A Novel CXCR4-Targeted Diphtheria Toxin Nanoparticle Inhibits Invasion and Metastatic Dissemination in a Head and Neck Squamous Cell Carcinoma Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production, Purification, and Characterization of Nanoparticles
2.2. Cell Lines and Culture
2.3. In Vivo Experiments
2.4. Histopathology, Immunofluorescence, and Immunohistochemical Analysis
2.5. Statistical Analysis
3. Results
3.1. CXCR4+ Tumor Cells Are Enriched in the Tumor Budding in a HNSCC Mouse Model
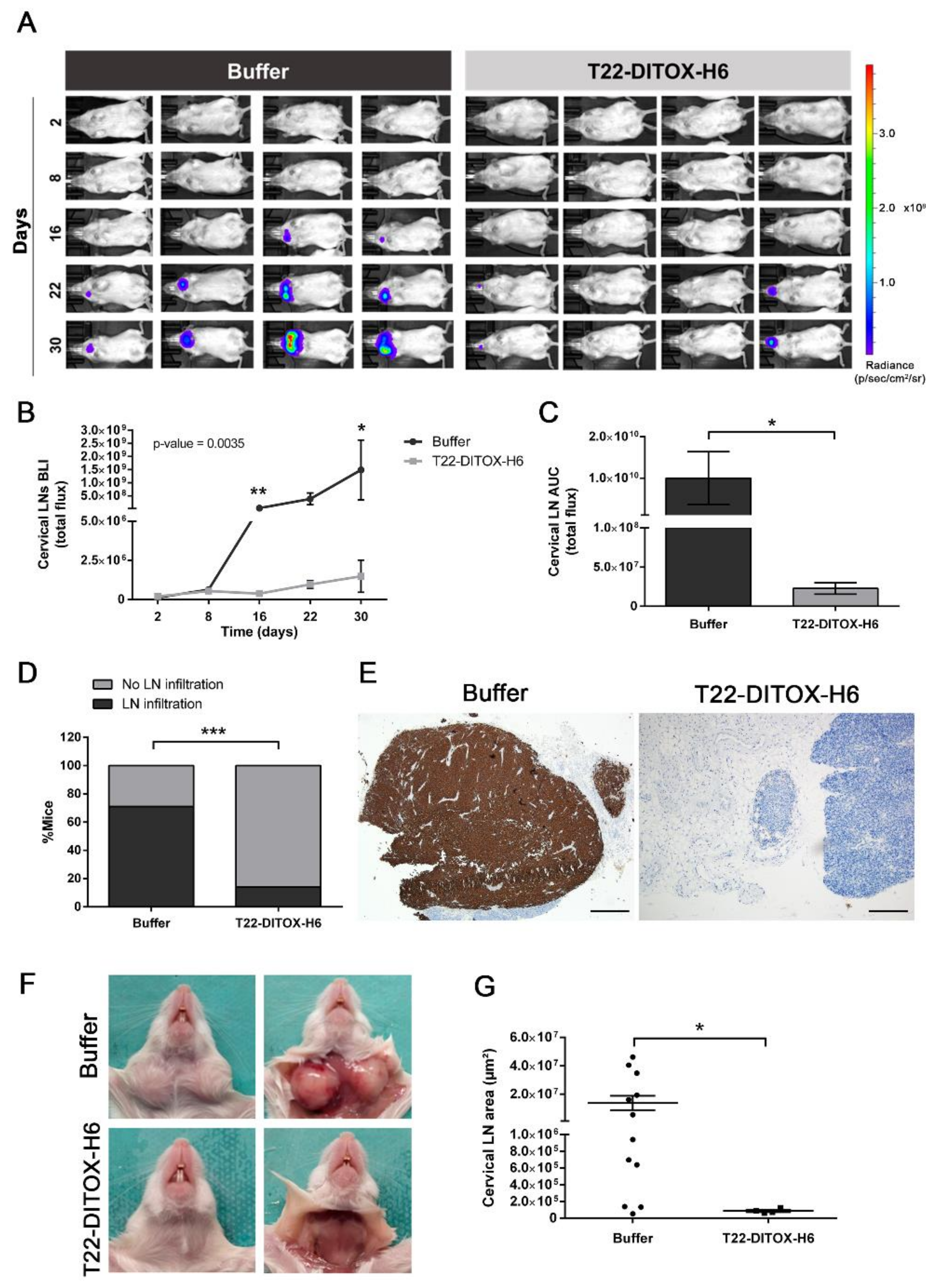
3.2. T22-DITOX-H6 Nanotoxin Treatment Abrogates Tumor-Cell Invasion In Vivo
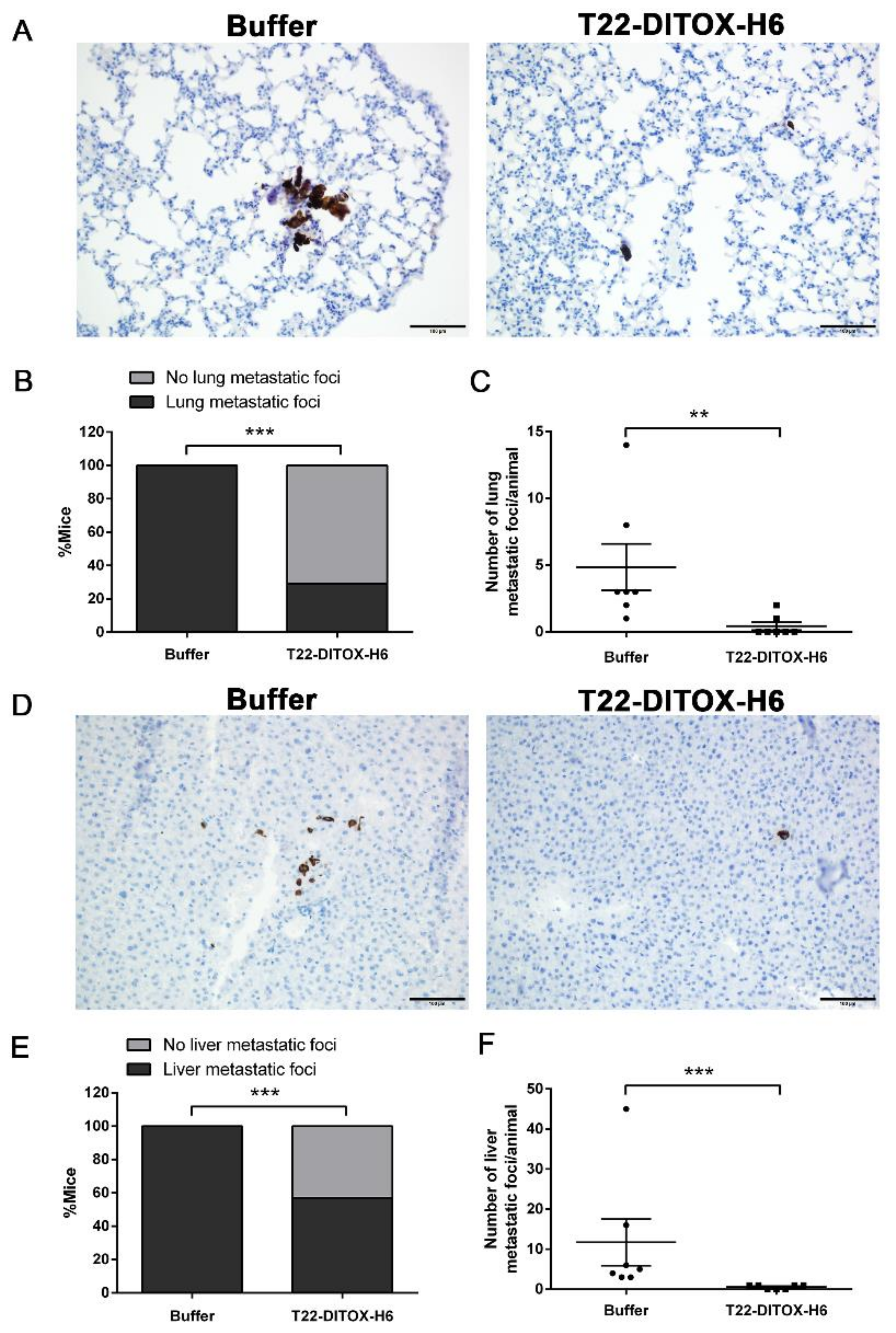
3.3. T22-DITOX-H6 Repeated Dosage Inhibits Metastatic Dissemination in a HNSCC Orthotopic Mouse Model in the Absence of Systemic Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sacco, A.G.; Cohen, E.E. Current Treatment Options for Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 3305–3313. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, C.; Zhang, C.; Li, Z.; Zhu, T.; Chen, J.; Ren, Y.; Wang, X.; Zhang, L.; Zhou, X. TGF-β-Induced STAT3 Overexpression Promotes Human Head and Neck Squamous Cell Carcinoma Invasion and Metastasis through Malat1/MiR-30a Interactions. Cancer Lett. 2018, 436, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Tang, Y.L.; Liang, X.H. Transforming Growth Factor-β Signaling in Head and Neck Squamous Cell Carcinoma: Insights into Cellular Responses. Oncol. Lett. 2018, 16, 4799–4806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.; Huang, W.; Lu, H.; Zhang, B.; Ma, J.; Zhao, D.; Wang, Y.; Yu, D.; He, X. Identification and Validation a TGF-β-Associated Long Non-Coding RNA of Head and Neck Squamous Cell Carcinoma by Bioinformatics Method. J. Transl. Med. 2018, 16, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ileana Dumbrava, E.; Alfattal, R.; Miller, V.A.; Tsimberidou, A.M. Complete Response to a Fibroblast Growth Factor Receptor Inhibitor in a Patient With Head and Neck Squamous Cell Carcinoma Harboring FGF Amplifications. JCO Precis. Oncol. 2018, 2, 1–7. [Google Scholar] [CrossRef]
- Chen, Q.; Chu, L.; Li, X.; Li, H.; Zhang, Y.; Cao, Q.; Zhuang, Q. Investigation of an FGFR-Signaling-Related Prognostic Model and Immune Landscape in Head and Neck Squamous Cell Carcinoma. Front. Cell Dev. Biol. 2022, 9, 801715. [Google Scholar] [CrossRef]
- Pernas, S.; Martin, M.; Kaufman, P.A.; Gil-Martin, M.; Gomez Pardo, P.; Lopez-Tarruella, S.; Manso, L.; Ciruelos, E.; Perez-Fidalgo, J.A.; Hernando, C.; et al. Balixafortide plus Eribulin in HER2-Negative Metastatic Breast Cancer: A Phase 1, Single-Arm, Dose-Escalation Trial. Lancet Oncol. 2018, 19, 812–824. [Google Scholar] [CrossRef]
- León, X.; Diez, S.; García, J.; Lop, J.; Sumarroca, A.; Quer, M.; Camacho, M. Expression of the CXCL12/CXCR4 Chemokine Axis Predicts Regional Control in Head and Neck Squamous Cell Carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 4525–4533. [Google Scholar] [CrossRef]
- De-Colle, C.; Menegakis, A.; Mönnich, D.; Welz, S.; Boeke, S.; Sipos, B.; Fend, F.; Mauz, P.-S.; Tinhofer, I.; Budach, V.; et al. SDF-1/CXCR4 Expression Is an Independent Negative Prognostic Biomarker in Patients with Head and Neck Cancer after Primary Radiochemotherapy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2018, 126, 125–131. [Google Scholar] [CrossRef]
- Albert, S.; Hourseau, M.; Halimi, C.; Serova, M.; Descatoire, V.; Barry, B.; Couvelard, A.; Riveiro, M.E.; Tijeras-Raballand, A.; De Gramont, A.; et al. Prognostic Value of the Chemokine Receptor CXCR4 and Epithelial-to-Mesenchymal Transition in Patients with Squamous Cell Carcinoma of the Mobile Tongue. Oral Oncol. 2012, 48, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Luker, G.D.; Yang, J.; Richmond, A.; Scala, S.; Festuccia, C.; Schottelius, M.; Wester, H.J.; Zimmermann, J. At the Bench: Pre-Clinical Evidence for Multiple Functions of CXCR4 in Cancer. J. Leukoc. Biol. 2021, 109, 969–989. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Oupický, D. Effect of Biodegradability on CXCR4 Antagonism, Transfection Efficacy and Antimetastatic Activity of Polymeric Plerixafor. Biomaterials 2014, 35, 5572–5579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhu, Y.; Hazeldine, S.T.; Li, C.; Oupický, D. Dual-Function CXCR4 Antagonist Polyplexes to Deliver Gene Therapy and Inhibit Cancer Cell Invasion. Angew. Chemie-Int. Ed. 2012, 51, 8740–8743. [Google Scholar] [CrossRef] [Green Version]
- Albert, S.; Riveiro, M.E.; Halimi, C.; Hourseau, M.; Couvelard, A.; Serova, M.; Barry, B.; Raymond, E.; Faivre, S. Focus on the Role of the CXCL12/CXCR4 Chemokine Axis in Head and Neck Squamous Cell Carcinoma. Head Neck 2013, 35, 1819–1828. [Google Scholar] [CrossRef]
- Domanska, U.M.; Kruizinga, R.C.; Nagengast, W.B.; Timmer-Bosscha, H.; Huls, G.; De Vries, E.G.E.; Walenkamp, A.M.E. A Review on CXCR4/CXCL12 Axis in Oncology: No Place to Hide. Eur. J. Cancer 2013, 49, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer Nanomedicine: Progress, Challenges and Opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Mangues, R.; Vázquez, E.; Villaverde, A. Targeting in Cancer Therapies. Med. Sci. 2016, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-García, L.; Serna, N.; Álamo, P.; Sala, R.; Céspedes, M.V.; Roldan, M.; Sánchez-Chardi, A.; Unzueta, U.; Casanova, I.; Mangues, R.; et al. Self-Assembling Toxin-Based Nanoparticles as Self-Delivered Antitumoral Drugs. J. Control. Release 2018, 274, 81–92. [Google Scholar] [CrossRef]
- Brenner, J.C.; Graham, M.P.; Kumar, B.; Saunders, L.M.; Kupfer, R.; Lyons, R.H.; Bradford, C.R.; Carey, T.E. Genotyping of 73 UM-SCC Head and Neck Squamous Cell Carcinoma Cell Lines. Head Neck 2010, 32, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Rioja-Blanco, E.; Arroyo-Solera, I.; Álamo, P.; Casanova, I.; Gallardo, A.; Unzueta, U.; Serna, N.; Sánchez-García, L.; Quer, M.; Villaverde, A.; et al. Self-Assembling Protein Nanocarrier for Selective Delivery of Cytotoxic Polypeptides to CXCR4+ Head and Neck Squamous Cell Carcinoma Tumors. Acta Pharm. Sin. B 2021, 12, 2595–2608. [Google Scholar] [CrossRef]
- Rioja-Blanco, E.; Arroyo-Solera, I.; Álamo, P.; Casanova, I.; Gallardo, A.; Unzueta, U.; Serna, N.; Sánchez-García, L.; Quer, M.; Villaverde, A.; et al. CXCR4-Targeted Nanotoxins Induce GSDME-Dependent Pyroptosis in Head and Neck Squamous Cell Carcinoma. J. Exp. Clin. Cancer Res. 2022, 41, 49. [Google Scholar] [CrossRef] [PubMed]
- Pontes, F.; Garcia, A.R.; Domingues, I.; João Sousa, M.; Felix, R.; Amorim, C.; Salgueiro, F.; Mariano, M.; Teixeira, M. Survival Predictors and Outcomes of Patients with Recurrent and/or Metastatic Head and Neck Cancer Treated with Chemotherapy plus Cetuximab as First-Line Therapy: A Real-World Retrospective Study. Cancer Treat. Res. Commun. 2021, 27, 100375. [Google Scholar] [CrossRef] [PubMed]
- Beckham, T.H.; Leeman, J.E.; Xie, P.; Li, X.; Goldman, D.A.; Zhang, Z.; Sherman, E.; McBride, S.; Riaz, N.; Lee, N.; et al. Long-Term Survival in Patients with Metastatic Head and Neck Squamous Cell Carcinoma Treated with Metastasis-Directed Therapy. Br. J. Cancer 2019, 121, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Bhave, S.L.; Teknos, T.N.; Pan, Q. Molecular Parameters of Head and Neck Cancer Metastasis. Crit. Rev. Eukaryot. Gene Expr. 2011, 21, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Hermann, P.C.; Huber, S.L.; Herrler, T.; Aicher, A.; Ellwart, J.W.; Guba, M.; Bruns, C.J.; Heeschen, C. Distinct Populations of Cancer Stem Cells Determine Tumor Growth and Metastatic Activity in Human Pancreatic Cancer. Cell Stem Cell 2007, 1, 313–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cioffi, M.; D’Alterio, C.; Camerlingo, R.; Tirino, V.; Consales, C.; Riccio, A.; Ieranò, C.; Cecere, S.C.; Losito, N.S.; Greggi, S.; et al. Identification of a Distinct Population of CD133+CXCR4+ Cancer Stem Cells in Ovarian Cancer. Sci. Rep. 2015, 5, 10357. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Bu, W.; Meng, L.; Liu, X.; Wang, S.; Jiang, L.; Ren, M.; Fan, Y.; Sun, H. CXCL12/CXCR4 Pathway Orchestrates CSC-like Properties by CAF Recruited Tumor Associated Macrophage in OSCC. Exp. Cell Res. 2019, 378, 131–138. [Google Scholar] [CrossRef]
- Vandergaast, R.; Khongwichit, S.; Jiang, H.; DeGrado, T.R.; Peng, K.-W.; Smith, D.R.; Russell, S.J.; Suksanpaisan, L. Enhanced Noninvasive Imaging of Oncology Models Using the NIS Reporter Gene and Bioluminescence Imaging. Cancer Gene Ther. 2020, 27, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Marcu, S.D.E.-L.G. Local Metastasis in Head and Neck Cancer-an Overview. In Contemporary Issues in Head and Neck Cancer Management; IntechOpen: London, UK, 2015; p. 6. [Google Scholar]
- Sproll, C.; Freund, A.K.; Hassel, A.; Hölbling, M.; Aust, V.; Storb, S.H.; Handschel, J.; Teichmann, C.; Depprich, R.; Behrens, B.; et al. Immunohistochemical Detection of Lymph Node-DTCs in Patients with Node-Negative HNSCC. Int. J. Cancer 2017, 140, 2112–2124. [Google Scholar] [CrossRef] [Green Version]
- Borcoman, E.; Marret, G.; Le Tourneau, C. Paradigm Change in First-Line Treatment of Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma. Cancers 2021, 13, 2573. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.; Yang, W.; Li, K.-Y.; Su, Y. Systemic Therapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma-A Systematic Review and Meta-Analysis. Crit. Rev. Oncol. Hematol. 2020, 153, 102984. [Google Scholar] [CrossRef] [PubMed]
- Tejani, M.A.; Cohen, R.B.; Mehra, R. The Contribution of Cetuximab in the Treatment of Recurrent and/or Metastatic Head and Neck Cancer. Biologics 2010, 4, 173–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehra, R.; Seiwert, T.Y.; Gupta, S.; Weiss, J.; Gluck, I.; Eder, J.P.; Burtness, B.; Tahara, M.; Keam, B.; Kang, H.; et al. Efficacy and Safety of Pembrolizumab in Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma: Pooled Analyses after Long-Term Follow-up in KEYNOTE-012. Br. J. Cancer 2018, 119, 153–159. [Google Scholar] [CrossRef]
- Lin, A.; Giuliano, C.J.; Palladino, A.; John, K.M.; Abramowicz, C.; Yuan, M.L.; Sausville, E.L.; Lukow, D.A.; Liu, L.; Chait, A.R.; et al. Off-Target Toxicity Is a Common Mechanism of Action of Cancer Drugs Undergoing Clinical Trials. Sci. Transl. Med. 2019, 11, eaaw8412. [Google Scholar] [CrossRef]
- Livshits, Z.; Rao, R.B.; Smith, S.W. An Approach to Chemotherapy-Associated Toxicity. Emerg. Med. Clin. N. Am. 2014, 32, 167–203. [Google Scholar] [CrossRef]
- Picon, H.; Guddati, A.K. Mechanisms of Resistance in Head and Neck Cancer. Am. J. Cancer Res. 2020, 10, 2742–2751. [Google Scholar]
- López-Verdín, S.; Lavalle-Carrasco, J.; Carreón-Burciaga, R.G.; Serafín-Higuera, N.; Molina-Frechero, N.; González-González, R.; Bologna-Molina, R. Molecular Markers of Anticancer Drug Resistance in Head and Neck Squamous Cell Carcinoma: A Literature Review. Cancers 2018, 10, 376. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Zhu, J. Recent Development and Optimization of Pseudomonas Aeruginosa Exotoxin Immunotoxins in Cancer Therapeutic Applications. Int. Immunopharmacol. 2021, 96, 107759. [Google Scholar] [CrossRef]
- Havaei, S.M.; Aucoin, M.G.; Jahanian-Najafabadi, A. Pseudomonas Exotoxin-Based Immunotoxins: Over Three Decades of Efforts on Targeting Cancer Cells with the Toxin. Front. Oncol. 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Vallera, D.A.; Kreitman, R.J. Immunotoxins Targeting B Cell Malignancy-Progress and Problems with Immunogenicity. Biomedicines 2018, 7, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-S.; Jun, S.-Y.; Kim, Y.-S. Critical Issues in the Development of Immunotoxins for Anticancer Therapy. J. Pharm. Sci. 2020, 109, 104–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Apriceno, A.; Sipin, M.; Scarpa, E.; Rodriguez-Arco, L.; Poma, A.; Marchello, G.; Battaglia, G.; Angioletti-Uberti, S. Combinatorial Entropy Behaviour Leads to Range Selective Binding in Ligand-Receptor Interactions. Nat. Commun. 2020, 11, 4836. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rioja-Blanco, E.; Gallardo, A.; Arroyo-Solera, I.; Álamo, P.; Casanova, I.; Unzueta, U.; Serna, N.; Sánchez-García, L.; Quer, M.; Villaverde, A.; et al. A Novel CXCR4-Targeted Diphtheria Toxin Nanoparticle Inhibits Invasion and Metastatic Dissemination in a Head and Neck Squamous Cell Carcinoma Mouse Model. Pharmaceutics 2022, 14, 887. https://doi.org/10.3390/pharmaceutics14040887
Rioja-Blanco E, Gallardo A, Arroyo-Solera I, Álamo P, Casanova I, Unzueta U, Serna N, Sánchez-García L, Quer M, Villaverde A, et al. A Novel CXCR4-Targeted Diphtheria Toxin Nanoparticle Inhibits Invasion and Metastatic Dissemination in a Head and Neck Squamous Cell Carcinoma Mouse Model. Pharmaceutics. 2022; 14(4):887. https://doi.org/10.3390/pharmaceutics14040887
Chicago/Turabian StyleRioja-Blanco, Elisa, Alberto Gallardo, Irene Arroyo-Solera, Patricia Álamo, Isolda Casanova, Ugutz Unzueta, Naroa Serna, Laura Sánchez-García, Miquel Quer, Antonio Villaverde, and et al. 2022. "A Novel CXCR4-Targeted Diphtheria Toxin Nanoparticle Inhibits Invasion and Metastatic Dissemination in a Head and Neck Squamous Cell Carcinoma Mouse Model" Pharmaceutics 14, no. 4: 887. https://doi.org/10.3390/pharmaceutics14040887
APA StyleRioja-Blanco, E., Gallardo, A., Arroyo-Solera, I., Álamo, P., Casanova, I., Unzueta, U., Serna, N., Sánchez-García, L., Quer, M., Villaverde, A., Vázquez, E., León, X., Alba-Castellón, L., & Mangues, R. (2022). A Novel CXCR4-Targeted Diphtheria Toxin Nanoparticle Inhibits Invasion and Metastatic Dissemination in a Head and Neck Squamous Cell Carcinoma Mouse Model. Pharmaceutics, 14(4), 887. https://doi.org/10.3390/pharmaceutics14040887








