Local Anesthetic Plasma Concentrations as a Valuable Tool to Confirm the Diagnosis of Local Anesthetic Systemic Toxicity? A Report of 10 Years of Experience
Abstract
1. Introduction
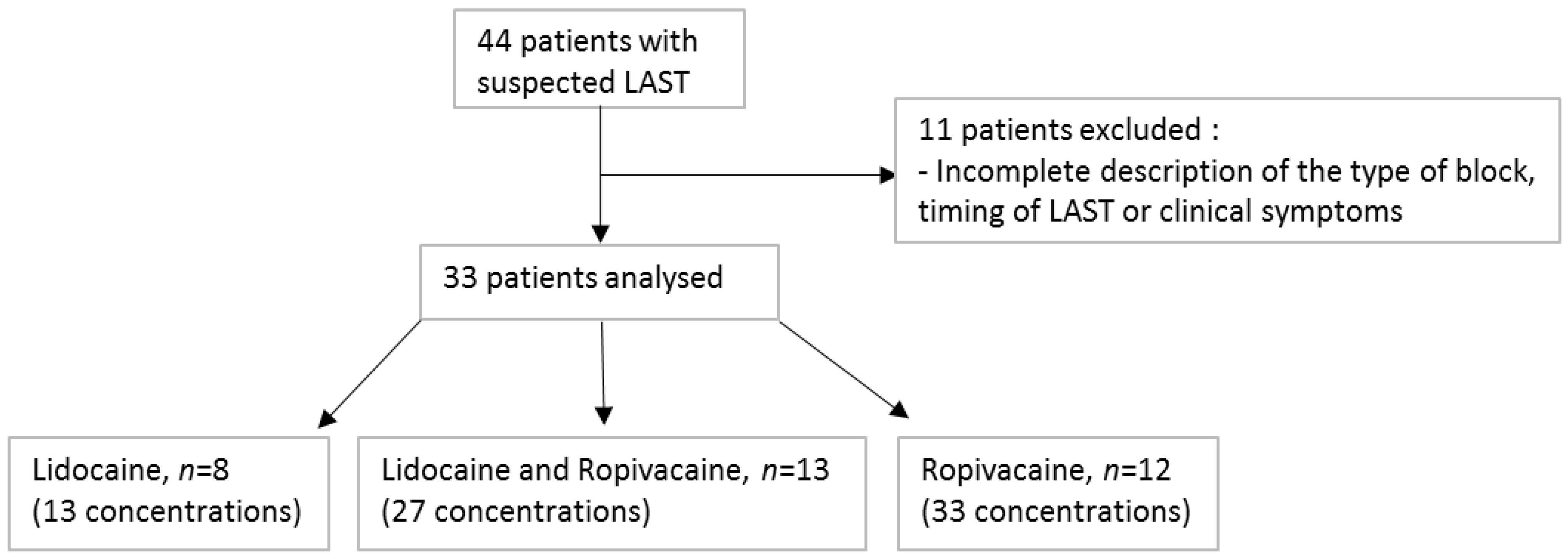
2. Materials and Methods
3. Results
3.1. Symptom Timing and Presentation
3.2. LA Observed Concentrations
3.3. Treatment and Patient Outcome
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neal, J.M.; Mulroy, M.F. American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity: 2012 version. Reg. Anesth. Pain Med. 2012, 37, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Salonen, M.H.; Haasio, J. Evaluation of efficacy and plasma concentrations of ropivacaine in continuous axillary brachial plexus block: High dose for surgical anesthesia and low dose for postoperative analgesia. Reg. Anesth. Pain Med. 2000, 25, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Mulroy, M.F. Systemic toxicity and cardiotoxicity from local anesthetics: Incidence and preventive measures. Reg. Anesth. Pain Med. 2002, 27, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Vadi, M.G.; Patel, N.; Stiegler, M.P. Local anesthetic systemic toxicity after combined psoas compartment-sciatic nerve block: Analysis of decision factors and diagnostic delay. Anesthesiology 2014, 120, 987–996. [Google Scholar] [CrossRef]
- Knudsen, K.; Beckman Suurküla, M.; Blomberg, S.; Sjövall, J.; Edvardsson, N. Central nervous and cardiovascular effects of i.v. infusions of ropivacaine, bupivacaine and placebo in volunteers. Br. J. Anaesth. 1997, 78, 507–514. [Google Scholar] [CrossRef]
- Di Gregorio, G.; Neal, J.M.; Rosenquist, R.W.; Weinberg, G.L. Clinical presentation of local anesthetic systemic toxicity: A review of published cases, 1979 to 2009. Reg. Anesth. Pain Med. 2010, 35, 181–187. [Google Scholar] [CrossRef]
- Müller, M.; Litz, R.J.; Hüler, M.; Albrecht, D.M. Grand mal convulsion and plasma concentrations after intravascular injection of ropivacaine for axillary brachial plexus blockade. Br. J. Anaesth. 2001, 87, 784–787. [Google Scholar] [CrossRef]
- Kimura, Y.; Kamada, Y.; Kimura, A.; Orimo, K. Ropivacaine-induced toxicity with overdose suspected after axillary brachial plexus block. J. Anesth. 2007, 21, 413–416. [Google Scholar] [CrossRef]
- Calenda, E.; Baste, J.M.; Hajjej, R.; Danielou, E.; Peillon, C. Toxic plasma concentration of ropivacaine after a paravertebral block in a patient suffering from severe hypoalbuminemia. J. Clin. Anesth. 2014, 26, 149–151. [Google Scholar] [CrossRef]
- Griffiths, J.D.; Le, N.V.; Grant, S.; Bjorksten, A.; Hebbard, P.; Royse, C. Symptomatic local anaesthetic toxicity and plasma ropivacaine concentrations after transversus abdominis plane block for Caesarean section. Br. J. Anaesth. 2013, 110, 996–1000. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on Bioanalytical Method Validation 2011, EMEA/CHMP/EWP/192217/2009 Rev. 1 Corr. 2. Available online: https://www.ema.europa.eu/en/documents/scientificguideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 23 January 2022).
- Lorec, A.M.; Bruguerolle, B.; Attolini, L.; Roucoules, X. Rapid simultaneous determination of lidocaine, bupivacaine, and their two main metabolites using capillary gas-liquid chromatography with nitrogen phosphorus detector. Ther. Drug Monit. 1994, 16, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Collinsworth, K.A.; Kalman, S.M.; Harrison, D.C. The clinical pharmacology of lidocaine as an antiarrhythymic drug. Circulation 1974, 50, 1217–1230. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.B.; Jebson, P.J.; Braid, D.P.; Ortengren, B.; Frisch, P. Factors affecting plasma levels of lignocaine and prilocaine. Br. J. Anaesth. 1972, 44, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Schoenmakers, K.P.W.; Vree, T.B.; Jack, N.T.M.; van den Bemt, B.; van Limbeek, J.; Stienstra, R. Pharmacokinetics of 450 mg ropivacaine with and without epinephrine for combined femoral and sciatic nerve block in lower extremity surgery. A pilot study. Br. J. Clin. Pharmacol. 2013, 75, 1321–1327. [Google Scholar] [CrossRef]
- Hübler, M.; Planitz, M.C.; Vicent, O. Early pharmacokinetic of ropivacaine without epinephrine after injection into the psoas compartment. Br. J. Anaesth. 2015, 114, 130–135. [Google Scholar] [CrossRef][Green Version]
- Vainionpää, V.A.; Haavisto, E.T.; Huha, T.M.; Korpi, K.J.; Nuutinen, L.S.; Hollmén, A.I.; Jozwiak, H.M.; Magnusson, A.A. A clinical and pharmacokinetic comparison of ropivacaine and bupivacaine in axillary plexus block. Anesth. Analg. 1995, 81, 534–538. [Google Scholar]
- Pere, P.; Salonen, M.; Jokinen, M.; Rosenberg, P.H.; Neuvonen, P.J.; Haasio, J. Pharmacokinetics of ropivacaine in uremic and nonuremic patients after axillary brachial plexus block. Anesth. Analg. 2003, 96, 563–569. [Google Scholar] [CrossRef]
- Blanco, L.J.; Reid, P.R.; King, T.M. Plasma lidocaine levels following paracervical infiltration for aspiration abortion. Obstet. Gynecol. 1982, 60, 506–508. [Google Scholar]
- Scavone, J.M.; Greenblatt, D.J.; Fraser, D.G. The bioavailability of intranasal lignocaine. Br. J. Clin. Pharmacol. 1989, 28, 722–724. [Google Scholar] [CrossRef]
- Dillane, D.; Finucane, B.T. Local anesthetic systemic toxicity. Can. J. Anaesth. 2010, 57, 368–380. [Google Scholar] [CrossRef]
- Ruetsch, Y.A.; Fattinger, K.E.; Borgeat, A. Ropivacaine-induced convulsions and severe cardiac dysrhythmia after sciatic block. Anesthesiology 1999, 90, 1784–1786. [Google Scholar] [CrossRef] [PubMed]
- Vasques, F.; Behr, A.U.; Weinberg, G.; Ori, C.; Di Gregorio, G. A Review of Local Anesthetic Systemic Toxicity Cases Since Publication of the American Society of Regional Anesthesia Recommendations: To Whom It May Concern. Reg. Anesth. Pain Med. 2015, 40, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Satsumae, T.; Tanaka, M.; Saito, S.; Inomata, S. Convulsions after ropivacaine 300 mg for brachial plexus block. Br. J. Anaesth. 2008, 101, 860–862. [Google Scholar] [CrossRef]
- Chazalon, P.; Tourtier, J.P.; Villevielle, T.; Giraud, D.; Saïssy, J.M.; Mion, G.; Benhamou, D. Ropivacaine-induced cardiac arrest after peripheral nerve block: Successful resuscitation. Anesthesiology 2003, 99, 1449–1451. [Google Scholar] [CrossRef]
- Hersh, E.V.; Helpin, M.L.; Evans, O.B. Local anesthetic mortality: Report of case. ASDC J. Dent. Child. 1991, 58, 489–491. [Google Scholar] [PubMed]
- Moore, P.A.; Hersh, E.V. Local anesthetics: Pharmacology and toxicity. Dent. Clin. N. Am. 2010, 54, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Tsen, L.C.; Tarshis, J.; Denson, D.D.; Datta, S.; Bader, A.M. Measurements of maternal protein binding of bupivacaine throughout pregnancy. Anesth. Analg. 1999, 89, 965–968. [Google Scholar] [CrossRef]
- Simon, M.J.; Veering, B.T.; Vletter, A.A.; Stienstra, R.; van Kleef, J.W.; Burm, A.G. The effect of age on the systemic absorption and systemic disposition of ropivacaine after epidural administration. Anesth. Analg. 2006, 102, 276–282. [Google Scholar] [CrossRef]
- Goodson, J.M.; Moore, P.A. Life threatening reactions after pedodontic sedation: An assessment of narcotic, local anesthetic, and antiemetic drug interaction. J. Am. Dent. Assoc. 1983, 107, 239–245. [Google Scholar] [CrossRef]


| Characteristics | n (%) |
|---|---|
| Demographic | |
| Male/female | 6/27 |
| Body weight (kg) * | 65 (45–116) |
| Age (years) | 47 (17–93) |
| Risk factors | |
| Age > 60 years | 6 (18%) |
| Pregnant | 10 (31%) |
| Overweight | 7 (21%) |
| Regional blocks | |
| Upper extremity | 11 |
| Torso | 12 |
| Lower extremity | 9 |
| Subcutaneous | 1 |
| Surgery | |
| Gynecological/obstetrics | 15 |
| Limb surgery | 7 |
| Heart | 4 |
| Others | 5 |
| NA | 2 |
| LA concentrations (n = 74) | |
| Ropivacaine | 33 |
| Lidocaine | 13 |
| Ropivacaine and lidocaine | 27 |
| Single sample | 11 |
| Multiple samples | 22 |
| Observed Signs of Toxicity | n |
|---|---|
| Prodromes | 13 |
| Loss of consciousness | 4 |
| Seizures | 7 |
| Tachycardia/hypertension | 5 |
| Bradycardia/hypotension/shock | 7 |
| Cardiac arrest | 3 |
| Ventricular tachycardia/ventricular fibrillation | 3 |
| Ventricular ectopics | 1 |
| Bundle branch block | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riff, C.; Le Caloch, A.; Dupouey, J.; Allanioux, L.; Leone, M.; Blin, O.; Bourgoin, A.; Guilhaumou, R. Local Anesthetic Plasma Concentrations as a Valuable Tool to Confirm the Diagnosis of Local Anesthetic Systemic Toxicity? A Report of 10 Years of Experience. Pharmaceutics 2022, 14, 708. https://doi.org/10.3390/pharmaceutics14040708
Riff C, Le Caloch A, Dupouey J, Allanioux L, Leone M, Blin O, Bourgoin A, Guilhaumou R. Local Anesthetic Plasma Concentrations as a Valuable Tool to Confirm the Diagnosis of Local Anesthetic Systemic Toxicity? A Report of 10 Years of Experience. Pharmaceutics. 2022; 14(4):708. https://doi.org/10.3390/pharmaceutics14040708
Chicago/Turabian StyleRiff, Camille, Axel Le Caloch, Julien Dupouey, Laurent Allanioux, Marc Leone, Olivier Blin, Aurélie Bourgoin, and Romain Guilhaumou. 2022. "Local Anesthetic Plasma Concentrations as a Valuable Tool to Confirm the Diagnosis of Local Anesthetic Systemic Toxicity? A Report of 10 Years of Experience" Pharmaceutics 14, no. 4: 708. https://doi.org/10.3390/pharmaceutics14040708
APA StyleRiff, C., Le Caloch, A., Dupouey, J., Allanioux, L., Leone, M., Blin, O., Bourgoin, A., & Guilhaumou, R. (2022). Local Anesthetic Plasma Concentrations as a Valuable Tool to Confirm the Diagnosis of Local Anesthetic Systemic Toxicity? A Report of 10 Years of Experience. Pharmaceutics, 14(4), 708. https://doi.org/10.3390/pharmaceutics14040708






