Ganciclovir Pharmacokinetics and Individualized Dosing Based on Covariate in Lung Transplant Recipients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Bioanalytical Assay
2.3. Pharmacokinetic Analysis
2.4. Statistical Analysis
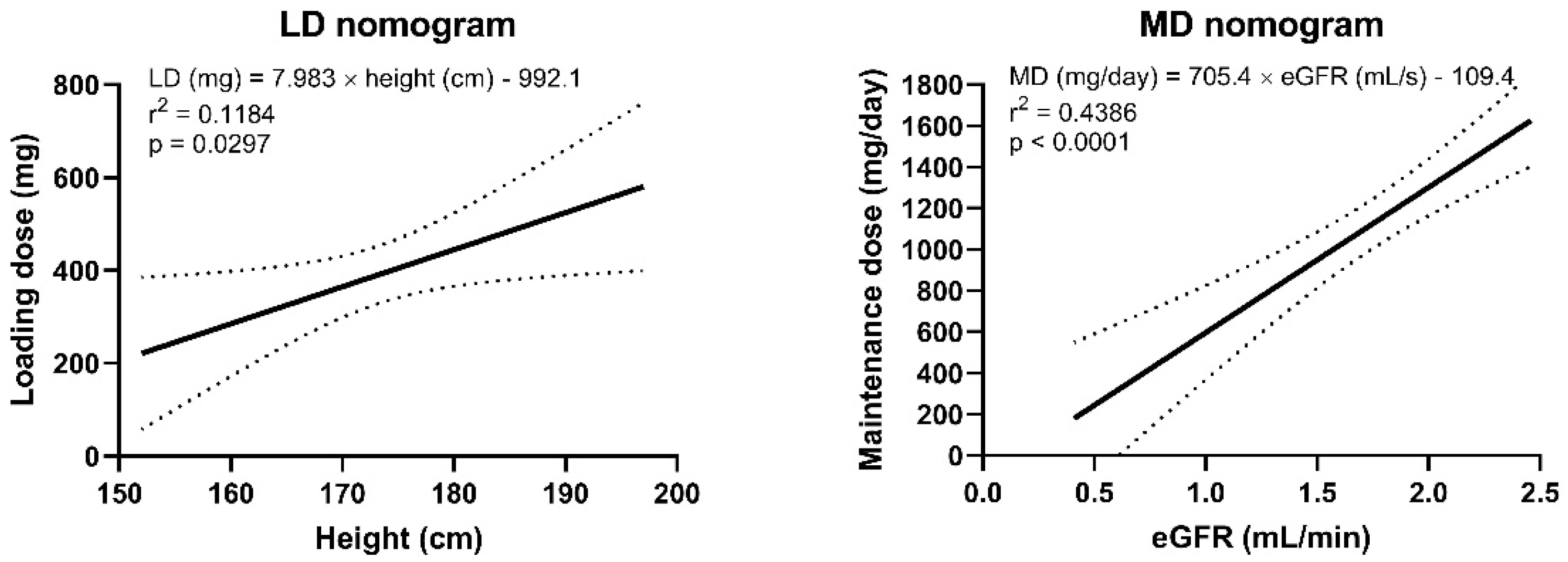
2.5. Loading and Maintenance Dose Calculation
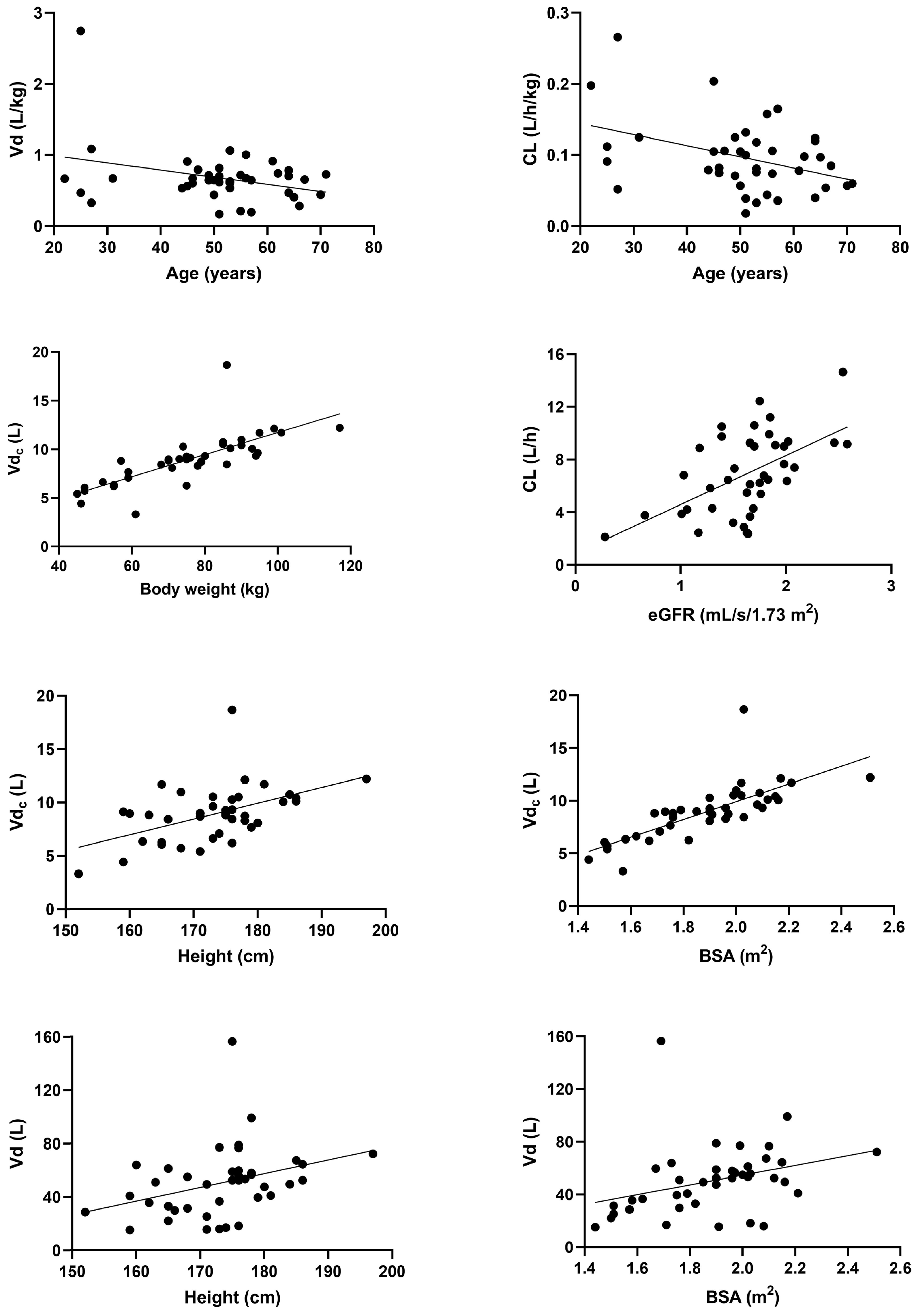
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hubacek, P.; Virgili, A.; Ward, K.N.; Pohlreich, D.; Keslova, P.; Goldova, B.; Markova, M.; Zajac, M.; Cinek, O.; Nacheva, E.P.; et al. HHV-6 DNA throughout the tissues of two stem cell transplant patients with chromosomally integrated HHV-6 and fatal CMV pneumonitis. Br. J. Haematol. 2009, 145, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, C.; Fernandez, R.; Safaeinili, N.; Akbarpour, M.; Wu, Q.; Budinger, G.R.S.; Bharat, A. Long-Term Impact of Cytomegalovirus Serologic Status on Lung Transplantation in the United States. Ann. Thorac. Surg. 2019, 107, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Kotton, C.N.; Kumar, D.; Caliendo, A.M.; Huprikar, S.; Chou, S.; Danziger-Isakov, L.; Humar, A.; The Transplantation Society International CMV Consensus Group. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation 2018, 102, 900–931. [Google Scholar] [PubMed] [Green Version]
- Ho, S.A.; Slavin, M.; Roberts, J.A.; Yong, M. Optimization of Ganciclovir use in allogeneic hematopoietic cell transplant recipients—The role of therapeutic drug monitoring. Expert Rev. Anti-Infect. Ther. 2021, 19, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Perrottet, N.; Decosterd, L.A.; Meylan, P.; Pascual, M.; Biollaz, J.; Buclin, T. Valganciclovir in adult solid organ transplant recipients: Pharmacokinetic and pharmacodynamic characteristics and clinical interpretation of plasma concentration measurements. Clin. Pharmacokinet. 2009, 48, 399–418. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, C.; Boivin, G. Human cytomegalovirus resistance to antiviral drugs. Antimicrob. Agents Chemother. 2005, 49, 873–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razonable, R.R. Management strategies for cytomegalovirus infection and disease in solid organ transplant recipients. Infect. Dis. Clin. N. Am. 2013, 27, 317–342. [Google Scholar] [CrossRef]
- Davis, C.L.; Springmeyer, S.; Gmerek, B.J. Central nervous system side effects of ganciclovir. N. Engl. J. Med. 1990, 322, 933–934. [Google Scholar]
- Chambers, D.C.; Zuckermann, A.; Cherikh, W.S.; Harhay, M.O.; Hayes, D., Jr.; Hsich, E.; Khush, K.K.; Potena, L.; Sadavarte, A.; Singh, T.P.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: 37th adult lung transplantation report—2020; focus on deceased donor characteristics. J. Heart Lung Transplant. 2020, 39, 1016–1027. [Google Scholar] [CrossRef]
- Snell, G.I.; Kotsimbos, T.C.; Levvey, B.J.; Skiba, M.; Rutherford, D.M.; Kong, D.C.; Williams, T.J.; Krum, H. Pharmacokinetic assessment of oral ganciclovir in lung transplant recipients with cystic fibrosis. J. Antimicrob. Chemother. 2000, 45, 511–516. [Google Scholar] [CrossRef]
- Crumpacker, C.S. Ganciclovir. N. Engl. J. Med. 1996, 335, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Faulds, D.; Heel, R.C. Ganciclovir: A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in cytomegalovirus infections. Drugs 1990, 39, 597–638. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known. Nutrition 1989, 5, 303–311; discussion 312–313. [Google Scholar]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrow, J.S. Quetelet index as indicator of obesity. Lancet 1986, 1, 1219. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services, Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation. U.S. 2018. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 3 February 2022).
- Sommadossi, J.P.; Bevan, R.; Ling, T.; Lee, F.; Mastre, B.; Chaplin, M.D.; Nerenberg, C.; Koretz, S.; Buhles, W.C., Jr. Clinical pharmacokinetics of ganciclovir in patients with normal and impaired renal function. Rev. Infect. Dis. 1988, 10 (Suppl. 3), S507–S514. [Google Scholar] [CrossRef]
- Bonett, D.G.; Price, R.M. Statistical inference for a linear function of medians: Confidence intervals, hypothesis testing, and sample size requirements. Psychol. Methods 2002, 7, 370–383. [Google Scholar] [CrossRef]
- Sima, M.; Pokorna, P.; Hronova, K.; Slanar, O. Effect of co-medication on the pharmacokinetic parameters of phenobarbital in asphyxiated newborns. Physiol. Res. 2015, 64, S513–S519. [Google Scholar] [CrossRef]
- Ritchie, B.M.; Barreto, J.N.; Barreto, E.F.; Crow, S.A.; Dierkhising, R.A.; Jannetto, P.J.; Tosh, P.K.; Razonable, R.R. Relationship of Ganciclovir Therapeutic Drug Monitoring with Clinical Efficacy and Patient Safety. Antimicrob. Agents Chemother. 2019, 63, e01855-18. [Google Scholar] [CrossRef] [Green Version]
- Trachuk, P.; Bartash, R.; Abbasi, M.; Keene, A. Infectious Complications in Lung Transplant Recipients. Lung 2020, 198, 879–887. [Google Scholar] [CrossRef]
- Lurain, N.S.; Chou, S. Antiviral drug resistance of human cytomegalovirus. Clin. Microbiol. Rev. 2010, 23, 689–712. [Google Scholar] [CrossRef] [Green Version]
- Stockmann, C.; Roberts, J.K.; Knackstedt, E.D.; Spigarelli, M.G.; Sherwin, C.M. Clinical pharmacokinetics and pharmacodynamics of ganciclovir and valganciclovir in children with cytomegalovirus infection. Expert Opin. Drug Metab. Toxicol. 2015, 11, 205–219. [Google Scholar] [CrossRef]
- Galar, A.; Valerio, M.; Catalan, P.; Garcia-Gonzalez, X.; Burillo, A.; Fernandez-Cruz, A.; Zatarain, E.; Sousa-Casasnovas, I.; Anaya, F.; Rodriguez-Ferrero, M.L.; et al. Valganciclovir-Ganciclovir Use and Systematic Therapeutic Drug Monitoring. An Invitation to Antiviral Stewardship. Antibiotics 2021, 10, 77. [Google Scholar] [PubMed]
- Martson, A.G.; Edwina, A.E.; Burgerhof, J.G.M.; Berger, S.P.; de Joode, A.; Damman, K.; Verschuuren, E.A.M.; Blokzijl, H.; Bakker, M.; Span, L.F.; et al. Ganciclovir therapeutic drug monitoring in transplant recipients. J. Antimicrob. Chemother. 2021, 76, 2356–2363. [Google Scholar] [CrossRef] [PubMed]
- De Sutter, P.J.; Gasthuys, E.; Van Braeckel, E.; Schelstraete, P.; Van Biervliet, S.; Van Bocxlaer, J.; Vermeulen, A. Pharmacokinetics in Patients with Cystic Fibrosis: A Systematic Review of Data Published Between 1999 and 2019. Clin. Pharmacokinet. 2020, 59, 1551–1573. [Google Scholar] [CrossRef] [PubMed]
- Jouret, F.; Bernard, A.; Hermans, C.; Dom, G.; Terryn, S.; Leal, T.; Lebecque, P.; Cassiman, J.J.; Scholte, B.J.; de Jonge, H.R.; et al. Cystic fibrosis is associated with a defect in apical receptor-mediated endocytosis in mouse and human kidney. J. Am. Soc. Nephrol. 2007, 18, 707–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, J.C.; Partovi, N.; Ensom, M.H. Ganciclovir in solid organ transplant recipients: Is there a role for clinical pharmacokinetic monitoring? Ther. Drug Monit. 2004, 26, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Lefeuvre, S.; Chevalier, P.; Charpentier, C.; Zekkour, R.; Havard, L.; Benammar, M.; Amrein, C.; Boussaud, V.; Lillo-Le Louet, A.; Guillemain, R.; et al. Valganciclovir prophylaxis for cytomegalovirus infection in thoracic transplant patients: Retrospective study of efficacy, safety, and drug exposure. Transpl. Infect. Dis. 2010, 12, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.M. Management of cytomegalovirus infection in solid-organ transplant recipients. Immunol. Investig. 1997, 26, 243–255. [Google Scholar] [CrossRef]
- Palacio-Lacambra, M.E.; Comas-Reixach, I.; Blanco-Grau, A.; Sune-Negre, J.M.; Segarra-Medrano, A.; Montoro-Ronsano, J.B. Comparison of the Cockcroft-Gault, MDRD and CKD-EPI equations for estimating ganciclovir clearance. Br. J. Clin. Pharmacol. 2018, 84, 2120–2128. [Google Scholar] [CrossRef] [Green Version]
- Sima, M.; Hartinger, J.; Cikankova, T.; Slanar, O. Estimation of once-daily amikacin dose in critically ill adults. J. Chemother. 2018, 30, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Sima, M.; Hartinger, J.; Stenglova Netikova, I.; Slanar, O. Creatinine Clearance Estimations for Vancomycin Maintenance Dose Adjustments. Am. J. Ther. 2018, 25, e602–e604. [Google Scholar] [CrossRef] [PubMed]
- Wiltshire, H.; Paya, C.V.; Pescovitz, M.D.; Humar, A.; Dominguez, E.; Washburn, K.; Blumberg, E.; Alexander, B.; Freeman, R.; Heaton, N.; et al. Pharmacodynamics of oral ganciclovir and valganciclovir in solid organ transplant recipients. Transplantation 2005, 79, 1477–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rousseau, A.; Monchaud, C.; Debord, J.; Vervier, I.; Estenne, M.; Thiry, P.; Marquet, P. Bayesian forecasting of oral cyclosporin pharmacokinetics in stable lung transplant recipients with and without cystic fibrosis. Ther. Drug Monit. 2003, 25, 28–35. [Google Scholar] [CrossRef] [PubMed]
| Time (min) | A (%) | B (%) | Flow (mL/min) |
|---|---|---|---|
| 0 | 95 | 5 | 0.2 |
| 1 | 0 | 100 | 0.4 |
| 3 | 0 | 100 | 0.4 |
| 3.1 | 95 | 5 | 0.4 |
| 3.5 | 95 | 5 | 0.4 |
| Sample | QC 1 | QC 2 | QC 3 | QC 4 |
|---|---|---|---|---|
| 0.1 (ng/mL) | 0.5 (ng/mL) | 2.5 (ng/mL) | 10 (ng/mL) | |
| Intra-Day Accuracy and Precision | ||||
| 1 | 0.107 | 0.47 | 2.29 | 9.42 |
| 2 | 0.108 | 0.47 | 2.26 | 9.43 |
| 3 | 0.111 | 0.47 | 2.26 | 9.40 |
| 4 | 0.109 | 0.47 | 2.28 | 9.34 |
| 5 | 0.105 | 0.47 | 2.30 | 9.24 |
| 6 | 0.109 | 0.47 | 2.30 | 9.32 |
| 7 | 0.106 | 0.48 | 2.26 | 9.19 |
| 8 | 0.109 | 0.47 | 2.25 | 9.27 |
| 9 | 0.103 | 0.47 | 2.28 | 9.40 |
| 10 | 0.104 | 0.46 | 2.30 | 9.35 |
| Mean (ng/mL) | 0.107 | 0.47 | 2.28 | 9.34 |
| SD (ng/mL) | 0.003 | 0.005 | 0.021 | 0.081 |
| CV (%) | 2.39 | 1.16 | 0.91 | 0.86 |
| BIAS (%) | 7.04 | 6.21 | 8.92 | 6.64 |
| Inter-Day Accuracy and Precision | ||||
| 1 | 0.107 | 0.47 | 2.23 | 9.87 |
| 2 | 0.114 | 0.48 | 2.45 | 10.21 |
| 3 | 0.119 | 0.46 | 2.26 | 10.55 |
| 4 | 0.116 | 0.51 | 2.79 | 9.92 |
| 5 | 0.115 | 0.52 | 2.36 | 9.78 |
| 6 | 0.112 | 0.49 | 2.48 | 10.23 |
| 7 | 0.104 | 0.53 | 2.74 | 10.46 |
| 8 | 0.116 | 0.51 | 2.77 | 9.48 |
| 9 | 0.119 | 0.48 | 2.69 | 10.33 |
| 10 | 0.119 | 0.47 | 2.61 | 10.56 |
| Mean (ng/mL) | 0.114 | 0.49 | 2.54 | 10.14 |
| SD (ng/mL) | 0.005 | 0.024 | 0.211 | 0.36 |
| CV (%) | 5.54 | 4.81 | 8.32 | 3.58 |
| BIAS (%) | 14.02 | 1.50 | 1.50 | 1.39 |
| Median | Interquartile Range | Range | |
|---|---|---|---|
| Age (years) | 52 | 46–58 | 22–71 |
| Body weight (kg) | 76 | 61–86 | 45–117 |
| Height (cm) | 175 | 168–178 | 152–197 |
| BSA (m2) | 1.90 | 1.73–2.03 | 1.44–2.51 |
| BMI (kg/m2) | 25.5 | 21.7–27.8 | 15.4–34.9 |
| eGFR creatinine (mL/s/1.73 m2) | 1.66 | 1.39–1.84 | 0.28–2.58 |
| eGFR cystatin C * (mL/s/1.73 m2) | 0.83 | 0.56–1.01 | 0.30–1.88 |
| ALT (μkat/L) | 0.56 | 0.31–1.08 | 0.15–7.87 |
| GGT (μkat/L) | 0.67 | 0.41–1.17 | 0.18–15.52 |
| White blood cell count (×109/L) | 9.55 | 7.30–13.25 | 2.70–23.00 |
| Platelet count (×109/L) | 266 | 187–377 | 63–530 |
| Total count | Percentage (%) | ||
| Sex (M/F) | 28/12 | 70/30 |
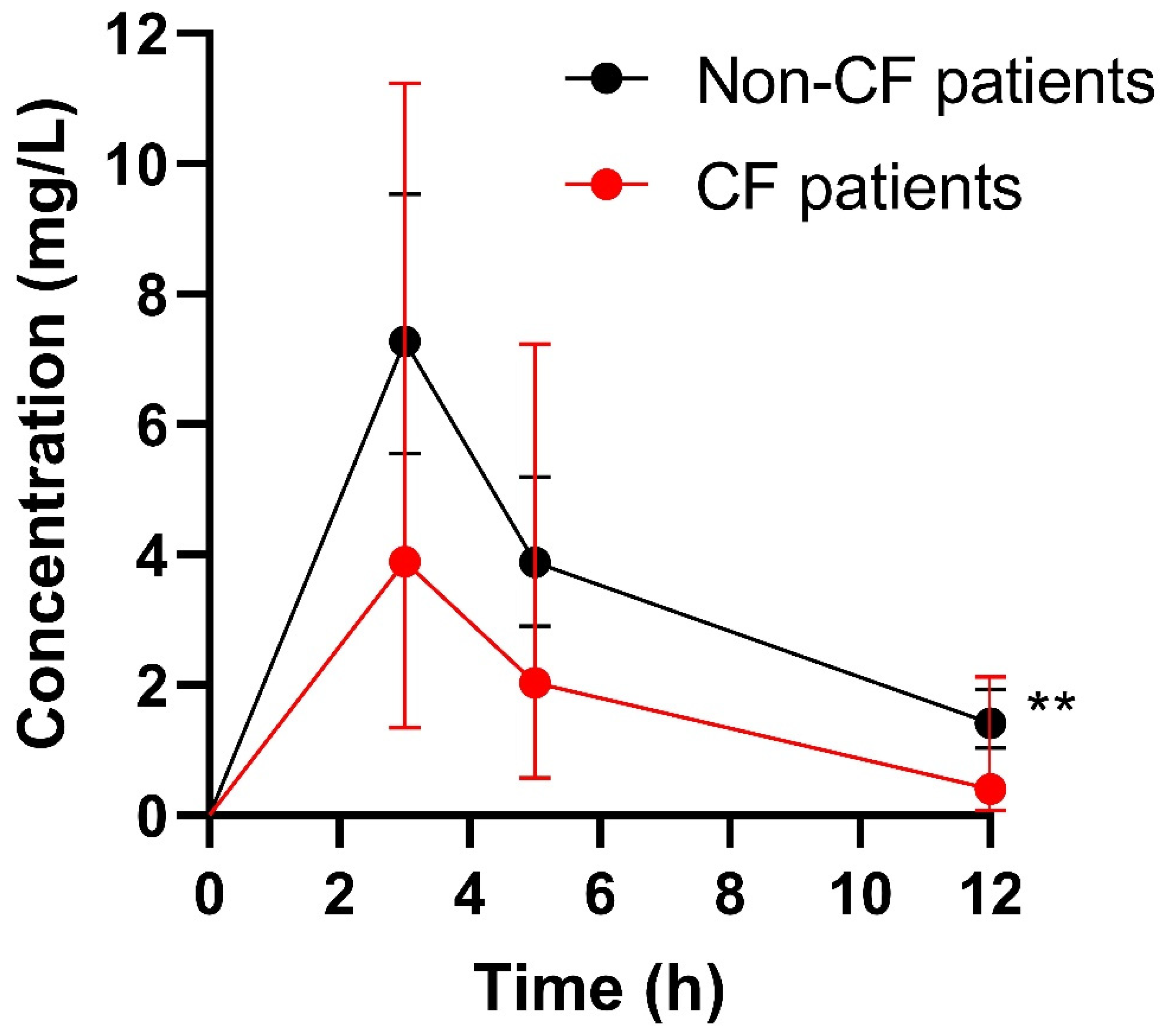
| PK Parameter | Whole Study Population (n = 40) | CF Patients (n = 5) | Non-CF Patients (n = 35) | p-Value CF vs. Non-CF |
|---|---|---|---|---|
| Vdc (L) | 8.96 (7.51–10.30) | 6.19 (6.05–7.65) | 9.13 (8.36–10.46) | - |
| Vdc (L/kg) | 0.120 (0.110–0.124) | 0.129 (0.121–0.130) | 0.119 (0.109–0.124) | 0.069 |
| Vd (L) | 51.7 (32.7–60.1) | 39.5 (31.5–59.7) | 52.5 (34.3–60.1) | - |
| Vd (L/kg) | 0.65 (0.52–0.73) | 0.67 (0.67–1.08) | 0.65 (0.50–0.72) | 0.1987 |
| CL (L/h) | 6.64 (4.27–9.20) | 7.39 (6.38–9.29) | 6.49 (4.04–9.14) | - |
| CL (L/h/kg) | 0.088 (0.059–0.118) | 0.125 (0.112–0.198) | 0.081 (0.057–0.106) | 0.0127 * |
| t1/2α (h) | 0.20 (0.18–0.37) | 0.18 (0.16–0.18) | 0.20 (0.18–0.44) | 0.0318 * |
| t1/2β (h) | 4.7 (3.6–5.8) | 3.6 (2.8–3.7) | 5.2 (3.7–5.9) | 0.1448 |
| AUC24 (mg × h/L) | 104.9 (76.1–154.0) | 53.8 (40.9–78.0) | 114.2 (85.5–155.9) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dvořáčková, E.; Šíma, M.; Petrus, J.; Klapková, E.; Hubáček, P.; Pozniak, J.; Havlín, J.; Lischke, R.; Slanař, O. Ganciclovir Pharmacokinetics and Individualized Dosing Based on Covariate in Lung Transplant Recipients. Pharmaceutics 2022, 14, 408. https://doi.org/10.3390/pharmaceutics14020408
Dvořáčková E, Šíma M, Petrus J, Klapková E, Hubáček P, Pozniak J, Havlín J, Lischke R, Slanař O. Ganciclovir Pharmacokinetics and Individualized Dosing Based on Covariate in Lung Transplant Recipients. Pharmaceutics. 2022; 14(2):408. https://doi.org/10.3390/pharmaceutics14020408
Chicago/Turabian StyleDvořáčková, Eliška, Martin Šíma, Jakub Petrus, Eva Klapková, Petr Hubáček, Jiří Pozniak, Jan Havlín, Robert Lischke, and Ondřej Slanař. 2022. "Ganciclovir Pharmacokinetics and Individualized Dosing Based on Covariate in Lung Transplant Recipients" Pharmaceutics 14, no. 2: 408. https://doi.org/10.3390/pharmaceutics14020408
APA StyleDvořáčková, E., Šíma, M., Petrus, J., Klapková, E., Hubáček, P., Pozniak, J., Havlín, J., Lischke, R., & Slanař, O. (2022). Ganciclovir Pharmacokinetics and Individualized Dosing Based on Covariate in Lung Transplant Recipients. Pharmaceutics, 14(2), 408. https://doi.org/10.3390/pharmaceutics14020408







