Cell-Penetrating Peptides with Unexpected Anti-Amyloid Properties
Abstract
1. Introduction
2. Amyloid Processes in Alzheimer’s Disease Involving the Amyloid-β Peptide
3. Anti-Prion Properties of Signal Peptide Derived CPPs
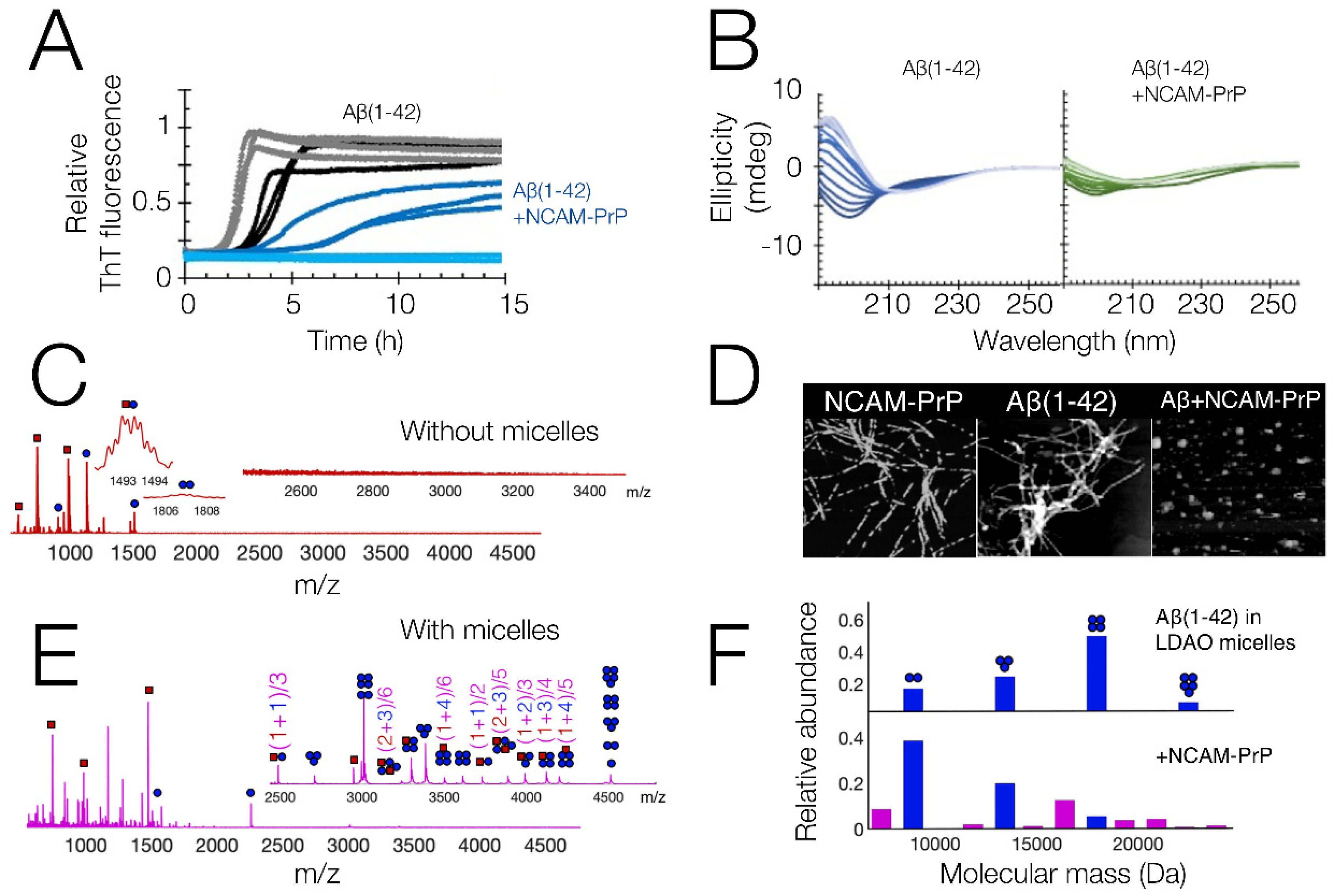
4. Anti-Amyloid Properties of Signal Peptide Derived CPPs
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Löfgren, K.; Wahlström, A.; Lundberg, P.; Langel, U.; Gräslund, A.; Bedecs, K. Antiprion properties of prion protein-derived cell-penetrating peptides. FASEB J. 2008, 22, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Henning-Knechtel, A.; Kumar, S.; Wallin, C.; Król, S.; Wärmländer, S.K.T.S.; Jarvet, J.; Esposito, G.; Kirmizialtin, S.; Gräslund, A.; Hamilton, A.D.; et al. Designed Cell-Penetrating Peptide Inhibitors of Amyloid-beta Aggregation and Cytotoxicity. Cell Rep. Phys. Sci. 2020, 1, 100014. [Google Scholar] [CrossRef]
- Gräslund, A.; Eriksson, L.E.G. Biophysical Studies of Cell-Penetrating Peptides. In Cell-Penetrating Peptides; CRC Press: Boca Raton, FL, USA, 2002; pp. 223–244. [Google Scholar] [CrossRef]
- Xie, J.; Shen, Z.; Anraku, Y.; Kataoka, K.; Chen, X. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials 2019, 224, 119491. [Google Scholar] [CrossRef]
- Sormanni, P.; Vendruscolo, M. Protein Solubility Predictions Using the CamSol Method in the Study of Protein Homeostasis. Cold Spring Harb. Perspect. Biol. 2019, 11, a033845. [Google Scholar] [CrossRef]
- Nielsen, H.; Engelbrecht, J.; Brunak, S.; Von Heijne, G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997, 10, 1–6. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; Von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Król, S.; Österlund, N.; Vosough, F.; Jarvet, J.; Wärmländer, S.; Barth, A.; Ilag, L.L.; Magzoub, M.; Gräslund, A.; Mörman, C. The amyloid-inhibiting NCAM-PrP peptide targets Aβ peptide aggregation in membrane-mimetic environments. Science 2021, 24, 102852. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Abelein, A.; Abrahams, J.P.; Danielsson, J.; Gräslund, A.; Jarvet, J.; Luo, J.; Tiiman, A.; Wärmländer, S.K.T.S. The hairpin conformation of the amyloid β peptide is an important structural motif along the aggregation pathway. J. Biol. Inorg. Chem. 2014, 19, 623–634. [Google Scholar] [CrossRef]
- Mroczko, B.; Groblewska, M.; Litman-Zawadzka, A.; Kornhuber, J.; Lewczuk, P. Amyloid β oligomers (AβOs) in Alzheimer’s disease. J. Neural Transm. 2018, 125, 177–191. [Google Scholar] [CrossRef]
- Sandberg, A.; Luheshi, L.M.; Sollvander, S.; de Barros, T.P.; Macao, B.; Knowles, T.P.J.; Biverstal, H.; Lendel, C.; Ekholm-Petterson, F.; Dubnovitsky, A.; et al. Stabilization of neurotoxic Alzheimer amyloid-oligomers by protein engineering. Proc. Natl. Acad. Sci. USA 2010, 107, 15595–15600. [Google Scholar] [CrossRef] [PubMed]
- Sciacca, M.F.M.; Kotler, S.A.; Brender, J.R.; Chen, J.; Lee, D.K.; Ramamoorthy, A. Two-step mechanism of membrane disruption by Aβ through membrane fragmentation and pore formation. Biophys. J. 2012, 103, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Österlund, N.; Moons, R.; Ilag, L.L.; Sobott, F.; Gräslund, A. Native Ion Mobility-Mass Spectrometry Reveals the Formation of β-Barrel Shaped Amyloid-β Hexamers in a Membrane-Mimicking Environment. J. Am. Chem. Soc. 2019, 141, 10440–10450. [Google Scholar] [CrossRef] [PubMed]
- Michaels, T.C.T.; Šarić, A.; Curk, S.; Bernfur, K.; Arosio, P.; Meisl, G.; Dear, A.J.; Cohen, S.I.A.; Dobson, C.M.; Vendruscolo, M.; et al. Dynamics of oligomer populations formed during the aggregation of Alzheimer’s Aβ42 peptide. Nat. Chem. 2020, 12, 445–451. [Google Scholar] [CrossRef]
- Tolar, M.; Abushakra, S.; Hey, J.A.; Porsteinsson, A.; Sabbagh, M. Aducanumab, gantenerumab, BAN2401, and ALZ-801—The first wave of amyloid-targeting drugs for Alzheimer’s disease with potential for near term approval. Alzheimer’s Res. Ther. 2020, 12, 95. [Google Scholar] [CrossRef]
- Biancalana, M.; Koide, S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim. Biophys. Acta Proteins Proteom. 2010, 1804, 1405–1412. [Google Scholar] [CrossRef]
- Linse, S.; Thalberg, K.; Knowles, T.P.J. The unhappy chaperone. QRB Discov. 2021, 2, e7. [Google Scholar] [CrossRef]
- Meisl, G.; Kirkegaard, J.B.; Arosio, P.; Michaels, T.C.T.; Vendruscolo, M.; Dobson, C.M.; Linse, S.; Knowles, T.P. Molecular mechanisms of protein aggregation from global fitting of kinetic models. Nat. Protoc. 2016, 11, 252–272. [Google Scholar] [CrossRef]
- Pansieri, J.; Ostojić, L.; Iashchishyn, I.A.; Magzoub, M.; Wallin, C.; Wärmländer, S.K.T.S.; Gräslund, A.; Ngoc, M.N.; Smirnovas, V.; Svedruzic, Z.; et al. Pro-Inflammatory S100A9 Protein Aggregation Promoted by NCAM1 Peptide Constructs. ACS Chem. Biol. 2019, 14, 1410–1417. [Google Scholar] [CrossRef]
- Lieblein, T.; Zangl, R.; Martin, J.; Hoffmann, J.; Hutchison, M.J.; Stark, T.; Stirnal, E.; Schrader, T.; Schwalbe, H.; Morgner, N. Structural rearrangement of amyloid-β upon inhibitor binding suppresses formation of Alzheimer’s disease related oligomers. eLife 2020, 9, e59306. [Google Scholar] [CrossRef]
- Österlund, N.; Lundqvist, M.; Ilag, L.L.; Gräslund, A.; Emanuelsson, C. Amyloid-β oligomers are captured by the DNAJB6 chaperone: Direct detection of interactions that can prevent primary nucleation. J. Biol. Chem. 2020, 295, 8135–8144. [Google Scholar] [CrossRef] [PubMed]
- Puig, E.; Tolchard, J.; Riera, A.; Carulla, N. Somatostatin, an In Vivo Binder to Aβ Oligomers, Binds to βPFOAβ(1–42) Tetramers. ACS Chem. Neurosci. 2020, 11, 3358–3365. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, P.; Magzoub, M.; Lindberg, M.; Hällbrink, M.; Jarvet, J.; Eriksson, L.E.G.; Langel, U.; Gräslund, A. Cell membrane translocation of the N-terminal (1–28) part of the prion protein. Biochem. Biophys. Res. Commun. 2002, 299, 85–90. [Google Scholar] [CrossRef]
- Magzoub, M.; Sandgren, S.; Lundberg, P.; Oglęcka, K.; Lilja, J.; Wittrup, A.; Eriksson, L.E.G.; Langel, U.; Belting, M.; Gräslund, A. N-terminal peptides from unprocessed prion proteins enter cells by macropinocytosis. Biochem. Biophys. Res. Commun. 2006, 348, 379–385. [Google Scholar] [CrossRef]
- Söderberg, K.L.; Guterstam, P.; Langel, U.; Gräslund, A. Targeting prion propagation using peptide constructs with signal sequence motifs. Arch. Biochem. Biophys. 2014, 564, 254–261. [Google Scholar] [CrossRef]
- Gielnik, M.; Zhukova, L.; Zhukov, I.; Gräslund, A.; Kozak, M.; Wärmländer, S.K.T.S. The engineered peptide construct NCAM1-Aβ inhibits aggregation of the human prion protein (PrP). Acta Biochim. Pol. 2022, 69, 257–261. [Google Scholar]
- Tjernberg, L.O.; Näslund, J.; Lindqvist, F.; Johansson, J.; Karlström, A.R.; Thyberg, J.; Terenius, L.; Nordstedt, C. Arrest of -Amyloid Fibril Formation by a Pentapeptide Ligand. J. Biol. Chem. 1996, 271, 8545–8548. [Google Scholar] [CrossRef]
- Lowe, T.L.; Strzelec, A.; Kiessling, A.L.L.; Murphy, R.M. Structure-Function Relationships for Inhibitors of β-Amyloid Toxicity Containing the Recognition Sequence KLVFF. Biochemistry 2001, 40, 7882–7889. [Google Scholar] [CrossRef]
- Ciudad, S.; Puig, E.; Botzanowski, T.; Meigooni, M.; Arango, A.S.; Do, J.; Mayzel, M.; Bayoumi, M.; Chaignepain, S.; Maglia, G.; et al. Aβ(1–42) tetramer and octamer structures reveal edge conductivity pores as a mechanism for membrane damage. Nat. Commun. 2020, 11, 3014. [Google Scholar] [CrossRef]
- Österlund, N.; Luo, J.; Wärmländer, S.K.T.S.; Gräslund, A. Membrane-mimetic systems for biophysical studies of the amyloid-β peptide. Biochim. Biophys. Acta Proteins Proteom. 2018, 1867, 492–501. [Google Scholar] [CrossRef]
- Bett, C.K.; Serem, W.K.; Fontenot, K.R.; Hammer, R.P.; Garno, J.C. Effects of Peptides Derived from Terminal Modifications of the Aβ Central Hydrophobic Core on Aβ Fibrillization. ACS Chem. Neurosci. 2010, 1, 661–678. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Assarsson, A.; Hellstrand, E.; Cabaleiro-Lago, C.; Linse, S. Charge dependent retardation of amyloid β aggregation by hydrophilic proteins. ACS Chem. Neurosci. 2014, 5, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Österlund, N.; Kulkarni, Y.S.; Misiaszek, A.D.; Wallin, C.; Krüger, D.M.; Liao, Q.; Rad, F.M.; Jarvet, J.; Strodel, B.; Wärmländer, S.K.T.S.; et al. Amyloid-β Peptide Interactions with Amphiphilic Surfactants: Electrostatic and Hydrophobic Effects. ACS Chem. Neurosci. 2018, 9, 1680–1692. [Google Scholar] [CrossRef]
- Van Groen, T.; Schemmert, S.; Brener, O.; Gremer, L.; Ziehm, T.; Tusche, M.; Nagel-Steger, L.; Kadish, I.; Schartmann, E.; Elfgen, A.; et al. The Aβ oligomer eliminating D-enantiomeric peptide RD2 improves cognition without changing plaque pathology. Sci. Rep. 2017, 7, 16275. [Google Scholar] [CrossRef]
- Olubiyi, O.O.; Frenzel, D.; Bartnik, D.; Gluck, J.M.; Brener, O.; Nagel-Steger, L.; Funke, S.A.; Willbold, D.; Strodel, B. Amyloid aggregation inhibitory mechanism of arginine-rich D-peptides. Curr. Med. Chem. 2014, 21, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Willbold, D.; Kutzsche, J.; Willuweit, A.; Windisch, M.; Jürgens, D. Clinical phase I data of the first orally available anti-aβ-prionic drug PRI-002 that reverses behavioral and cognitive deficits, and decelerates neurodegeneration in AD animal models. Alzheimer’s Dement. 2020, 16, e12001. [Google Scholar] [CrossRef]
- Zhang, T.; Gering, I.; Kutzsche, J.; Nagel-Steger, L.; Willbold, D. Toward the Mode of Action of the Clinical Stage All-d-Enantiomeric Peptide RD2 on Aβ42 Aggregation. ACS Chem. Neurosci. 2019, 10, 4800–4809. [Google Scholar] [CrossRef]
- Neddenriep, B.; Calciano, A.; Conti, D.; Sauve, E.; Paterson, M.; Bruno, E.; Moffet, D.A. Short Peptides as Inhibitors of Amyloid Aggregation. Open Biotechnol. J. 2011, 5, 39–46. [Google Scholar] [CrossRef]
- Roterman, I.; Banach, M.; Konieczny, L. Towards the design of anti-amyloid short peptide helices. Bioinformation 2018, 14, 1–7. [Google Scholar] [CrossRef]
- Riek, R.; Eisenberg, D.S. The activities of amyloids from a structural perspective. Nature 2016, 539, 227–235. [Google Scholar] [CrossRef]
- Taylor, B.N.; Mehta, R.R.; Yamada, T.; Lekmine, F.; Christov, K.; Chakrabarty, A.M.; Green, A.; Bratescu, L.; Shilkaitis, A.; Beattie, C.W.; et al. Noncationic Peptides Obtained from Azurin Preferentially Enter Cancer Cells. Cancer Res. 2009, 69, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, A.; Khazaei, M.; Avan, A.; Hasanian, S.M.; Cho, W.C.; Soleimanpour, S. p28 Bacterial Peptide, as an Anticancer Agent. Front. Oncol. 2020, 10, 1303. [Google Scholar] [CrossRef] [PubMed]
- Horsley, J.R.; Jovcevski, B.; Wegener, K.L.; Yu, J.; Pukala, T.L.; Abell, A.D. Rationally designed peptide-based inhibitor of Aβ42 fibril formation and toxicity: A potential therapeutic strategy for Alzheimer’s disease. Biochem. J. 2019, 477, 2039–2054. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Österlund, N.; Wärmländer, S.K.T.S.; Gräslund, A. Cell-Penetrating Peptides with Unexpected Anti-Amyloid Properties. Pharmaceutics 2022, 14, 823. https://doi.org/10.3390/pharmaceutics14040823
Österlund N, Wärmländer SKTS, Gräslund A. Cell-Penetrating Peptides with Unexpected Anti-Amyloid Properties. Pharmaceutics. 2022; 14(4):823. https://doi.org/10.3390/pharmaceutics14040823
Chicago/Turabian StyleÖsterlund, Nicklas, Sebastian K. T. S. Wärmländer, and Astrid Gräslund. 2022. "Cell-Penetrating Peptides with Unexpected Anti-Amyloid Properties" Pharmaceutics 14, no. 4: 823. https://doi.org/10.3390/pharmaceutics14040823
APA StyleÖsterlund, N., Wärmländer, S. K. T. S., & Gräslund, A. (2022). Cell-Penetrating Peptides with Unexpected Anti-Amyloid Properties. Pharmaceutics, 14(4), 823. https://doi.org/10.3390/pharmaceutics14040823




