Photosensitive EGFR-Targeted Nanocarriers for Combined Photodynamic and Local Chemotherapy
Abstract
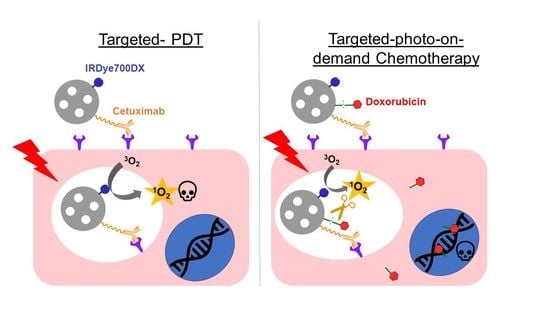
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Mesoporous Silica Nanoparticles’ Precursors
2.2.1. Synthesis of the 1O2-Cleavable Linker
2.2.2. Conjugation of Doxorubicin to the 1O2-Cleavable Linker
2.2.3. Conjugation between NHS-PEG5kDa-COOH and Cet
2.3. Synthesis and Derivatization of Mesoporous Silica Nanoparticles
2.3.1. IRDye700DX-Loaded Nanoparticles
2.3.2. Doxorubicin-Loaded Nanoparticles
2.4. Physico-Chemical Characterization of the Nanoparticles
2.5. Determination of the Drug Concentration in the Nanoparticles
2.6. Photo-Responsive Release of Doxorubicin from Nanoparticles
2.7. Cell Lines and Culture Conditions
2.8. Photophysical Characterization of the Nanoparticles
2.9. Confocal Microscopy
2.10. Singlet Oxygen Generation in Cell Cultures
2.11. Cellular Uptake Assays
2.12. In Vitro Dark- and Phototoxicity Assays
3. Results and Discussion
3.1. Synthesis of Precursors
3.1.1. Synthesis of the 1O2-Cleavable Linker
3.1.2. Orthogonal Conjugation of Doxorubicin to the 1O2-Cleavable Linker
3.1.3. Conjugation between Cet and PEG
3.2. Synthesis and Derivatization of Mesoporous Silica Nanoparticles
3.2.1. IRDye700DX-Cet-MSNP
- 1.
- Modification of the surface of blank MSNPs with amino groups (MSNP1). A high amount of APTES was employed in the reaction to ensure a high degree of surface’s derivatization.
- 2.
- Attachment of IRDye700DX-NHS to the MSNP1 nanoparticles via N-acylation (MSNP2), whereby the NHS group from IRDye700DX reacted with the MSNP- amino groups.
- 3.
- Anchoring of PEG-Cet (conjugate 7) via N-acylation to MSNP2 nanoparticles. The activated carboxyl group of PEG reacted with an amino group on the surface of the MSNP. Unlike in the previous steps, the final product of this reaction was not washed since the resuspension of the pellet required harsh sonication that could have damaged the antibody.
3.2.2. IRDye700DX-Cet-DOX-MSNP
- 1.
- Modification of the surface of blank MSNPs with amino groups (MSNP1).
- 2.
- Attachment of IRDye700DX-NHS to MSNP1 nanoparticles via N-acylation (MSNP2).
- 3.
- Derivatization of MSNP2 nanoparticles with the DOX conjugate 4 via N-acylation (MSNP3). The activated carboxyl of the conjugate 4 reacts with an amino group on the surface of MSNP2.
- 4.
- Anchoring of the conjugate PEG-Cet (compound 7) to the MSNP3 nanoparticles via N-acylation.
3.3. Physicochemical Characterization of the Nanoparticles
3.4. Photophysical Characterization of the Nanoparticles
3.4.1. Light Absorption and Steady-State Fluorescence
3.4.2. Singlet Oxygen Generation
- MSNP2 and MSNP3 nanoparticles
- IRDye700DX-Cet-MSNP and IRDye700DX-Cet-DOX-MSNP nanoparticles
- MSNP4 nanoparticles: Effect of DOX-IRDye700DX distance
3.4.3. Photo-Release of DOX
3.5. Biological Studies
3.5.1. In Vitro Uptake of IRDye700-Cet-MSNPs
3.5.2. In Vitro Singlet Oxygen Generation
3.5.3. In Vitro Phototoxicity Studies
- Free IRDye700DX and DOX
- IRDye700DX-MSNP and IRDye700DX-Cet-MSNP nanoparticles
- IRDye700DX-Cetuximab-DOX-MSNPs nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic Therapy. JNCI J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy for the Treatment and Diagnosis of Cancer–A Review of the Current Clinical Status. Front. Chem. 2021, 9, 686303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, L. Photodynamic combinational therapy in cancer treatment. J. BUON 2018, 23, 561–567. [Google Scholar]
- Sandland, J.; Boyle, R.W. Photosensitizer Antibody–Drug Conjugates: Past, Present, and Future. Bioconj. Chem. 2019, 30, 975–993. [Google Scholar] [CrossRef]
- Ghosh, S.; Gul, A.R.; Xu, P.; Lee, S.Y.; Rafique, R.; Kim, Y.H.; Park, T.J. Target delivery of photo-triggered nanocarrier for externally activated chemo-photodynamic therapy of prostate cancer. Mater. Today Chem. 2022, 23, 100688. [Google Scholar] [CrossRef]
- Hao, Y.; Chung, C.K.; Yu, Z.; Huis in ‘t Veld, R.V.; Ossendorp, F.A.; ten Dijke, P.; Cruz, L.J. Combinatorial Therapeutic Approaches with Nanomaterial-Based Photodynamic Cancer Therapy. Pharmaceutics 2022, 14, 120. [Google Scholar] [CrossRef]
- Liu, Z.; Xie, Z.; Li, W.; Wu, X.; Jiang, X.; Li, G.; Cao, L.; Zhang, D.; Wang, Q.; Xue, P.; et al. Photodynamic immunotherapy of cancers based on nanotechnology: Recent advances and future challenges. J. Nanobiotechnol. 2021, 19, 160. [Google Scholar] [CrossRef]
- Wong, R.C.H.; Ng, D.K.P.; Fong, W.-P.; Lo, P.-C. Glutathione- and light-controlled generation of singlet oxygen for triggering drug release in mesoporous silica nanoparticles. J. Mater. Chem. B 2020, 8, 4460–4468. [Google Scholar] [CrossRef]
- Lee, J.; Park, J.; Singha, K.; Kim, W.J. Mesoporous silica nanoparticle facilitated drug release through cascade photosensitizer activation and cleavage of singlet oxygen sensitive linker. Chem. Commun. 2013, 49, 1545–1547. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, J.; Liu, B. Conjugated-Polyelectrolyte-Based Polyprodrug: Targeted and Image-Guided Photodynamic and Chemotherapy with On-Demand Drug Release upon Irradiation with a Single Light Source. Angew. Chem. Int. Ed. 2014, 53, 7163–7168. [Google Scholar] [CrossRef]
- Bio, M.; Rajaputra, P.; Nkepang, G.; You, Y. Far-Red Light Activatable, Multifunctional Prodrug for Fluorescence Optical Imaging and Combinational Treatment. J. Med. Chem. 2014, 57, 3401–3409. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Zhang, C.; Alfranca, G.; Yang, Y.; Jiang, X.; Yang, Y.; Pan, F.; de la Fuente, J.M.; Cui, D. Near-Infrared Light Triggered ROS-activated Theranostic Platform based on Ce6-CPT-UCNPs for Simultaneous Fluorescence Imaging and Chemo-Photodynamic Combined Therapy. Theranostics 2016, 6, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cui, D.; Jiang, Y.; Huang, J.; Cheng, P.; Pu, K. Near-Infrared Photoactivatable Semiconducting Polymer Nanoblockaders for Metastasis-Inhibited Combination Cancer Therapy. Adv. Mater. 2019, 31, 1905091. [Google Scholar] [CrossRef] [PubMed]
- Tabero, A.; Planas, O.; Gallavardin, T.; Nieves, I.; Nonell, S.; Villanueva, A. Smart Dual-Functionalized Gold Nanoclusters for Spatio-Temporally Controlled Delivery of Combined Chemo- and Photodynamic Therapy. Nanomaterials 2020, 10, 2474. [Google Scholar] [CrossRef] [PubMed]
- von Felbert, V.; Bauerschlag, D.; Maass, N.; Bräutigam, K.; Meinhold-Heerlein, I.; Woitok, M.; Barth, S.; Hussain, A.F. A specific photoimmunotheranostics agent to detect and eliminate skin cancer cells expressing EGFR. J. Cancer Res. Clin. Oncol. 2016, 142, 1003–1011. [Google Scholar] [CrossRef]
- Sadraeian, M.; Bahou, C.; da Cruz, E.F.; Janini, L.M.R.; Sobhie Diaz, R.; Boyle, R.W.; Chudasama, V.; Eduardo Gontijo Guimarães, F. Photoimmunotherapy Using Cationic and Anionic Photosensitizer-Antibody Conjugates against HIV Env-Expressing Cells. Int. J. Mol. Sci. 2020, 21, 9151. [Google Scholar] [CrossRef]
- Boss, M.; Bos, D.; Frielink, C.; Sandker, G.; Bronkhorst, P.; van Lith, S.A.M.; Brom, M.; Buitinga, M.; Gotthardt, M. Receptor-Targeted Photodynamic Therapy of Glucagon-Like Peptide 1 Receptor–Positive Lesions. J. Nucl. Med. 2020, 61, 1588–1593. [Google Scholar] [CrossRef]
- Ngen, E.J.; Chen, Y.; Azad, B.B.; Boinapally, S.; Jacob, D.; Lisok, A.; Shen, C.; Hossain, M.S.; Jin, J.; Bhujwalla, Z.M.; et al. Prostate-specific membrane antigen (PSMA)-targeted photodynamic therapy enhances the delivery of PSMA-targeted magnetic nanoparticles to PSMA-expressing prostate tumors. Nanotheranostics 2021, 5, 182–196. [Google Scholar] [CrossRef]
- Kobayashi, H.; Furusawa, A.; Rosenberg, A.; Choyke, P.L. Near-infrared photoimmunotherapy of cancer: A new approach that kills cancer cells and enhances anti-cancer host immunity. Int. Immunol. 2021, 33, 7–15. [Google Scholar] [CrossRef]
- Wang, H.; Han, R.-L.; Yang, L.-M.; Shi, J.-H.; Liu, Z.-J.; Hu, Y.; Wang, Y.; Liu, S.-J.; Gan, Y. Design and Synthesis of Core–Shell–Shell Upconversion Nanoparticles for NIR-Induced Drug Release, Photodynamic Therapy, and Cell Imaging. ACS Appl. Mater. Interfaces 2016, 8, 4416–4423. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques, 3rd ed.; Academic Press: Cambridge, MA, USA, 2013; ISBN 9780123822390. [Google Scholar]
- Grabarek, Z.; Gergely, J. Zero-length crosslinking procedure with the use of active esters. Anal. Biochem. 1990, 185, 131–135. [Google Scholar] [CrossRef]
- Nakajima, N.; Ikada, Y. Mechanism of Amide Formation by Carbodiimide for Bioconjugation in Aqueous Media. Bioconj. Chem. 1995, 6, 123–130. [Google Scholar] [CrossRef]
- Er, Ö.; Colak, S.G.; Ocakoglu, K.; Ince, M.; Bresolí-Obach, R.; Mora, M.; Sagristá, M.L.; Yurt, F.; Nonell, S. Selective Photokilling of Human Pancreatic Cancer Cells Using Cetuximab-Targeted Mesoporous Silica Nanoparticles for Delivery of Zinc Phthalocyanine. Molecules 2018, 23, 2749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, X.; Nomoto, T.; Takemoto, H.; Matsui, M.; Tomoda, K.; Nishiyama, N. Effect of multiple cyclic RGD peptides on tumor accumulation and intratumoral distribution of IRDye 700DX-conjugated polymers. Sci. Rep. 2018, 8, 8126. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, Y.; Mao, C.; Kong, Y.; Ming, X. RGD-Modified Albumin Nanoconjugates for Targeted Delivery of a Porphyrin Photosensitizer. Mol. Pharm. 2017, 14, 2793–2804. [Google Scholar] [CrossRef]
- Deken, M.M.; Kijanka, M.M.; Beltrán Hernández, I.; Slooter, M.D.; de Bruijn, H.S.; van Diest, P.J.; van Bergen En Henegouwen, P.M.P.; Lowik, C.W.G.M.; Robinson, D.J.; Vahrmeijer, A.L.; et al. Nanobody-targeted photodynamic therapy induces significant tumor regression of trastuzumab-resistant HER2-positive breast cancer, after a single treatment session. J. Control. Release 2020, 323, 269–281. [Google Scholar] [CrossRef]
- Rehm, D.; Weller, A. Kinetics of Fluorescence Quenching by Electron and H-Atom Transfer. Isr. J. Chem. 1970, 8, 259–271. [Google Scholar] [CrossRef]
- Bandera, Y.; Burdette, M.; Shetzline, J.A.; Jenkins, R.; Creager, S.E.; Foulger, S. Synthesis of water soluble axially disubstituted silicon (IV) phthalocyanines with alkyne & azide functionality. Dye Pigment. 2016, 125, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Guin, P.S.; Das, S. Exploration of Electrochemical Intermediates of the Anticancer Drug Doxorubicin Hydrochloride Using Cyclic Voltammetry and Simulation Studies with an Evaluation for Its Interaction with DNA. Int. J. Electrochem. 2014, 2014, 517371. [Google Scholar] [CrossRef] [Green Version]



















| Sample | NP Formulation | Size/nm | PDI | Zeta-Potential/mV | [IRDye700DX]/μM | [DOX]/μM | LE |
|---|---|---|---|---|---|---|---|
| Blank | MSNP | 165 ± 3 | 0.07 ± 0.04 a | −22 ±1 | - | - | - |
| With IRDye700DX and without DOX | MSNP1 | 165 ± 1 | 0.03 ± 0.03 a | 6 ± 1 | - | - | - |
| MSNP2 | 172 ± 2 | 0.13 ± 0.02 a | 6 ± 1 | 9.2 | - | 100% | |
| IRDye700DX-MSNP | 243 ± 4 | 0.11 ± 0.09 a | 10 ± 1 | 9.2 | - | - | |
| IRDye700DX-Cet-MSNP | 410 ± 38 | 0.95 ± 0.10 b | - | 9.2 | - | - | |
| With IRDye700DX and with releasable DOX | MSNP3 | 196 ± 3 | 0.10 ± 0.05 a | 8 ± 1 | 9.2 | 18.7 21.7 134.4 | 68% 59% 29% |
| IRDye700DX-DOX-MSNP | 255 ± 6 | 0.12 ± 0.04 a | 6 ± 1 | 9.2 | 18.7 21.7 134.4 | - | |
| IRDye700DX-Cet-DOX-MSNP | 430 ± 52 | 0.89 ± 0.04 b | - | 9.2 | 18.7 21.7 134.4 | - |
| Sample | ΦF a | τT/µs | τΔ/µs | ΦΔ a |
|---|---|---|---|---|
| MSNP2 | 1 | 1.0 | 14.2 | 1 |
| MSNP3 (18 µM of DOX) | - | 0.9 | 14.0 | 0.48 |
| MSNP3 (134 µM of DOX) | 0.40 | 1.2 | 14.5 | 0.51 |
| Sample | ΦF a | τT/µs | τΔ/µs | ΦΔ a |
|---|---|---|---|---|
| IRDye700DX | - | 2.6 | 63.8 | 2.0 |
| IRDye700DX-Cet-MSNP | 1 | 7.2 | 54.7 | 1 |
| IRDye700DX-Cet-DOX-MSNP (18 µM of DOX) | 0.70 | 2.9 | 53.6 | 0.24 |
| IRDye700DX-Cet-DOX-MSNP (134 µM of DOX) | 0.30 | 0.3 | 42.5 | 0.12 |
| Sample | τT/µs | τΔ/µs | ΦΔ a |
|---|---|---|---|
| IRDye700DX-Cet-MSNP | 0.23 | 17.5 | 1 |
| IRDye700DX-Cet-DOX-MSNP (134 µM of DOX) | 0.29 | 18.3 | 0.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de las Heras, E.; Sagristá, M.L.; Agut, M.; Nonell, S. Photosensitive EGFR-Targeted Nanocarriers for Combined Photodynamic and Local Chemotherapy. Pharmaceutics 2022, 14, 405. https://doi.org/10.3390/pharmaceutics14020405
de las Heras E, Sagristá ML, Agut M, Nonell S. Photosensitive EGFR-Targeted Nanocarriers for Combined Photodynamic and Local Chemotherapy. Pharmaceutics. 2022; 14(2):405. https://doi.org/10.3390/pharmaceutics14020405
Chicago/Turabian Stylede las Heras, Elena, M. Lluïsa Sagristá, Montserrat Agut, and Santi Nonell. 2022. "Photosensitive EGFR-Targeted Nanocarriers for Combined Photodynamic and Local Chemotherapy" Pharmaceutics 14, no. 2: 405. https://doi.org/10.3390/pharmaceutics14020405
APA Stylede las Heras, E., Sagristá, M. L., Agut, M., & Nonell, S. (2022). Photosensitive EGFR-Targeted Nanocarriers for Combined Photodynamic and Local Chemotherapy. Pharmaceutics, 14(2), 405. https://doi.org/10.3390/pharmaceutics14020405