Melittin from Bee Venom Encapsulating Electrospun Fibers as a Potential Antimicrobial Wound Dressing Patches for Skin Infections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fibers Using Electrospinning
2.3. Melittin-Loaded Fibers Morphology and Diameter Assessment
2.4. Fourier Transform Infrared (FTIR) of Melittin-Loaded Fibers
2.5. Melittin Quantification Using Ultra-High-Performance Liquid Chromatography-–MS/MS (UHPLC–MS/MS)
2.6. Drug Loading (DL) and Entrapment Efficiency (EE%) of the Melittin-Loaded Fibers Determination
2.7. Drug Release of the Melittin-Loaded Fibers
2.8. In Vitro Cytotoxicity Assessment of Melittin
2.9. Bacterial Inoculums and Culture Medium Preparation
2.10. Minimum Inhibitory Concentration (MIC) of Melittin
2.11. Zone of Inhibition Assessment of Melittin-Loaded Fibers
3. Results and Discussion
3.1. Fibers’ Morphology and Diameter Analysis
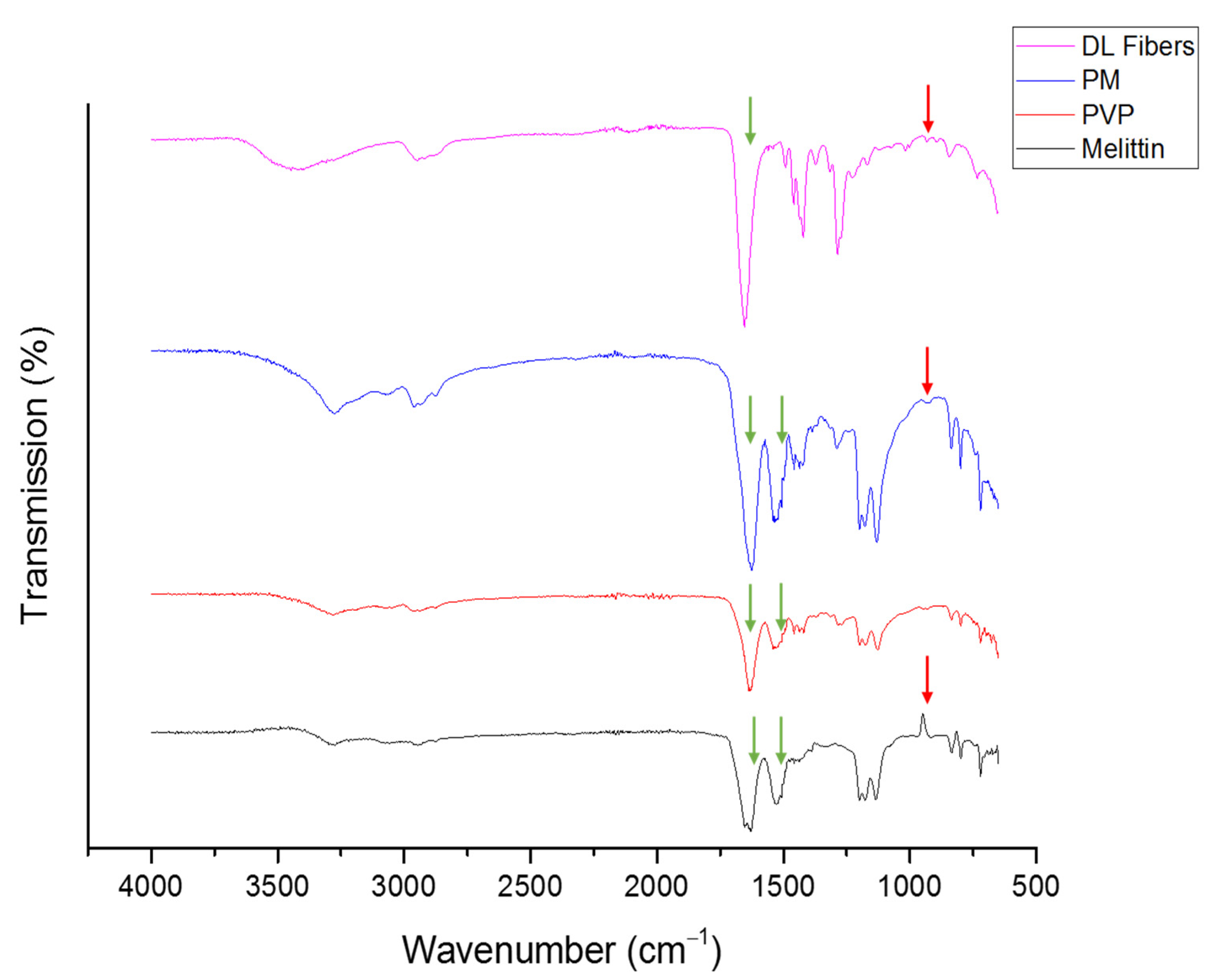
3.2. Fourier Transform Infrared (FTIR) of Melittin-Loaded Fibers
3.3. Drug Loading (DL) and Entrapment Efficiency (EE%) of the Melittin-Loaded Fibers Determination
3.4. Drug Release of the Melittin-Loaded Fibers
3.5. In Vitro Cytotoxicity Assessment of Melittin
3.6. Minimum Inhibitory Concentration (MIC) of Melittin
3.7. Zone of Inhibition Assessment of Melittin-Loaded Fibers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, B.D.; Brooks, A.E. Therapeutic strategies to combat antibiotic resistance. Adv. Drug Deliv. Rev. 2014, 78, 14–27. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Sahl, H.-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Kjos, M.; Nes, I.F.; Diep, D.B.; Lotfipour, F. Natural antimicrobial peptides from bacteria: Characteristics and potential applications to fight against antibiotic resistance. J. Appl. Microbiol. 2012, 113, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.-J.; Park, S.J.; Mishig-Ochir, T.; Lee, B.-J. Antimicrobial peptides: Therapeutic potentials. Expert Rev. Anti-Infect. Ther. 2014, 12, 1477–1486. [Google Scholar] [CrossRef]
- Yeung, A.T.Y.; Gellatly, S.L.; Hancock, R.E.W. Multifunctional cationic host defence peptides and their clinical applications. Cell. Mol. Life Sci. 2011, 68, 2161–2176. [Google Scholar] [CrossRef] [PubMed]
- Marr, A.K.; Gooderham, W.J.; Hancock, R.E.W. Antibacterial peptides for therapeutic use: Obstacles and realistic outlook. Curr. Opin. Pharmacol. 2006, 6, 468–472. [Google Scholar] [CrossRef]
- Nordström, R.; Malmsten, M. Delivery systems for antimicrobial peptides. In Advances in Colloid and Interface Science; Elsevier B.V.: Amsterdam, The Netherlands, 2017; Volume 242, pp. 17–34. [Google Scholar] [CrossRef]
- Piras, A.M.; Maisetta, G.; Sandreschi, S.; Gazzarri, M.; Bartoli, C.; Grassi, L.; Esin, S.; Chiellini, F.; Batoni, G. Chitosan nanoparticles loaded with the antimicro-bial peptide temporin B exert a long-term antibacterial activity in vitro against clinical isolates of Staphylococcus epidermidis. Front. Microbiol. 2015, 6, 372. [Google Scholar] [CrossRef] [Green Version]
- Biswaro, L.S.; da Costa Sousa, M.G.; Rezende, T.M.B.; Dias, S.C.; Franco, O.L. Antimicrobial Peptides and Nanotechnology, Recent Advances and Challenges. Front. Microbiol. 2018, 9, 855. [Google Scholar] [CrossRef] [Green Version]
- Gallarate, M.; Battaglia, L.; Peira, E.; Trotta, M. Peptide-loaded solid lipid nanoparticles prepared through coacervation tech-nique. Int. J. Chem. Eng. 2011, 2011, 132435. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi-Samani, S.; Taghipour, B. PLGA micro and nanoparticles in delivery of peptides and proteins; problems and ap-proaches. Pharm. Dev. Technol. 2015, 20, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Wickremasinghe, N.C.; Kumar, V.A.; Shi, S.; Hartgerink, J.D. Controlled Angiogenesis in Peptide Nanofiber Composite Hydrogels. ACS Biomater. Sci. Eng. 2015, 1, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Taylor, N.L.; Shi, S.; Wang, B.K.; Jalan, A.A.; Kang, M.K.; Wickremasinghe, N.C.; Hartgerink, J. Highly Angiogenic Peptide Nanofibers. ACS Nano 2015, 9, 860–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudra, J.S.; Tian, Y.F.; Jung, J.P.; Collier, J.H. A self-assembling peptide acting as an immune adjuvant. Proc. Natl. Acad. Sci. USA 2009, 107, 622–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudalla, G.A.; Modica, J.A.; Tian, Y.F.; Rudra, J.S.; Chong, A.S.; Sun, T.; Mrksich, M.; Collier, J.H. A Self-Adjuvanting Supramolecular Vaccine Carrying a Folded Protein Antigen. Adv. Health Mater. 2013, 2, 1114–1119. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.; Collier, J.H. Supramolecular peptide vaccines: Tuning adaptive immunity. Curr. Opin. Immunol. 2015, 35, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Galler, K.M.; Aulisa, L.; Regan, K.R.; D’Souza, R.N.; Hartgerink, J.D. Self-assembling multidomain peptide hydrogels: Designed sus-ceptibility to enzymatic cleavage allows enhanced cell migration and spreading. J. Am. Chem. Soc. 2010, 132, 3217–3223. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, X.; Horii, A.; Wang, X.; Qiao, L.; Zhang, S.; Cui, F.-Z. In vivo studies on angiogenic activity of two designer self-assembling peptide scaffold hydrogels in the chicken embryo chorioallantoic membrane. Nanoscale 2012, 4, 2720–2727. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Zolfagharian, H.; Mohajeri, M.; Babaie, M. Bee venom (apis mellifera) an effective potential alternative to gentamicin for specific bacteria strains-Bee venom an effective potential for bacteria. J. Pharmacopunct. 2016, 19, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Kadajji, V.G.; Betageri, G.V. Water soluble polymers for pharmaceutical applications. Polymers 2011, 3, 1972–2009. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, A.; Elesawy, B.H.; Ali, T.M.; Ahmed, O.M. Bee Venom: From Venom to Drug. Molecules 2021, 26, 4941. [Google Scholar] [CrossRef] [PubMed]
- Malanovic, N.; Lohner, K. Antimicrobial Peptides Targeting Gram-Positive Bacteria. Pharmaceuticals 2016, 9, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebbensgaard, A.E.; Mordhorst, H.; Aarestrup, F.; Hansen, E.B. The Role of Outer Membrane Proteins and Lipopolysaccharides for the Sensitivity of Escherichia coli to Antimicrobial Peptides. Front. Microbiol. 2018, 9, 2153. [Google Scholar] [CrossRef] [Green Version]
- El-Seedi, H.; El-Wahed, A.A.; Yosri, N.; Musharraf, S.G.; Chen, L.; Moustafa, M.; Zou, X.; Al-Mousawi, S.; Guo, Z.; Khatib, A.; et al. Antimicrobial Properties of Apis mellifera’s Bee Venom. Toxins 2020, 12, 451. [Google Scholar] [CrossRef]
- Cheon, S.Y.; Chung, K.S.; Roh, S.S.; Cha, Y.Y.; An, H.J. Bee venom suppresses the differentiation of preadipocytes and high fat di-et-induced obesity by inhibiting adipogenesis. Toxins 2018, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Aburayan, W.S.; Booq, R.Y.; Binsaleh, N.S.; Alfassam, H.A.; Bakr, A.A.; Bukhary, H.A.; Alyamani, E.J.; Tawfik, E.A. The Delivery of the Novel Drug ‘Halicin’ Using Electrospun Fibers for the Treatment of Pressure Ulcer against Pathogenic Bacteria. Pharmaceutics 2020, 12, 1189. [Google Scholar] [CrossRef]
- Huang, S.; Wang, J.; Guo, Z.; Wang, Y.; Liu, C. Quantitative Measurement of Melittin in Asian Honeybee Venom Using a New Method Including UPLC-QqTOF-MS. Toxins 2020, 12, 437. [Google Scholar] [CrossRef]
- Alkahtani, M.; Alsofyani, N.; Alfahd, A.; Almuqhim, A.A.; Almughem, F.A.; Alshehri, A.A.; Qasem, H.; Hemmer, P.R. Engineering red-enhanced and bi-ocompatible upconversion nanoparticles. Nanomaterials 2021, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.P. An informational supplement for global application developed through the Clinical and Laboratory Standards Institute. In Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2011; Volume 33, pp. 1–35. [Google Scholar]
- Gizaw, M.; Thompson, J.; Faglie, A.; Lee, S.-Y.; Neuenschwander, P.; Chou, S.-F. Electrospun Fibers as a Dressing Material for Drug and Biological Agent Delivery in Wound Healing Applications. Bioengineering 2018, 5, 9. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhou, S.; Gao, Y.; Zhai, Y. Electrospun nanofibers as a wound dressing for treating diabetic foot ulcer. Asian J. Pharm. Sci. 2019, 14, 130–143. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Bukhary, H.; Williams, G.R.; Orlu, M. Electrospun fixed dose formulations of amlodipine besylate and valsartan. Int. J. Pharm. 2018, 549, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guan, S.-M.; Sun, W.; Fu, H. Melittin, the Major Pain-Producing Substance of Bee Venom. Neurosci. Bull. 2016, 32, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Al-Rabia, M.W.; Alhakamy, N.A.; Ahmed, O.A.; Eljaaly, K.; Aloafi, A.L.; Mostafa, A.; Asfour, H.Z.; Aldarmahi, A.A.; Darwish, K.M.; Ibrahim, T.S.; et al. Repurposing of sitagliptin-melittin opti-mized nanoformula against sars-cov-2: Antiviral screening and molecular docking studies. Pharmaceutics 2021, 13, 307. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Mo, X.; He, C.; Wang, H. Intermolecular interactions in electrospun collagen–chitosan complex nanofibers. Carbohydr. Polym. 2008, 72, 410–418. [Google Scholar] [CrossRef]
- Alkahtani, M.; Aodah, A.; Abu Asab, O.; Basit, A.; Orlu, M.; Tawfik, E. Fabrication and Characterization of Fast-Dissolving Films Containing Escitalopram/Quetiapine for the Treatment of Major Depressive Disorder. Pharmaceutics 2021, 13, 891. [Google Scholar] [CrossRef] [PubMed]
- Alshaya, H.A.; Alfahad, A.J.; Alsulaihem, F.M.; Aodah, A.H.; Alshehri, A.A.; Almughem, F.A.; Alfassam, H.A.; Aldossary, A.M.; Halwani, A.A.; Bukhary, H.A.; et al. Fast-Dissolving Nifedipine and Atorvastatin Calcium Electrospun Nanofibers as a Potential Buccal Delivery System. Pharmaceutics 2022, 14, 358. [Google Scholar] [CrossRef] [PubMed]
- Kamble, R.N.; Gaikwad, S.; Maske, A.; Patil, S.S. Fabrication of electrospun nanofibres of BCS II drug for enhanced dissolution and permeation across skin. J. Adv. Res. 2016, 7, 483–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramaswamy, R.; Mani, G.; Jang, H.T. Fabrication of buccal dissolving tetrahydro curcumin loaded polyvidone fiber mat: Synthe-sis, characterization, and in vitro evaluations. J. Appl. Pharm. Sci. 2018, 8, 026–031. [Google Scholar]
- Li, J.; Pan, H.; Ye, Q.; Shi, C.; Zhang, X.; Pan, W. Carvedilol-loaded polyvinylpyrrolidone electrospun nanofiber film for sublingual delivery. J. Drug Deliv. Sci. Technol. 2020, 58, 101726. [Google Scholar] [CrossRef]
- Sriyanti, I.; Edikresnha, D.; Rahma, A.; Munir, M.M.; Rachmawati, H.; Khairurrijal, K. Mangosteen pericarp extract embedded in electrospun PVP nanofiber mats: Physicochemical properties and release mechanism of α-mangostin. Int. J. Nanomed. 2018, 13, 4927–4941. [Google Scholar] [CrossRef] [Green Version]
- Askari, P.; Namaei, M.H.; Ghazvini, K.; Hosseini, M. In vitro and in vivo toxicity and antibacterial efficacy of melittin against clinical extensively drug-resistant bacteria. BMC Pharmacol. Toxicol. 2021, 22, 42. [Google Scholar] [CrossRef]
- Dart, A.; Bhave, M.; Kingshott, P. Antimicrobial Peptide-Based Electrospun Fibers for Wound Healing Applications. Macromol. Biosci. 2019, 19, e1800488. [Google Scholar] [CrossRef]
- Khosravimelal, S.; Chizari, M.; Farhadihosseinabadi, B.; Moghaddam, M.M.; Gholipourmalekabadi, M. Fabrication and characterization of an antibacterial chitosan/silk fibroin electrospun nanofiber loaded with a cationic peptide for wound-dressing application. J. Mater. Sci. Mater. Med. 2021, 32, 32. [Google Scholar] [CrossRef]
- Zekry, S.S.A.; Abdellatif, A.; Azzazy, H.M. Fabrication of pomegranate/honey nanofibers for use as antibacterial wound dressings. Wound Med. 2020, 28, 100181. [Google Scholar] [CrossRef]
- Fennell, J.F.; Shipman, W.H.; Cole, L.J. Antibacterial action of a bee venom fraction (melittin) against a penicillin-resistant staphy-lococcus and other microorganisms. Res. Dev. Tech. Rep. 1967, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sarhan, W.A.; Azzazy, H.M. Apitherapeutics and phage-loaded nanofibers as wound dressings with enhanced wound healing and antibacterial activity. Nanomedicine 2017, 12, 2055–2067. [Google Scholar] [CrossRef]




| Microorganism | Melittin MIC (μg/mL) |
|---|---|
| E. coli ATCC 25922 | 5 |
| E. coli MDR 1060 | 20 |
| A. baumannii ATCC BAA 747 | 5 |
| A. baumannii MDR 3087 | 2.5 |
| P. aeruginosa ATCC 27853 | 20 |
| P. aeruginosa MDR 7067 | 20 |
| S. aureus ATCC 29213 | 5 |
| S. aureus ATCC 976 | 5 |
| S. aureus ATCC 977 | 5 |
| MRSA ATCC 43300 | 5 |
| C. albicans ATCC 66027 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aburayan, W.S.; Alajmi, A.M.; Alfahad, A.J.; Alsharif, W.K.; Alshehri, A.A.; Booq, R.Y.; Alsudir, S.A.; Alsulaihem, F.M.; Bukhary, H.A.; Badr, M.Y.; et al. Melittin from Bee Venom Encapsulating Electrospun Fibers as a Potential Antimicrobial Wound Dressing Patches for Skin Infections. Pharmaceutics 2022, 14, 725. https://doi.org/10.3390/pharmaceutics14040725
Aburayan WS, Alajmi AM, Alfahad AJ, Alsharif WK, Alshehri AA, Booq RY, Alsudir SA, Alsulaihem FM, Bukhary HA, Badr MY, et al. Melittin from Bee Venom Encapsulating Electrospun Fibers as a Potential Antimicrobial Wound Dressing Patches for Skin Infections. Pharmaceutics. 2022; 14(4):725. https://doi.org/10.3390/pharmaceutics14040725
Chicago/Turabian StyleAburayan, Walaa S., Areej M. Alajmi, Ahmed J. Alfahad, Wijdan K. Alsharif, Abdullah A. Alshehri, Rayan Y. Booq, Samar A. Alsudir, Fatemah M. Alsulaihem, Haitham A. Bukhary, Moutaz Y. Badr, and et al. 2022. "Melittin from Bee Venom Encapsulating Electrospun Fibers as a Potential Antimicrobial Wound Dressing Patches for Skin Infections" Pharmaceutics 14, no. 4: 725. https://doi.org/10.3390/pharmaceutics14040725
APA StyleAburayan, W. S., Alajmi, A. M., Alfahad, A. J., Alsharif, W. K., Alshehri, A. A., Booq, R. Y., Alsudir, S. A., Alsulaihem, F. M., Bukhary, H. A., Badr, M. Y., Alyamani, E. J., & Tawfik, E. A. (2022). Melittin from Bee Venom Encapsulating Electrospun Fibers as a Potential Antimicrobial Wound Dressing Patches for Skin Infections. Pharmaceutics, 14(4), 725. https://doi.org/10.3390/pharmaceutics14040725






