Vehicle-Free Nanotheranostic Self-Assembled from Clinically Approved Dyes for Cancer Fluorescence Imaging and Photothermal/Photodynamic Combinational Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
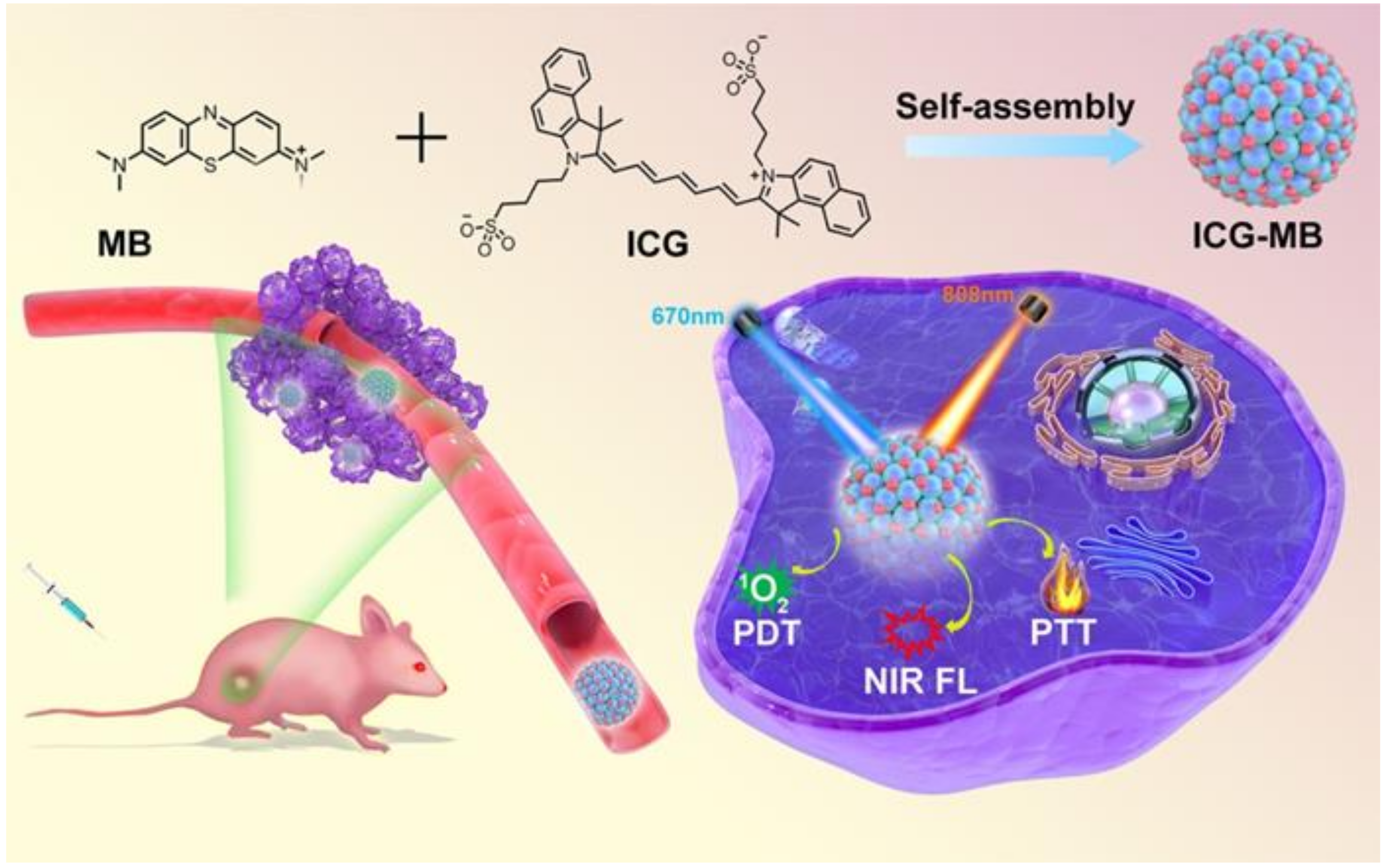
2.3. Synthesis of ICG-MB NPs
2.4. Characterization of ICG-MB NPs
2.5. Evaluation of the Photothermal Ability of ICG-MB NPs
2.6. Photodynamic Performance of ICG-MB NPs
2.7. Cellular Internalization
2.8. Intracellular ROS Detection
2.9. In Vitro Cytotoxicity Assay
2.10. In Vitro Cell Killing Ability
2.11. Animal Experiments
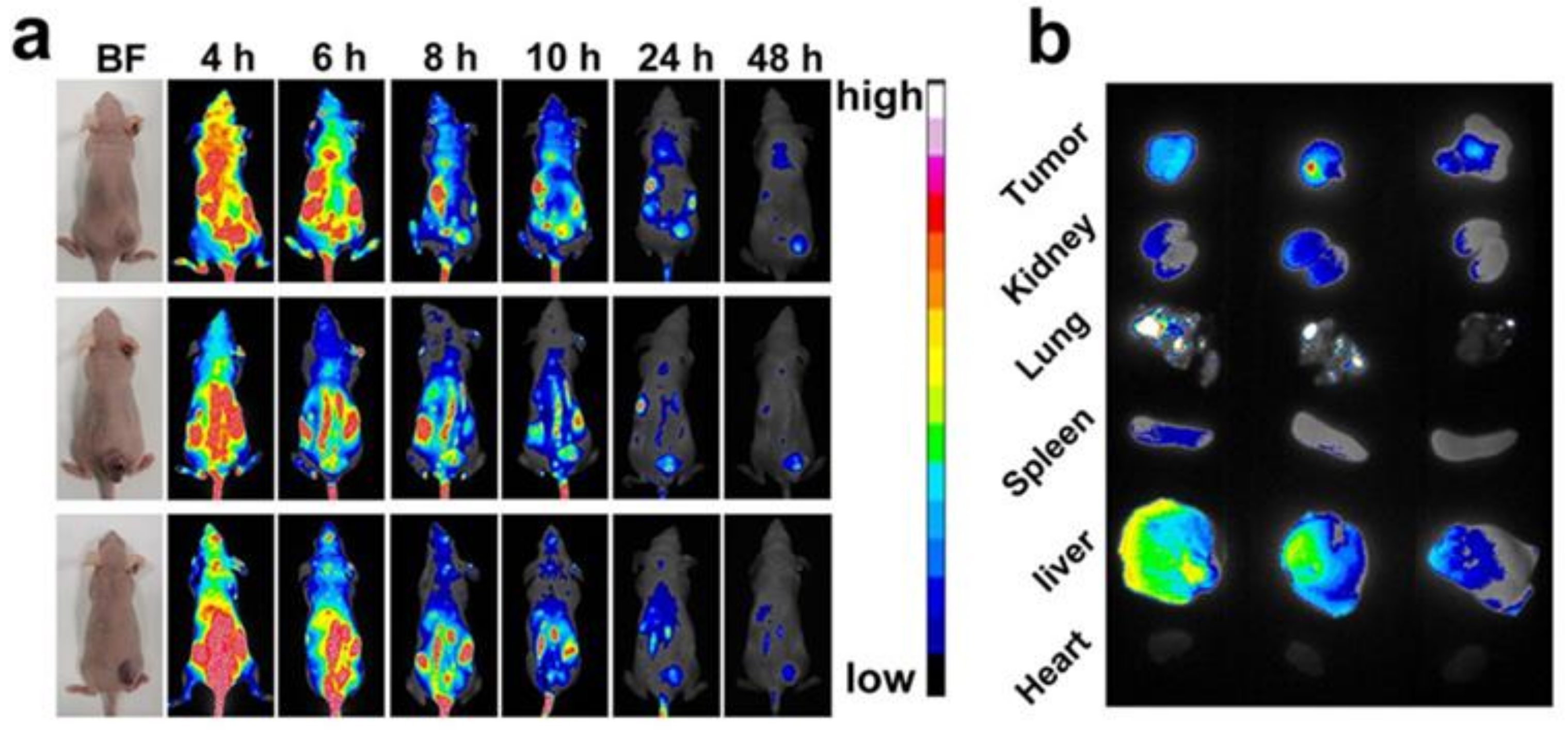
2.12. Biodistribution of ICG-MB NPs
2.13. In Vivo Anti-tumor Ability of ICG-MB NPs
2.14. Statistical Analysis
3. Results and Discussion
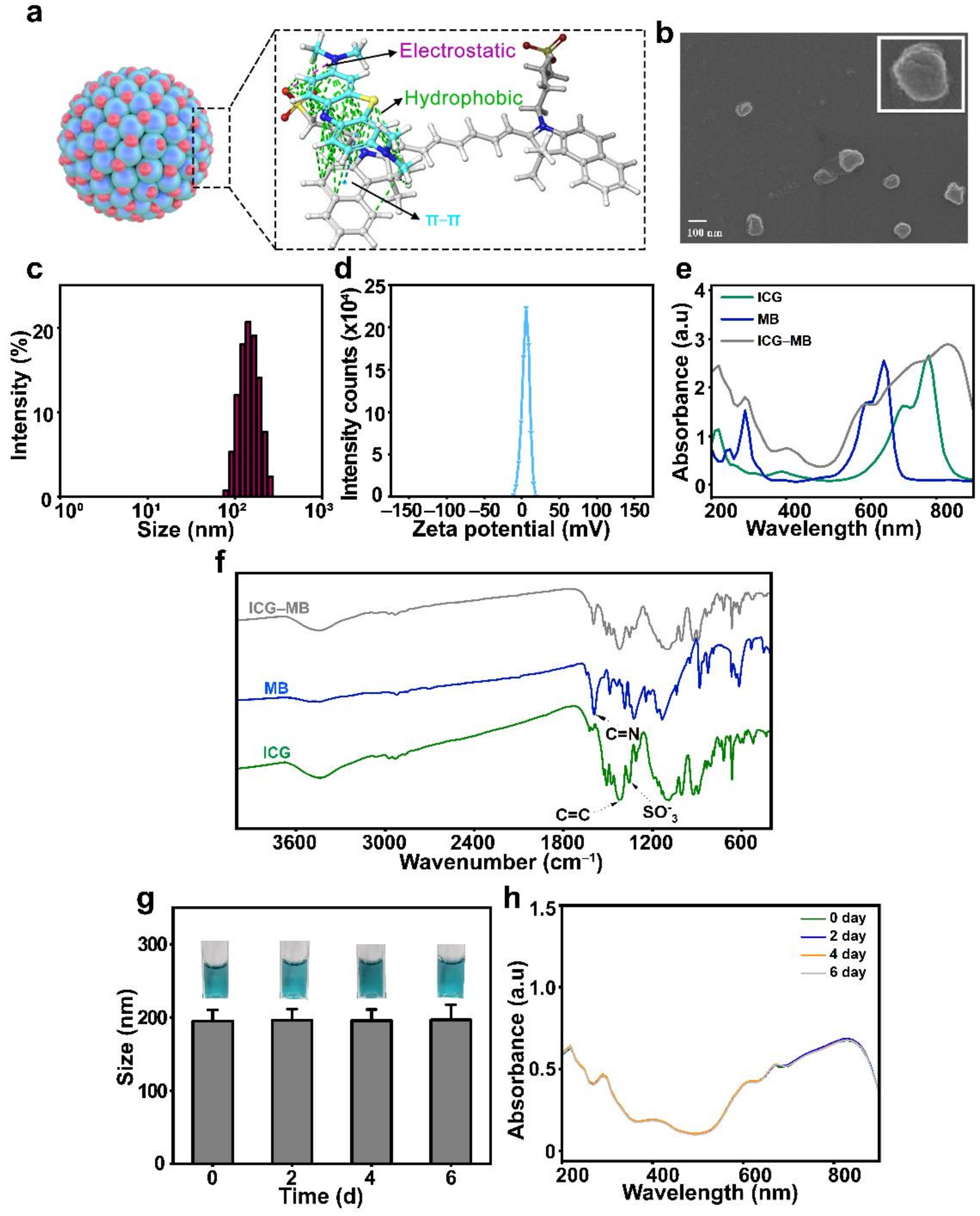
3.1. Synthesis and Characterization of ICG-MB NPs
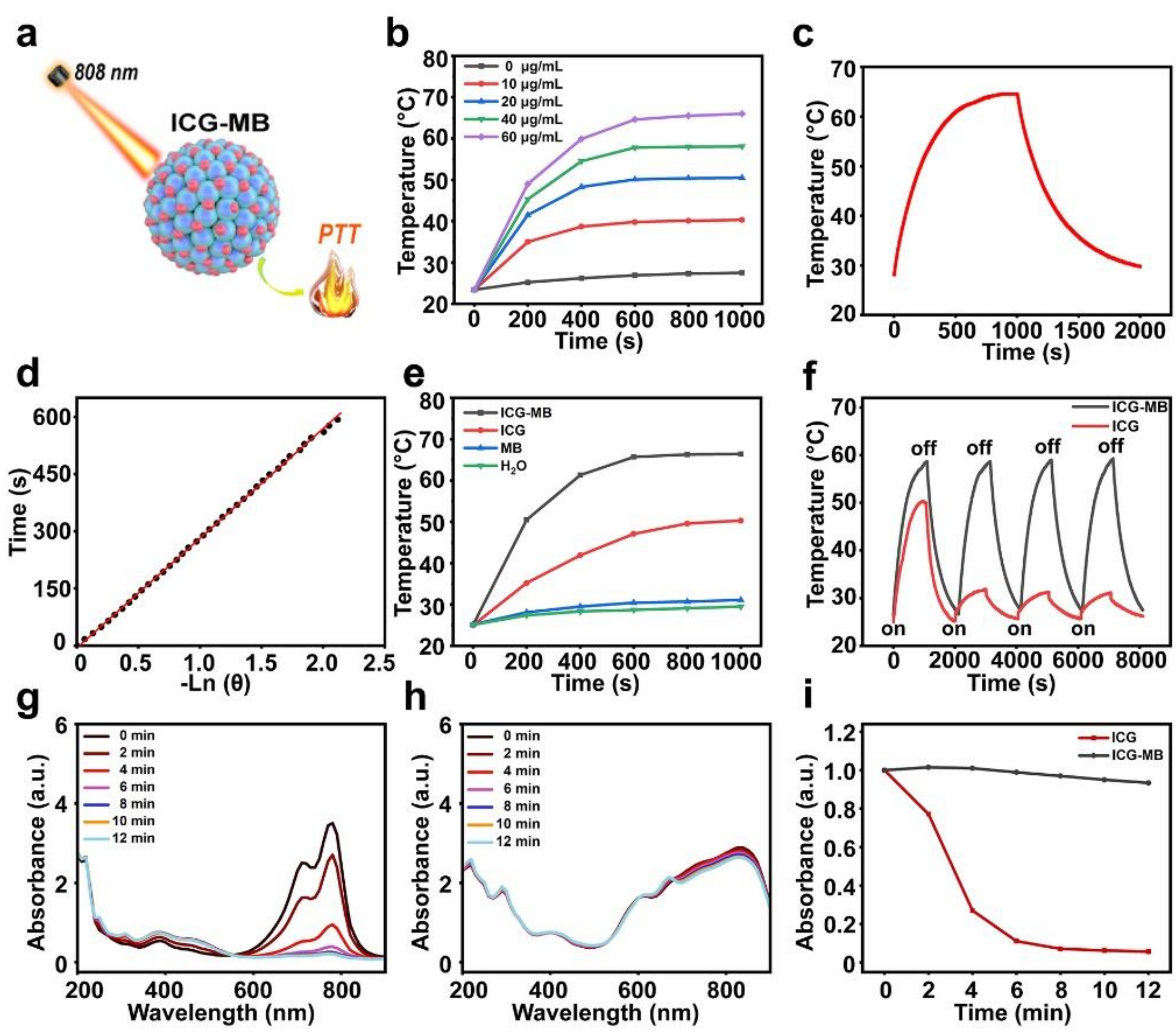
3.2. Photothermal Properties of ICG-MB
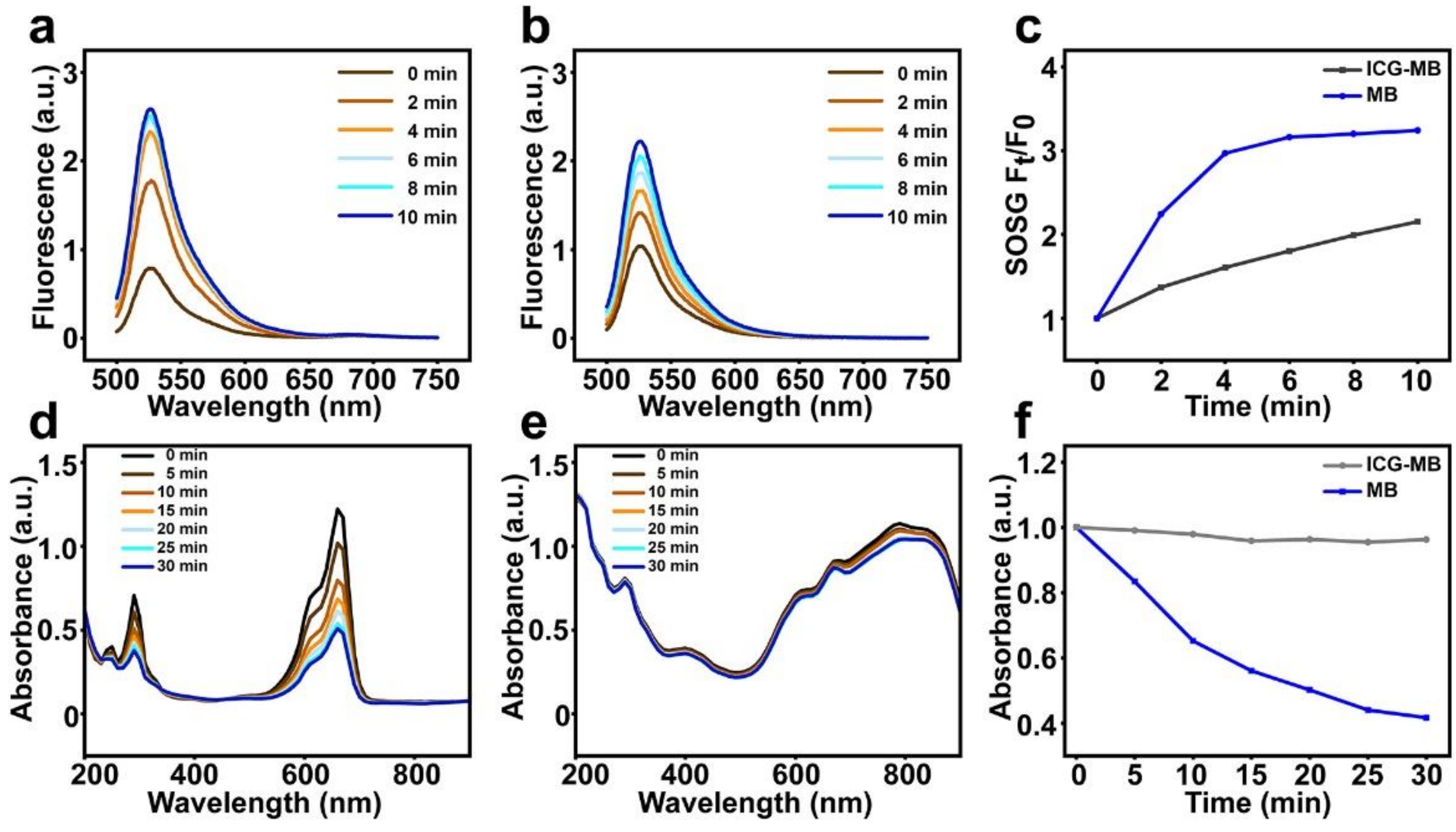
3.3. Photodynamic Properties of ICG-MB
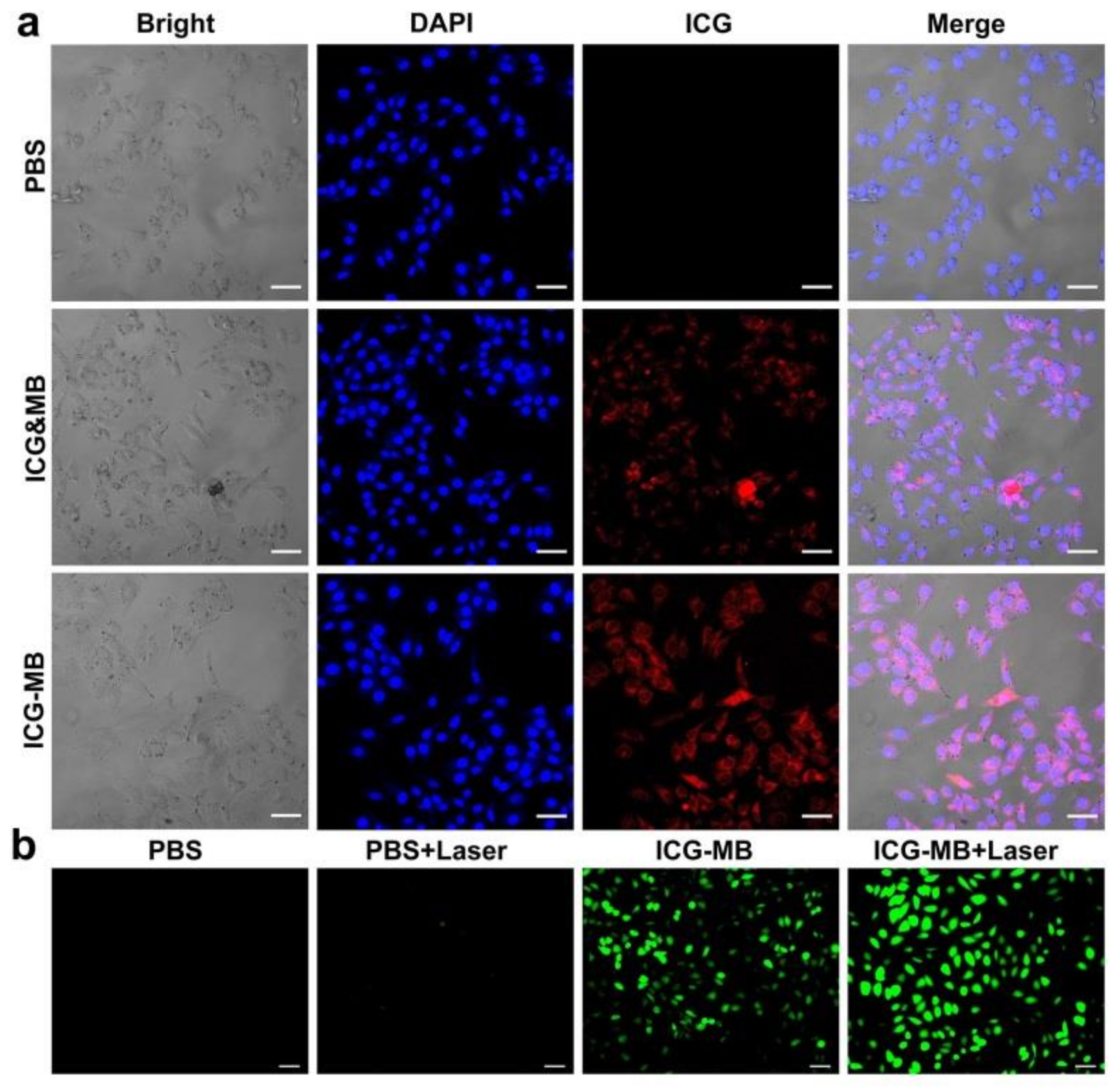
3.4. Intracellular Internalization and ROS Production
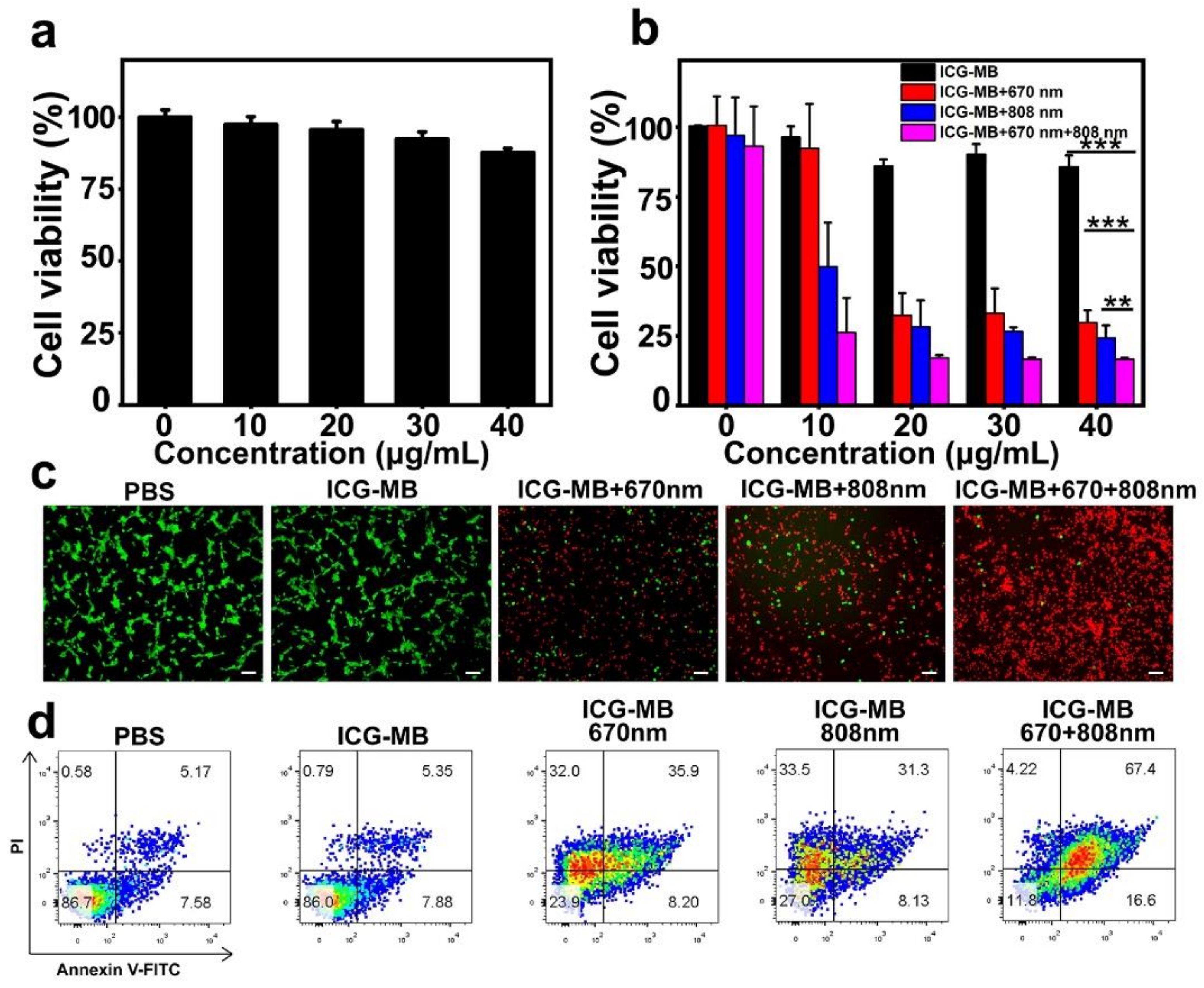
3.5. In Vitro Cell Cytotoxicity and Phototherapy Effect
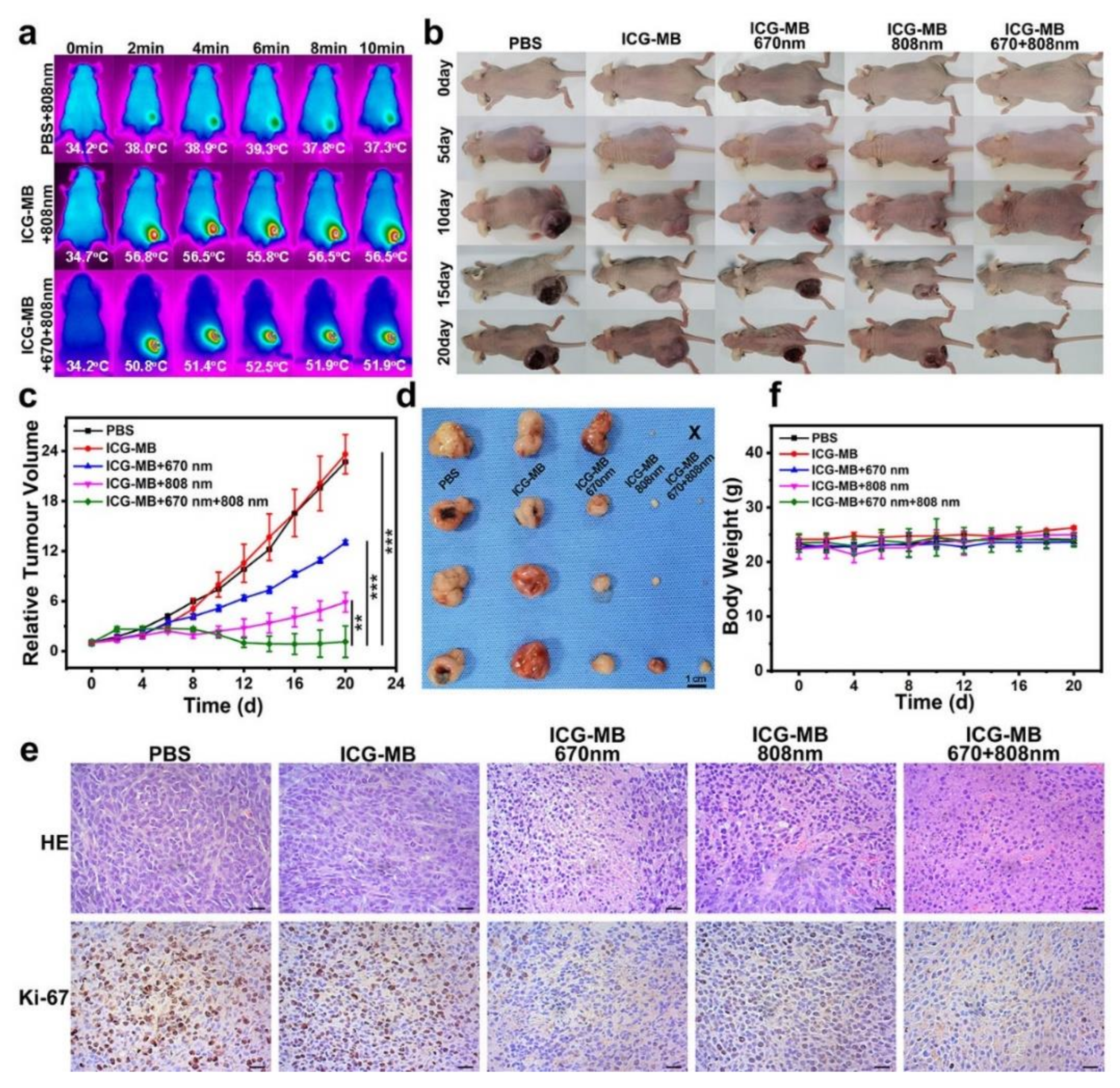
3.6. In Vivo Phototherapy Effect
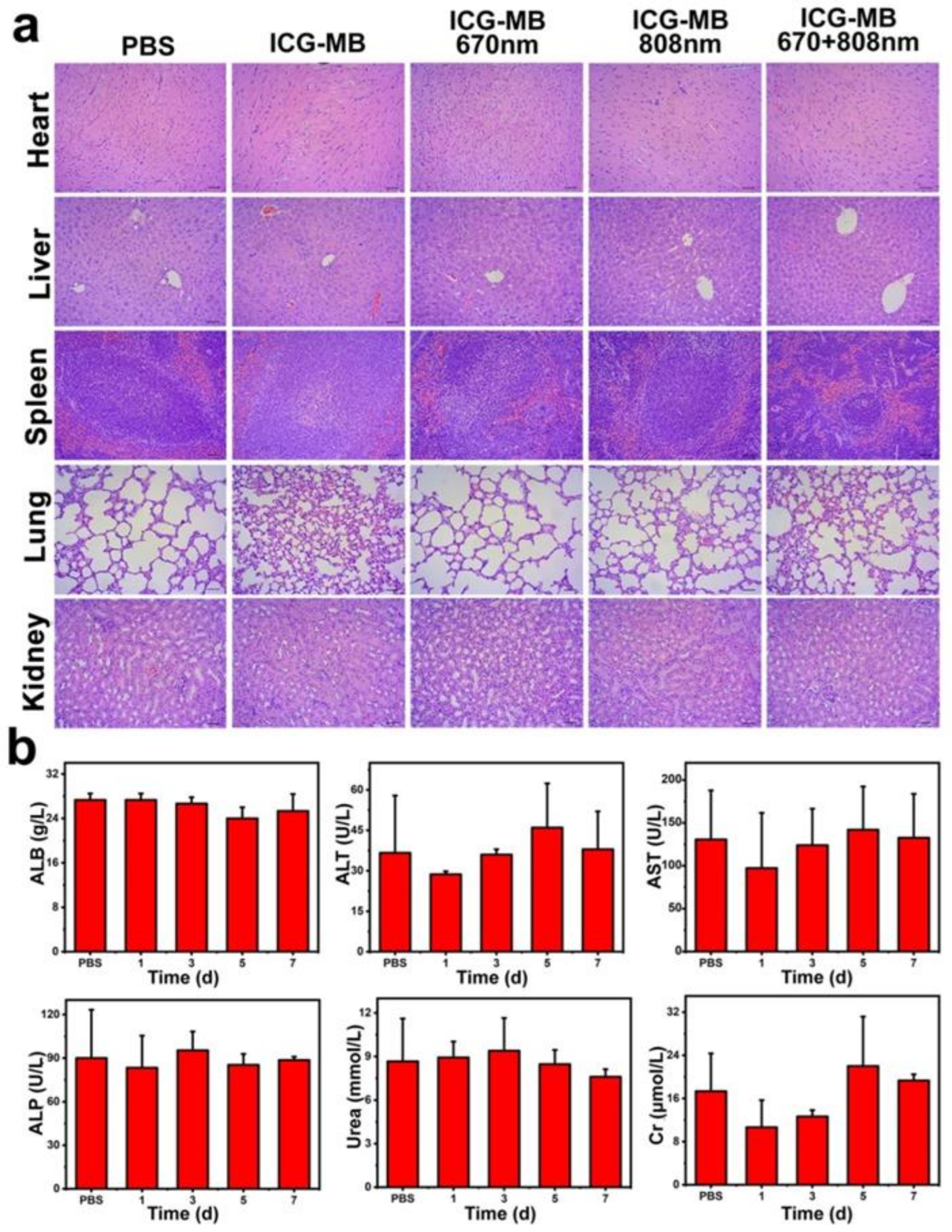
3.7. Biocompatibility Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Wain, S.L.; Kier, R.; Vollmer, R.T.; Bossen, E.H. Basaloid-squamous carcinoma of the tongue, hypopharynx, and larynx: Report of 10 cases. Hum. Pathol. 1986, 17, 1158–1166. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Lordick, F.; Siewert, J.R. Recent advances in multimodal treatment for gastric cancer: A review. Gastric Cancer 2005, 8, 78–85. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bale, R.; Putzer, D.; Schullian, P. Local treatment of breast cancer liver metastasis. Cancers 2019, 11, 1341. [Google Scholar] [CrossRef]
- Connell, P.P.; Hellman, S. Advances in radiotherapy and implications for the next century: A historical perspective. Cancer Res. 2009, 69, 383–392. [Google Scholar] [CrossRef]
- Thangudu, S.; Kaur, N.; Korupalli, C.; Sharma, V.; Kalluru, P.; Vankayala, R. Recent advances in near infrared light responsive multi-functional nanostructures for phototheranostic applications. Biomater. Sci. 2021, 9, 5472–5483. [Google Scholar] [CrossRef]
- Hiremath, N.; Kumar, R.; Hwang, K.C.; Banerjee, I.; Thangudu, S.; Vankayala, R. Near-infrared light activatable two-dimensional nanomaterials for theranostic applications: A comprehensive review. ACS Appl. Nano Mater. 2022, 5, 1719–1733. [Google Scholar] [CrossRef]
- Wei, Z.; Wu, M.; Lan, S.; Li, J.; Zhang, X.; Zhang, D.; Liu, X.; Liu, J. Semiconducting polymer-based nanoparticles for photothermal therapy at the second near-infrared window. Chem. Commun. 2018, 54, 13599–13602. [Google Scholar] [CrossRef]
- Wei, Z.; Xin, F.; Zhang, J.; Wu, M.; Qiu, T.; Lan, Y.; Qiao, S.; Liu, X.; Liu, J. Donor-acceptor conjugated polymer-based nanoparticles for highly effective photoacoustic imaging and photothermal therapy in the NIR-II window. Chem. Commun. 2020, 56, 1093–1096. [Google Scholar] [CrossRef]
- Qiao, S.; Xin, F.; Wu, M.; Zheng, Y.; Zhao, B.; Zhang, C.; Liu, X.; Wei, Z.; Liu, J. A remotely controlled NIR-II photothermal-sensitive transgene system for hepatocellular carcinoma synergistic therapy. J. Mater. Chem. B 2021, 9, 5083–5091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zheng, A.; Li, J.; Wu, M.; Cai, Z.; Wu, L.; Wei, Z.; Yang, H.; Liu, X.; Liu, J. Tumor microenvironment activable self-assembled DNA hybrids for pH and redox dual-responsive chemotherapy/PDT treatment of jepatocellular carcinoma. Adv. Sci 2017, 4, 1600460. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zou, Y.; He, H.; Rao, J.; Ji, S.; Cui, X.; Ke, H.; Deng, Y.; Yang, H.; Chen, C.; et al. Bifunctional platinated nanoparticles for photoinduced tumor ablation. Adv. Mater. 2016, 28, 10155–10164. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, J.; Nie, W.; Wang, S.; Liu, G.; Cai, K. Recent progress in the development of multifunctional nanoplatform for precise tumor phototherapy. Adv. Healthc. Mater. 2021, 10, 2001207. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wu, Y.; Wu, M.; Zhao, Q.; Li, H.; Liu, J.; Liu, X.; Zhang, X.; Zeng, Y. Engineered red blood cell biomimetic nanovesicle with oxygen self-supply for near-infrared-II fluorescence-guided synergetic chemo-photodynamic therapy against hypoxic tumors. ACS Appl. Mater. Interfaces 2021, 13, 52435–52449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zheng, Y.; Lin, Z.; Lan, S.; Zhang, X.; Zheng, A.; Li, J.; Liu, G.; Yang, H.; Liu, X. Artificial engineered natural killer cells combined with antiheat endurance as a powerful strategy for enhancing photothermal-immunotherapy efficiency of solid tumors. Small 2019, 15, 1902636. [Google Scholar] [CrossRef]
- Guan, Q.; Zhou, L.-L.; Li, Y.-A.; Dong, Y.-B. Diiodo-bodipy-encapsulated nanoscale metal-organic framework for pH-driven selective and mitochondria targeted photodynamic therapy. Inorg. Chem. 2018, 57, 10137–10145. [Google Scholar] [CrossRef]
- Yan, N.; Wang, X.J.; Lin, L.; Song, T.J.; Sun, P.J.; Tian, H.Y.; Liang, H.J.; Chen, X.S. Gold nanorods electrostatically binding nucleic acid probe for in vivo microRNA amplified detection and photoacoustic imaging-guided photothermal therapy. Adv. Funct. Mater. 2018, 28, 1800490. [Google Scholar] [CrossRef]
- Mao, F.; Wen, L.; Sun, C.; Zhang, S.; Wang, G.; Zeng, J.; Wang, Y.; Ma, J.; Gao, M.; Li, Z. Ultrasmall biocompatible Bi2Se3 nanodots for multimodal imaging-guided synergistic radiophotothermal therapy against cancer. ACS Nano 2016, 10, 11145–11155. [Google Scholar] [CrossRef]
- Hagan, S.A.; Coombes, A.G.A.; Garnett, M.C.; Dunn, S.E.; Davies, M.C.; Illum, L.; Davis, S.S.; Harding, S.E.; Purkiss, S.; Gellert, P.R. Polylactide-poly(ethylene glycol) copolymers as drug delivery systems. 1. Characterization of water dispersible micelle-forming systems. Langmuir 1996, 12, 2153–2161. [Google Scholar] [CrossRef]
- Zhao, L.P.; Zheng, R.R.; Chen, H.Q.; Liu, L.S.; Zhao, X.Y.; Liu, H.H.; Qiu, X.Z.; Yu, X.Y.; Cheng, H.; Li, S.-Y. Self-delivery nanomedicine for O2-economized photodynamic tumor therapy. Nano Lett. 2020, 20, 2062–2071. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, J.; Cai, Z.; Wang, P.; Luo, Q.; Yao, C.; Zhang, Y.; Hou, Z.; Liu, J.; Liu, X. Tumor microenvironment-activated self-recognizing nanodrug through directly tailored assembly of small-molecules for targeted synergistic chemotherapy. J. Control. Release 2020, 321, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, M.; Cai, Z.; Liao, N.; Ke, K.; Liu, H.; Li, M.; Liu, G.; Yang, H.; Liu, X.; et al. Chemotherapeutic drug based metal-organic particles for microvesicle-mediated deep penetration and programmable pH/NIR/hypoxia activated cancer photochemotherapy. Adv. Sci. 2018, 5, 1700648. [Google Scholar] [CrossRef]
- van Manen, L.; Handgraaf, H.J.; Diana, M.; Dijkstra, J.; Ishizawa, T.; Vahrmeijer, A.L.; Mieog, J.S.D. A practical guide for the use of indocyanine green and methylene blue in fluorescence-guided abdominal surgery. J. Surg. Oncol. 2018, 118, 283–300. [Google Scholar] [CrossRef]
- Fang, S.; Lin, J.; Li, C.; Huang, P.; Hou, W.; Zhang, C.; Liu, J.; Huang, S.; Luo, Y.; Fan, W.; et al. Dual-stimuli responsive nanotheranostics for multimodal imaging guided trimodal synergistic therapy. Small 2017, 13, 1602580. [Google Scholar] [CrossRef]
- Park, T.; Lee, S.; Amatya, R.; Cheong, H.; Moon, C.; Kwak, H.D.; Min, K.A.; Shin, M.C. ICG-loaded PEGylated BSA-silver nanoparticles for effective photothermal cancer therapy. Int. J. Nanomed. 2020, 15, 5459–5471. [Google Scholar] [CrossRef]
- Beytollahi, L.; Pourhajibagher, M.; Chiniforush, N.; Ghorbanzadeh, R.; Raoofian, R.; Pourakbari, B.; Bahador, A. The efficacy of photodynamic and photothermal therapy on biofilm formation of streptococcus mutans: An in vitro study. Photodiagn. Photodyn. Ther. 2017, 17, 56–60. [Google Scholar] [CrossRef]
- Ren, S.; Cheng, X.; Chen, M.; Liu, C.; Zhao, P.; Huang, W.; He, J.; Zhou, Z.; Miao, L. Hypotoxic and rapidly metabolic PEG-PCL-C3-ICG nanoparticles for fluorescence-guided photothermal/photodynamic therapy against OSCC. ACS Appl. Mater. Interfaces 2017, 9, 31509–31518. [Google Scholar] [CrossRef]
- Campu, A.; Focsan, M.; Lerouge, F.; Borlan, R.; Tie, L.; Rugina, D.; Astilean, S. ICG-loaded gold nano-bipyramids with NIR activatable dual PTT-PDT therapeutic potential in melanoma cells. Colloids Surf. B 2020, 194, 111213. [Google Scholar] [CrossRef]
- dos Santos, A.F.; de Almeida, D.R.Q.; Terra, L.F.; Wailemann, R.A.M.; Gomes, V.M.; Arini, G.S.; Ravagnani, F.G.; Baptista, M.S.; Labriola, L. Fluence rate determines PDT efficiency in breast cancer cells displaying different GSH levels. Photochem. Photobiol. 2020, 96, 658–667. [Google Scholar] [CrossRef]
- dos Santos, A.F.; Terra, L.F.; Wailemann, R.A.M.; Oliveira, T.C.; Gomes, V.d.M.; Mineiro, M.F.; Meotti, F.C.; Bruni-Cardoso, A.; Baptista, M.S.; Labriola, L. Methylene blue photodynamic therapy induces selective and massive cell death in human breast cancer cells. BMC Cancer 2017, 17, 194. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, Q.; Zhang, D.; Liao, N.; Wu, L.; Huang, A.; Liu, X. Magnetite nanocluster@poly(dopamine)-PEG@indocyanine green nanobead with magnetic field-targeting enhanced MR imaging and photothermal therapy in vivo. Colloids Surf. B 2016, 141, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, D.; Wu, M.; Liu, Y.; Zhang, X.; Li, L.; Li, Z.; Han, X.; Wei, X.; Liu, X. Lipid-AuNPs@ PDA nanohybrid for MRI/CT imaging and photothermal therapy of hepatocellular carcinoma. ACS Appl. Mater. Interfaces 2014, 6, 14266–14277. [Google Scholar] [CrossRef] [PubMed]
- Jesus, V.; Raniero, L.; Lemes, G.; Bhattacharjee, T.; Júnior, P.C.; Castilho, M. Nanoparticles of methylene blue enhance photodynamic therapy. Photodiagn. Photodyn. Ther. 2018, 23, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Deng, F.A.; Zheng, R.R.; Liu, L.S.; Liu, Y.B.; Kong, R.J.; Chen, A.L.; Yu, X.Y.; Li, S.Y.; Cheng, H. Carrier free photodynamic synergists for oxidative damage amplified tumor therapy. Small 2021, 17, 2102470. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Hou, M.; Gao, Y.; Zhang, L.; Xu, Z.; Kang, Y.; Xue, P. Indocyanine green-modified hollow mesoporous Prussian blue nanoparticles loading doxorubicin for fluorescence-guided tri-modal combination therapy of cancer. Nanoscale 2019, 11, 5717–5731. [Google Scholar] [CrossRef]
- Wojtoniszak, M.; Rogińska, D.; Machaliński, B.; Drozdzik, M.; Mijowska, E. Graphene oxide functionalized with methylene blue and its performance in singlet oxygen generation. Mater. Res. Bull. 2013, 48, 2636–2639. [Google Scholar] [CrossRef]
- Zeng, J.; Goldfeld, D.; Xia, Y. A plasmon-assisted optofluidic (PAOF) system for measuring the photothermal conversion efficiencies of gold nanostructures and controlling an electrical switch. Angew. Chem. Int. Ed. 2013, 125, 4263–4267. [Google Scholar] [CrossRef]
- Hessel, C.M.; Pattani, V.P.; Rasch, M.; Panthani, M.G.; Koo, B.; Tunnell, J.W.; Korgel, B.A. Copper selenide nanocrystals for photothermal therapy. Nano Lett. 2011, 11, 2560–2566. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, X.; Yan, L.; Zhou, L.; Tian, G.; Yin, W.; Wang, L.; Liu, Y.; Hu, Z.; Gu, Z. Bismuth sulfide nanorods as a precision nanomedicine for in vivo multimodal imaging-guided photothermal therapy of tumor. ACS Nano 2015, 9, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, S.; Lin, X.; Cao, Y.; Cai, Z.; Wang, J.; Zhang, Z.; Liu, X.; Wu, M.; Yao, C. Red blood cell-mimic nanocatalyst triggering radical storm to augment cancer immunotherapy. Nano-Micro Lett. 2022, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.D.; Nishiyama, N.; Zhang, G.D.; Harada, A.; Jiang, D.L.; Kawauchi, S.; Morimoto, Y.; Kikuchi, M.; Koyama, H.; Aida, T. Supramolecular nanocarrier of anionic dendrimer porphyrins with cationic block copolymers modified with polyethylene glycol to enhance intracellular photodynamic efficacy. Angew. Chem. Int. Ed. 2005, 44, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhao, Y.; Yang, F.; Lou, X.; Wu, F.; Li, H.; Xing, X.; Peng, T.; Menze, B.; Huang, J.; et al. Preoperative prediction of lymph node metastasis in colorectal cancer with deep learning. BME Front. 2022, 2022, 9860179. [Google Scholar] [CrossRef]
- Jian, W.H.; Yu, T.W.; Chen, C.J.; Huang, W.C.; Chiu, H.C.; Chiang, W.-H. Indocyanine green-encapsulated hybrid polymeric nanomicelles for photothermal cancer therapy. Langmuir 2015, 31, 6202–6210. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Xu, C.; Yang, S.; Zhang, Z.; Wei, Z.; Wu, M.; Xue, F. Vehicle-Free Nanotheranostic Self-Assembled from Clinically Approved Dyes for Cancer Fluorescence Imaging and Photothermal/Photodynamic Combinational Therapy. Pharmaceutics 2022, 14, 1074. https://doi.org/10.3390/pharmaceutics14051074
Huang M, Xu C, Yang S, Zhang Z, Wei Z, Wu M, Xue F. Vehicle-Free Nanotheranostic Self-Assembled from Clinically Approved Dyes for Cancer Fluorescence Imaging and Photothermal/Photodynamic Combinational Therapy. Pharmaceutics. 2022; 14(5):1074. https://doi.org/10.3390/pharmaceutics14051074
Chicago/Turabian StyleHuang, Mingbin, Chao Xu, Sen Yang, Ziqian Zhang, Zuwu Wei, Ming Wu, and Fangqin Xue. 2022. "Vehicle-Free Nanotheranostic Self-Assembled from Clinically Approved Dyes for Cancer Fluorescence Imaging and Photothermal/Photodynamic Combinational Therapy" Pharmaceutics 14, no. 5: 1074. https://doi.org/10.3390/pharmaceutics14051074
APA StyleHuang, M., Xu, C., Yang, S., Zhang, Z., Wei, Z., Wu, M., & Xue, F. (2022). Vehicle-Free Nanotheranostic Self-Assembled from Clinically Approved Dyes for Cancer Fluorescence Imaging and Photothermal/Photodynamic Combinational Therapy. Pharmaceutics, 14(5), 1074. https://doi.org/10.3390/pharmaceutics14051074





