Genipin-Crosslinked, Proteosaccharide Scaffolds for Potential Neural Tissue Engineering Applications
Abstract
:1. Introduction
2. Materials and Methods
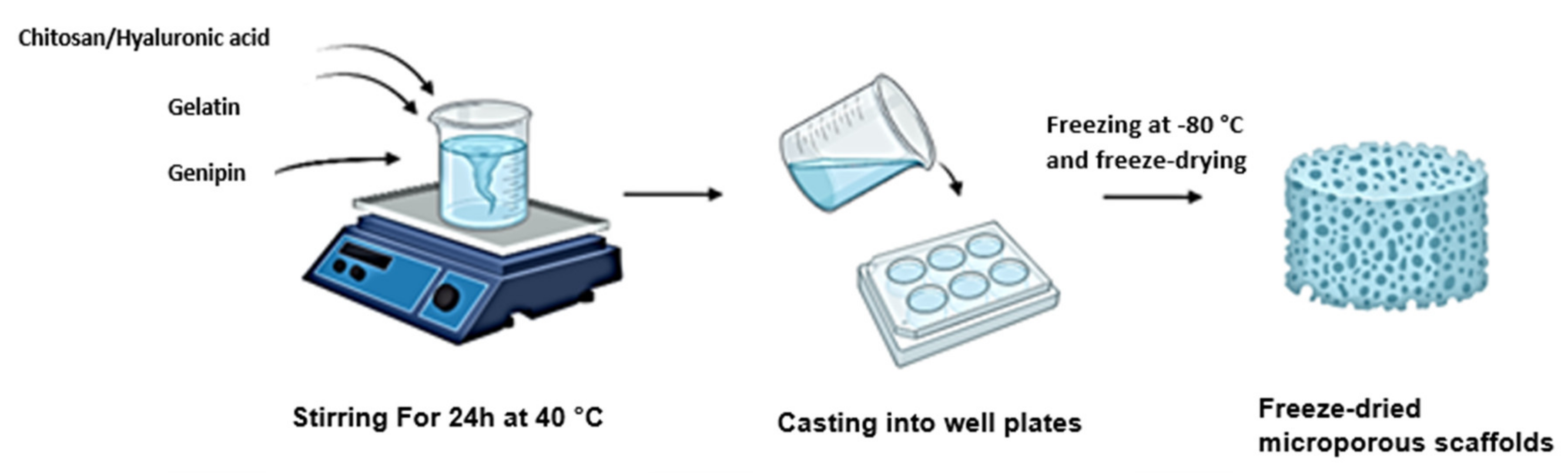
2.1. Synthesis of the Full IPN and Semi-IPN
2.2. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy (ATR-FTIR) of Pristine Polymers and Their Crosslinked Networks
2.3. Rheological Analysis of the Proteosaccharide Hydrogels
2.4. Analysis of Crystallinity of the Proteosaccharide Scaffolds
2.5. Thermoanalytical Analysis of the Lyophilised Proteosaccharide Scaffolds
2.6. Swelling Potential of Scaffolds under Physiological Conditions
2.7. Morphological Analysis of Proteosaccharide Scaffolds
2.8. Determination of Bulk Density of Each Scaffold
2.9. Degradation of Proteosaccharide Scaffolds
2.10. Drug Release of Dexamethasone-21-Phosphate from Proteosaccharide Scaffolds
2.11. In Vitro Cytotoxicity Evaluation of the Full IPN and Semi-IPN
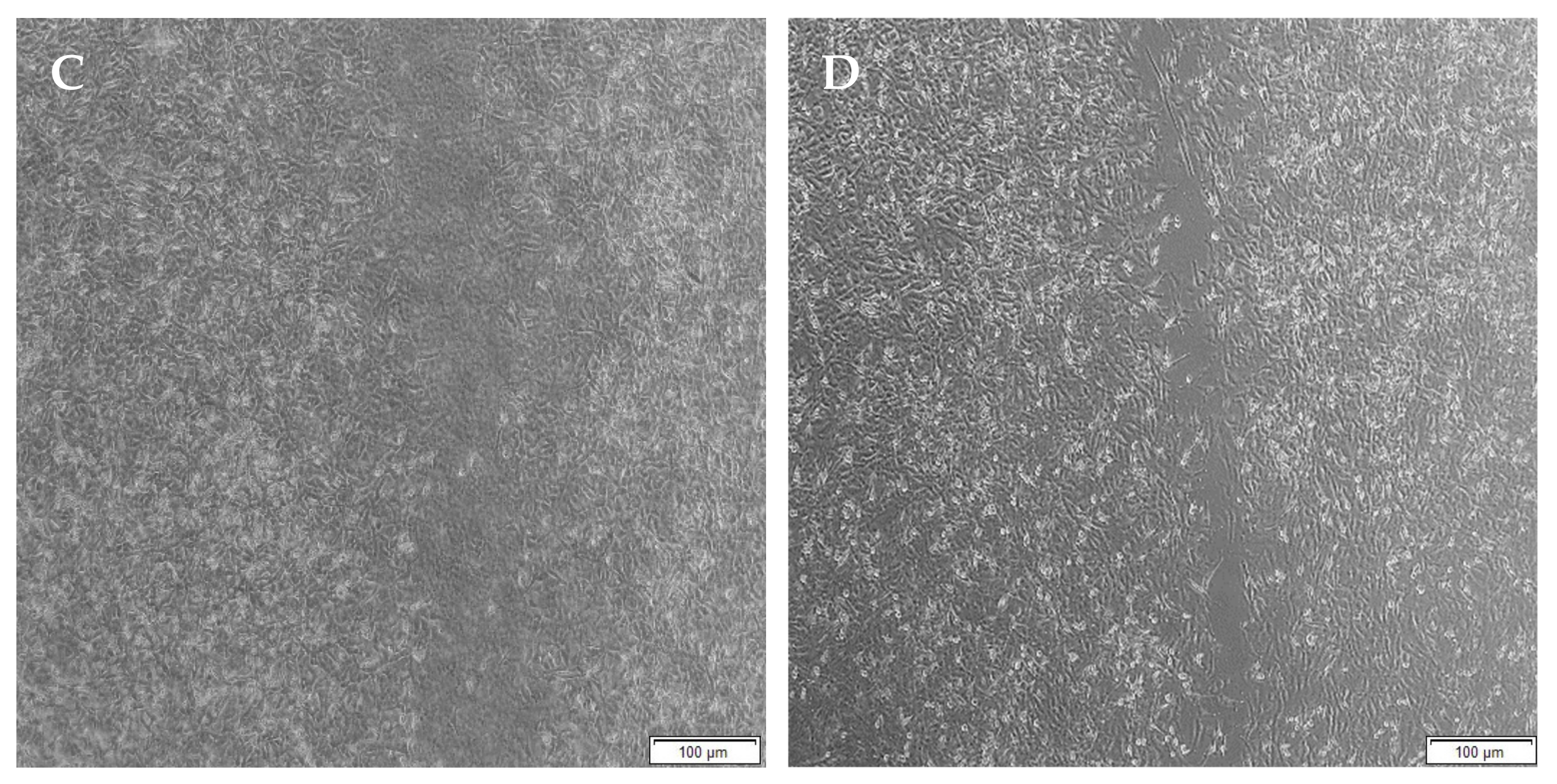
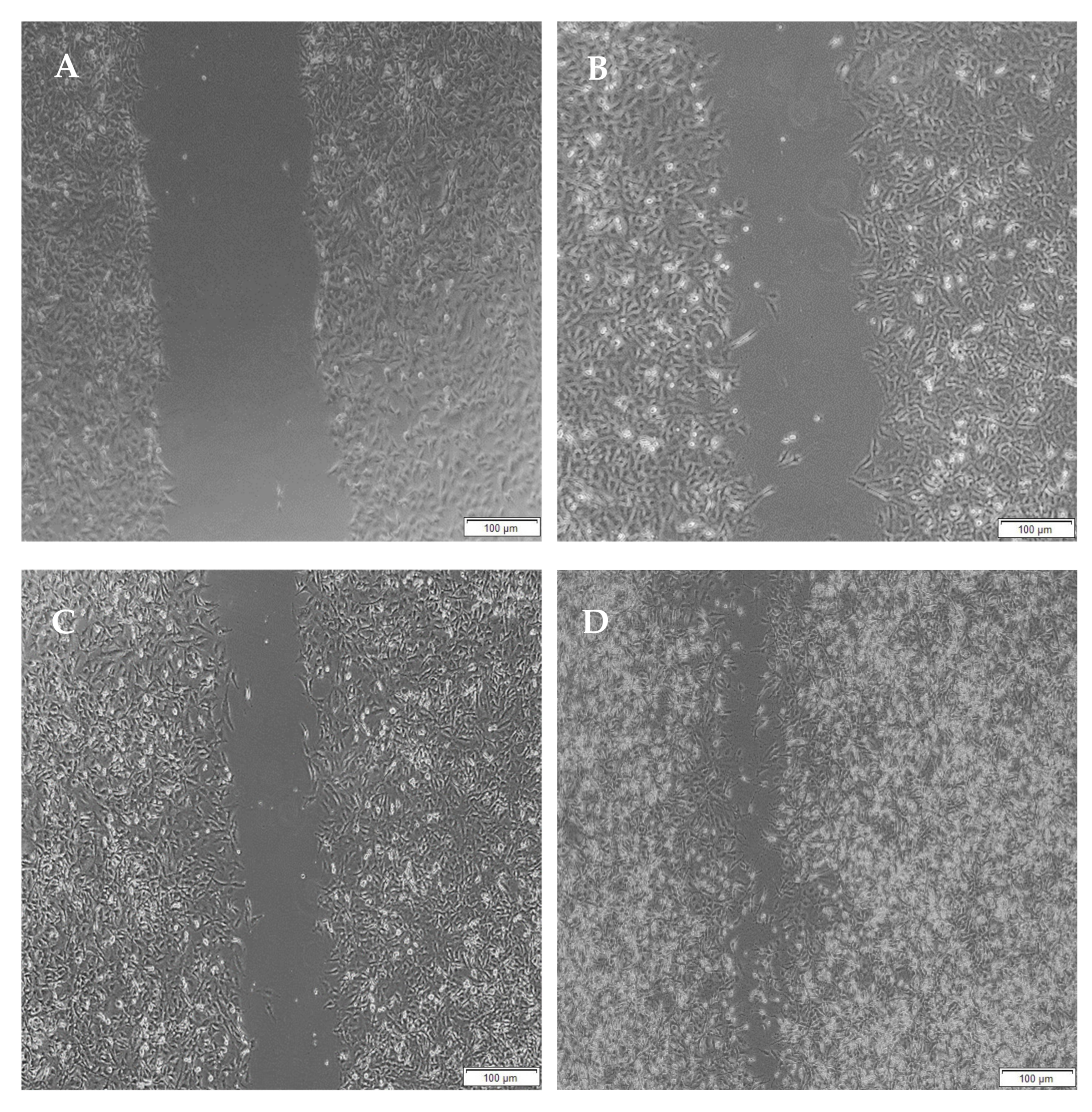
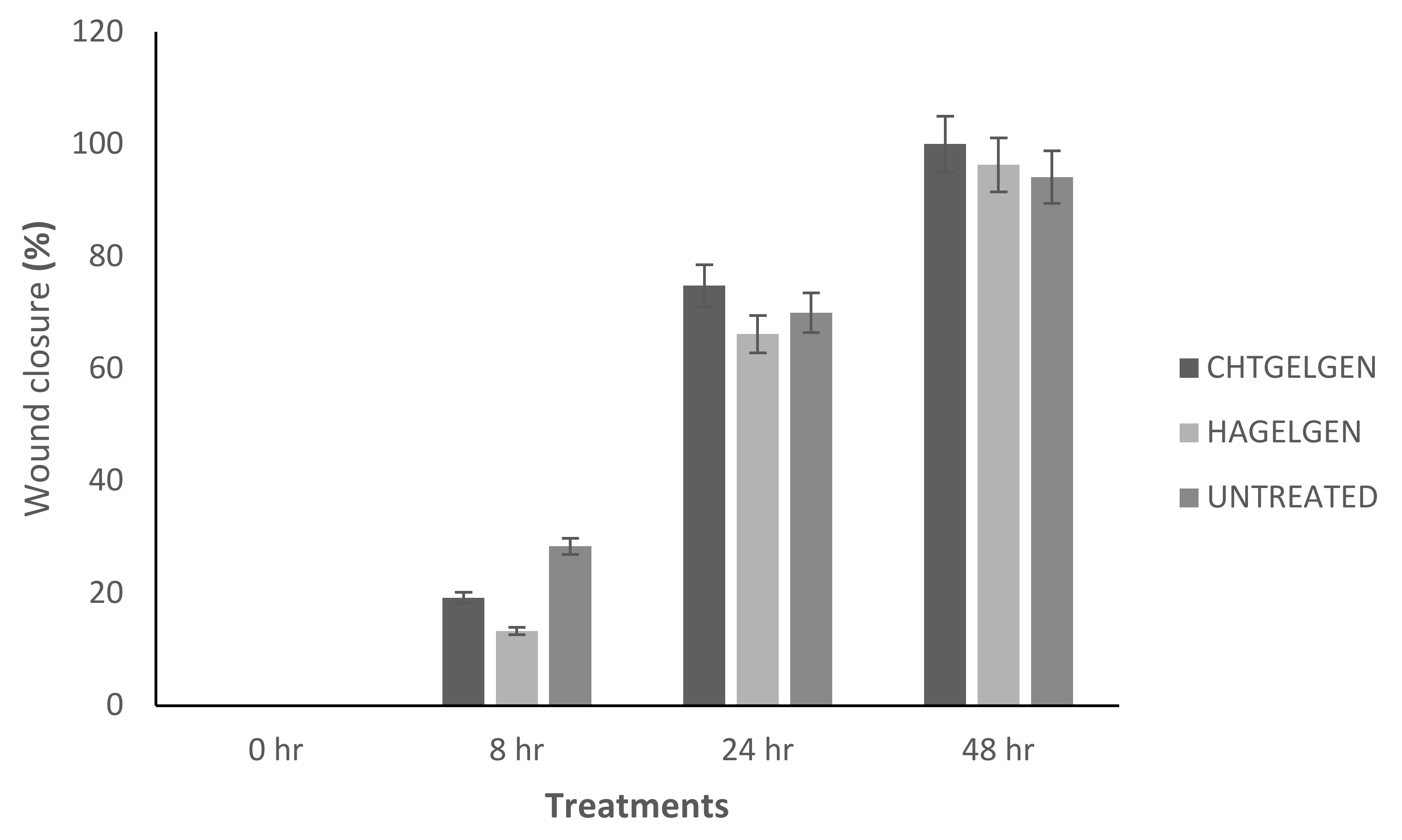
2.12. Migration of A172 Cells in Response to the Semi- and Full IPN
2.13. Statistical Analysis
3. Results
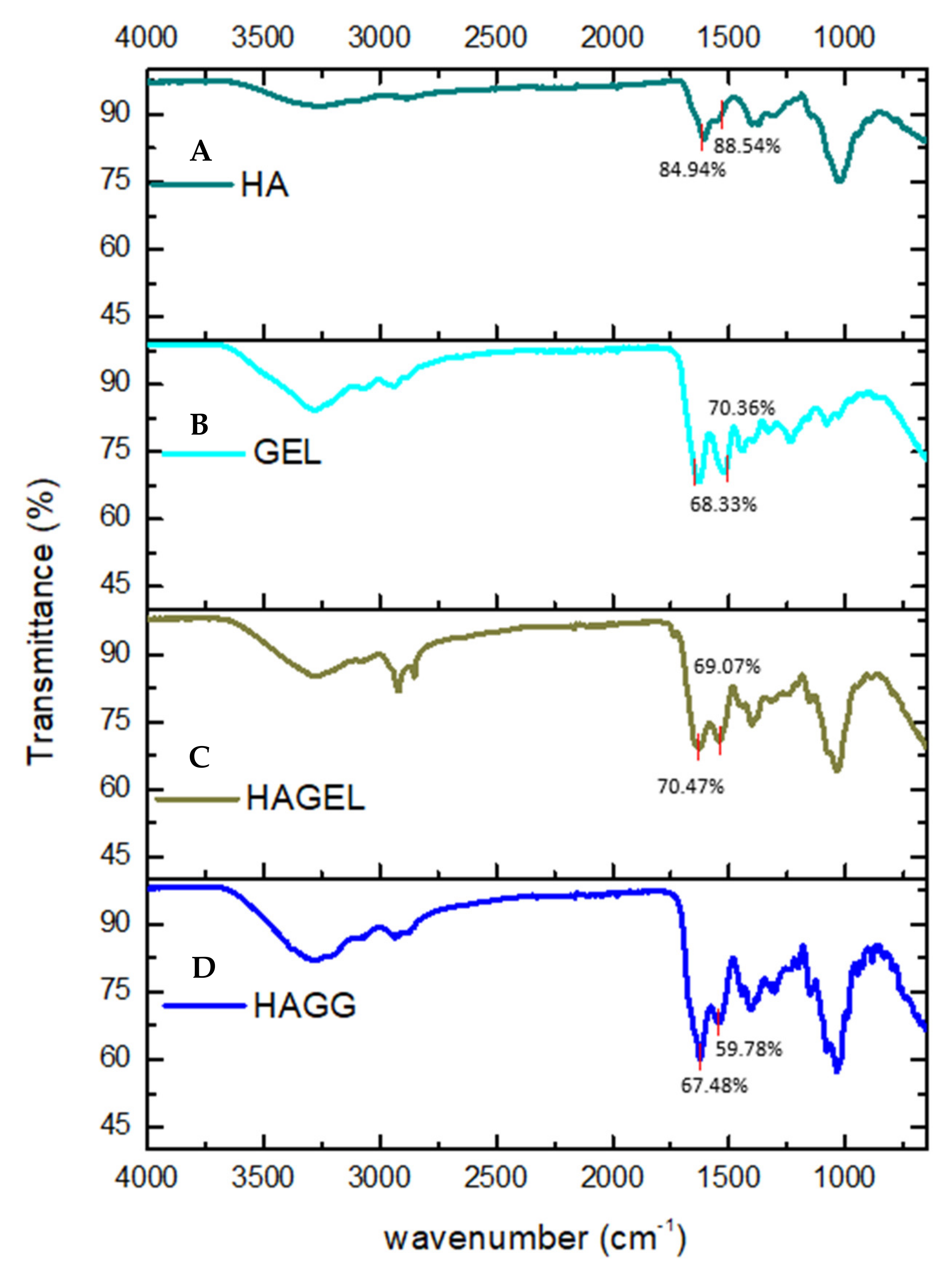
3.1. FTIR Analysis
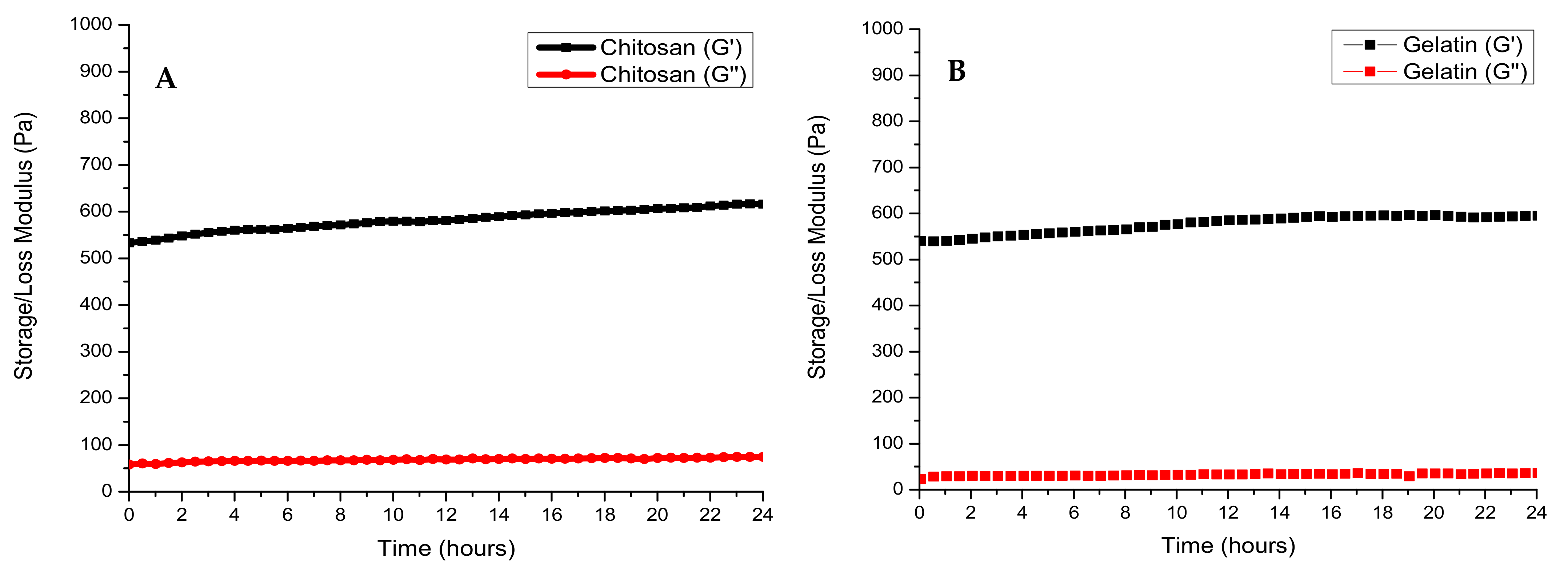
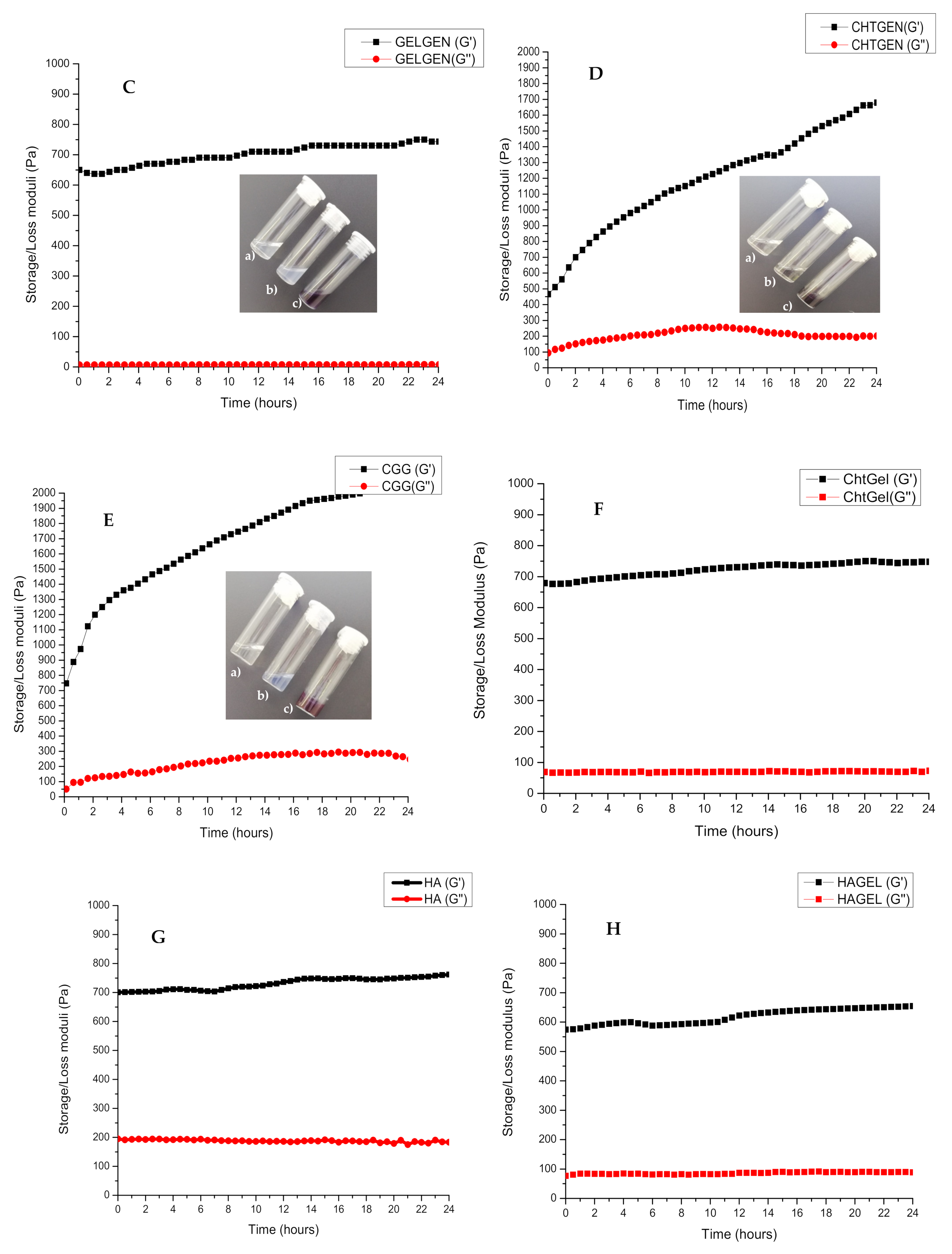
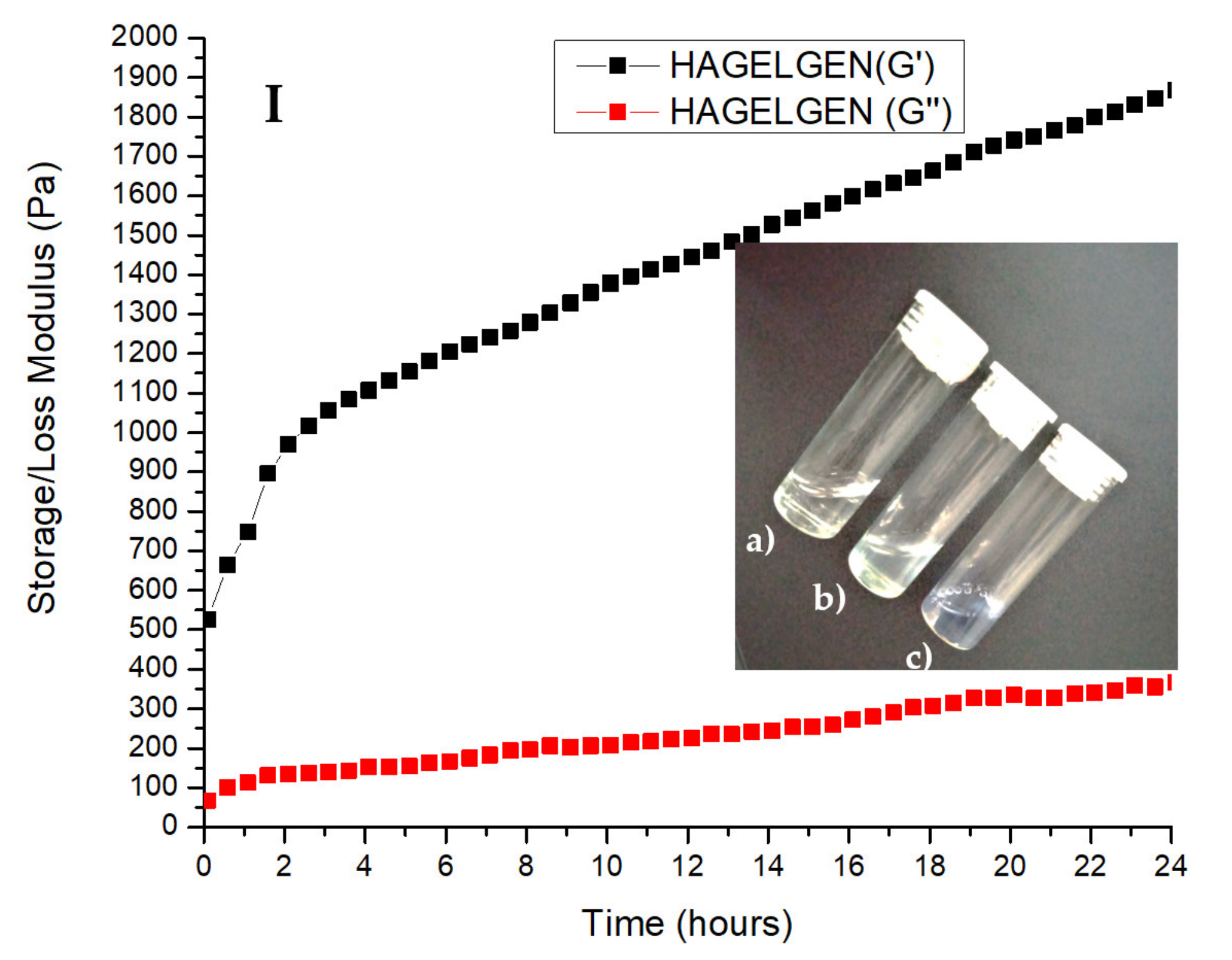
3.2. Rheological Characterisation of the Crosslinking Reaction
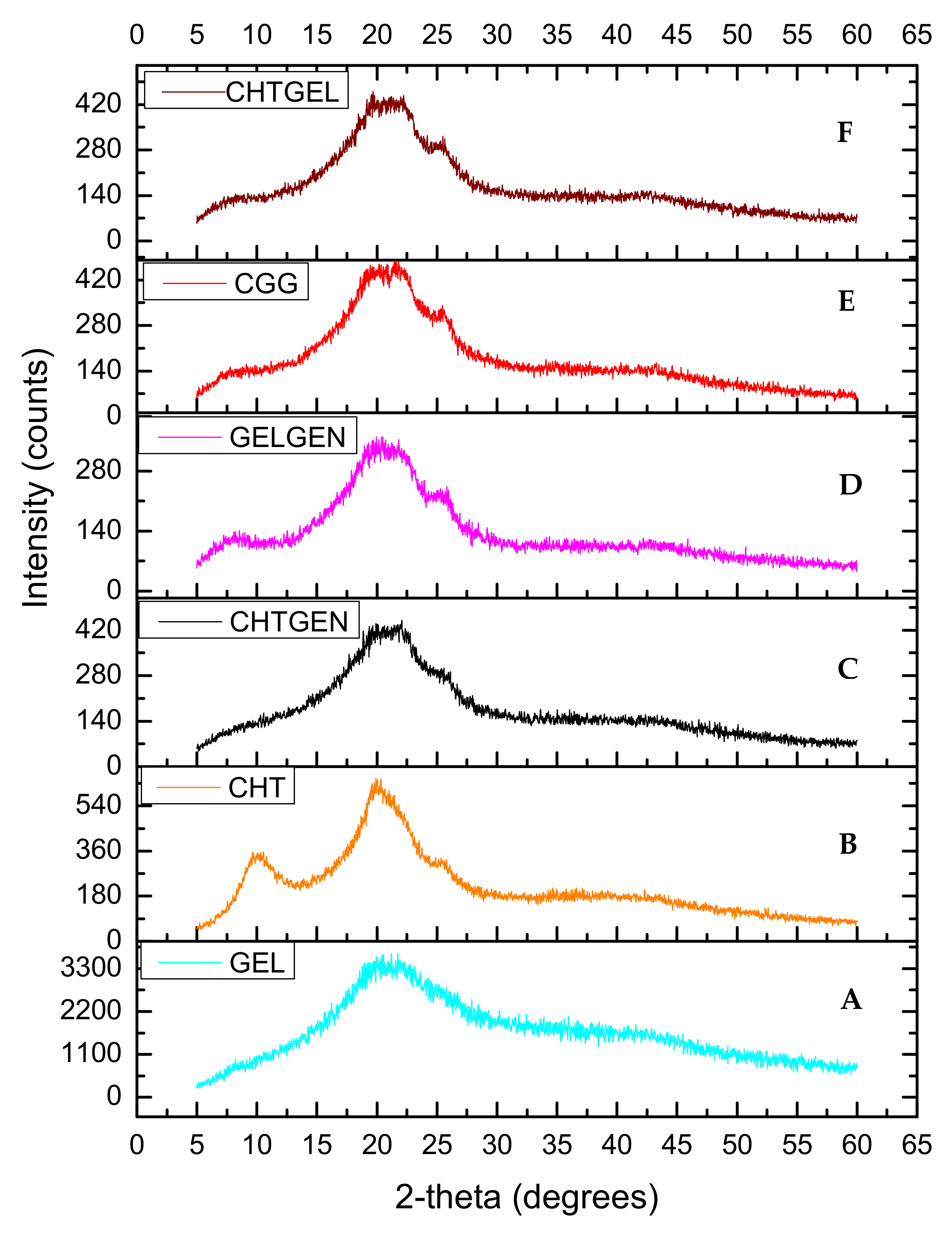
3.3. XRD of Each Scaffold
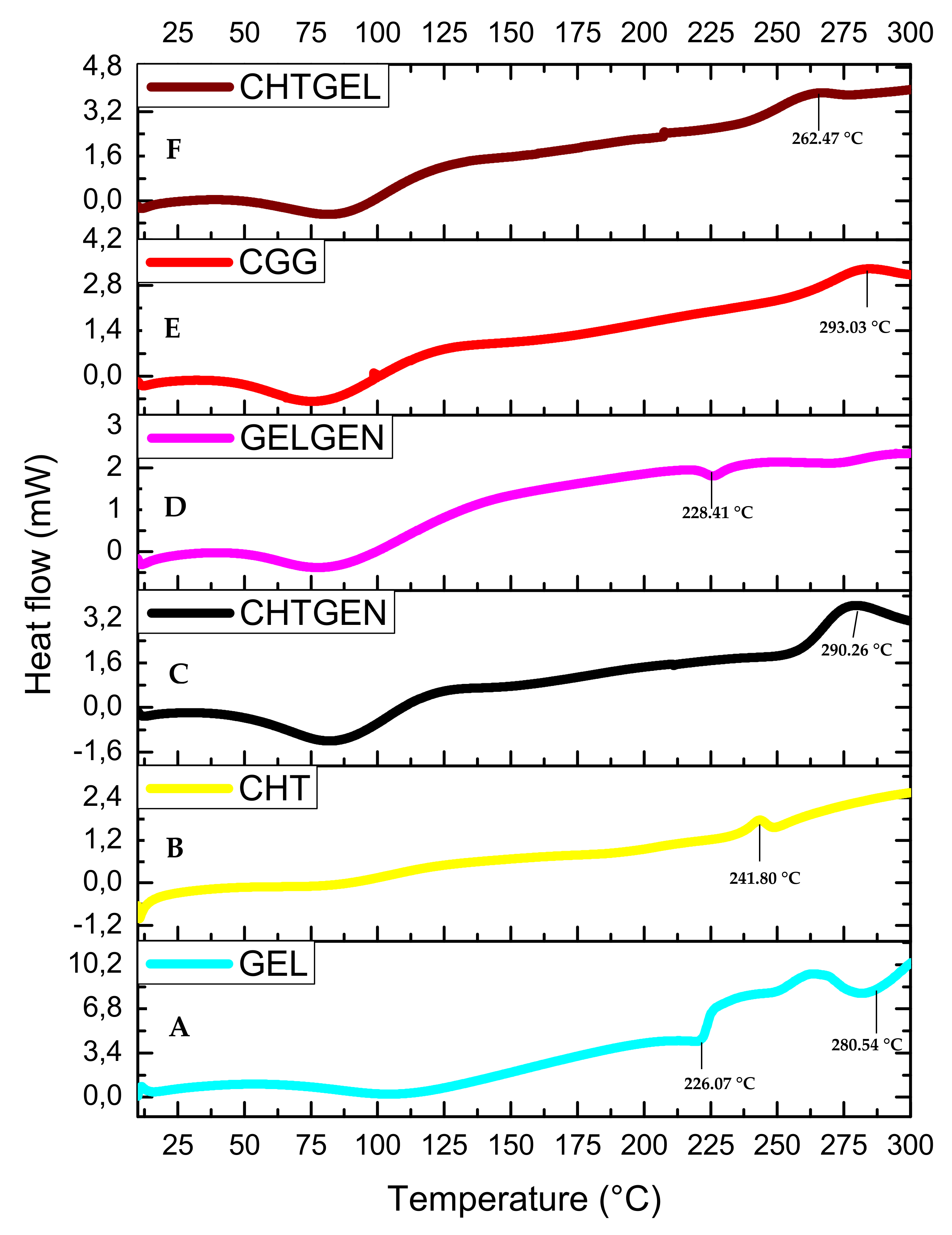
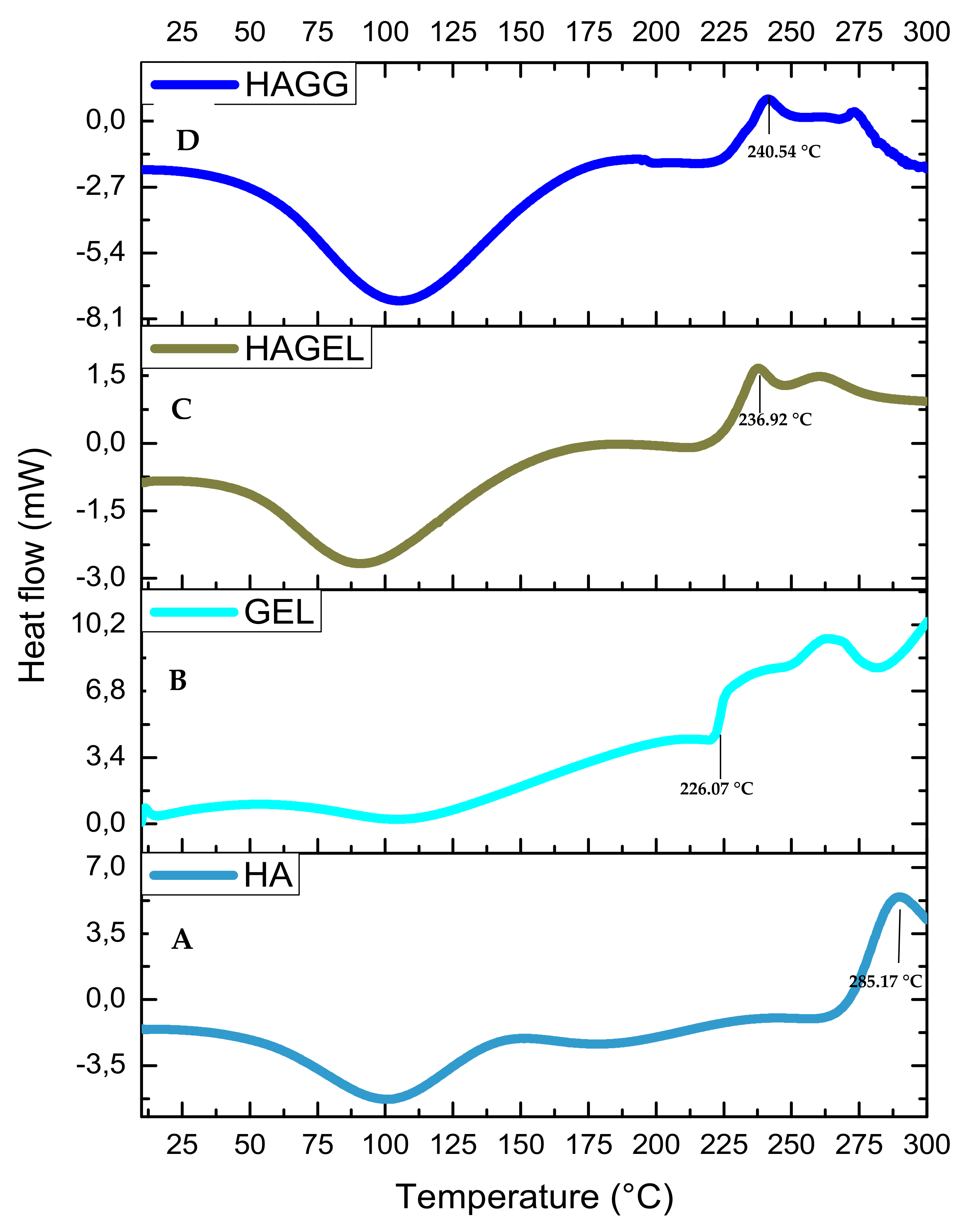
3.4. Thermal Stability of Each Scaffold
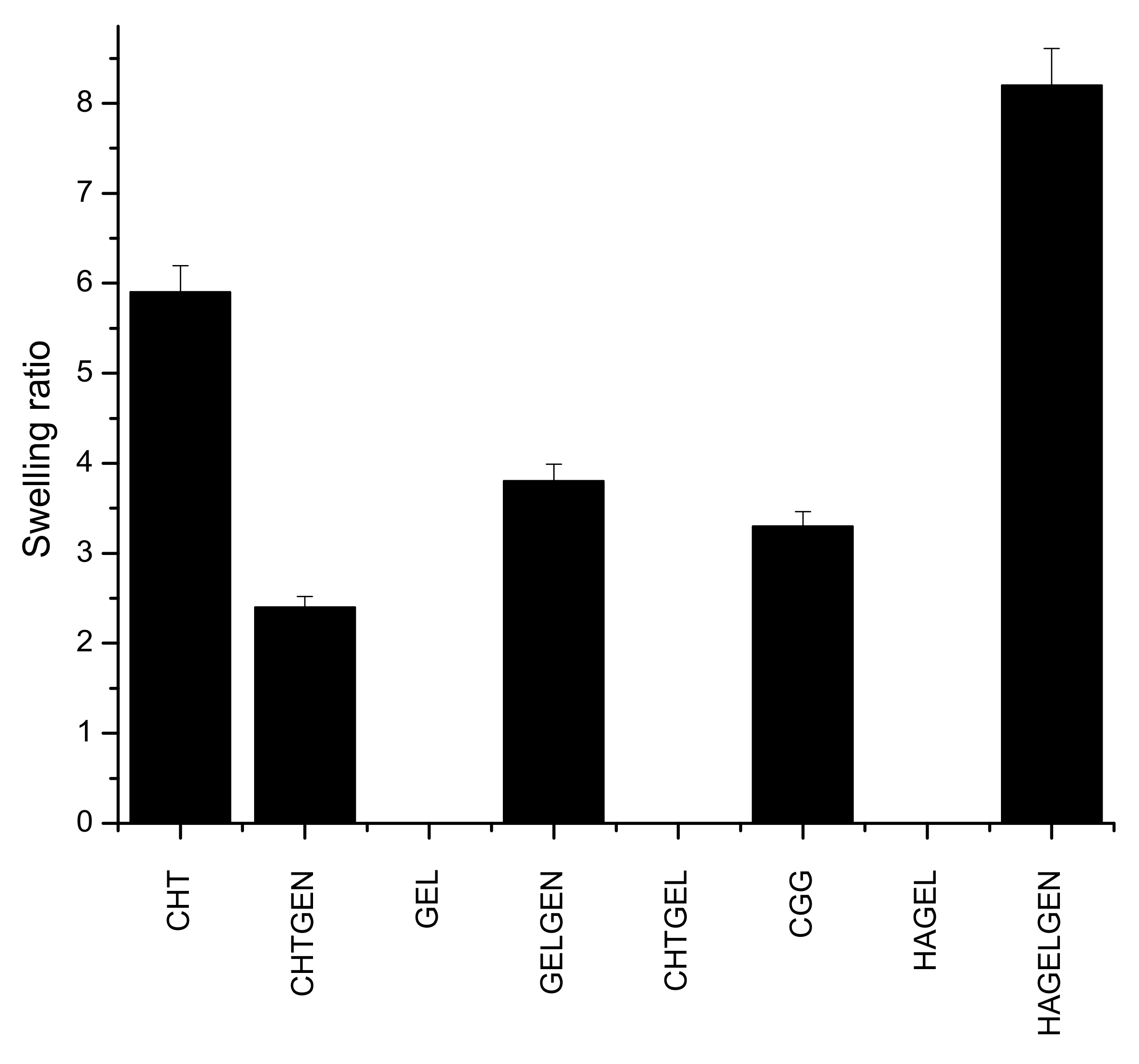
3.5. Swelling Potential of Each Scaffold
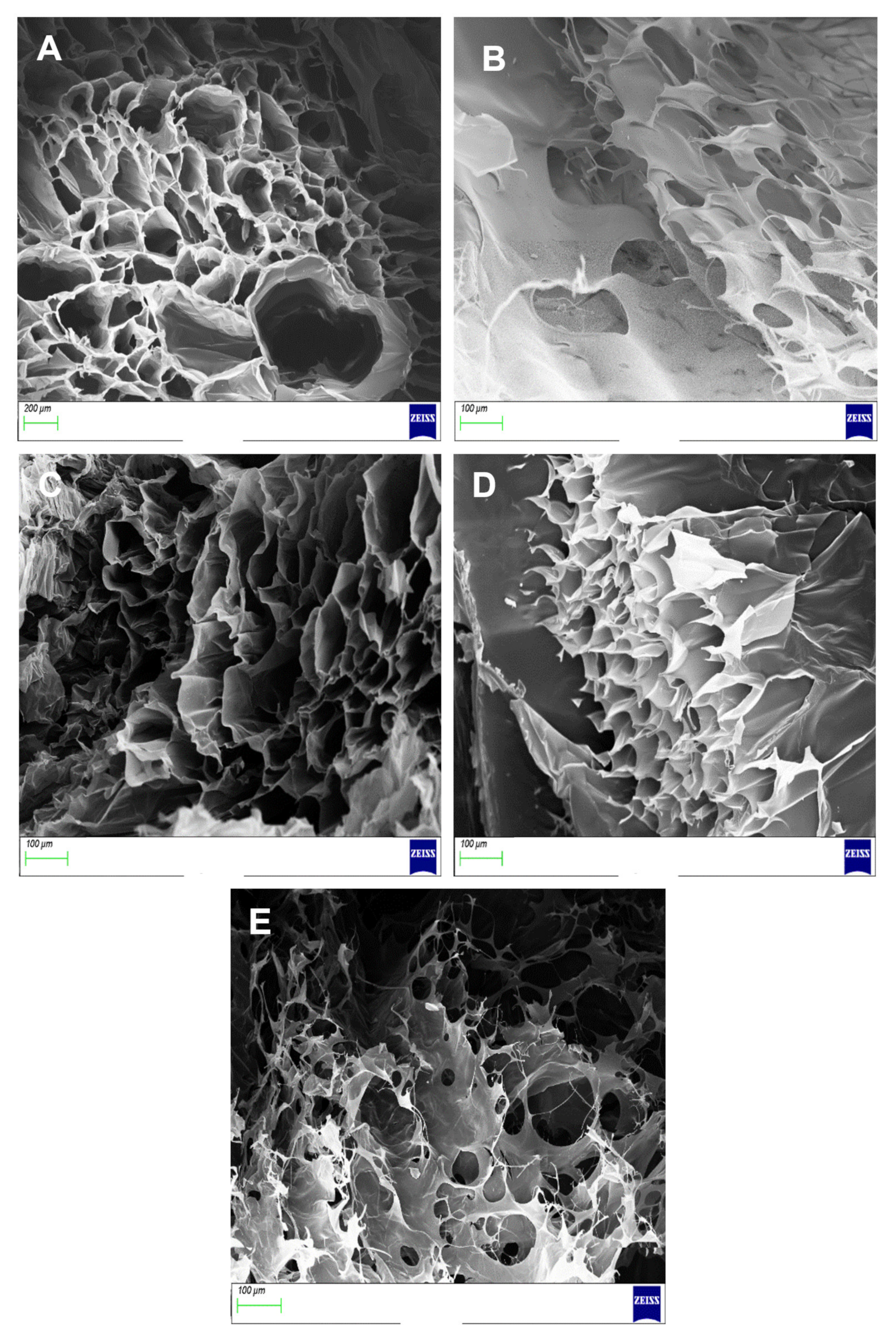
3.6. Morphological Analysis of Proteosaccharide Scaffolds
3.7. Density of Scaffolds
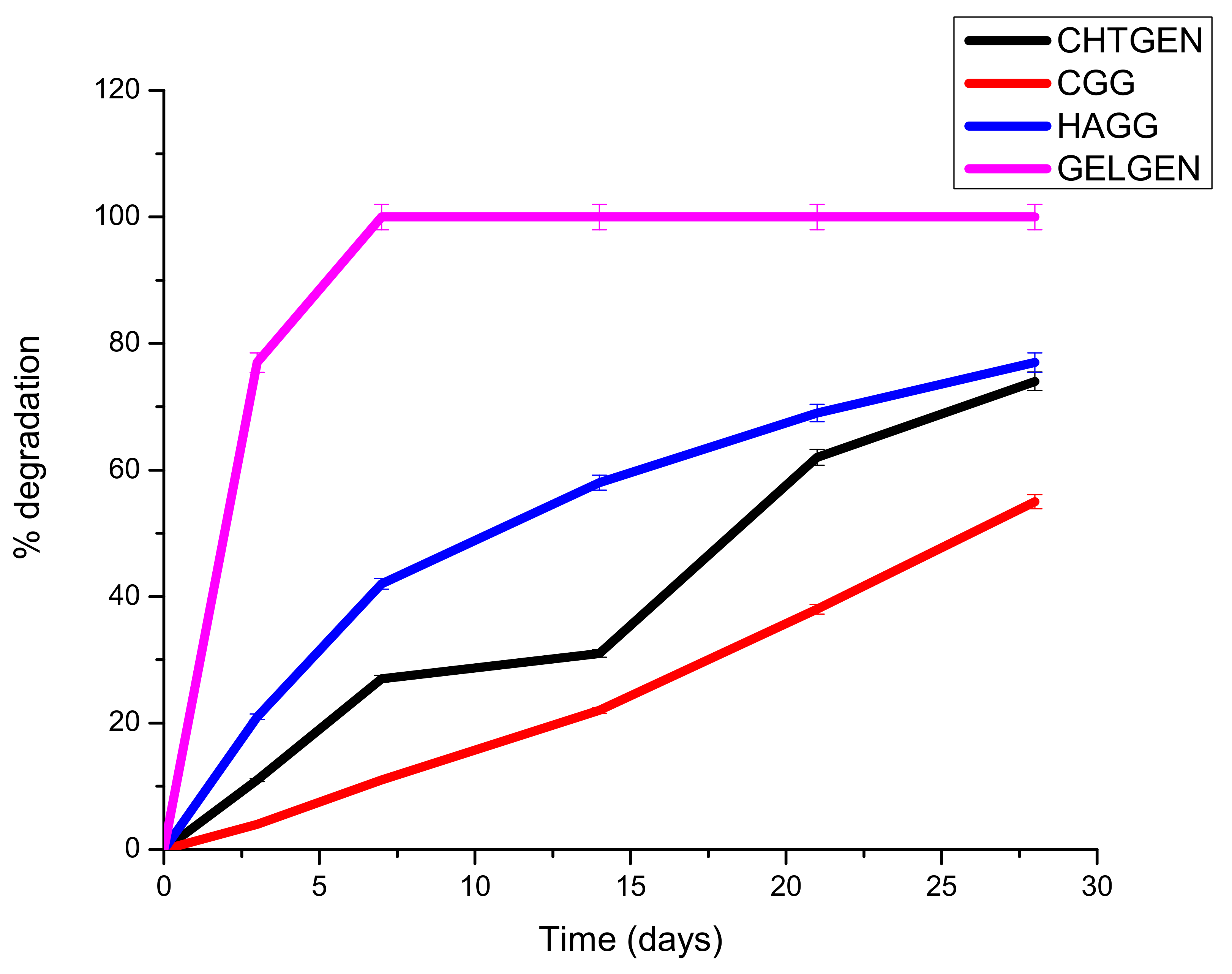
3.8. In Vitro Degradation of Proteosaccharide Scafffolds
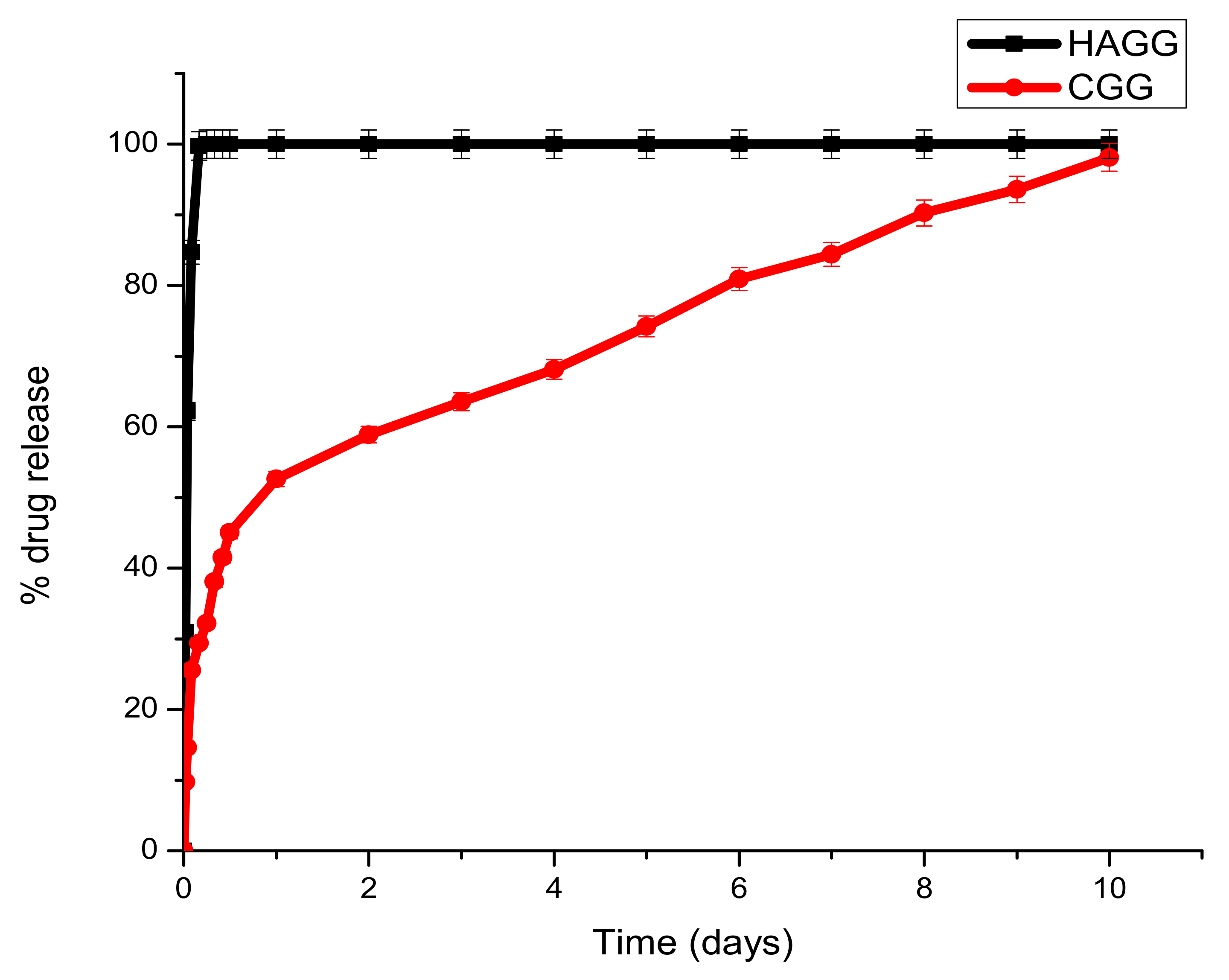
3.9. Drug Release of Dexamethasone-21-Phosphate from the IPN and Semi-IPN Hydrogel Scaffolds
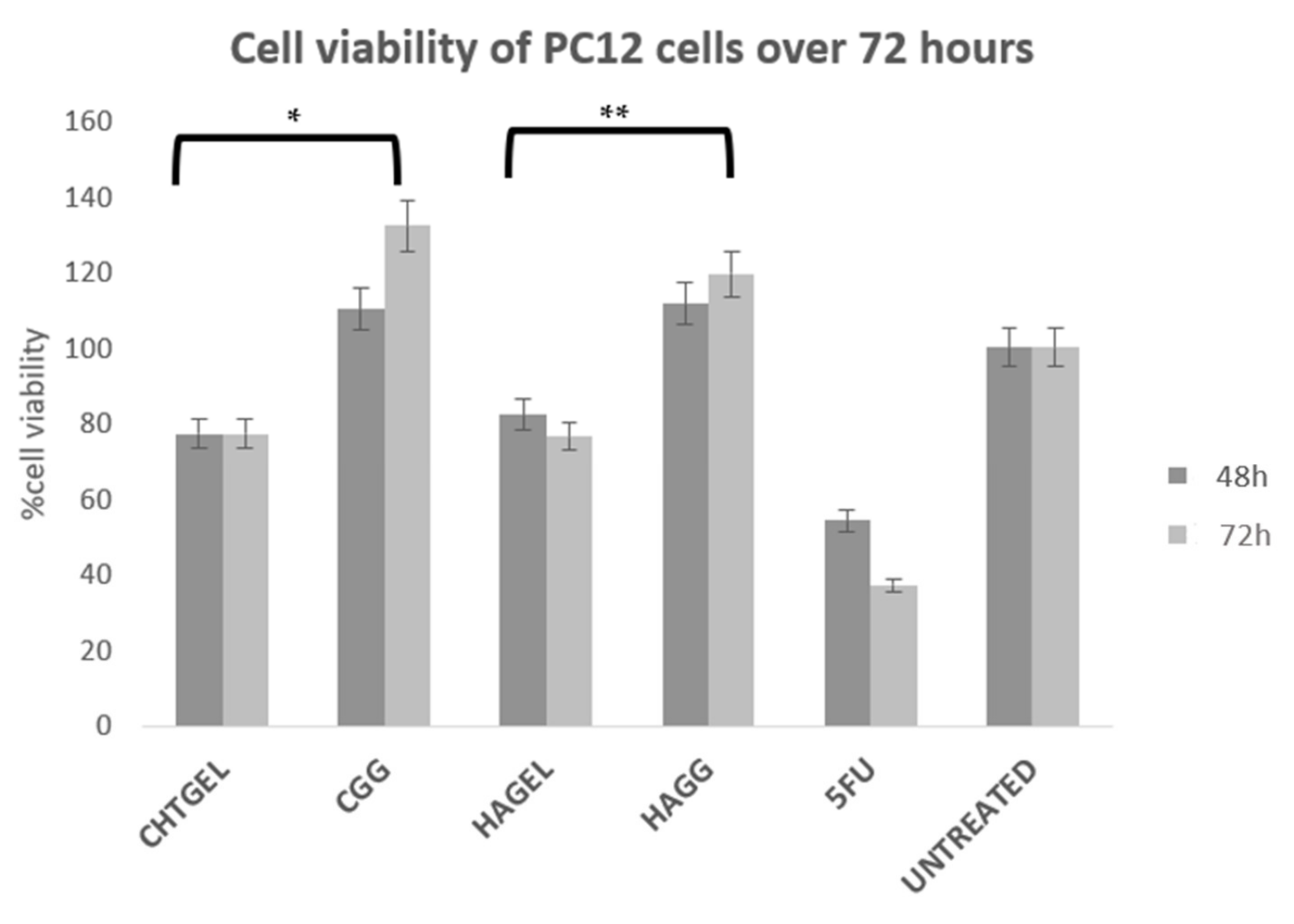
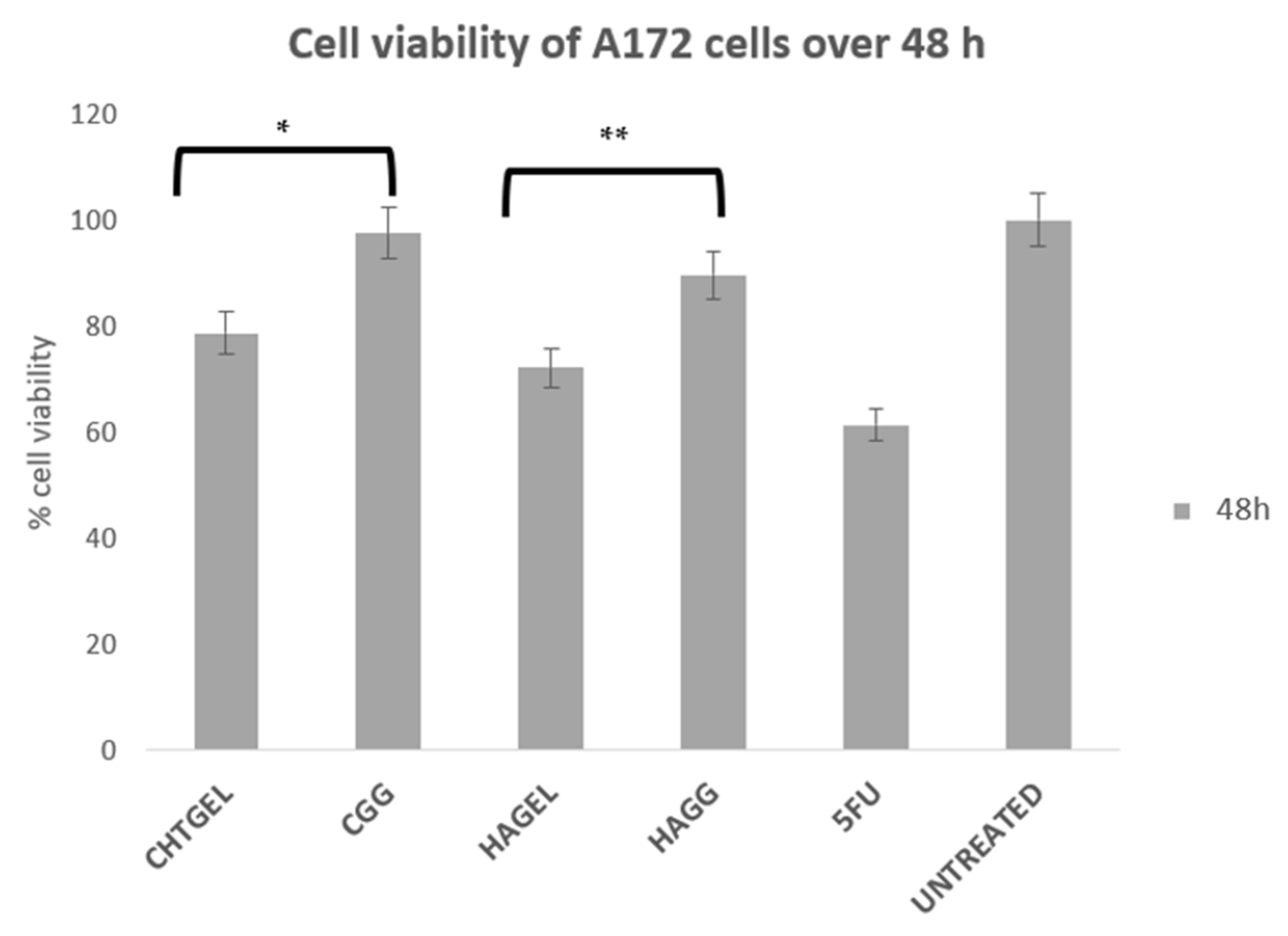
3.10. Cytotoxicity Analysis of Proteosaccharide Hydrogels (IPN and Semi-IPN)
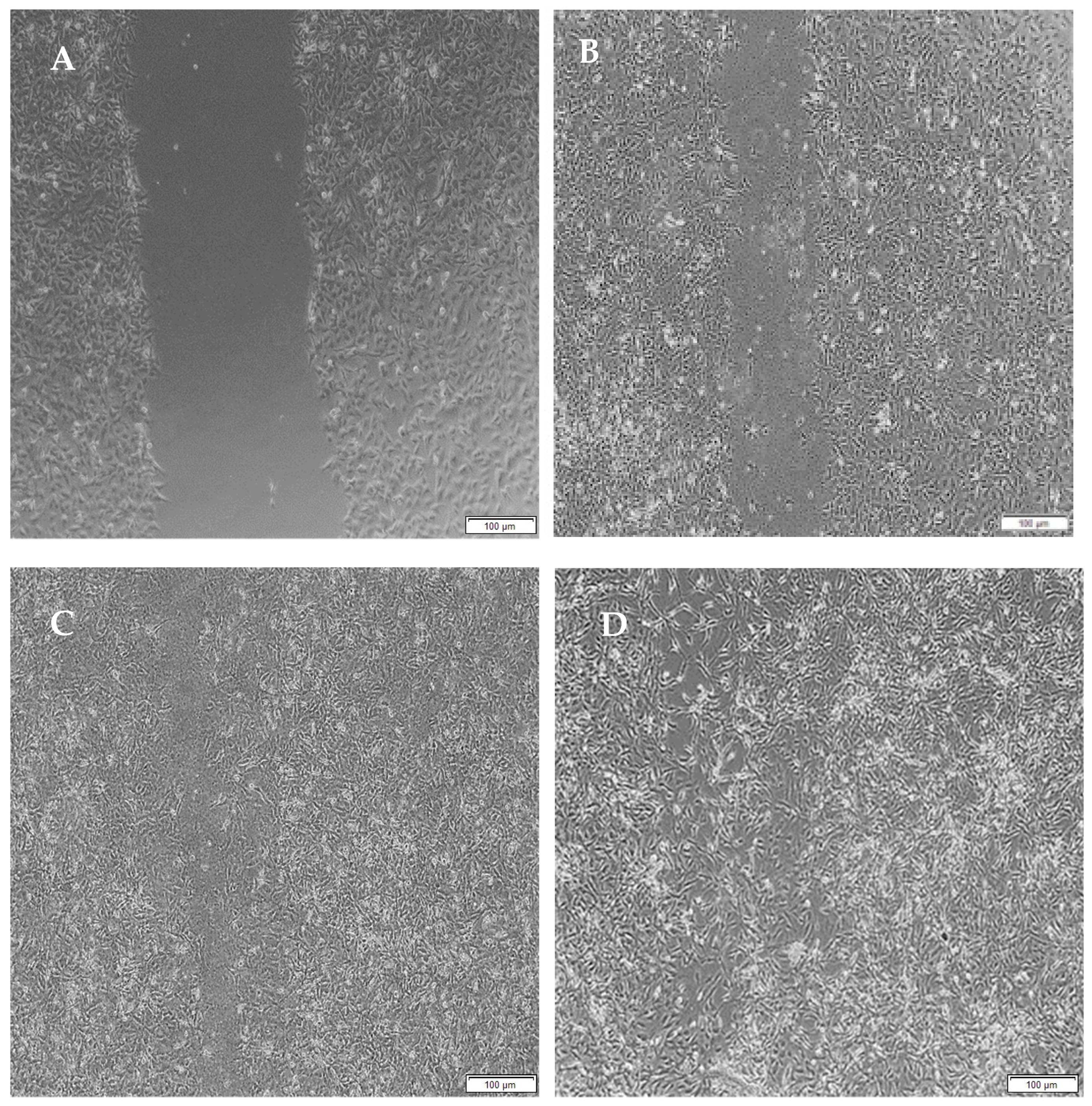
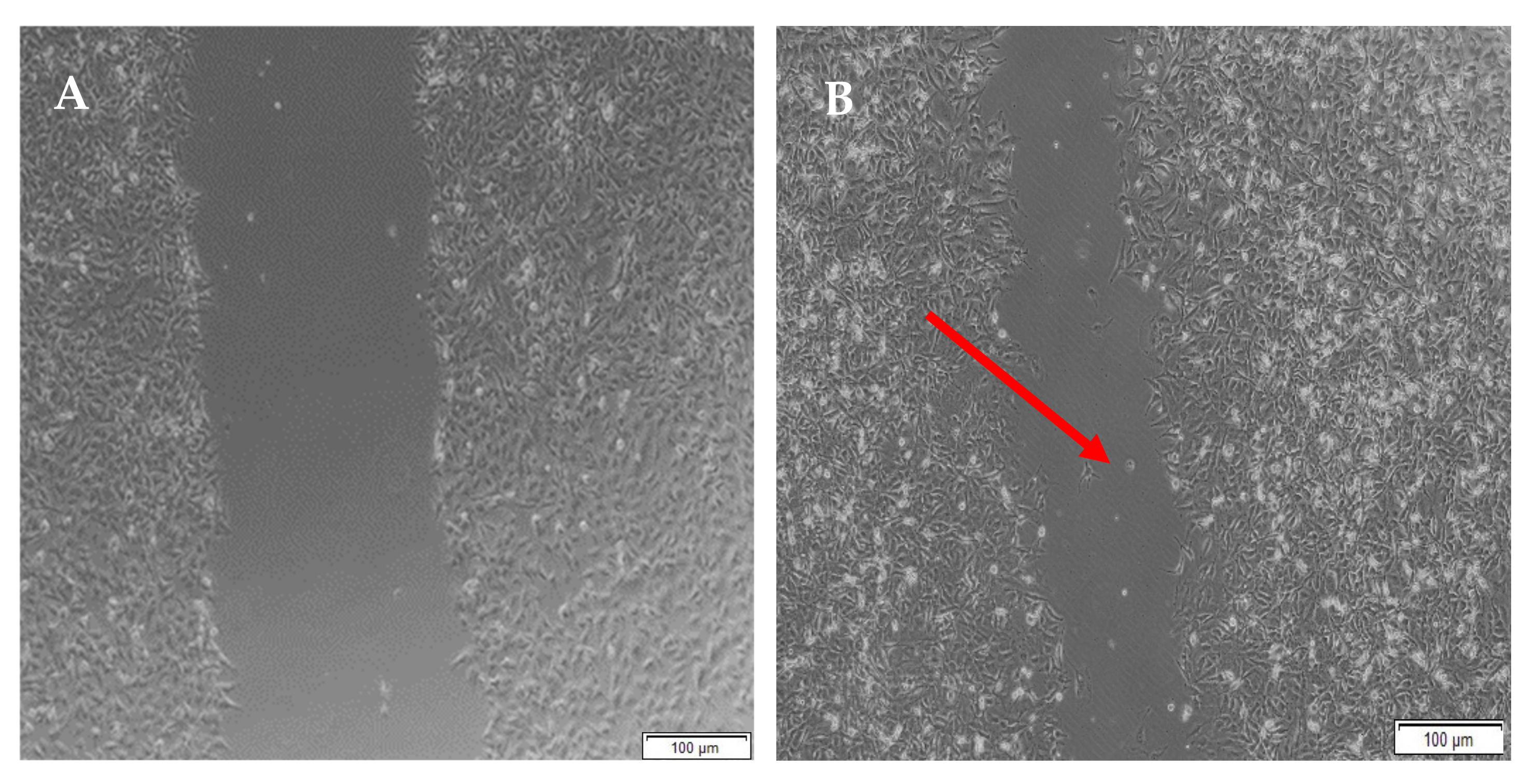
3.11. Migration of A172 Cells in Response to the Full IPN and Semi-IPN
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E. Advanced Strategies for Tissue Engineering in Regenerative Medicine: A Biofabrication and Biopolymer Perspective. Molecules 2021, 26, 2518. [Google Scholar] [CrossRef]
- Chedly, J.; Soares, S.; Montembault, A.; von Boxberg, Y.; Veron-Ravaille, M.; Mouffle, C.; Benassy, M.-N.; Taxi, J.; David, L.; Nothias, F. Physical chitosan microhydrogels as scaffolds for spinal cord injury restoration and axon regeneration. Biomaterials 2017, 138, 91–107. [Google Scholar] [CrossRef] [Green Version]
- Mo, L.; Yang, Z.; Zhang, A.; Li, X. The repair of the injured adult rat hippocampus with NT-3-chitosan carriers. Biomaterials 2010, 31, 2184–2192. [Google Scholar] [CrossRef]
- Chandy, T.; Sharma, C.P. Chitosan-as a Biomaterial. Biomater. Artif. Cells Artif. Organs 1990, 18, 1–24. [Google Scholar] [CrossRef]
- Roy, J.C.; Salaün, F.; Giraud, S.; Ferri, A.; Chen, G.; Guan, J. Solubility of Chitin: Solvents, Solution Behaviors and Their Related Mechanisms. Solubility Polysacch. 2017, 3, 20–60. [Google Scholar]
- Tiwari, S.; Patil, R.; Bahadur, P. Polysaccharide Based Scaffolds for Soft Tissue Engineering Applications. Polymers 2018, 11, 1. [Google Scholar] [CrossRef] [Green Version]
- Cheng, R.; Cao, Y.; Yan, Y.; Shen, Z.; Zhao, Y.; Zhang, Y.; Sang, S.; Han, Y. Fabrication and characterization of chitosan-based composite scaffolds for neural tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2021, 1–11. [Google Scholar] [CrossRef]
- Sionkowska, A. Molecular interactions in collagen and chitosan blends. Biomaterials 2004, 25, 795–801. [Google Scholar] [CrossRef]
- Levenberg, S.; Langer, R. Advances in Tissue Engineering. Curr. Top. Dev. Biol. 2004, 61, 113–134. [Google Scholar] [CrossRef]
- Voron’Ko, N.G.; Derkach, S.R.; Kuchina, Y.A.; Sokolan, N.I. The chitosan–gelatin (bio)polyelectrolyte complexes formation in an acidic medium. Carbohydr. Polym. 2016, 138, 265–272. [Google Scholar] [CrossRef]
- Re, F.; Sartore, L.; Moulisova, V.; Cantini, M.; Almici, C.; Bianchetti, A.; Chinello, C.; Dey, K.; Agnelli, S.; Manferdini, C.; et al. 3D gelatin-chitosan hybrid hydrogels combined with human platelet lysate highly support human mesenchymal stem cell proliferation and osteogenic differentiation. J. Tissue Eng. 2019, 10, 2041731419845852. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.A.; Radhakrishnan, S.; Nagarajan, S.; Muthukoori, S.; Dueñas, J.M.; Ribelles, J.L.; Lakshmi, B.S.; Nivethaa, E.A.; Gómez-Tejedor, J.A.; Reddy, M.S.; et al. An innovative bioresorbable gelatin based 3D scaffold that maintains the stemness of adipose tissue derived stem cells and the plasticity of differentiated neurons. RSC Adv. 2019, 9, 14452–14464. [Google Scholar] [CrossRef] [Green Version]
- Doblado, L.R.; Martínez-Ramos, C.; Pradas, M.M. Biomaterials for Neural Tissue Engineering. Front. Nanotechnol. 2021, 3, 21. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar] [CrossRef]
- Liu, Y.; An, M.; Wang, L.; Qiu, H. Preparation and Characterization of Chitosan-Gelatin/Glutaraldehyde Scaffolds. J. Macromol. Sci. Part B 2014, 53, 309–325. [Google Scholar] [CrossRef]
- Georgopoulou, A.; Papadogiannis, F.; Batsali, A.; Marakis, J.; Alpantaki, K.; Eliopoulos, A.G.; Pontikoglou, C.; Chatzinikolaidou, M. Chitosan/gelatin scaffolds support bone regeneration. J. Mater. Sci. Mater. Med. 2018, 29, 59. [Google Scholar] [CrossRef]
- England, S.; Rajaram, A.; Schreyer, D.J.; Chen, X. Bioprinted fibrin-factor XIII-hyaluronate hydrogel scaffolds with encapsulated Schwann cells and their in vitro characterization for use in nerve regeneration. Bioprinting 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Guo, Y.; Yuan, T.; Xiao, Z.; Tang, P.; Xiao, Y.; Fan, Y.; Zhang, X. Hydrogels of collagen/chondroitin sulfate/hyaluronan interpenetrating polymer network for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2012, 23, 2267–2279. [Google Scholar] [CrossRef]
- Wolf, K.J.; Kumar, S. Hyaluronic Acid: Incorporating the Bio into the Material. ACS Biomater. Sci. Eng. 2019, 5, 3753–3765. [Google Scholar] [CrossRef]
- Rayahin, J.E.; Buhrman, J.S.; Zhang, Y.; Koh, T.J.; Gemeinhart, R.A. High and Low Molecular Weight Hyaluronic Acid Differentially Influence Macrophage Activation. ACS Biomater. Sci. Eng. 2015, 1, 481–493. [Google Scholar] [CrossRef] [Green Version]
- Khaing, Z.Z.; Milman, B.D.; Vanscoy, J.E.; Seidlits, S.K.; Grill, R.J.; Schmidt, C.E. High molecular weight hyaluronic acid limits astrocyte activation and scar formation after spinal cord injury. J. Neural Eng. 2011, 8, 046033. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Tang, S.; Spector, M. Incorporation of hyaluronic acid into collagen scaffolds for the control of chondrocyte-mediated contraction and chondrogenesis. Biomed. Mater. 2007, 2, S135–S141. [Google Scholar] [CrossRef]
- Brännvall, K.; Bergman, K.; Wallenquist, U.; Svahn, S.; Bowden, T.; Hilborn, J.; Forsberg-Nilsson, K. Enhanced neuronal differentiation in a three-dimensional collagen-hyaluronan matrix. J. Neurosci. Res. 2007, 85, 2138–2146. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, S.; Li, S.; Pan, H. Genipin-cross-linked hydrogels based on biomaterials for drug delivery: A review. Biomater. Sci. 2020, 9, 1583–1597. [Google Scholar] [CrossRef]
- Frohbergh, M.E.; Katsman, A.; Botta, G.P.; Lazarovici, P.; Schauer, C.L.; Wegst, U.G.; Lelkes, P.I. Electrospun hydroxyapatite-containing chitosan nanofibers crosslinked with genipin for bone tissue engineering. Biomaterials 2012, 33, 9167–9178. [Google Scholar] [CrossRef] [Green Version]
- Madhusudanan, P.; Raju, G.; Shankarappa, S. Hydrogel systems and their role in neural tissue engineering. J. R. Soc. Interface 2020, 17, 20190505. [Google Scholar] [CrossRef] [Green Version]
- Zamproni, L.N.; Mundim, M.T.V.V.; Porcionatto, M.A. Neurorepair and Regeneration of the Brain: A Decade of Bioscaffolds and Engineered Microtissue. Front. Cell Dev. Biol. 2021, 9, 649891. [Google Scholar] [CrossRef]
- Georges, P.; Miller, W.J.; Meaney, D.; Sawyer, E.S.; Janmey, P.A. Matrices with Compliance Comparable to that of Brain Tissue Select Neuronal over Glial Growth in Mixed Cortical Cultures. Biophys. J. 2006, 90, 3012–3018. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Duan, Q.; Yu, L.; Xie, F. Thermomechanically processed chitosan:gelatin films being transparent, mechanically robust and less hygroscopic. Carbohydr. Polym. 2021, 272, 118522. [Google Scholar] [CrossRef]
- Paajanen, A.; Vaari, J.; Verho, T. Crystallization of cross-linked polyethylene by molecular dynamics simulation. Polymer 2019, 171, 80–86. [Google Scholar] [CrossRef]
- Snyder, T.N.; Madhavan, K.; Intrator, M.; Dregalla, R.C.; Park, D. A fibrin/hyaluronic acid hydrogel for the delivery of mesenchymal stem cells and potential for articular cartilage repair. J. Biol. Eng. 2014, 8, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velasco-Rodriguez, B.; Diaz-Vidal, T.; Rosales-Rivera, L.; García-González, C.; Alvarez-Lorenzo, C.; Al-Modlej, A.; Domínguez-Arca, V.; Prieto, G.; Barbosa, S.; Martínez, J.S.; et al. Hybrid Methacrylated Gelatin and Hyaluronic Acid Hydrogel Scaffolds. Preparation and Systematic Characterization for Prospective Tissue Engineering Applications. Int. J. Mol. Sci. 2021, 22, 6758. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.D.; Hexiu, J.; Patel, D.K.; Ganguly, K.; Lim, K.-T. 3D-printed bioactive and biodegradable hydrogel scaffolds of alginate/gelatin/cellulose nanocrystals for tissue engineering. Int. J. Biol. Macromol. 2021, 167, 644–658. [Google Scholar] [CrossRef]
- Mahumane, G.D.; Kumar, P.; du Toit, L.C.; Choonara, Y.E.; Pillay, V. 3D scaffolds for brain tissue regeneration: Architectural challenges. Biomater. Sci. 2018, 6, 2812–2837. [Google Scholar] [CrossRef]
- Koc, F.E.; Altıncekic, T.G. Investigation of gelatin/chitosan as potential biodegradable polymer films on swelling behavior and methylene blue release kinetics. Polym. Bull. 2021, 78, 3383–3398. [Google Scholar] [CrossRef]
- Kildeeva, N.; Chalykh, A.; Belokon, M.; Petrova, T.; Matveev, V.; Svidchenko, E.; Surin, N.; Sazhnev, N. Influence of Genipin Crosslinking on the Properties of Chitosan-Based Films. Polymer 2020, 12, 1086. [Google Scholar] [CrossRef]
- Ji, W.; Yang, F.; Seyednejad, H.; Chen, Z.; Hennink, W.E.; Anderson, J.M.; Beucken, J.J.V.D.; Jansen, J.A. Biocompatibility and degradation characteristics of PLGA-based electrospun nanofibrous scaffolds with nanoapatite incorporation. Biomaterials 2012, 33, 6604–6614. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, N.; Xu, Q.; Zhang, L.; Zhang, C.; Liu, H.; Yu, Z.; Zhou, S.; Feng, G.; Huang, F. Decellularized nerve extracellular matrix/chitosan crosslinked by genipin to prepare a moldable nerve repair material. Cell Tissue Bank. 2021, 22, 419–430. [Google Scholar] [CrossRef]
- Sirivisoot, S.; Pareta, R.; Webster, T.J. Electrically controlled drug release from nanostructured polypyrrole coated on titanium. Nanotechnology 2011, 22, 085101. [Google Scholar] [CrossRef]
- Jeong, D.U.; Bae, S.; Macks, C.; Whitaker, J.; Lynn, M.; Webb, K.; Lee, J.S. Hydrogel-mediated local delivery of dexamethasone reduces neuroinflammation after traumatic brain injury. Biomed. Mater. 2021, 16, 035002. [Google Scholar] [CrossRef]
- Moll, A.; Lara, M.; Pomar, J.; Orozco, M.; Frontera, G.; Llompart-Pou, J.A.; Moratinos, L.; González, V.; Ibáñez, J.; Pérez-Bárcena, J. Effects of dexamethasone in traumatic brain injury patients with pericontusional vasogenic edema. Medicine 2020, 99, e22879. [Google Scholar] [CrossRef]
- Faupel, G.; Reulen, H.J.; Muller, D.; Schurmann, K. Dexamethasone in severe head injuries. Neurosurg. Rev. 1979, 2, 105–111. [Google Scholar] [CrossRef]
- Bryś, M.; Urbańska, K.; Olas, B. Novel Findings regarding the Bioactivity of the Natural Blue Pigment Genipin in Human Diseases. Int. J. Mol. Sci. 2022, 23, 902. [Google Scholar] [CrossRef]
- Ahani, N.; Sangtarash, M.H.; Houshmand, M.; Eskandani, M.A. Genipin induces cell death via intrinsic apoptosis pathways in human glioblastoma cells. J. Cell. Biochem. 2019, 120, 2047–2057. [Google Scholar] [CrossRef]


| Scaffold | Ci | Scaffold | Ci |
|---|---|---|---|
| Chitosan | 0.61821 ± 0.0599 | Hyaluronic acid | 0.43432 ± 0.0152 |
| Gelatin | 0.51431 ± 0.0212 | Hyaluronic acid–Gelatin | 0.51289 ± 0.0178 |
| Chitosan–Gelatin | 0.62533 ± 0.0374 | Hyaluronic acid Gelatin–Genipin | 0.49226 ± 0.0210 |
| Chitosan–Genipin | 0.46459 ± 0.0165 | Chitosan–Gelatin–Genipin | 0.50380 ± 0.0410 |
| Gelatin–Genipin | 0.52687 ± 0.0138 |
| Scaffold | Average Pore Diameter (µm) | Porosity (%) | Scaffold | Average Pore Diameter (µm) | Porosity (%) |
|---|---|---|---|---|---|
| CHTGEN | 148.920 μm ± 6.335 µm | 52.791% | HAGEL | 107.54 μm ± 13.52 µm | 73.614% |
| GELGEN | 65.298 μm ± 9.936 µm | 76.985% | HAGELGEN | 84.289 μm ± 7.658 μm | 46.257% |
| CGG | 72.789 μm ± 16.85 µm | 71.335% |
| Combination | Density (mg/mm3) | Combination | Density (mg/mm3) |
|---|---|---|---|
| CHT | 0.048 ± 0.002 | CHTGEN | 0.1345 ± 0.037 |
| GEL | 0.0323 ± 0.016 | GELGEN | 0.0572 ± 0.028 |
| HA | 0.0457 ± 0.021 | HAGG | 0.1967 ± 0.059 |
| CHTGEL | 0.0573 ± 0.013 | CGG | 0.1476 ± 0.065 |
| HAGEL | 0.0276 ± 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cassimjee, H.; Kumar, P.; Ubanako, P.; Choonara, Y.E. Genipin-Crosslinked, Proteosaccharide Scaffolds for Potential Neural Tissue Engineering Applications. Pharmaceutics 2022, 14, 441. https://doi.org/10.3390/pharmaceutics14020441
Cassimjee H, Kumar P, Ubanako P, Choonara YE. Genipin-Crosslinked, Proteosaccharide Scaffolds for Potential Neural Tissue Engineering Applications. Pharmaceutics. 2022; 14(2):441. https://doi.org/10.3390/pharmaceutics14020441
Chicago/Turabian StyleCassimjee, Henna, Pradeep Kumar, Philemon Ubanako, and Yahya E. Choonara. 2022. "Genipin-Crosslinked, Proteosaccharide Scaffolds for Potential Neural Tissue Engineering Applications" Pharmaceutics 14, no. 2: 441. https://doi.org/10.3390/pharmaceutics14020441
APA StyleCassimjee, H., Kumar, P., Ubanako, P., & Choonara, Y. E. (2022). Genipin-Crosslinked, Proteosaccharide Scaffolds for Potential Neural Tissue Engineering Applications. Pharmaceutics, 14(2), 441. https://doi.org/10.3390/pharmaceutics14020441







