Reptiles as Promising Sources of Medicinal Natural Products for Cancer Therapeutic Drugs
Abstract
1. Introduction
2. Natural Sources of Bioactive Cancer Therapeutic Components
2.1. Plants
2.2. Marine Organisms
3. Cancer Therapeutic Research Using Reptile-Derived Components
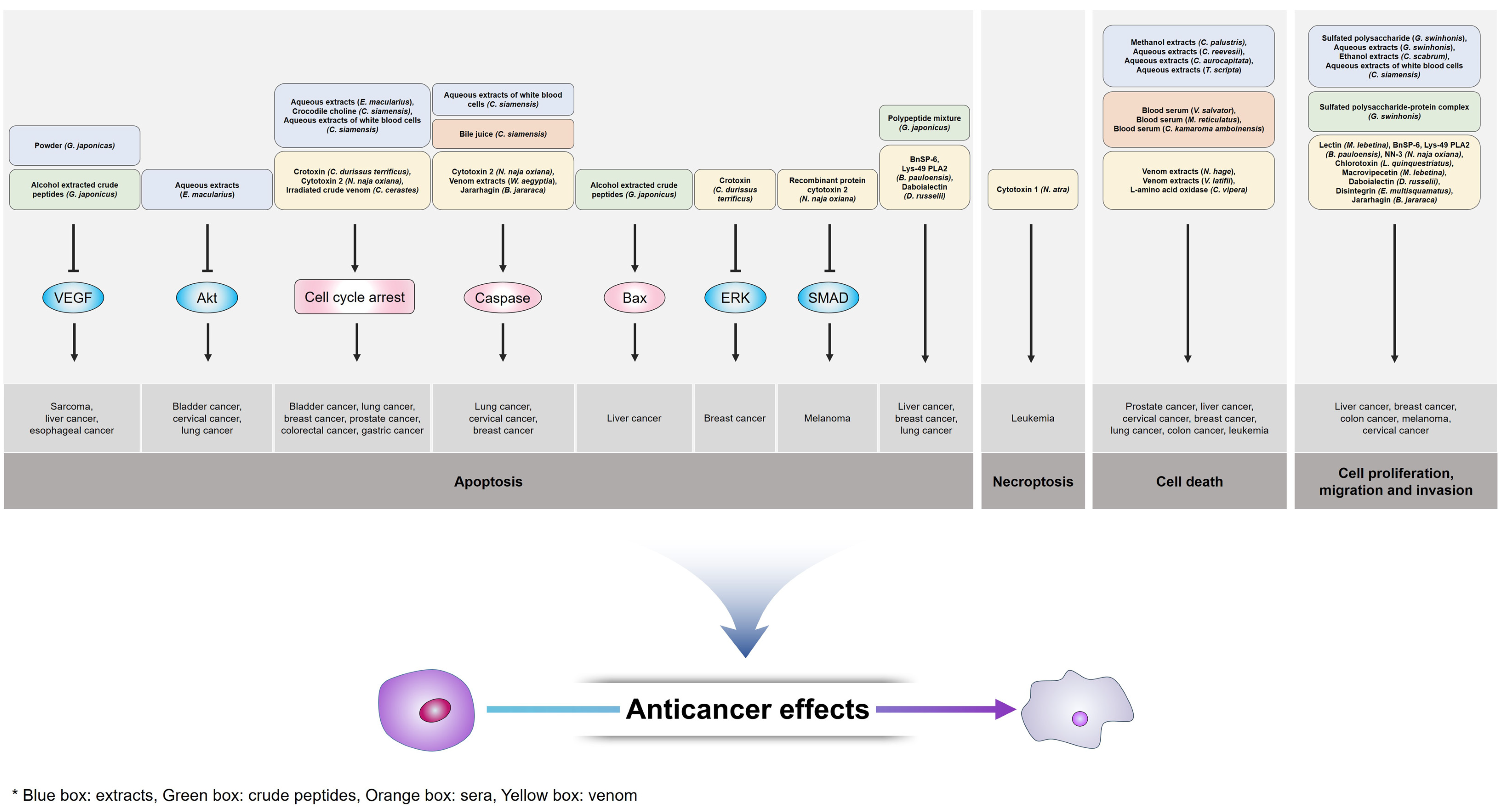
3.1. Extracts
3.2. Crude Peptides
3.3. Sera and Bile
3.4. Venom
4. Conclusions and Perspectives Regarding Reptile-Derived Products as Natural Pharmaceutical Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Curtius, K.; Wright, N.A.; Graham, T.A. An evolutionary perspective on field cancerization. Nat. Rev. Cancer 2018, 18, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Nia, H.T.; Munn, L.L.; Jain, R.K. Physical traits of cancer. Science 2020, 370, eaaz0868. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T. The matrix environmental and cell mechanical properties regulate cell migration and contribute to the invasive phenotype of cancer cells. Rep. Prog. Phys. 2019, 82, 064602. [Google Scholar] [CrossRef] [PubMed]
- Zanotelli, M.R.; Reinhart-King, C.A. Mechanical forces in tumor angiogenesis. Adv. Exp. Med. Biol. 2018, 1092, 91–112. [Google Scholar] [PubMed]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Lev, S. Targeted therapy and drug resistance in triple-negative breast cancer: The EGFR axis. Biochem. Soc. Trans. 2020, 48, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Ulfo, L.; Costantini, P.E.; Giosia, M.; Danielli, A.; Calvaresi, M. EGFR-targeted photodynamic therapy. Pharmaceutics 2022, 14, 241. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, Y.; Zhang, Z.; Tang, B.; Zhou, Z.; Chen, H. Nanoparticle-based RNAi therapeutics targeting cancer stem cells: Update and prospective. Pharmaceutics 2021, 13, 2116. [Google Scholar] [CrossRef]
- Zafar, A.; Wang, W.; Liu, G.; Wang, X.; Xian, W.; Mckeon, F.; Foster, J.; Zhou, J.; Zhang, R. Molecular targeting therapies for neuroblastoma: Progress and challenges. Med. Res. Rev. 2021, 41, 961–1021. [Google Scholar] [CrossRef]
- Kijanka, M.; Dorresteijn, B.; Oliveira, S.; van Bergen en Henegouwen, P.M. Nanobody-based cancer therapy of solid tumors. Nanomedicine 2015, 10, 161–174. [Google Scholar] [CrossRef]
- Curigliano, G.; Criscitiello, C. Successes and limitations of targeted cancer therapy in breast cancer. Prog. Tumor. Res. 2014, 41, 15–35. [Google Scholar] [PubMed]
- Gu, Z.; Da Silva, C.G.; Van der Maaden, K.; Ossendorp, F.; Cruz, L.J. Liposome-based drug delivery systems in cancer immunotherapy. Pharmaceutics 2020, 12, 1054. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.B.; Salama, A.K.S. A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 2020, 70, 86–104. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 141–167. [Google Scholar] [CrossRef] [PubMed]
- Trapani, J.A.; Darcy, P.K. Immunotherapy of cancer. Aust. Fam. Physician. 2017, 46, 194–199. [Google Scholar] [PubMed]
- Gong, J.; Chehrazi-Raffle, A.; Reddi, S.; Salgia, R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J. Immunother. Cancer. 2018, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Xu, A.; Xu, J. Roles of PD-1/PD-L1 pathway: Signaling, cancer, and beyond. Adv. Exp. Med. Biol. 2020, 1248, 33–59. [Google Scholar]
- Chamoto, K.; Hatae, R.; Honjo, T. Current issues and perspectives in PD-1 blockade cancer immunotherapy. Int. J. Clin. Oncol. 2020, 25, 790–800. [Google Scholar] [CrossRef]
- Nair, R.; Westin, J. CAR T-cells. Adv. Exp. Med. Biol. 2020, 1244, 215–233. [Google Scholar]
- Mas, S.; Li, X.; Wang, X.; Cheng, L.; Li, Z.; Zhang, C.; Ye, Z.; Qian, Q. Current progress in CAR-T cell therapy for solid tumors. Int. J. Biol. Sci. 2019, 15, 2548–2560. [Google Scholar]
- Hong, M.; Clubb, J.D.; Chen, Y.Y. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell 2020, 38, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.; Bltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, W.; Dong, X.; Fu, C.; Yuan, J.; Xu, M.; Liang, Z.; Qiu, C.; Xu, C. A natural product solution to aging and aging-associated diseases. Pharmacol. Ther. 2020, 216, 107673. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Choudhary, S.; Singh, P.K.; Verma, H.; Singh, H.; Silakari, O. Success stories of natural product-based hybrid molecules for multi-factorial diseases. Eur. J. Med. Chem. 2018, 151, 62–97. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef]
- Papon, N.; Copp, B.R.; Courdavault, V. Marine drugs: Biology, pipelines, current and future prospects for production. Biotechnol. Adv. 2022, 54, 107871. [Google Scholar] [CrossRef]
- Liang, X.; Luo, D.; Luesch, H. Advances in exploring the therapeutic potential of marine natural products. Pharmacol. Res. 2019, 147, 104373. [Google Scholar] [CrossRef]
- Yun, C.W.; Kim, H.J.; Lee, S.H. Therapeutic application of diverse marine-derived natural products in cancer therapy. Anticancer Res. 2019, 39, 5261–5284. [Google Scholar] [CrossRef] [PubMed]
- Khalif, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.F.; Moustafa, M.S.; Abd Ei-Wahed, A.; Ai-Mousawi, S.M.; Musharraf, S.G.; et al. Marine natural products: A source of novel anticancer drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef] [PubMed]
- Napavichayanun, S.; Aramwit, P. Effect of animal products and extracts on wound healing promotion in topical apllications: A review. J. Biomater. Sci. Plym. Ed. 2017, 28, 703–729. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Donga, C.; Zhang, J.; Su, L.; Wang, X.; Cui, H.; Chen, Z. Combination therapy of anti-cancer bioactive peptide with Cisplatin decreases chemotherapy dosing and toxicity to improve the quality of life in xenograft nude mice bearing human gastric cancer. Cell. Biosci. 2014, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.; Jo, C.; Kang, K.; Lee, M. Antimicrobial and human cancer cell cytotoxic effect of synthetic angiotensin-converting enzyme (ACE) inhibitory peptides. Food Chem. 2008, 107, 327–336. [Google Scholar] [CrossRef]
- Wang, L.; Dong, C.; Li, X.; Han, W.; Su, X. Anticancer potential of bioactive peptides from animal sources (Review). Oncol. Rep. 2017, 38, 637–651. [Google Scholar] [CrossRef]
- Amiche, M. Amphibian skin as a source of therapeutic peptides. Biol. Aujourdhui 2016, 210, 101–117. [Google Scholar] [CrossRef]
- Conclon, J.M.; Mechkarska, M.; Lukic, M.L.; Flatt, P.R. Potential therapeutic applications of multifunctional host-defense peptides from frog skin as anti-cancer, anti-viral, immunomodulatory, and anti-diabetic agents. Peptides 2014, 57, 67–77. [Google Scholar] [CrossRef]
- Lu, C.X.; Nan, K.J.; Lei, Y. Agents from amphibians with anticancer properties. Anticancer Drugs 2008, 19, 931–939. [Google Scholar] [CrossRef]
- Li, L.; Huang, J.; Lin, Y. Snake venoms in cancer therapy: Past, present and future. Toxins 2018, 10, 346. [Google Scholar] [CrossRef]
- Calderon, L.A.; Sobrinho, J.C.; Zaqueo, K.D.; Moura, A.A.; Grabner, A.N.; Mazzi, M.V.; Marcussi, S.; Nomizo, A.; Fernandes, C.F.C.; Zuliani, J.P.; et al. Antitumoral activity of snake venom proteins: New trends in cancer therapy. Biomed. Res. Int. 2014, 2014, 203639. [Google Scholar] [CrossRef] [PubMed]
- Skubnik, J.; Pavlickova, V.S.; Ruml, T.; Rimpelova, S. Vincristine in combination therapy of cancer: Emerging trends in clinics. Biology 2021, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Salerni, B.L.; Bates, D.J.; Albershardt, T.C.; Lowrey, C.H.; Eastman, A. Vinblastine induces acute, cell cycle phase-independent apoptosis in some leukemias and lymphomas and can induce acute apoptosis in others when Mcl-1 is suppressed. Mol. Cancer Ther. 2010, 9, 791–802. [Google Scholar] [CrossRef]
- Yang, Y.H.; Mao, J.W.; Tan, X.L. Research progress on the souce, production, and anti-cancer mechanisms of paclitaxel. Chin. J. Nat. Med. 2020, 18, 890–897. [Google Scholar] [PubMed]
- Yang, Y.; Paik, J.H.; Cho, D.; Cho, J.A.; Kim, C.W. Resveratrol induces the suppression of tumor-derived CD4+CD25+ regulatory T cells. Int. Immunopharmacol. 2008, 8, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Wu, H.; Wang, Y.; Peng, H. Capsaicin induces immunogenic cell death in human osteosarcoma cells. Exp. Ther. Med. 2016, 12, 765–770. [Google Scholar] [CrossRef]
- Lecumberri, E.; Dupertuis, Y.M.; Miralbell, R.; Pichard, C. Green tea polyphenol epigallocatechin-3-gallate (EGCG) as adjuvant in cancer therapy. Clin. Nutr. 2013, 32, 893–903. [Google Scholar] [CrossRef]
- Sztiller-Sikorska, M.; Czyz, M. Parthenolide as cooperating agent for anti-cancer treatment of various malignancies. Pharmaceuticals 2020, 13, 194. [Google Scholar] [CrossRef]
- Li, X.; Huang, R.; Li, M.; Zhu, Z.; Chen, Z.; Cui, L.; Luo, H.; Luo, L. Parthenolide inhibits the growth on non-small cell lung cancer by targeting epidermal growth factor receptor. Cancer Cell Int. 2020, 20, 561. [Google Scholar] [CrossRef]
- Sun, M.; Ye, Y.; Xiao, L.; Duan, X.; Zhang, Y.; Zhang, H. Anticancer effects of ginsenoside Rg3 (Review). Int. J. Mol. Med. 2017, 39, 507–518. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Di, A.; Zhang, L.; Zhao, G. Effects of wogonin on the growth and metastasis of colon cancer through the Hippo signaling pathway. Bioengineered 2022, 13, 2586–2597. [Google Scholar] [CrossRef] [PubMed]
- Ruibin, J.; Danying, W.; Chihong, Z.; Jianguo, F.; Linhui, G. Therapy effects of wogonin on ovarian cancer cells. Biomed. Res. Int. 2017, 2017, 9381513. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Qi, X.; Liu, H.; Xue, K.; Xu, S.; Tian, Z. The anti-cancer effects of fucoidan: A review of both in vivo and in vitro investigations. Cancer Cell Int. 2020, 20, 154. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Minami, M.; Kobayashi, M. Antitumor activity of TZT-1027 (Soblidotin). Anticancer Res. 2006, 36, 1973–1981. [Google Scholar]
- Natsume, T.; Watanabe, J.; Koh, Y.; Fuijo, N.; Ohe, Y.; Horiuchi, T.; Saijo, N.; Nishio, K.; Kobayashi, M. Antitumor activity of TZT-1027 (Soblidotin) against vascular endothelial growth factor-secreting human lung cancer in vivo. Cancer Sci. 2003, 94, 826–833. [Google Scholar] [CrossRef]
- Atallah, J.; Khachfe, H.H.; Berro, J.; Assi, H.I. The use of heparin and heparin-like molecules in cancer treatment: A review. Cancer Treat. Res. Commun. 2020, 24, 100192. [Google Scholar] [CrossRef]
- Niers, T.M.H.; Klerk, C.P.W.; DiNisio, M.; Noorden, C.J.F.V.; Buller, H.R.; Reitsma, P.H.; Richel, D.J. Mechanisms of heparin induced anti-cancer activity in experimental cancer models. Crit. Rev. Oncol. Hematol. 2007, 61, 195–207. [Google Scholar] [CrossRef]
- Magalhaes, K.D.; Costa, L.S.; Fidelis, G.P.; Oliveira, R.M.; Nobre, L.T.D.B.; Dantas-Santos, N.; Camara, R.B.G.; Albuquerque, I.R.L.; Cordeiro, S.L.; Sabry, D.A.; et al. Anticoagulant, antioxidant and antitumor activities of heterofucans from the seaweed Dictyopteris delicatula. Int. J. Mol. Sci. 2011, 12, 3352–3365. [Google Scholar] [CrossRef]
- Heiferman, M.J.; Salabat, M.R.; Ujiki, M.B.; Strouch, M.J.; Cheon, E.C.; Silverman, R.B.; Bentrem, D.J. Sansalvamide induces pancreatic cancer growth arrest through changes in the cell cycle. Anticancer Res. 2010, 30, 73–78. [Google Scholar]
- Vasko, R.C.; Rodriguez, R.A.; Cunningham, C.N.; Ardi, V.C.; Agard, D.A.; McAlpine, S.R. Mechanistic studies of Sansalvamide A-amide: An allosteric modulator of Hsp90. ACS Med. Chem. Lett. 2010, 1, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Galmarini, C.M.; D’Incalci, M.; Allavena, P. Trabectedin and plitidepsin: Drugs from the sea that strike the tumor microenvironment. Mar. Drugs. 2014, 12, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.; Jackson, W.H.; Pettit, G.R.; Wells, A.; Kraft, A.S. Treatment of human prostate cancer cells with dolastatin 10, a peptide isolated from a marine shell-less mollusk. Prostate 1998, 34, 175–181. [Google Scholar] [CrossRef]
- Gao, G.; Wang, Y.; Hua, H.; Li, D.; Tang, C. Marine antitumor peptide dolastatin 10: Biological activity, structural modification and synthetic chemistry. Mar. Drugs 2021, 19, 363. [Google Scholar] [CrossRef]
- Swami, U.; Shah, U.; Goel, S. Eribulin in cancer treatment. Mar. Drugs 2015, 13, 5016–5058. [Google Scholar] [CrossRef]
- Fenical, W.; Jensen, P.R.; Palladino, M.A.; Lam, K.S.; Lloyd, G.K.; Potts, B.C. Discovery and development of the anticancer agent salinosporamide A (NPI-0052). Bioorg. Med. Chem. 2009, 17, 2175–2180. [Google Scholar] [CrossRef]
- Gulder, T.A.M.; Moore, B.S. Salinosporamide natural products: Potent 20 S proteasome inhibitors as promising cancer chemotherapeutics. Angew. Chem. Int. Ed. Engl. 2010, 49, 9346–9367. [Google Scholar] [CrossRef]
- Wu, L.; Ye, K.; Jiang, S.; Zhou, G. Marine power on cancer: Drugs, lead compounds, and mechanisms. Mar. Drugs 2021, 19, 488. [Google Scholar] [CrossRef]
- Raninga, P.V.; Lee, A.; Sinha, D.; Dong, L.F.; Datta, K.K.; Lu, X.; Croft, P.K.; Dutt, M.; Hill, M.; Pouliot, N.; et al. Marizomib suppresses triple-negative breast cancer via proteasome and oxidative phosphorylation inhibition. Theranostics 2020, 1, 5259–5275. [Google Scholar] [CrossRef]
- Chhikara, B.S.; Parang, K. Dvelopment of cytarabine prodrugs and delivery systems for leukemia treatment. Expert Opin. Drug Deliv. 2010, 7, 1399–1414. [Google Scholar] [CrossRef]
- Ocio, E.M.; Maiso, P.; Chen, X.; Garayoa, M.; Alvarez-Fernandez, G.; San-Segundo, L.; Vilanova, D.; Lopez-Corral, L.; Montero, J.C.; Hernandez-Iglesias, T.; et al. Zalypsis: A novel marine-derived compound with potent antimyeloma activity that reveals high sensitivity of malignant plasma cells to DNA double-strand breaks. Blood 2009, 113, 3781–3791. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, D.; Xie, G.R. Effects of Gekko sulfated polysaccharide on the proliferation and differentiation of hepatic cancer cell line. Cell Biol. Int. 2006, 30, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Gu, X.X.; Geng, D.; Sun, H.Y.; Wang, C.M.; Jiang, G.X.; Hou, X.N.; Ma, C.H. Differentiation of bel-7402 human hepatocarcinoma cells induced by aqueous extracts of fresh gecko (AG) and its anti-tumor activity in vivo. J. Ethnopharmacol. 2014, 155, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, J.G.; Wang, S.Y.; Li, Y.; Wu, Y.P.; Xi, S.M. Antitumor effect and mechanism of Gecko on human esophageal carcinoma cell lines in vitro and xenografted sarcoma 180 in Kunming mice. World J. Gastroenterol. 2008, 14, 3990–3996. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Namavari, M.; Rashidi, M.; Fahmidehkar, M.A.; Seghatoleslam, A. Inhibitory effects of Cyrtopodion scabrum extract on growth of human breast and colorectal cancer cells. Asian Pac. J. Cancer Prev. 2015, 16, 5465–5570. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, G.Y.; Park, S.Y.; Jo, A.; Kim, M.; Leem, S.H.; Jun, W.J.; Shim, S.I.; Lee, S.C.; Chung, J.W. Gecko proteins induce the apoptosis of bladder cancer 5637 cells by inhibiting Akt and activating the intrinsic caspase cascade. BMB Rep. 2015, 48, 531–536. [Google Scholar] [CrossRef]
- Jeong, A.J.; Chung, C.N.; Kim, H.J.; Bae, K.S.; Choi, S.; Jun, W.J.; Shim, S.I.; Kang, T.H.; Leem, S.H.; Chung, J.W. Gecko proteins exert anti-tumor effect against cervical cancer cells via PI3-kinase/Akt pathway. Korean J. Physiol. Pharmacol. 2012, 16, 361–365. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, D.G.; Park, S.Y.; Jo, A.; Kim, H.K.; Han, J.; Min, J.K.; Chung, J.W. Gekkonidae, lizard tail extracts elicit apoptotic response against non-small lung cancer via inhibiting Akt signaling. Biomed. Pharmacother. 2019, 116, 109050. [Google Scholar]
- Mao, X.M.; Fu, Q.R.; Li, H.L.; Zheng, Y.H.; Chen, S.M.; Hy, X.Y.; Chen, Q.X.; Chen, Q.H. Crocodile choline from Crocodylus siamensis induces apoptosis of human gastric cancer. Tumour Biol. 2017, 39, 1010428317694320. [Google Scholar] [CrossRef]
- Siddiqui, R.; Jeyamogan, S.; Ali, S.M.; Abbas, F.; Sagatheva, K.A.; Khan, N.A. Crocodiles and alligators: Antiamoebic and antitumor compounds of crocodiles. Exp. Parasitol. 2017, 183, 194–200. [Google Scholar] [CrossRef]
- Patathananone, S.; Thammasirirak, S.; Daduang, J.; Chung, J.G.; Temsiripong, Y.; Daduang, S. Bioactive compounds from crocodile (Crocodylus siamensis) white blood cells induced apoptotic cell death in HeLa cells. Environ. Toxicol. 2016, 31, 986–987. [Google Scholar] [CrossRef] [PubMed]
- Patathananone, S.; Thammasirirak, S.; Daduang, J.; Chung, J.G.; Temsiripong, Y.; Daduang, S. Inhibition of HeLa cells metastasis by bioactive compounds in crocodile (Crocodylus siamensis) white blood cells extract. Environ. Toxicol. 2016, 31, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Phosri, S.; Jangpromma, N.; Chang, L.C.; Tan, G.T.; Wongwiwatthananukit, S.; Maijaroen, S.; Anwised, P.; Payoungkiattikun, W.; Klaynongsruang, S. Siamese crocodile white blood cell extract inhibits cell proliferation and promotes autophagy in multiple cancer cell lines. J. Microbiol. Biotechnol. 2018, 28, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cheung, H. Turtle shell extract as a functional food and its component-based comparison among different species. Hong Kong Pharm. J. 2012, 19, 33–37. [Google Scholar]
- Chen, D.; Yao, W.J.; Zhang, X.L.; Han, X.Q.; Qu, X.Y.; Ka, W.B.; Sun, D.G.; Wu, X.Z.; Wen, W.Y. Effects of Gekko sulfated polysaccharide-protein complex on human hepatoma SMMC-7721 cells: Inhibition of proliferation and migration. J. Ethnopharmacol. 2010, 127, 702–708. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.G.; Li, R.F.; Li, Y.; Cui, Z.C.; Duan, L.X.; Lu, F. Gecko crude peptides induce apoptosis in human liver carcinoma cells in vitro and exert antitumor activity in a mouse ascites H22 xenograft model. J. Biomed. Biotechnol. 2012, 2012, 743573. [Google Scholar] [CrossRef]
- Duan, Y.M.; Jin, Y.; Guo, M.L.; Duan, L.X.; Wang, J.G. Differentially expressed genes of HepG2 cells treated with gecko polypeptide mixture. J. Cancer 2018, 9, 2723–2733. [Google Scholar] [CrossRef]
- Jeyamogan, S.; Khan, N.A.; Sagathevan, K.; Siddiqui, R. Anticancer properties of Asian water monitor lizard (Varanus salvator), python (Malayophyon reticulatus) and tortoise (Cuora kamaroma amboinensis). Anticancer Agents Med. Chem. 2020, 20, 1558–1570. [Google Scholar] [CrossRef]
- Tian, L.; Deng, Y.T.; Dong, X.; Fan, J.Y.; Li, H.L.; Ding, Y.M.; Peng, W.X.; Chen, Q.X.; Shen, D.Y. Siamese crocodile bile induces apoptosis in NCI-H1299 human non-small cell lung cancer cells via a mitochondria-mediated intrinsic pathway and inhibits tumorigenesis. Mol. Med. Rep. 2017, 15, 1727–1737. [Google Scholar] [CrossRef]
- Jebali, J.; Fakhfekh, E.; Morgen, M.; Srairi-Abid, N.; Majdoub, J.; Gargour, A.; Ayeb, M.E.; Luis, J.; Marrakchi, N.; Sarray, S. Lebecin, a new C-type lectin like protein from Macrovipera lebetina venom with anti-tumor activity against the breast cancer cell line MDA-MB231. Toxicon 2014, 86, 16–27. [Google Scholar] [CrossRef]
- Azevedo, F.V.P.V.; Lopes, D.S.; Gimenes, S.N.C.; Ache, D.C.; Vecchi, L.; Alves, P.T.; Guimaraes, D.O.; Rodrigues, R.S.; Goulart, L.R.; Rodrigues, V.M.; et al. Human breast cancer cell death induced by BnSP-6, a Lys-49 PLA2 homologue from Bothrops pauloensis venom. Int. J. Biol. Macromol. 2016, 82, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Attarde, S.S.; Pandit, S.V. Cytotoxic activity of NN-32 toxin from Indian spectacled cobra venom on human breast cancer cell lines. BMC Complement Altern. Med. 2017, 17, 503. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, K.; Han, S.; Tian, Y.H.; Hu, P.C.; Xu, X.L.; He, Y.Q.; Pan, W.T.; Gao, Y.; Zhang, Z.; et al. Chlorotoxin targets ERα/VaSP signaling pathway to combat breast cancer. Cancer Med. 2019, 8, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, M.B.; Riahi-Chebbi, I.; Souid, S.; Othman, H.; Aloui, Z.; Srairi-Abid, N.; Karou, H.; Gasmi, A.; Magnenat, E.M.; Wells, T.N.C.; et al. Macrovipecetin, a C-type lectin from Macrovipera lebetina venom, inhibits proliferation migration and invasion of SK-MEL-28 human melanoma cells and enhances their sensitivity to cisplatin. Biochim. Biophys. Acta. Gen. Subj. 2018, 1862, 600–614. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.F.; Amaral, C.; Augusto, T.V.; Correia-da-Silva, G.; Andrade, C.M.; Torqueti, M.R.; Teixeira, N. The anti-cancer potential of crotoxin in estrogen receptor-positive breast cancer: Its effects and mechanism of action. Toxicon 2021, 200, 69–77. [Google Scholar] [CrossRef]
- Derakhshani, A.; Silverstris, N.; Hajiasgharzadeh, K.; Mahmoudzadeh, S.; Fereidouni, M.; Paradiso, A.V.; Brunetti, O.; Atarod, D.; Safarpour, H.; Baradaran, B. Expression and characterization of a novel recombinant cytotoxin II from Naja naja oxiana venom: A potential treatment for breast cancer. Int. J. Biol. Macromol. 2020, 162, 1283–1292. [Google Scholar] [CrossRef]
- Ye, B.; Xie, Y.; Qin, Z.H.; Wu, J.C.; Han, R.; He, J.K. Anti-tumor activity of CrTX in human lung adenocarcinoma cell line A549. Acta Pharmacol. Sin. 2011, 32, 1397–1401. [Google Scholar] [CrossRef]
- Pathan, J.; Mondal, S.; Sarkar, A.; Chakrabarty, D. Daboialectin, a C-type lectin from Russell’s viper venom induces cytoskeletal damage and apoptosis in human lung cancer cells in vitro. Toxicon 2017, 127, 11–21. [Google Scholar] [CrossRef]
- Liu, Y.; Ming, W.; Wang, Y.; Liu, S.; Qiu, Y.; Xiang, Y.; Hu, L.; Fan, L.; Peng, X.; Wang, H.; et al. Cytotoxin 1 from Naja atra Cantor venom induced necroptosis of leukemia cells. Toxicon 2019, 165, 110–115. [Google Scholar] [CrossRef]
- Chernyshenko, V.; Petruk, N.; Korolova, D.; Kasatkina, L.; Gornytska, O.; Platonova, T.; Chernyshenko, T.; Revriev, A.; Dzhus, O.; Garmanchuk, L.; et al. Antiplatelet and anti-proliferative action of disintegrin from Echis multisquamatis snake venom. Croat. Med. J. 2017, 58, 118–127. [Google Scholar] [CrossRef]
- Lafnoune, A.; Lee, S.Y.; Heo, J.Y.; Gourja, I.; Darkaoui, B.; Abdelkafi-Koubaa, Z.; Chgoury, F.; Daoudi, K.; Chakir, S.; Cadi, R.; et al. Anti-cancer effect of Moroccan cobra Naja hage venom and its fractions against hepatocellular carcinoma in 3D cell culture. Toxins 2021, 13, 402. [Google Scholar] [CrossRef] [PubMed]
- Moridikia, A.; Zargan, J.; Sobati, H.L.; Goodarzi, H.R.; Hajinourmohamadi, A. Anticancer and antibacterial effects of Iranian viper (Vipera latifii) venom; an in-vitro study. J. Cell. Physiol. 2018, 233, 6790–6797. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, K.A.; Mostafa, A.A.; Danny, M.R.; Gamal, B. Induction of apoptosis and growth arrest in human breast carcinoma cells by a snake (Walterinnesia aegyptia) venom combined with silica nanoparticles: Crosstalk between Bcl2 and Caspase 3. Cell. Physiol. Biochem. 2012, 30, 653–665. [Google Scholar]
- Derakhshani, A.; Silvestris, N.; Hemmat, N.; Asadzadeh, Z.; Shadbad, M.A.; Nourbakhsh, N.S.; Mobasheri, L.; Vahedi, P.; Shahmirzaie, M.; Brunetti, O.; et al. Targeting TGF-β-mediated SMAD signaling pathway via novel recombinant cytotoxin II: A potent protein from Naja naja oxiana venom in melanoma. Molecules 2020, 25, 5148. [Google Scholar] [CrossRef] [PubMed]
- Maria, D.A.; Silva, M.G.L.; Junior, M.C.C.; Ruiz, I.R.G. Antiproliferative effect of the jararhagin toxin on B16F10 murine melanoma. BMC Complement Altern. Med. 2014, 14, 446. [Google Scholar] [CrossRef] [PubMed]
- Salama, W.H.; Ibrahim, N.M.; Hakim, A.E.E.; Bassuiny, R.I.; Mohamed, M.M.; Mousa, F.M.; Ali, M.M. L-amino acid oxidase from Cerastes vipera snake venom: Isolation, characterization and biological effects on bacteria and tumor cell lines. Toxicon 2018, 150, 270–279. [Google Scholar] [CrossRef]
- Lu, W.; Hu, L.; Yang, J.; Sun, X.; Yan, H.; Liu, J.; Chen, J.; Cheng, X.; Zhou, Q.; Yu, Y.; et al. Isolation and pharmacological characterization of a new cytotoxic L-amino acid oxidase from Bungarus multicinctus snake venom. J. Ethnopharmacol. 2018, 213, 311–320. [Google Scholar] [CrossRef]
- Mostafa, I.A.; Sanaa, O.A.; Mohamed, A.E.; Mohammad, Y.A.; Serag, E.I.E.; Aly, F.M. Evaluation of the anticancer potential of crude, irradiated Cerastes cerastes snake venom and propolis ethanolic extract & related biological alterations. Molecules 2021, 26, 7057. [Google Scholar]
- Uddin, M.B.; Lee, B.H.; Nikapitiya, C.; Kim, J.H.; Kim, T.H.; Lee, H.C.; Kim, C.G.; Lee, J.S.; Kim, C.J. Inhibitory effects of bee venom and its components against viruses in vitro and in vivo. J. Microbiol. 2016, 54, 853–866. [Google Scholar] [CrossRef]
- Moreno, M.; Giralt, E. Three valuable peptides from bee and wasp venoms for therapeutic and biotechnological use: Melittin, apamin and mastoparan. Toxins 2015, 7, 1126–1150. [Google Scholar] [CrossRef]
- Chalk, C.H.; Benstead, T.J.; Pound, J.D.; Keezer, M.R. Medical treatment for botulism. Cochrane Database Syst. Rev. 2019, 4, CD008123. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.K.; Sobel, J.; Chatham-Stephens, K.; Luquez, C. Clinical guidelines for diagnosis and treatment of botulism, 2021. MMWR Recomm. Rep. 2021, 70, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Lonati, D.; Schicchi, A.; Crevani, M.; Buscaglia, E.; Scaravaggi, G.; Maida, F.; Cirronis, M.; Petrolini, V.M.; Locatelli, C.A. Foodborne botulism: Clinical diagnosis and medical treatment. Toxins 2020, 12, 509. [Google Scholar] [CrossRef] [PubMed]
- Falcao, C.B.; Radis-Baptista, G. Crotamine and crotalicidin, membrane active peptides from Crotalus durissus terrificus rattlesnake venom, and their structurally-minimized fragments for applications in medicine and biotechnology. Peptides 2020, 126, 170234. [Google Scholar] [CrossRef]
- Badr, G.; Al-Sadoon, M.K.; Rabah, D.M. Therapeutic efficacy and molecular mechanisms of snake (Walterinnesia aegyptia) venom-loaded silica nanoparticles in the treatment of breast cancer- and prostate cancer-bearing experimental mouse models. Free Radic. Biol. Med. 2013, 65, 175–189. [Google Scholar] [CrossRef]
- Badr, G.; Al-Saddon, M.K.; Abdel-Maksoud, M.A.; Rabah, D.M.; El-Toni, A. Cellular and molecular mechanisms underlie the anti-tumor activities exerted by Walterinnesia aegyptia venom combined with silica nanoparticles against multiple myeloma cancer cell types. PLoS ONE 2012, 7, e51661. [Google Scholar] [CrossRef]
- Ma, D.L.; Wu, C.; Cheng, S.S.; Lee, J.W.; Han, Q.B.; Leung, C.H. Development of natural product-conjugated metal complexes as cancer therapies. Int. J. Mol. Sci. 2019, 20, 341. [Google Scholar] [CrossRef]
- Baker, C.; Rodrigues, T.; Almeida, B.P.; Barbosa-Morais, N.L.; Bernardes, G.J.L. Natural product-drug conjugates for modulation of TRPV1-expressing tumors. Bioorg. Med. Chem. 2019, 27, 2531–2536. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, Z.Y.; Wang, P.Y.; Li, Y.J.; Wang, R.R.; Xie, S.Y. Nanoplatform-based natural products co-delivery system to surmount cancer multidrug-resistant. J. Control Release 2021, 336, 396–409. [Google Scholar] [CrossRef]
- Worsham, R.D.; Thomas, V.; Farid, S.S. Potential of continuous manufacturing for liposomal drug products. Biotechnol. J. 2019, 14, e1700740. [Google Scholar] [CrossRef]
- Billings, C.; Anderson, D.E. Role of implantable drug delivery devise with dual platform capabilities in the prevention and treatment of bacterial osteomyelitis. Bioengineering 2022, 9, 65. [Google Scholar] [CrossRef] [PubMed]
| Natural Components | Source | Type of Cancer | Mechanism | Refs. |
|---|---|---|---|---|
| Vincristine | Catharanthus roseus | Acute lymphocytic leukemia Acute myeloid leukemia Hodgkin’s disease Neuroblastoma Lung cancer | Induction of apoptosis via binding to β-tubulin during cell division | [42] |
| Vinblastine | Catharanthus roseus | Leukemia Lymphoma | Induction of apoptosis via microtubule interference during cell division | [43] |
| Paclitaxel | Taxus brevifolia | Breast cancer Kaposi’s sarcoma Pancreatic cancer Gastric cancer | Inhibition of mitotic spindle assembly during cell division | [44] |
| Resveratrol | Rheum rhaponticum | Lymphoma Breast cancer | Suppression of Treg cells Inhibition of TGF-β production Interference interaction of PD-1/PD-L1 | [45] |
| Curcumin | Curcuma longa | Breast cancer Lung cancer Gastric cancer Colon cancer | Induction of cell cycle arrest and apoptosis via inhibition of ERK, PI3K/Akt, Notch-1 and STAT-3 | [46] |
| Capsaicin | Capsicum annuum | Osteosarcoma | Promotion of immunogenic cell death by mediating phagocytosis | [47] |
| Epigallocatechin-3-gallate (EGCG) | Camelia sinensis | Prostate cancer Melanoma | Induction of apoptosis and anti-angiogenesis | [48] |
| Parthenolide | Tanacetum parthenium | Breast cancer Lung cancer | Inhibition of JAK/STAT signaling Downregulation of EGFR expression | [49,50] |
| Ginsenoside Rg3 | Panax ginseng | Breast cancer Colon cancer Gastric cancer Liver cancer | Induction of apoptosis via inhibition of ERK and Akt Inhibition of proliferation via G1 phase cell cycle arrest | [51] |
| Wogonin | Scutellaria baicalensis | Colon cancer Ovarian cancer | Inhibition of YAP1 expression Inhibition of VEGF, Bcl-2 and Akt signaling | [52,53] |
| Natural Components | Source | Type of Cancer | Mechanism | Refs. |
|---|---|---|---|---|
| Fucoidan | Ascophyllum nodosum | Colon cancer Breast cancer | Activation of macrophages and NK cells Induction of G1 phase cell cycle arrest | [54] |
| TZT-1027 (Soblidotin) | Dolabella auricularia | Lung cancer Colon cancer | Anti-angiogenesis Induction of apoptosis via microtubule interference during cell division | [55,56] |
| Heparin | Dictyopteris delicatula | Lung cancer Liver cancer Cervical cancer | Inhibition of PI3K/Akt signaling Anti-metastasis | [57,58,59] |
| Sansalvamide | Fusarium solani | Pancreatic cancer Colon cancer Prostate cancer Breast cancer | Induction of apoptosis via G1 phase cell cycle arrest | [60,61] |
| Plitidepsin | Aplidium albicans | Chronic lymphocytic leukemia | Inhibition of CXCL12 release from nurse-like cells (NLCs) | [62] |
| Dolastatin 10 | Dolabella auricularia | Breast cancer Lung cancer Prostate cancer | Induction of apoptosis via microtubule interference during cell division | [63,64] |
| Halichondrin B (Eribulin) | Halichondria okadai | Breast cancer Liposarcoma | Induction of apoptosis via microtubule interference during cell division | [65] |
| Salinosporamide A (Marizomib) | Salinispora tropica | Lymphoma Breast cancer | Induction of apoptosis via inhibition of proteasome activity | [66,67,68,69] |
| C-nucleoside (Cytarabine) | Cryptotheca crypta | Leukemia | Inhibition of DNA synthesis | [70] |
| Jorumycin (Zalypsis) | Jorunna funebris | Leukemia Lung cancer Colon cancer | Induction of apoptosis via G1 phase cell cycle arrest | [68,71] |
| Category | Natural Components | Source | Type of Cancer | Mechanism | Refs. |
|---|---|---|---|---|---|
| Extracts | Sulfated polysaccharide | Gekko swinhonis | Liver cancer | Inhibition of proliferation and differentiation | [72] |
| Aqueous extracts | Gekko swinhonis | Liver cancer | Inhibition of growth Reduction in alpha fetoprotein | [73] | |
| Powder | Gekko japonicus | Esophageal carcinoma Sarcoma | Induction of apoptosis via decrease in VEGF and bFGF expression | [74] | |
| Ethanol extracts | Cyrtopodion scabrum | Breast cancer Colon cancer | Inhibition of growth and migration | [75] | |
| Aqueous extracts | Eublepharis macularius | Bladder cancer Cervical cancer Lung cancer | Induction of apoptosis via inhibition of PI3K/Akt signaling Induction of caspase-dependent apoptosis via G2/M phase cell cycle arrest | [76,77,78] | |
| Crocodile choline | Crocodylus siamensis | Gastric cancer | Induction of apoptosis via G2/M phase cell cycle arrest | [79] | |
| Methanol extracts | Crocodylus palustris | Prostate cancer | Induction of cell death | [80] | |
| Aqueous extracts of white blood cells | Crocodylus siamensis | Cervical cancer | Induction of mitochondria/caspase-3/caspase-9-mediated apoptosis Inhibition of proliferation, migration and invasion | [81,82] | |
| Aqueous extracts of white blood cells | Crocodylus siamensis | Lung cancer Prostate cancer Breast cancer Colorectal cancer | Induction of apoptosis via G2/M phase cell cycle arrest | [83] | |
| Aqueous extracts | Chinemys reevesii | Leukemia Liver cancer | Induction of cell death | [84] | |
| Aqueous extracts | Cuora aurocapitata | Leukemia Liver cancer | Induction of cell death | [84] | |
| Aqueous extracts | Trachemys scripta | Leukemia Liver cancer | Induction of cell death | [84] | |
| Crude peptides | Sulfated polysaccharide–protein complex | Gekko swinhonis | Liver cancer | Inhibition of proliferation and migration | [85] |
| Alcohol extracted crude peptides | Gekko japonicus | Liver cancer | Induction of apoptosis via Bcl-2/Bax pathway regulation Reduction in VEGF expression | [86] | |
| Polypeptide mixture | Gekko japonicus | Liver cancer | Induction of apoptosis Promotion of ROS-related processes and UPR | [87] | |
| Sera | Blood serum | Varanus salvator | Cervical cancer Prostate cancer Breast cancer | Induction of cell death | [88] |
| Blood serum | Malayopython reticulatus | Cervical cancer Prostate cancer Breast cancer | Induction of cell death | [88] | |
| Blood serum | Cuora amboinensis karamoja | Cervical cancer Prostate cancer Breast cancer | Induction of cell death | [88] | |
| Bile juice | Crocodylus siamensis | Lung cancer | Induction of mitochondria/caspase-3/caspase-9-mediated apoptosis | [89] | |
| Venom | Lectin | Macrovipera lebetina | Breast cancer | Inhibition of integrin-mediated attachment and migration | [90] |
| BnSP-6 Lys-49 PLA2 | Bothrops pauloensis | Breast cancer | Induction of apoptosis Inhibition of adhesion, migration and angiogenesis | [91] | |
| NN-3 | Naja naja oxiana | Breast cancer | Inhibition of proliferation | [92] | |
| Chlorotoxin | Leiurus quinquestriatus | Breast cancer | Inhibition of proliferation, migration and invasion | [93] | |
| Macrovipecetin | Macrovipera lebetina | Melanoma | Inhibition of proliferation, migration and invasion | [94] | |
| Crotoxin | Crotalus durissus terrificus | Breast cancer | Induction of apoptosis via G2/M phase cell cycle arrest Inhibition of ERK signaling | [95] | |
| Cytotoxin 2 | Naja naja oxiana | Breast cancer Lung cancer | Induction of apoptosis via G1 phase cell cycle arrest Activation of caspase-3 and p38 signaling | [96,97] | |
| Daboialectin | Daboia russelii | Lung cancer | Induction of apoptosis Inhibition of migration | [98] | |
| Cytotoxin 1 | Naja atra | Leukemia | Induction of necroptosis | [99] | |
| Disintegrin | Echis multisquamatus | Cervical cancer | Inhibition of proliferation | [100] | |
| Venom extracts | Naja hage | Liver cancer | Induction of cell death | [101] | |
| Venom extracts | Vipera latifii | Liver cancer | Induction of cell death | [102] | |
| Venom extracts | Walterinnesia aegyptia | Breast cancer | Induction of apoptosis Activation of caspase-3 pathway | [103] | |
| Recombinant protein cytotoxin 2 | Naja naja oxiana | Melanoma | Induction of apoptosis via TGF-β-mediating SMAD signaling | [104] | |
| Jararhagin | Bothrops jararaca | Murine melanoma | Activation of caspase-3 pathway Suppression of tumor growth and metastasis | [105] | |
| L-Amino acid oxidase | Cerastes vipera | Breast cancer Liver cancer Lung cancer Prostate cancer Colon cancer | Induction of cell death | [106,107] | |
| Irradiated crude venom | Cerastes cerastes | Lung cancer Prostate cancer | Induction of apoptosis via G2/M phase cell cycle arrest | [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.Y.; Choi, H.; Chung, J.W. Reptiles as Promising Sources of Medicinal Natural Products for Cancer Therapeutic Drugs. Pharmaceutics 2022, 14, 874. https://doi.org/10.3390/pharmaceutics14040874
Park SY, Choi H, Chung JW. Reptiles as Promising Sources of Medicinal Natural Products for Cancer Therapeutic Drugs. Pharmaceutics. 2022; 14(4):874. https://doi.org/10.3390/pharmaceutics14040874
Chicago/Turabian StylePark, Soon Yong, Hyeongrok Choi, and Jin Woong Chung. 2022. "Reptiles as Promising Sources of Medicinal Natural Products for Cancer Therapeutic Drugs" Pharmaceutics 14, no. 4: 874. https://doi.org/10.3390/pharmaceutics14040874
APA StylePark, S. Y., Choi, H., & Chung, J. W. (2022). Reptiles as Promising Sources of Medicinal Natural Products for Cancer Therapeutic Drugs. Pharmaceutics, 14(4), 874. https://doi.org/10.3390/pharmaceutics14040874






