Influence of Formulation Factors, Process Parameters, and Selected Quality Attributes on Carvedilol Release from Roller-Compacted Hypromellose-Based Matrix Tablets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Composition of Tablet Formulation Tested
2.3. Compression Mixture Preparation and Tableting
2.4. Data Acquisition and Data Analysis
2.4.1. Spatial Filtering Technique
2.4.2. Tablet Weight and Hardness Evaluation
2.4.3. Drug Release Testing
2.4.4. Statistical Data Analysis
2.4.5. Laser Diffraction
2.4.6. Dynamic Image Analysis
2.4.7. True and Bulk Density
2.4.8. Compressibility and Compactibility Studies
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robinson, J.R.; Jantzen, G.M. Sustained and Controlled-Release Drug Delivery Systems. In Modern Pharmaceutics, 4th ed.; Banker, G.S., Rhodes, C.T., Gilbert, S., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2002; pp. 501–528. [Google Scholar]
- Sako, K.; Sawada, T.; Nakashima, H.; Yokohama, S.; Sonobe, T. Influence of water soluble fillers in hydroxypropylmethylcellulose matrices on in vitro and in vivo drug release. J. Control. Release 2002, 81, 165–172. [Google Scholar] [CrossRef]
- Vueba, M.L.; de Carvalho, L.A.E.B.; Veiga, F.; Sousa, J.J.; Pina, M.E. Influence of cellulose ether polymers on ketoprofen release from hydrophilic matrix tablets. Eur. J. Pharm. Biopharm. 2004, 58, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Caraballo, I. Critical points in the formulation of pharmaceutical swellable controlled release dosage forms—Influence of particle size. Particuology 2009, 7, 421–425. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Huang, Z.; Cui, Y.; Zhao, Z.; Yue, X.; Wang, G.; Liang, R.; Huang, Y.; Tan, W.; et al. Development and pharmacokinetics evaluation of quetiapine fumarate sustained-release tablets based on hydrophilic matrix. J. Drug Deliv. Sci. Technol. 2019, 54, 101322. [Google Scholar] [CrossRef]
- Sung, K.C.; Nixon, P.R.; Skoug, J.W.; Ju, T.R.; Gao, P.; Topp, E.M.; Patel, M.V. Effect of formulation variables on drug and polymer release from HPMC-based matrix tablets. Int. J. Pharm. 1996, 142, 53–60. [Google Scholar] [CrossRef]
- Maderuelo, C.; Zarzuelo, A.; Lanao, J.M. Critical factors in the release of drugs from sustained release hydrophilic matrices. J. Control. Release 2011, 154, 2–19. [Google Scholar] [CrossRef]
- Mašková, E.; Kubová, K.; Raimi-Abraham, B.T.; Vllasaliu, D.; Vohlídalová, E.; Turánek, J.; Mašek, J. Hypromellose—A traditional pharmaceutical excipient with modern applications in oral and oromucosal drug delivery. J. Control. Release 2020, 324, 695–727. [Google Scholar] [CrossRef]
- Li, C.L.; Martini, L.G.; Ford, J.L.; Roberts, M. The use of hypromellose in oral drug delivery. J. Pharm. Pharmacol. 2005, 57, 533–546. [Google Scholar] [CrossRef]
- Herder, J.; Adolfsson, Å.; Larsson, A. Initial studies of water granulation of eight grades of hypromellose (HPMC). Int. J. Pharm. 2006, 313, 57–65. [Google Scholar] [CrossRef]
- Leuenberger, H. New trends in the production of pharmaceutical granules: Batch versus continuous processing. Eur. J. Pharm. Biopharm. 2001, 52, 289–296. [Google Scholar] [CrossRef]
- Kleinebudde, P. Roll compaction/dry granulation: Pharmaceutical applications. Eur. J. Pharm. Biopharm. 2004, 58, 317–326. [Google Scholar] [CrossRef]
- Miller, R.W. Roller Compaction Technology. In Handbook of Pharmaceutical Granulation Technology, 2nd ed.; Parikh, D.M., Ed.; Taylor & Francis Group, LLC.: Boca Raton, FL, USA, 2005; pp. 159–190. [Google Scholar]
- Rowe, J.M.; Charlton, S.T.; McCann, R.J. Development, Scale-Up, and Optimization of Process Parameters: Roller Compaction Theory and Practice. In Developing Solid Oral Dosage Forms, 2nd ed.; Qiu, Y., Chen, Y., Zhang, G.G.Z., Yu, L., Mantri, R.V., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 869–915. [Google Scholar]
- Zhang, G.Z.; Law, D.; Schmitt, E.A.; Qiu, Y. Phase transformation considerations during process development and manufacture of solid oral dosage forms. Adv. Drug Deliv. Rev. 2004, 56, 371–390. [Google Scholar] [CrossRef]
- Perez-Gandarillas, L.; Perez-Gago, A.; Mazor, A.; Kleinebudde, P.; Lecoq, O.; Michrafy, A. Effect of roll-compaction and milling conditions on granules and tablet properties. Eur. J. Pharm. Biopharm. 2016, 106, 38–49. [Google Scholar] [CrossRef] [Green Version]
- Dular Vovko, A.; Vrečer, F. Process analytical technology tools for process control of roller compaction in solid pharmaceuticals manufacturing. Acta Pharm. 2020, 70, 443–463. [Google Scholar] [CrossRef]
- Kleinebudde, P. Improving Process Understanding in Roll Compaction. J. Pharm. Sci. 2022, 111, 552–558. [Google Scholar] [CrossRef]
- Hariharan, M.; Wowchuk, C.; Nkansah, P.; Gupta, V.K. Effect of formulation composition on the properties of controlled release tablets prepared by roller compaction. Drug Dev. Ind. Pharm. 2004, 30, 565–572. [Google Scholar] [CrossRef]
- Sheskey, P.J.; Hendren, J. The effects of roll compaction equipment variables, granulation technique, and HPMC polymer level on a controlled-release matrix model drug formulation. Pharm. Technol. 1999, 23, 90–106. [Google Scholar]
- Ohmori, S.; Makino, T. Sustained-release phenylpropanolamine hydrochloride bilayer caplets containing the hydroxypropylmethylcellulose 2208 matrix. II. Effects of filling order in bilayer compression and manufacturing method of the prolonged-release layer on compactibility of bilayer caplets. Chem. Pharm. Bull. 2000, 48, 678–682. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Himmelspach, M.W. Reduced tabletability of roller compacted granules as a result of granule size enlargement. J. Pharm. Sci. 2006, 95, 200–206. [Google Scholar] [CrossRef]
- Herting, M.G.; Kleinebudde, P. Studies on the reduction of tensile strength of tablets after roll compaction/dry granulation. Eur. J. Pharm. Biopharm. 2008, 70, 372–379. [Google Scholar] [CrossRef]
- Mosig, J.; Kleinebudde, P. Critical evaluation of root causes of the reduced compactability after roll compaction/dry granulation. J. Pharm. Sci. 2015, 104, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Heiman, J.; Tajarobi, F.; Gururajan, B.; Juppo, A.; Abrahmsen-Alami, S. Roller compaction of hydrophilic extended release tablets—Combined effects of processing variables and drug/matrix former particle size. AAPS PharmSciTechnol. 2015, 16, 267–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dular Vovko, A.; Hodžić, B.; Hudovornik, G.; Vrečer, F. Implementation of spatial filtering technique in monitoring roller compaction process. Int. J. Pharm. 2021, 606, 120896. [Google Scholar] [CrossRef] [PubMed]
- Košir, D.; Ojsteršek, T.; Baumgartner, S.; Vrečer, F. A study of critical functionality-related characteristics of HPMC for sustained-release tablets. Pharm. Dev. Technol. 2018, 23, 865–873. [Google Scholar] [CrossRef]
- Sathe, P.M.; Tsong, Y.; Shah, V.P. In-vitro dissolution profile comparison: Statistics and analysis, model dependent approach. Pharm Res. 1996, 13, 1799–1803. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Schaller, B.E.; Moroney, K.M.; Castro-Dominguez, B.; Cronin, P.; Belen-Girona, J.; Ruane, P.; Croker, D.M.; Walker, G.M. Systematic development of a high dosage formulation to enable direct compression of a poorly flowing API: A case study. Int. J. Pharm. 2019, 566, 615–630. [Google Scholar] [CrossRef]
- Xu, G.; Sunada, H. Influence of formulation change on drug release kinetics from hydroxypropylmethylcellulose matrix tablets. Chem. Pharm. Bull. 1995, 43, 483–487. [Google Scholar] [CrossRef]
- Velasco, M.V.; Ford, J.L.; Rowe, P.; Rajabi-Siahboomi, A.R. Influence of drug: Hydroxypropylmethylcellulose ratio, drug and polymer particle size and compression force on the release of diclofenac sodium from HPMC tablets. J. Control. Release 1999, 57, 75–85. [Google Scholar] [CrossRef]
- Lotfipour, F.; Nokhodchi, A.; Saeedi, M.; Norouzi-Sani, S.; Sharbafi, J.; Siahi-Shadbad, M.R. The effect of hydrophilic and lipophilic polymers and fillers on the release rate of atenolol from HPMC matrices. Farmaco 2004, 59, 819–825. [Google Scholar] [CrossRef]
- Bultmann, J.M. Multiple compaction of microcrystalline cellulose in a roller compactor. Eur. J. Pharm. Biopharm. 2002, 54, 59–64. [Google Scholar] [CrossRef]
- Skelbæk-Pedersen, A.L.; Vilhelmsen, T.K.; Rantanen, J.; Kleinebudde, P. The relevance of granule fragmentation on reduced tabletability of granules from ductile or brittle materials produced by roll compaction/dry granulation. Int. J. Pharm. 2021, 592, 120035. [Google Scholar] [CrossRef] [PubMed]
- Goldoozian, S.; Mohylyuk, V.; Dashevskiy, A.; Bodmeier, R. Gel Strength of Hydrophilic Matrix Tablets in Terms of In Vitro Robustness. Pharm. Res. 2021, 38, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
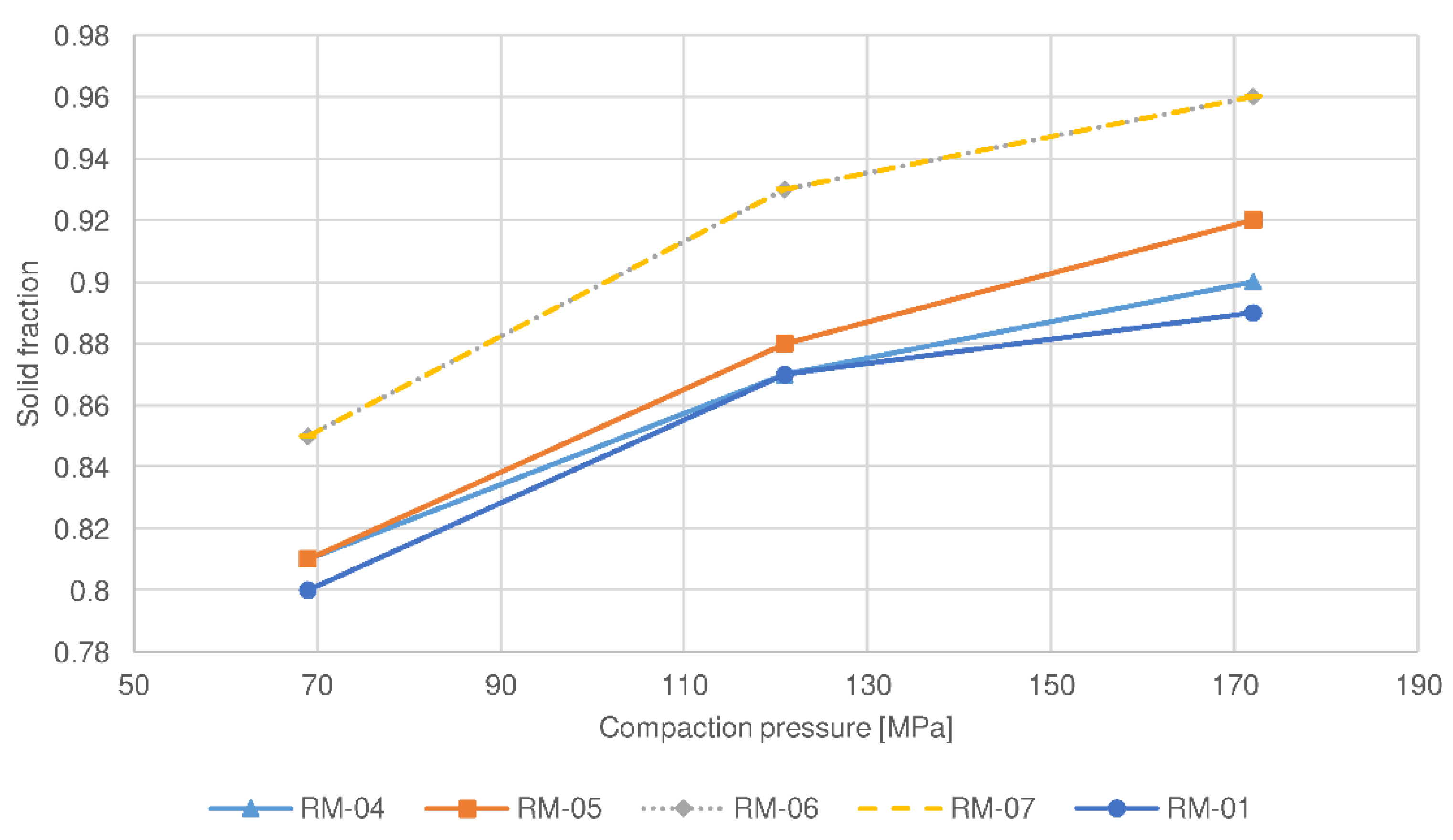
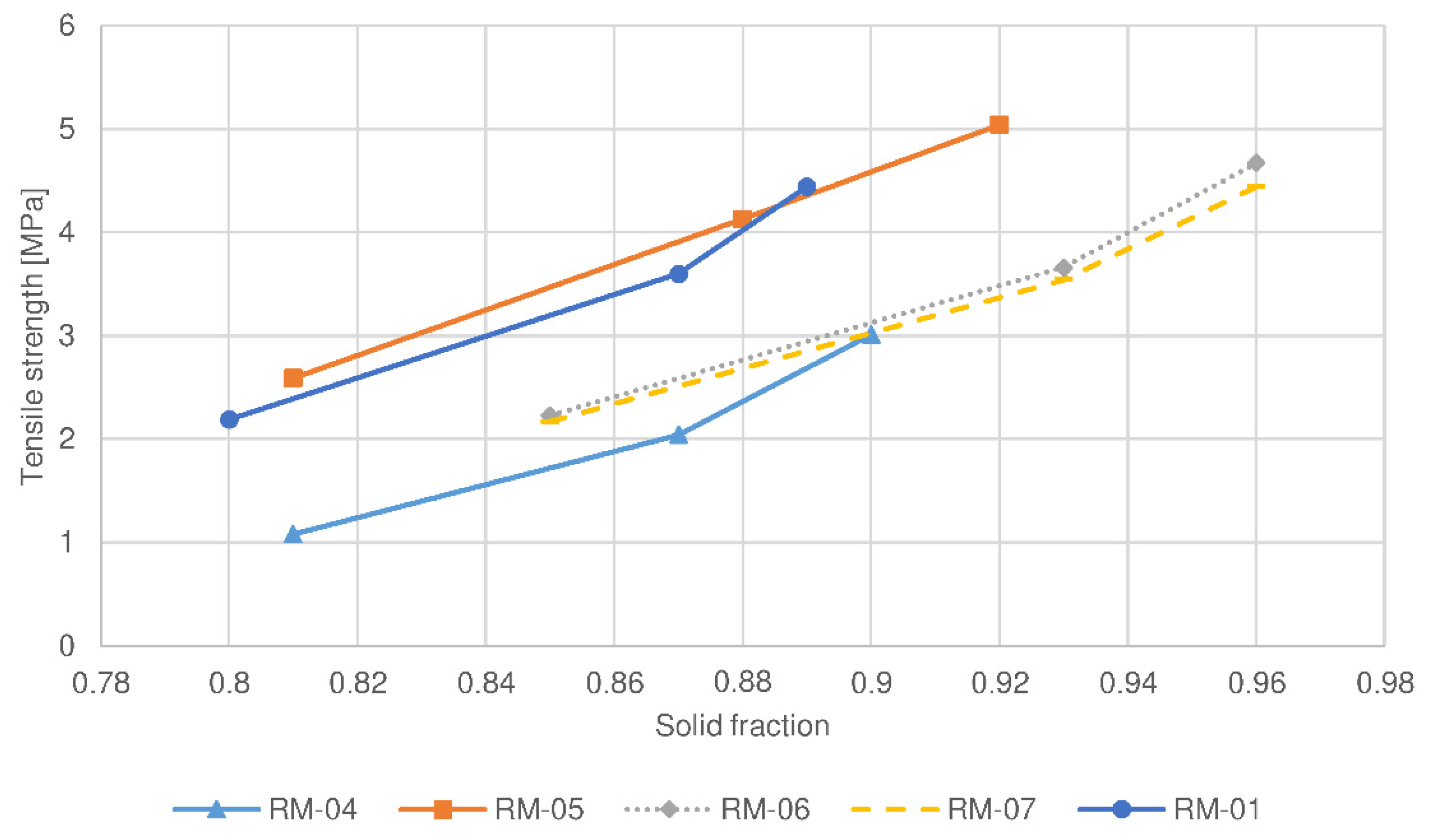

| Batch No. | Roll Force | Roll Speed | Screen Aperture Size |
|---|---|---|---|
| RL-01 *, RL-12 *, RL-10 **, RL-11 ***, RM-01 *, RM-11 *, RM-10 *** | 70 bar | 5 rpm | 1.00 mm |
| RL-02 *, RM-02 * | 30 bar | 5 rpm | 1.00 mm |
| RL-03 *, RM-03 * | 110 bar | 5 rpm | 1.00 mm |
| RL-04 *, RL-04 * | 70 bar | 3 rpm | 1.00 mm |
| RL-05 *, RM-05 * | 70 bar | 7 rpm | 1.00 mm |
| RL-06 *, RM-06 * | 70 bar | 5 rpm | 0.80 mm |
| RL-07 *, RM-07 * | 70 bar | 5 rpm | 1.25 mm |
| TL-01 *, TM-01 * | 70 bar | 5 rpm | 1.00 mm |
| TL-03 *, TM-03 * | 100 bar | 3 rpm | 1.00 mm |
| TL-04 *, TM-04 * | 50 bar | 7 rpm | 1.00 mm |
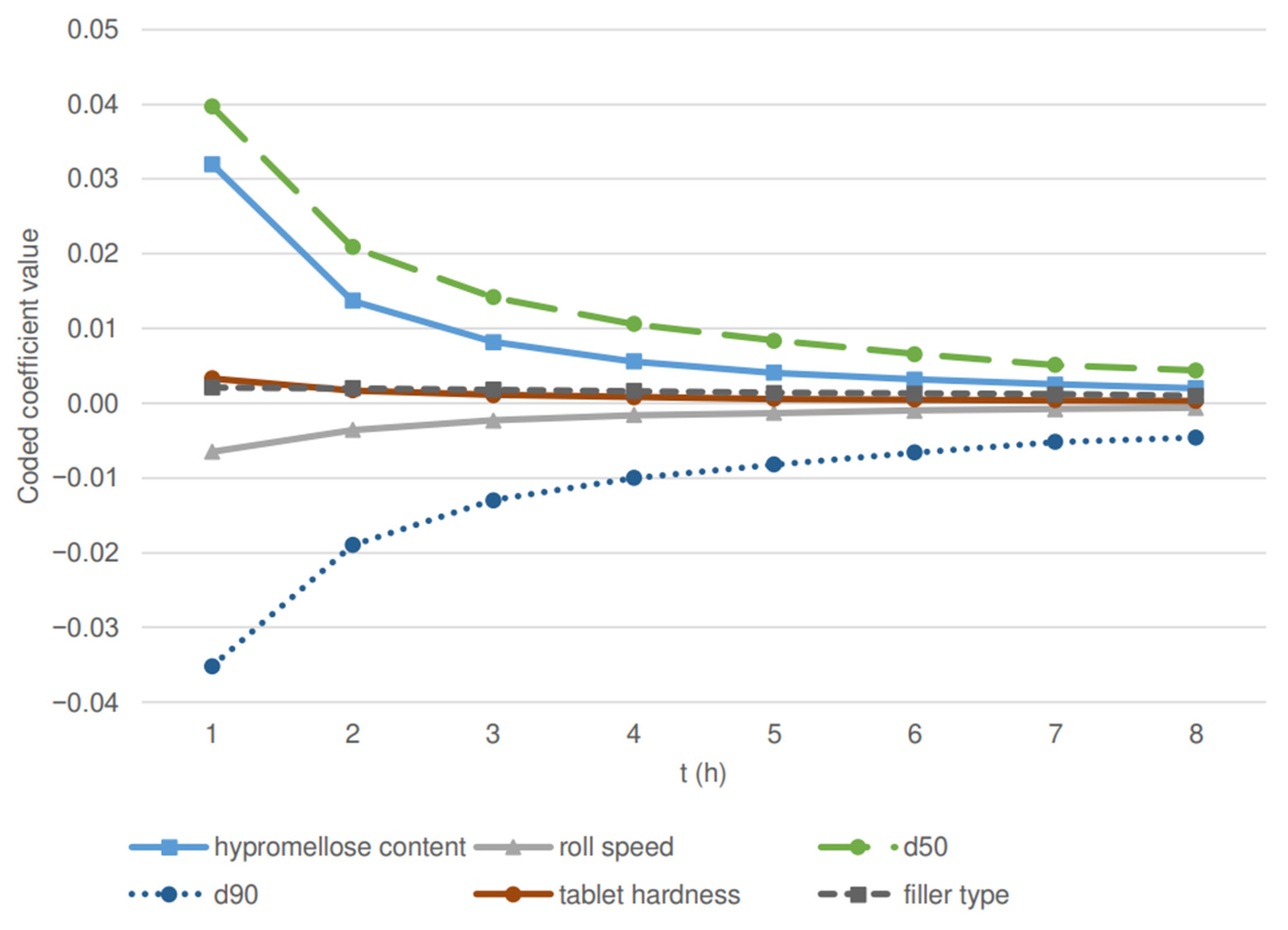
| Parameter | 1 h | 2 h | 3 h | 4 h | 5 h | 6 h | 7 h | 8 h |
|---|---|---|---|---|---|---|---|---|
| Model F-value | 70.27 | 57.66 | 52.44 | 51.02 | 47.86 | 46.97 | 47.54 | 47.08 |
| R2 | 0.89 | 0.87 | 0.86 | 0.85 | 0.85 | 0.84 | 0.85 | 0.84 |
| Adjusted R2 | 0.88 | 0.85 | 0.84 | 0.84 | 0.83 | 0.83 | 0.83 | 0.83 |
| Predicted R2 | 0.86 | 0.83 | 0.82 | 0.81 | 0.80 | 0.80 | 0.80 | 0.80 |
| p-values | ||||||||
| Hypromellose content | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Roll speed | 0.0027 | 0.0008 | 0.0010 | 0.0011 | 0.0017 | 0.0028 | 0.0025 | 0.0033 |
| d50 | 0.0301 | 0.0206 | 0.0161 | 0.0135 | 0.0132 | 0.0162 | 0.0245 | 0.0200 |
| d90 | 0.0157 | 0.0084 | 0.0057 | 0.0036 | 0.0026 | 0.0029 | 0.0038 | 0.0024 |
| Tablet hardness | 0.0004 | 0.0002 | 0.0004 | 0.0003 | 0.0007 | 0.0012 | 0.0014 | 0.0028 |
| Filler type | 0.0451 | 0.0002 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
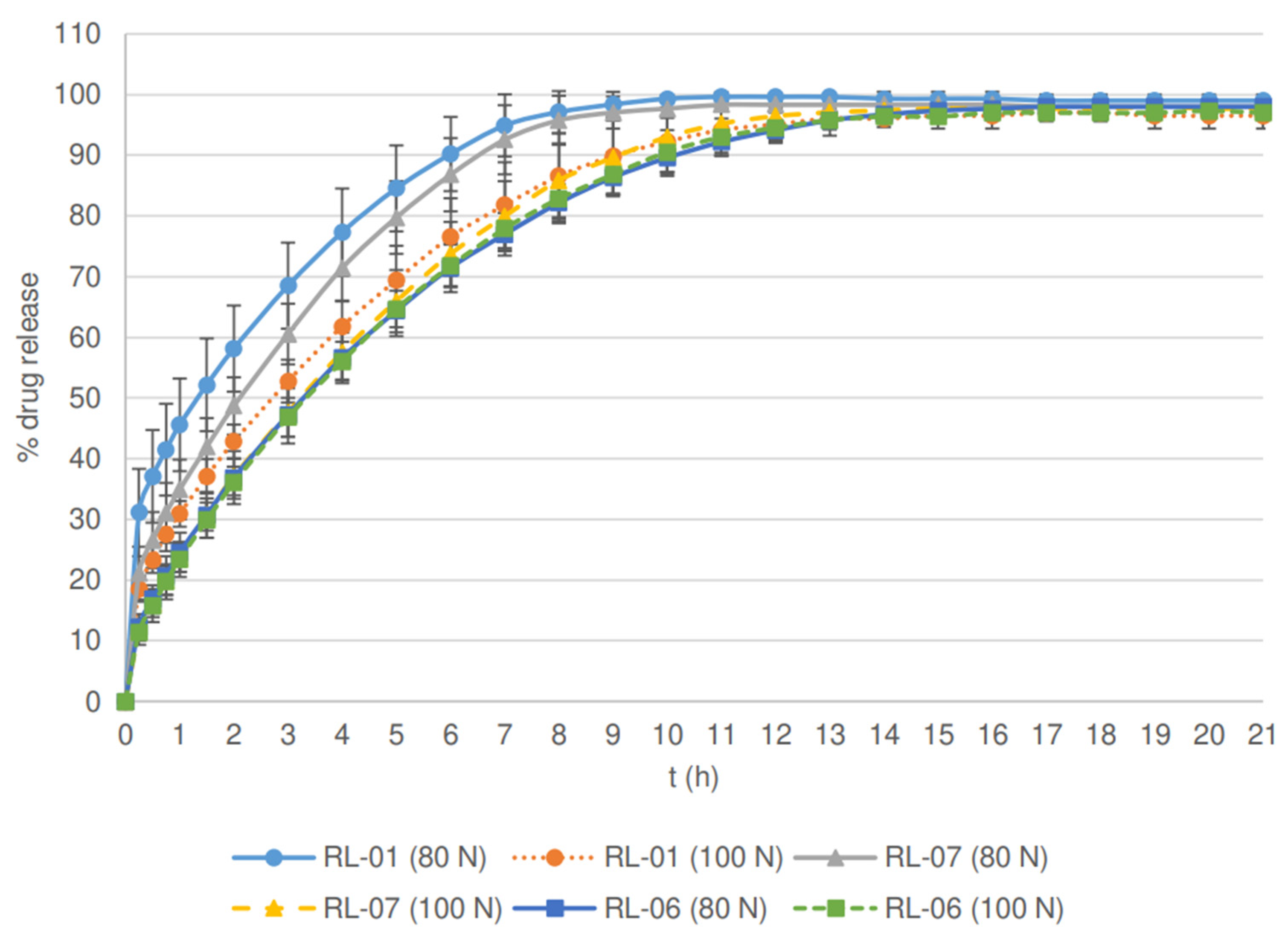
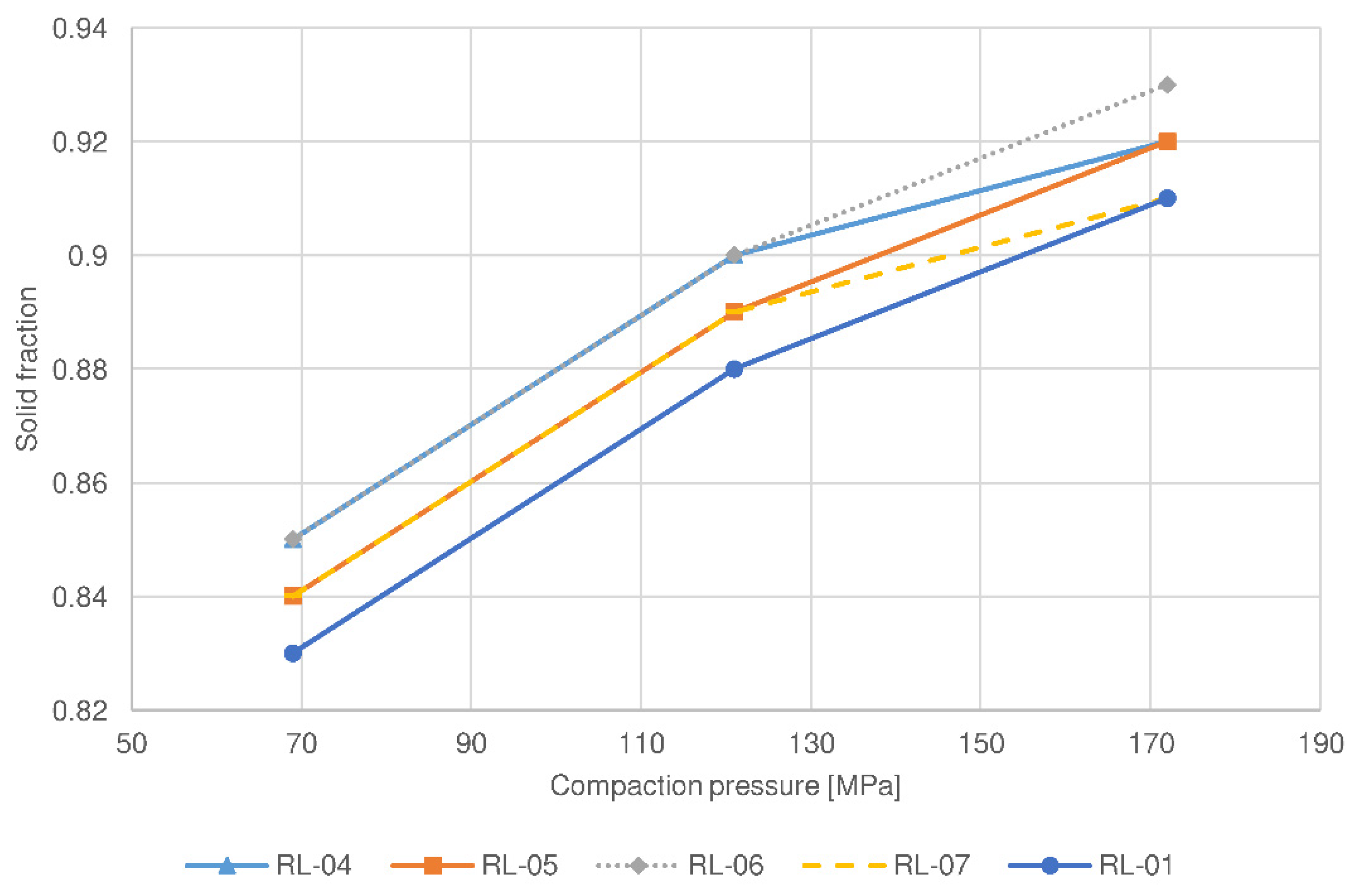
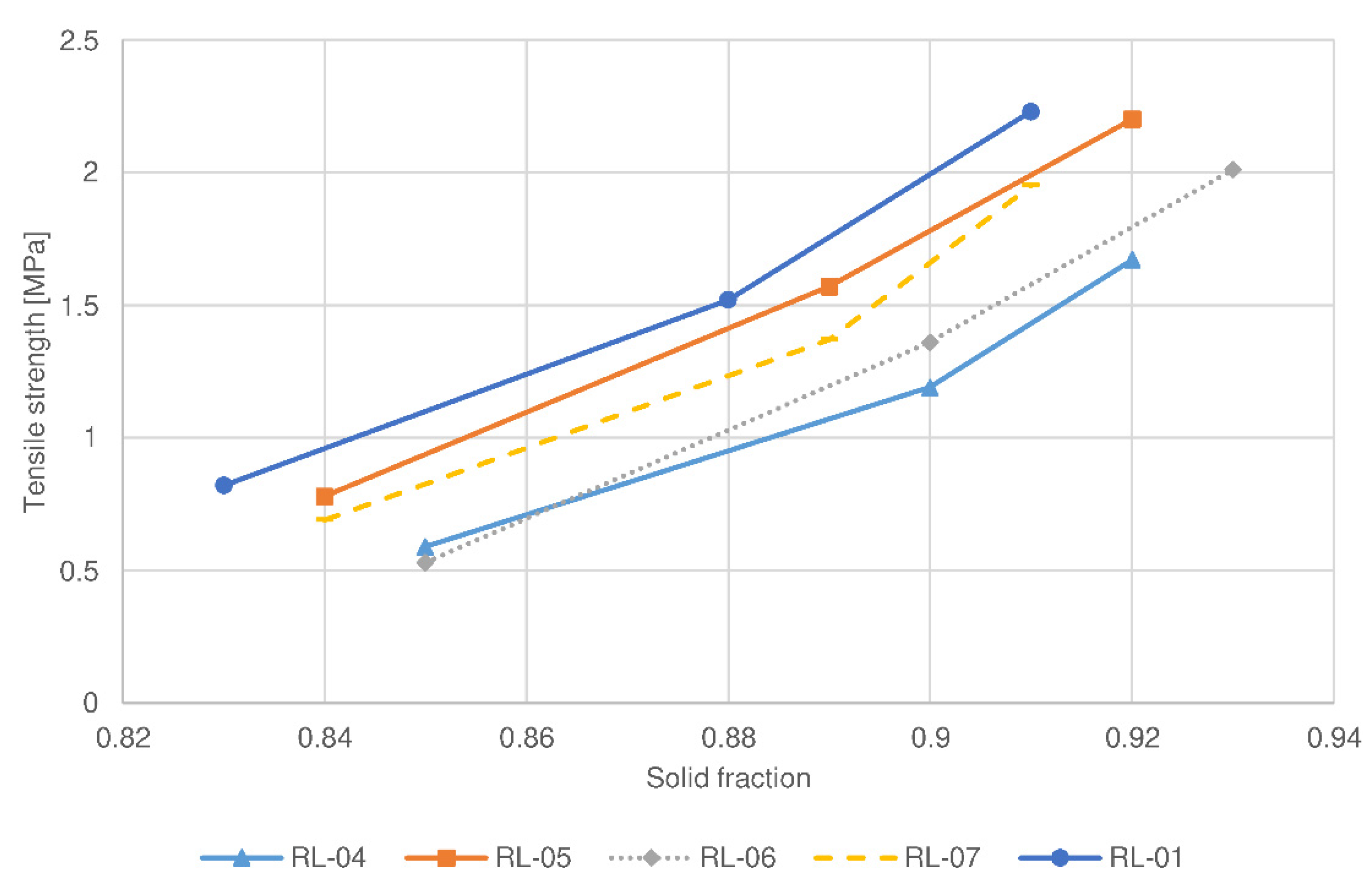
| Parameter | RL-01 | RL-04 | RL-05 | RL-06 | RL-07 | RM-01 | RM-04 | RM-05 | RM-06 | RM-07 |
|---|---|---|---|---|---|---|---|---|---|---|
| Filler type | lactose | lactose | lactose | lactose | lactose | MCC ** | MCC | MCC | MCC | MCC |
| Roll speed (rpm) | 5 | 3 | 7 | 5 | 5 | 5 | 3 | 7 | 5 | 5 |
| Screen aperture size (mm) | 1.00 | 1.00 | 1.00 | 0.80 | 1.25 | 1.00 | 1.00 | 1.00 | 0.80 | 1.25 |
| d50 (SFT *) (µm) | 695 | 715 | 252 | 500 | 877 | 378 | 718 | 113 | 151 | 659 |
| d90 (SFT *) (µm) | 1153 | 1392 | 854 | 959 | 1586 | 907 | 1248 | 772 | 690 | 1254 |
| Granule porosity (%) | 61 | 59 | 66 | 61 | 59 | 74 | 73 | 79 | 77 | 75 |
| Granule friability (LD *** d [4, 3], %) | 44 | 28 | 53 | 51 | 82 | 36 | 37 | 11 | 35 | 56 |
| Granule friability (LD d90, %) | 46 | 18 | 39 | 55 | 83 | 38 | 27 | 7 | 27 | 68 |
| Sphericity (DIA ****) | 0.74 | 0.73 | 0.77 | 0.75 | 0.72 | 0.70 | 0.68 | 0.70 | 0.69 | 0.68 |
| Aspect ratio (DIA) | 0.66 | 0.65 | 0.66 | 0.66 | 0.66 | 0.58 | 0.60 | 0.56 | 0.57 | 0.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dular Vovko, A.; Hodžić, B.; Brec, T.; Hudovornik, G.; Vrečer, F. Influence of Formulation Factors, Process Parameters, and Selected Quality Attributes on Carvedilol Release from Roller-Compacted Hypromellose-Based Matrix Tablets. Pharmaceutics 2022, 14, 876. https://doi.org/10.3390/pharmaceutics14040876
Dular Vovko A, Hodžić B, Brec T, Hudovornik G, Vrečer F. Influence of Formulation Factors, Process Parameters, and Selected Quality Attributes on Carvedilol Release from Roller-Compacted Hypromellose-Based Matrix Tablets. Pharmaceutics. 2022; 14(4):876. https://doi.org/10.3390/pharmaceutics14040876
Chicago/Turabian StyleDular Vovko, Aleša, Bor Hodžić, Tina Brec, Grega Hudovornik, and Franc Vrečer. 2022. "Influence of Formulation Factors, Process Parameters, and Selected Quality Attributes on Carvedilol Release from Roller-Compacted Hypromellose-Based Matrix Tablets" Pharmaceutics 14, no. 4: 876. https://doi.org/10.3390/pharmaceutics14040876
APA StyleDular Vovko, A., Hodžić, B., Brec, T., Hudovornik, G., & Vrečer, F. (2022). Influence of Formulation Factors, Process Parameters, and Selected Quality Attributes on Carvedilol Release from Roller-Compacted Hypromellose-Based Matrix Tablets. Pharmaceutics, 14(4), 876. https://doi.org/10.3390/pharmaceutics14040876






