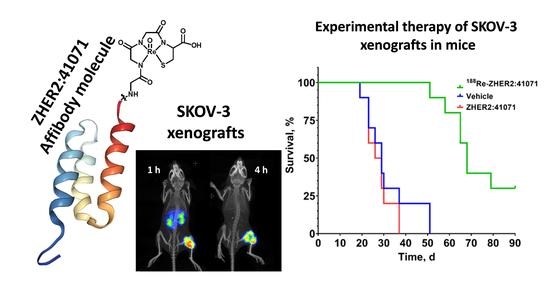
Experimental Therapy of HER2-Expressing Xenografts Using the Second-Generation HER2-Targeting Affibody Molecule 188Re-ZHER2:41071
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Labeling Chemistry
2.3. In Vitro Studies
2.4. In Vivo Studies
2.5. Experimental Radionuclide Therapy
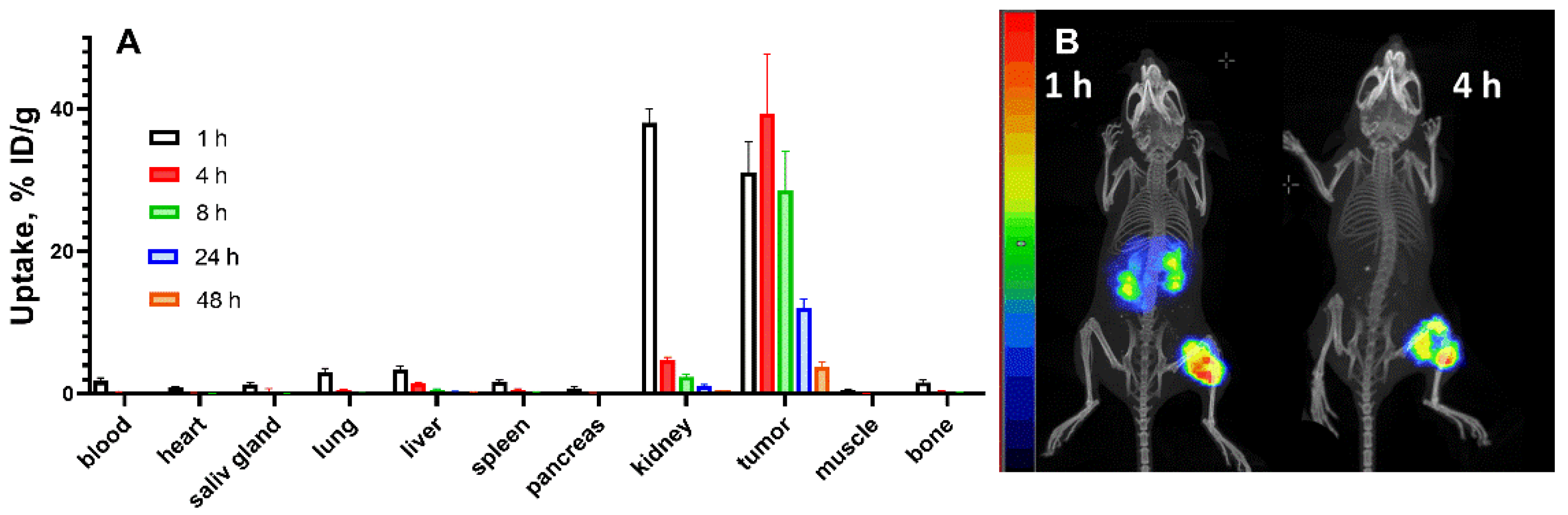
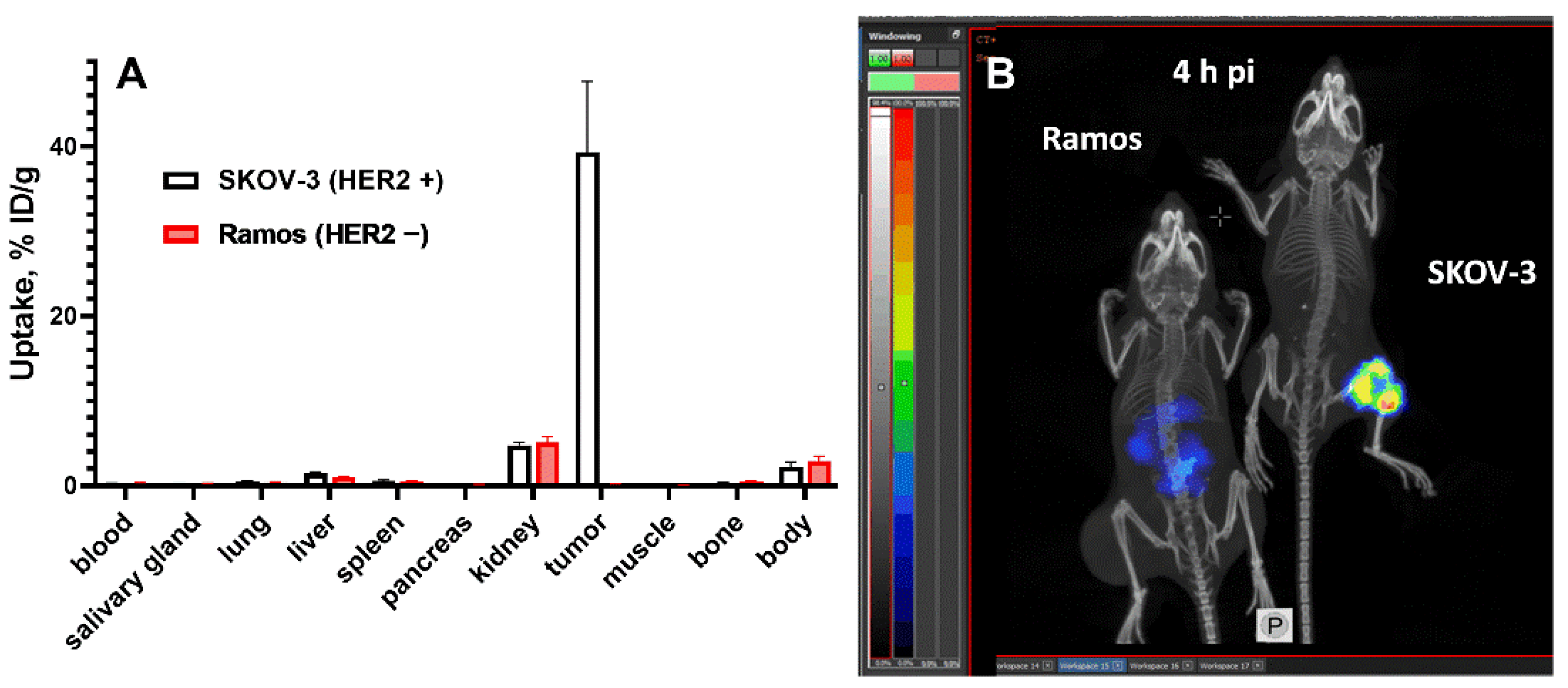
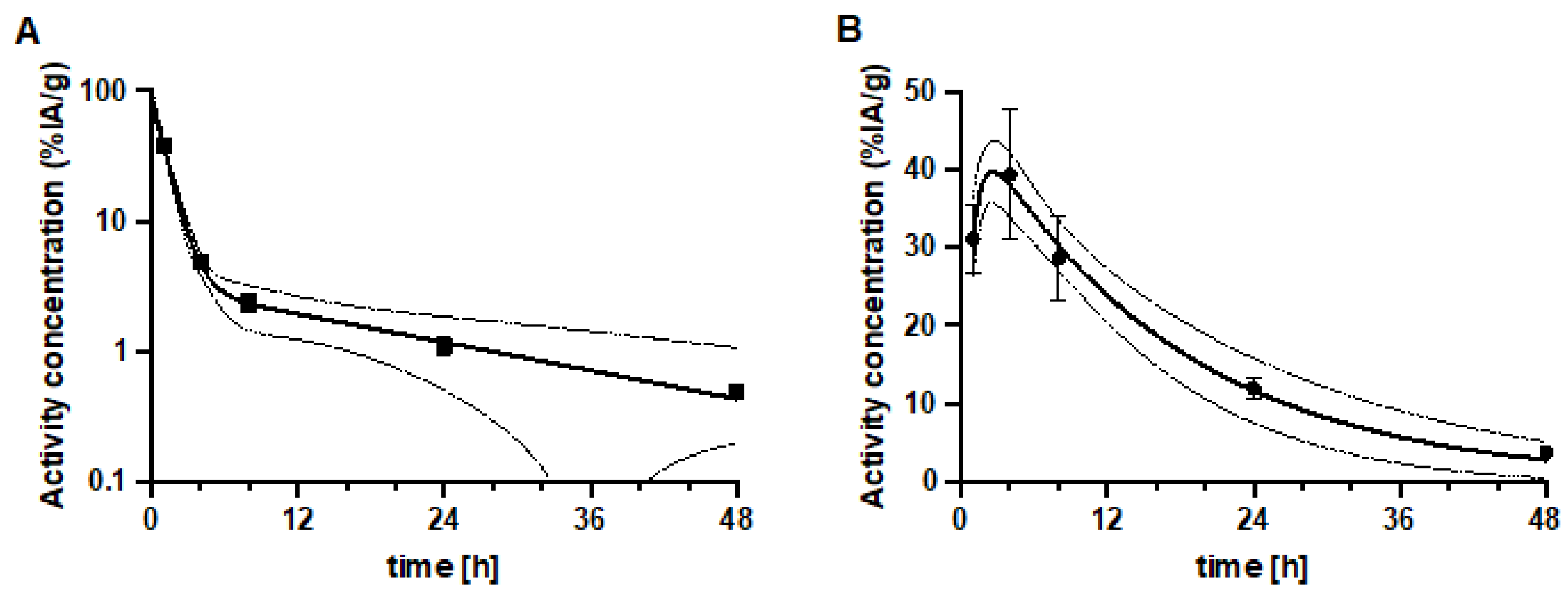
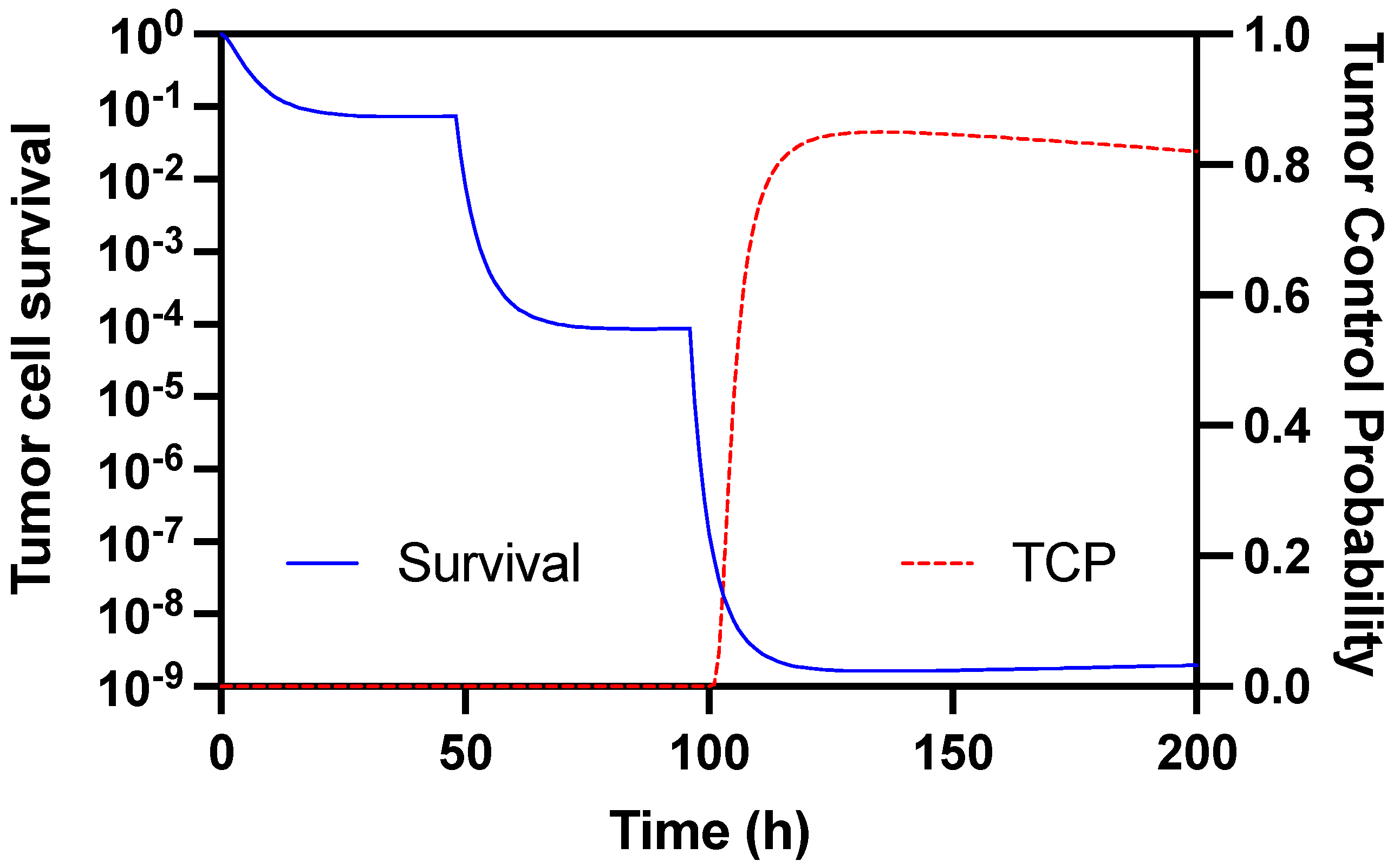
3. Results
3.1. Labeling Chemistry
3.2. In Vitro Studies
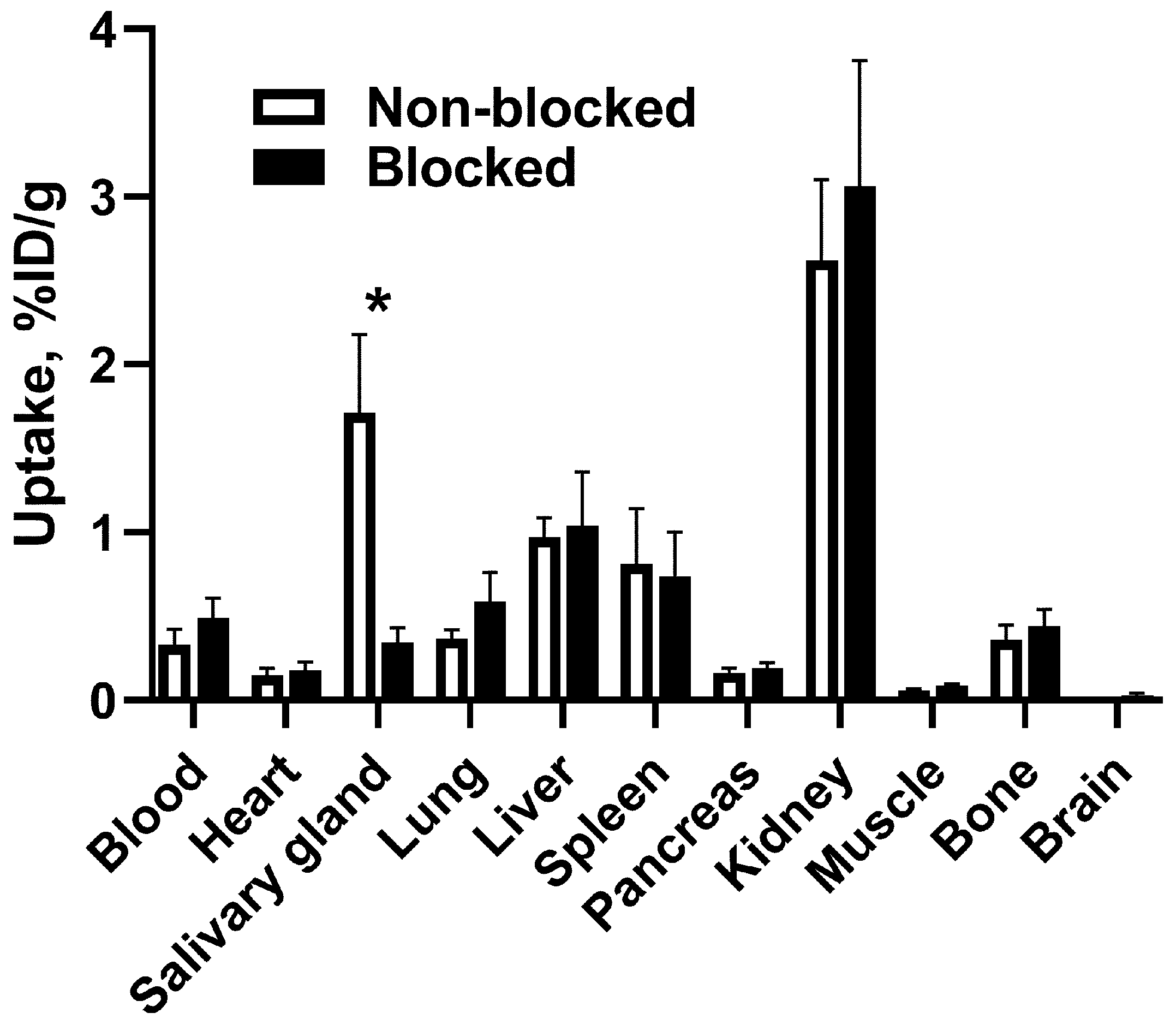
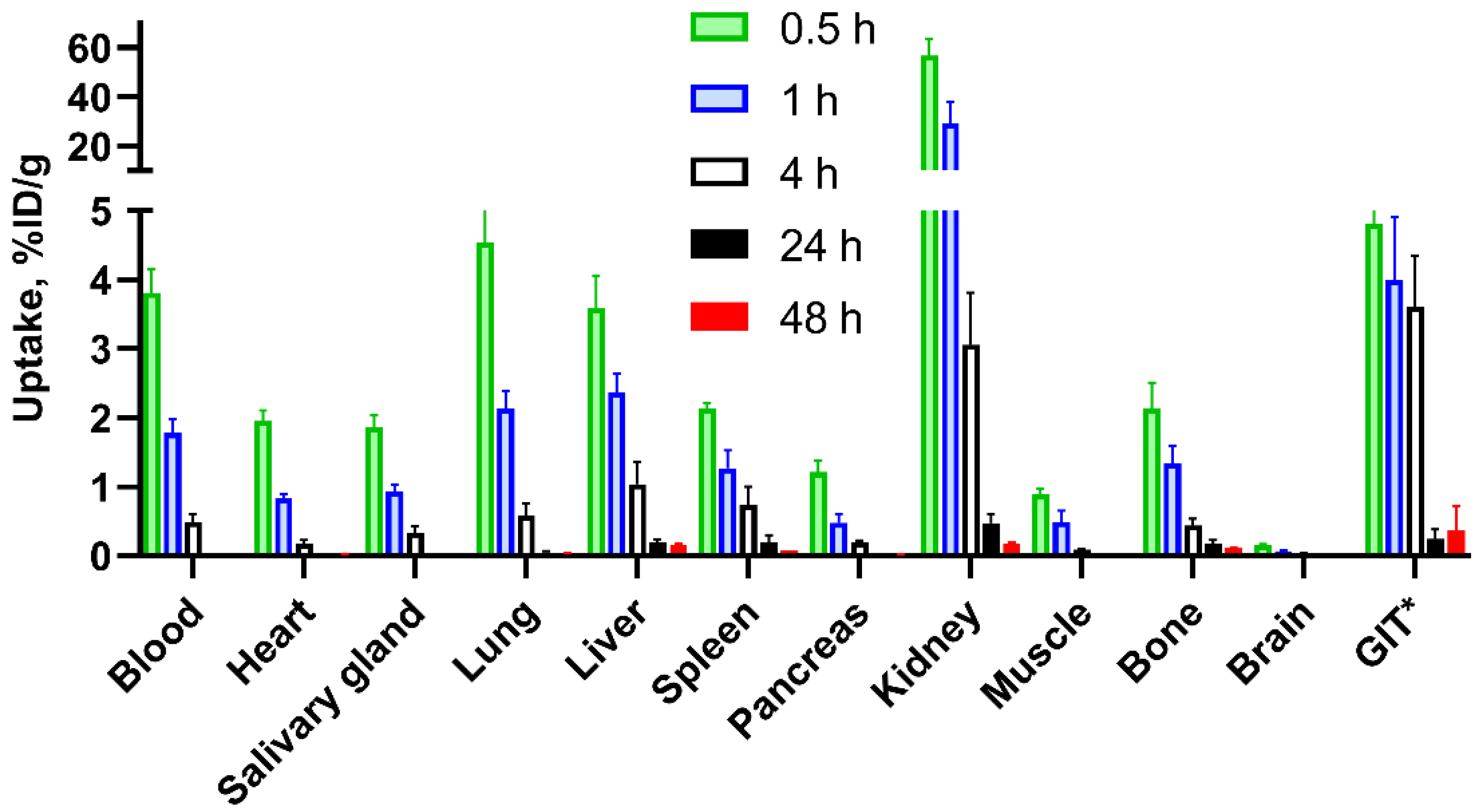
3.3. In Vivo Studies
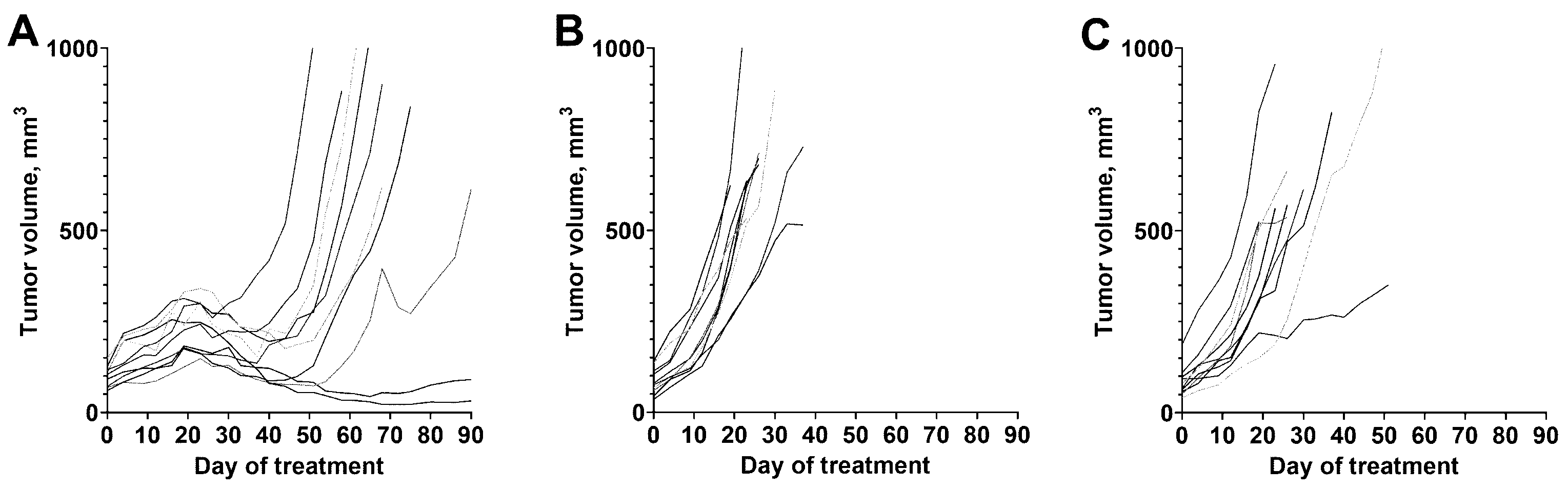
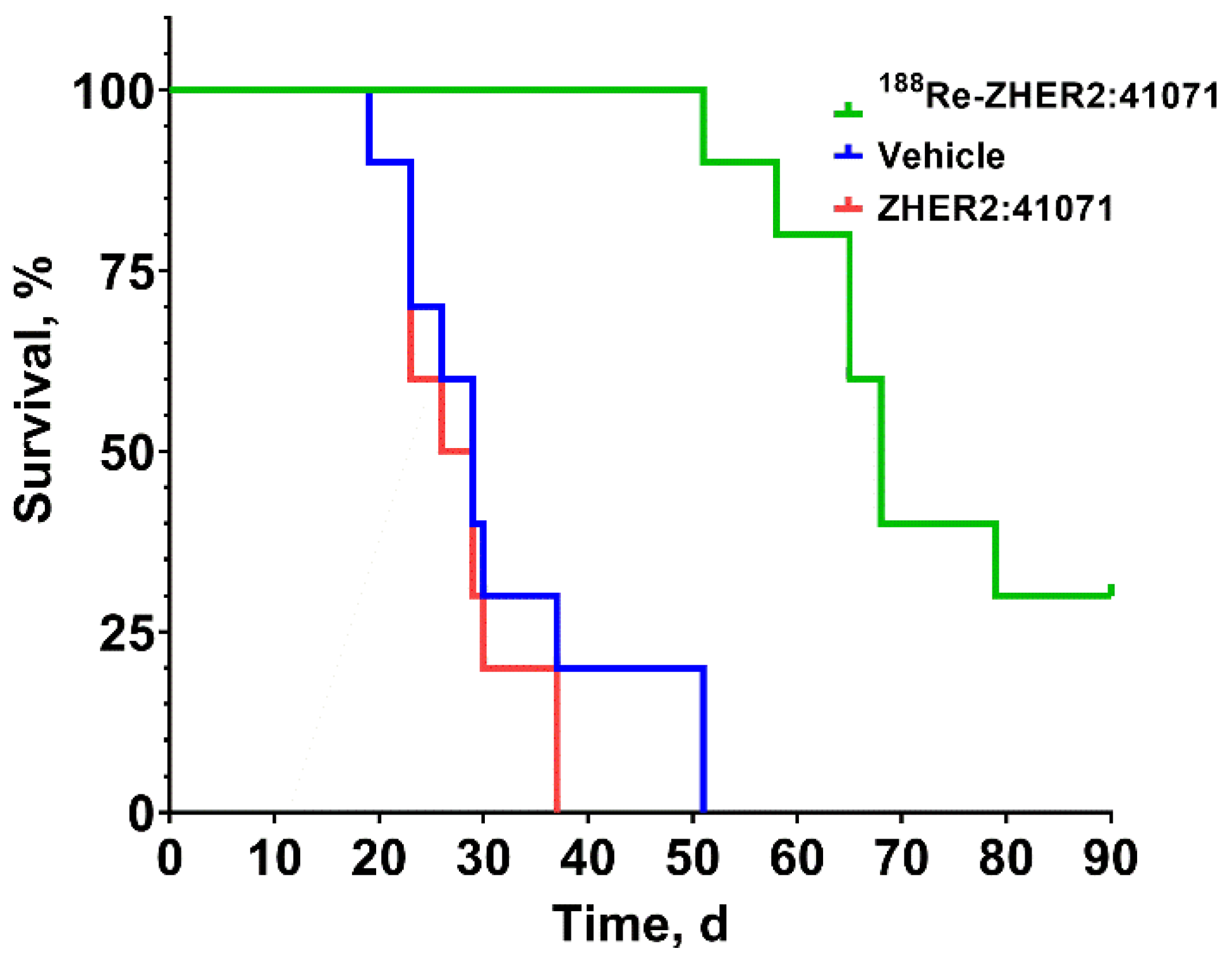
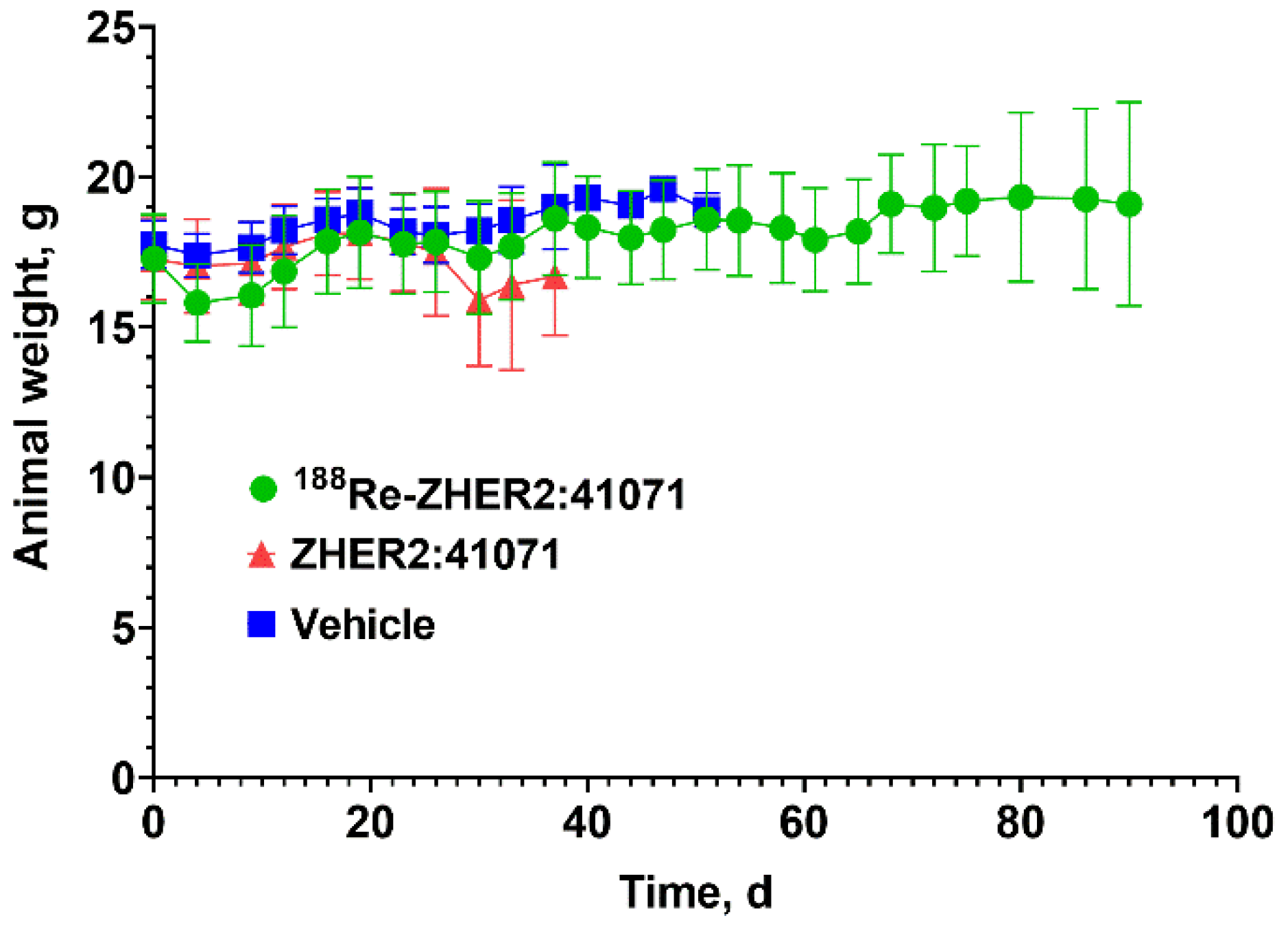
3.4. Experimental Radionuclide Therapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Kim, S.B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Panowski, S.; Bhakta, S.; Raab, H.; Polakis, P.; Junutula, J.R. Site-specific antibody drug conjugates for cancer therapy. mAbs 2014, 6, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.A., 3rd; Rugo, H.S.; Vukelja, S.J.; Vogel, C.L.; Borson, R.A.; Limentani, S.; Tan-Chiu, E.; Krop, I.E.; Michaelson, R.A.; Girish, S.; et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J. Clin. Oncol. 2011, 29, 398–405. [Google Scholar] [CrossRef]
- Krop, I.E.; LoRusso, P.; Miller, K.D.; Modi, S.; Yardley, D.; Rodriguez, G.; Guardino, E.; Lu, M.; Zheng, M.; Girish, S.; et al. A phase II study of trastuzumab emtansine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer who were previously treated with trastuzumab, lapatinib, an anthracycline, a taxane, and capecitabine. J. Clin. Oncol. 2012, 30, 3234–3241. [Google Scholar] [CrossRef]
- Hunter, F.W.; Barker, H.R.; Lipert, B.; Rothé, F.; Gebhart, G.; Piccart-Gebhart, M.J.; Sotiriou, C.; Jamieson, S. Mechanisms of resistance to trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. Br. J. Cancer 2020, 122, 603–612. [Google Scholar] [CrossRef]
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical therapy in cancer: Clinical advances and challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef]
- Ståhl, S.; Gräslund, T.; Eriksson Karlström, A.; Frejd, F.Y.; Nygren, P.Å.; Löfblom, J. Affibody Molecules in Biotechnological and Medical Applications. Trends Biotechnol. 2017, 35, 691–712. [Google Scholar] [CrossRef]
- Tolmachev, V.; Orlova, A. Affibody Molecules as Targeting Vectors for PET Imaging. Cancers 2020, 12, 651. [Google Scholar] [CrossRef]
- Löfblom, J.; Feldwisch, J.; Tolmachev, V.; Carlsson, J.; Ståhl, S.; Frejd, F.Y. Affibody molecules: Engineered proteins for therapeutic, diagnostic and biotechnological applications. FEBS Lett. 2010, 584, 2670–2680. [Google Scholar] [CrossRef] [PubMed]
- Orlova, A.; Magnusson, M.; Eriksson, T.L.; Nilsson, M.; Larsson, B.; Höidén-Guthenberg, I.; Widström, C.; Carlsson, J.; Tolmachev, V.; Ståhl, S.; et al. Tumor imaging using a picomolar affinity HER2 binding affibody molecule. Cancer Res. 2006, 66, 4339–4348. [Google Scholar] [CrossRef] [PubMed]
- Sörensen, J.; Sandberg, D.; Sandström, M.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Åström, G.; Lubberink, M.; Garske-Román, U.; Carlsson, J.; et al. First-in-human molecular imaging of HER2 expression in breast cancer metastases using the 111In-ABY-025 affibody molecule. J. Nucl. Med. 2014, 55, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Sörensen, J.; Velikyan, I.; Sandberg, D.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Orlova, A.; Sandström, M.; Lubberink, M.; Olofsson, H.; et al. Measuring HER2-Receptor Expression In Metastatic Breast Cancer Using [68Ga]ABY-025 Affibody PET/CT. Theranostics 2016, 6, 262–271. [Google Scholar] [CrossRef]
- Fortin, M.A.; Orlova, A.; Malmström, P.U.; Tolmachev, V. Labelling chemistry and characterization of [90Y/177Lu]-DOTA-ZHER2:342-3 Affibody molecule, a candidate agent for locoregional treatment of urinary bladder carcinoma. Int. J. Mol. Med. 2007, 19, 285–291. [Google Scholar] [CrossRef]
- Garousi, J.; Vorobyeva, A.; Altai, M. Influence of Several Compounds and Drugs on the Renal Uptake of Radiolabeled Affibody Molecules. Molecules 2020, 25, 2673. [Google Scholar] [CrossRef]
- Wållberg, H.; Orlova, A. Slow internalization of anti-HER2 synthetic affibody monomer 111In-DOTA-ZHER2:342-pep2: Implications for development of labeled tracers. Cancer Biother. Radiopharm. 2008, 23, 435–442. [Google Scholar] [CrossRef]
- Tolmachev, V.; Mume, E.; Sjöberg, S.; Frejd, F.Y.; Orlova, A. Influence of valency and labelling chemistry on in vivo targeting using radioiodinated HER2-binding Affibody molecules. Eur. J. Nucl. Med. Mol. Imaging 2008, 36, 692–701. [Google Scholar] [CrossRef]
- Wållberg, H.; Orlova, A.; Altai, M.; Hosseinimehr, S.J.; Widström, C.; Malmberg, J.; Ståhl, S.; Tolmachev, V. Molecular design and optimization of 99mTc-labeled recombinant affibody molecules improves their biodistribution and imaging properties. J. Nucl. Med. 2011, 52, 461–469. [Google Scholar] [CrossRef]
- Feldwisch, J.; Tolmachev, V.; Lendel, C.; Herne, N.; Sjöberg, A.; Larsson, B.; Rosik, D.; Lindqvist, E.; Fant, G.; Höidén-Guthenberg, I.; et al. Design of an optimized scaffold for affibody molecules. J. Mol. Biol. 2010, 398, 232–247. [Google Scholar] [CrossRef]
- Oroujeni, M.; Rinne, S.S.; Vorobyeva, A.; Loftenius, A.; Feldwisch, J.; Jonasson, P.; Chernov, V.; Orlova, A.; Frejd, F.Y.; Tolmachev, V. Preclinical Evaluation of 99mTc-ZHER2:41071, a Second-Generation Affibody-Based HER2-Visualizing Imaging Probe with a Low Renal Uptake. Int. J. Mol. Sci. 2021, 22, 2770. [Google Scholar] [CrossRef] [PubMed]
- Lepareur, N.; Lacœuille, F.; Bouvry, C.; Hindré, F.; Garcion, E.; Chérel, M.; Noiret, N.; Garin, E.; Knapp, F., Jr. Rhenium-188 Labeled Radiopharmaceuticals: Current Clinical Applications in Oncology and Promising Perspectives. Front. Med. 2019, 6, 132. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, E.; Libson, K.; Vanderheyden, J.L.; Ketring, A.R.; Maxon, H.R. The chemistry of rhenium and technetium as related to the use of isotopes of these elements in therapeutic and diagnostic nuclear medicine. Int. J. Radiat. Appl. Instrum. Part B Nucl. Med. Biol. 1986, 13, 465–477. [Google Scholar] [CrossRef]
- Ahlgren, S.; Wållberg, H.; Tran, T.A.; Widström, C.; Hjertman, M.; Abrahmsén, L.; Berndorff, D.; Dinkelborg, L.M.; Cyr, J.E.; Feldwisch, J.; et al. Targeting of HER2-expressing tumors with a site-specifically 99mTc-labeled recombinant affibody molecule, ZHER2:2395, with C-terminally engineered cysteine. J. Nucl. Med. 2009, 50, 781–789. [Google Scholar] [CrossRef]
- Tolmachev, V.; Orlova, A.; Andersson, K. Methods for radiolabelling of monoclonal antibodies. Methods Mol. Biol. 2014, 1060, 309–330. [Google Scholar]
- Altschuh, D.; Björkelund, H.; Strandgård, J.; Choulier, L.; Malmqvist, M.; Andersson, K. Deciphering complex protein interaction kinetics using Interaction Map. Biochem. Biophys. Res. Commun. 2012, 428, 74–79. [Google Scholar] [CrossRef]
- Keenan, M.A.; Stabin, M.G.; Segars, W.P.; Fernald, M.J. RADAR realistic animal model series for dose assessment. J. Nucl. Med. 2010, 51, 471–476. [Google Scholar] [CrossRef]
- Andersson, M.; Johansson, L.; Eckerman, K.; Mattsson, S. IDAC-Dose 2.1, an internal dosimetry program for diagnostic nuclear medicine based on the ICRP adult reference voxel phantoms. EJNMMI Res. 2017, 7, 88. [Google Scholar] [CrossRef]
- Tamborino, G.; Nonnekens, J.; De Saint-Hubert, M.; Struelens, L.; Feijtel, D.; de Jong, M.; Konijnenberg, M.W. Dosimetric Evaluation of the Effect of Receptor Heterogeneity on the Therapeutic Efficacy of Peptide Receptor Radionuclide Therapy: Correlation with DNA Damage Induction and In Vivo Survival. J. Nucl. Med. 2022, 63, 100–107. [Google Scholar] [CrossRef]
- Steffen, A.C.; Göstring, L.; Tolmachev, V.; Palm, S.; Stenerlöw, B.; Carlsson, J. Differences in radiosensitivity between three HER2 overexpressing cell lines. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1179–1191. [Google Scholar] [CrossRef]
- Altai, M.; Wållberg, H.; Honarvar, H.; Strand, J.; Orlova, A.; Varasteh, Z.; Sandström, M.; Löfblom, J.; Larsson, E.; Strand, S.E.; et al. 188Re-ZHER2:V2, a promising affibody-based targeting agent against HER2-expressing tumors: Preclinical assessment. J. Nucl. Med. 2014, 55, 1842–1848. [Google Scholar] [CrossRef] [PubMed]
- Altai, M.; Honarvar, H.; Wållberg, H.; Strand, J.; Varasteh, Z.; Rosestedt, M.; Orlova, A.; Dunås, F.; Sandström, M.; Löfblom, J.; et al. Selection of an optimal cysteine-containing peptide-based chelator for labeling of affibody molecules with (188)Re. Eur. J. Med. Chem. 2014, 87, 519–528. [Google Scholar] [CrossRef]
- Barta, P.; Malmberg, J.; Melicharova, L.; Strandgård, J.; Orlova, A.; Tolmachev, V.; Laznicek, M.; Andersson, K. Protein interactions with HER-family receptors can have different characteristics depending on the hosting cell line. Int. J. Oncol. 2012, 40, 1677–1682. [Google Scholar] [PubMed]
- Zuckier, L.S.; Dohan, O.; Li, Y.; Chang, C.J.; Carrasco, N.; Dadachova, E. Kinetics of perrhenate uptake and comparative biodistribution of perrhenate, pertechnetate, and iodide by NaI symporter-expressing tissues in vivo. J. Nucl. Med. 2004, 45, 500–507. [Google Scholar] [PubMed]
- Giammarile, F.; Chiti, A.; Lassmann, M.; Brans, B.; Flux, G.; EANM. EANM procedure guidelines for 131I-meta-iodobenzylguanidine (131I-mIBG) therapy. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Steel, G.G. Dose fractionation in radiotherapy. In Handbook of Radiotherapy Physics—Theory and Practice; Mayles, P., Nahum, A.E., Rosenwald, J.-C., Eds.; Taylor and Francis: London, UK, 2007; pp. 163–177. [Google Scholar]
- Ilan, E.; Sandström, M.; Wassberg, C.; Sundin, A.; Garske-Román, U.; Eriksson, B.; Granberg, D.; Lubberink, M. Dose response of pancreatic neuroendocrine tumors treated with peptide receptor radionuclide therapy using 177Lu-DOTATATE. J. Nucl. Med. 2015, 56, 177–182. [Google Scholar] [CrossRef]
- Westerlund, K.; Altai, M.; Mitran, B.; Konijnenberg, M.; Oroujeni, M.; Atterby, C.; de Jong, M.; Orlova, A.; Mattsson, J.; Micke, P.; et al. Radionuclide Therapy of HER2-Expressing Human Xenografts Using Affibody-Based Peptide Nucleic Acid-Mediated Pretargeting: In Vivo Proof of Principle. J. Nucl. Med. 2018, 59, 1092–1098. [Google Scholar] [CrossRef]
- Mitran, B.; Rinne, S.S.; Konijnenberg, M.W.; Maina, T.; Nock, B.A.; Altai, M.; Vorobyeva, A.; Larhed, M.; Tolmachev, V.; de Jong, M.; et al. Trastuzumab cotreatment improves survival of mice with PC-3 prostate cancer xenografts treated with the GRPR antagonist 177 Lu-DOTAGA-PEG2 -RM26. Int. J. Cancer 2019, 145, 3347–3358. [Google Scholar] [CrossRef] [PubMed]
- Garousi, J.; von Witting, E.; Borin, J.; Vorobyeva, A.; Altai, M.; Vorontsova, O.; Konijnenberg, M.W.; Oroujeni, M.; Orlova, A.; Tolmachev, V.; et al. Radionuclide therapy using ABD-fused ADAPT scaffold protein: Proof of Principle. Biomaterials 2021, 266, 120381. [Google Scholar] [CrossRef]
- Sandström, M.; Ilan, E.; Karlberg, A.; Johansson, S.; Freedman, N.; Garske-Román, U. Method dependence, observer variability and kidney volumes in radiation dosimetry of (177)Lu-DOTATATE therapy in patients with neuroendocrine tumours. EJNMMI Phys. 2015, 2, 2. [Google Scholar] [CrossRef][Green Version]

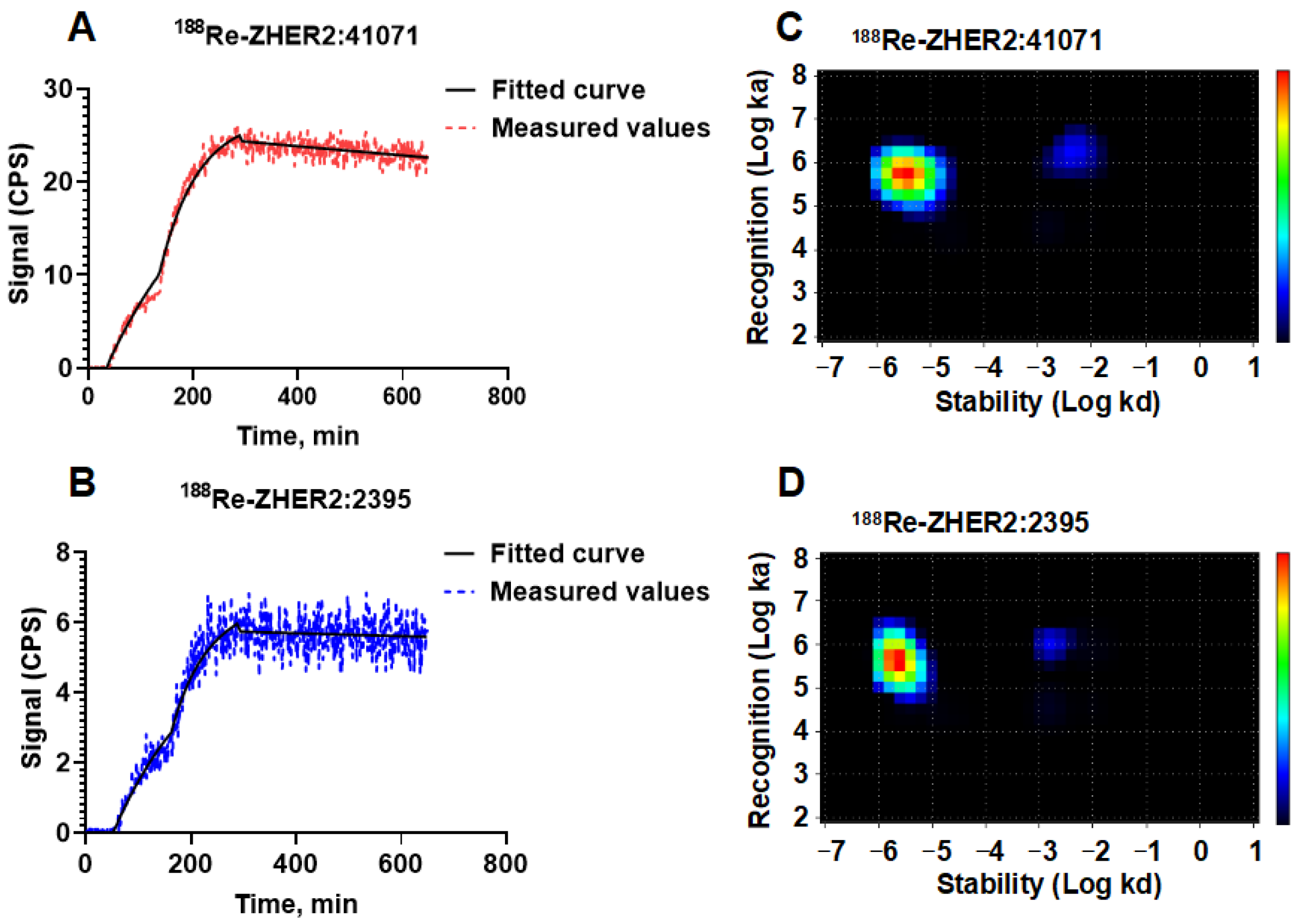


| Interaction | ka (M−1s−1) | kd (s−1) | KD (M) | Weight (%) |
|---|---|---|---|---|
| 188Re-ZHER2:41071 | ||||
| 1 | [5.7 ± 0.4] × 105 | [2.6 ± 1.3] × 10−6 | [5 ± 3] × 10−12 | 76 |
| 2 | [1.3 ± 0.3] × 106 | [5.5 ± 3.5] × 10−4 | [5 ± 4] × 10−9 | 6 |
| 188Re-ZHER2:2395 | ||||
| 1 | [5.4 ± 2.0] × 105 | [3.5 ± 1.5] × 10−6 | [7 ± 4] × 10−12 | 75 |
| 2 | [7.7 ± 6.5] × 105 | [2.5 ± 1.5] × 10−3 | [27 ± 18] × 10−9 | 8 |
| Organ | Absorbed Dose (mGy/MBq) | Organ | Absorbed Dose (mGy/MBq) |
|---|---|---|---|
| Adrenals | 0.007 | Muscle | 0.005 |
| Brain | 0.001 | Ovaries | 0.007 |
| Breasts | 0.007 | Pancreas | 0.010 |
| Gallbladder Wall | 0.007 | Red Marrow | 0.012 |
| Lower Large Intestines Wall | 0.227 | Osteogenic Cells | 0.062 |
| Small Intestine | 0.044 | Skin | 0.006 |
| Stomach Wall | 0.208 | Spleen | 0.020 |
| Upper Large Intestines Wall | 0.007 | Thymus | 0.007 |
| Heart Wall | 0.131 | Thyroid | 0.007 |
| Kidneys | 0.244 | Urinary Bladder Wall | 0.007 |
| Liver | 0.029 | Uterus | 0.007 |
| Lungs | 0.031 | Total Body | 0.015 |
| Effective Dose | 0.0683 (mSv/MBq) | ||
| Organ | TIACC (h/g) | Absorbed Dose per IA (mGy/MBq) | Absorbed Dose per 16 MBq (Gy) | Absorbed Dose after 3 Cycles of 16 MBq (Gy) |
|---|---|---|---|---|
| Brain | 0.0024 | 8 | 0.1 | 0.4 |
| Large Intestines | 0.0318 | 31 | 0.5 | 1.5 |
| Small Intestine | 0.0443 | 29 | 0.5 | 1.4 |
| Stomach Wall | 0.0329 | 40 | 0.6 | 1.9 |
| Heart | 0.0296 | 29 | 0.5 | 1.4 |
| Kidneys | 1.3013 | 341 | 5.4 | 16.3 |
| Liver | 0.1835 | 69 | 1.1 | 3.3 |
| Lungs | 0.0997 | 34 | 0.5 | 1.6 |
| Pancreas | 0.0234 | 43 | 0.7 | 2.1 |
| Skeleton | 0.0823 | 39 | 0.6 | 1.9 |
| Spleen | 0.0863 | 40 | 0.6 | 1.9 |
| Thyroid | 0.0457 | 16 | 0.3 | 0.8 |
| Total Body | 0.0157 | 34 | 0.5 | 1.6 |
| Tumor (100 mg) | 4.47 | 1014 | 16.2 | 48.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Vorobyeva, A.; Orlova, A.; Konijnenberg, M.W.; Xu, T.; Bragina, O.; Loftenius, A.; Rosander, E.; Frejd, F.Y.; Tolmachev, V. Experimental Therapy of HER2-Expressing Xenografts Using the Second-Generation HER2-Targeting Affibody Molecule 188Re-ZHER2:41071. Pharmaceutics 2022, 14, 1092. https://doi.org/10.3390/pharmaceutics14051092
Liu Y, Vorobyeva A, Orlova A, Konijnenberg MW, Xu T, Bragina O, Loftenius A, Rosander E, Frejd FY, Tolmachev V. Experimental Therapy of HER2-Expressing Xenografts Using the Second-Generation HER2-Targeting Affibody Molecule 188Re-ZHER2:41071. Pharmaceutics. 2022; 14(5):1092. https://doi.org/10.3390/pharmaceutics14051092
Chicago/Turabian StyleLiu, Yongsheng, Anzhelika Vorobyeva, Anna Orlova, Mark W. Konijnenberg, Tianqi Xu, Olga Bragina, Annika Loftenius, Erica Rosander, Fredrik Y. Frejd, and Vladimir Tolmachev. 2022. "Experimental Therapy of HER2-Expressing Xenografts Using the Second-Generation HER2-Targeting Affibody Molecule 188Re-ZHER2:41071" Pharmaceutics 14, no. 5: 1092. https://doi.org/10.3390/pharmaceutics14051092
APA StyleLiu, Y., Vorobyeva, A., Orlova, A., Konijnenberg, M. W., Xu, T., Bragina, O., Loftenius, A., Rosander, E., Frejd, F. Y., & Tolmachev, V. (2022). Experimental Therapy of HER2-Expressing Xenografts Using the Second-Generation HER2-Targeting Affibody Molecule 188Re-ZHER2:41071. Pharmaceutics, 14(5), 1092. https://doi.org/10.3390/pharmaceutics14051092