Adalimumab Decorated Nanoparticles Enhance Antibody Stability and Therapeutic Outcome in Epithelial Colitis Targeting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanoparticle Preparation
2.3. Immobilization of Adalimumab and BSA
2.4. Physicochemical Characterization of the Nanoparticles
2.5. Field Emission Scanning Electron Microscopy
2.6. Assessment of Adalimumab In Vitro Activity
2.7. Stability of Adalimumab against Proteolytic Activity
2.8. TNF-α Neutralization Efficiency in Cell Culture Models
2.9. Investigation of Cell–Nanoparticle Interactions
2.10. Investigation of the Potential Immunogenicity
2.11. Animals and In Vivo Study Design
2.12. Assessment of Therapeutic Efficacy
2.13. Statistical Analysis
3. Results
3.1. Particle Characterization
3.2. Adalimumab Immobilization
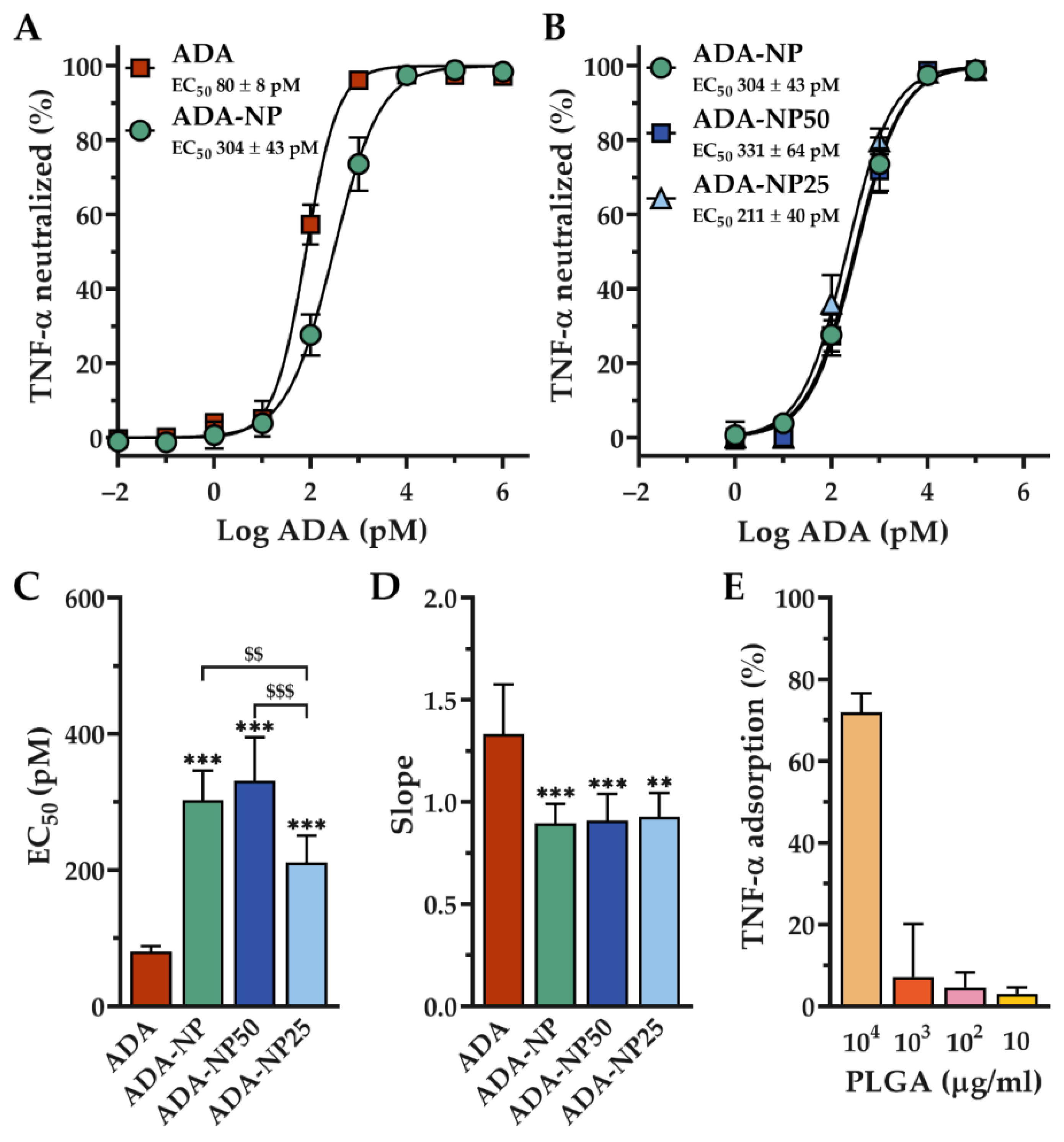
3.3. In Vitro Activity of Adalimumab
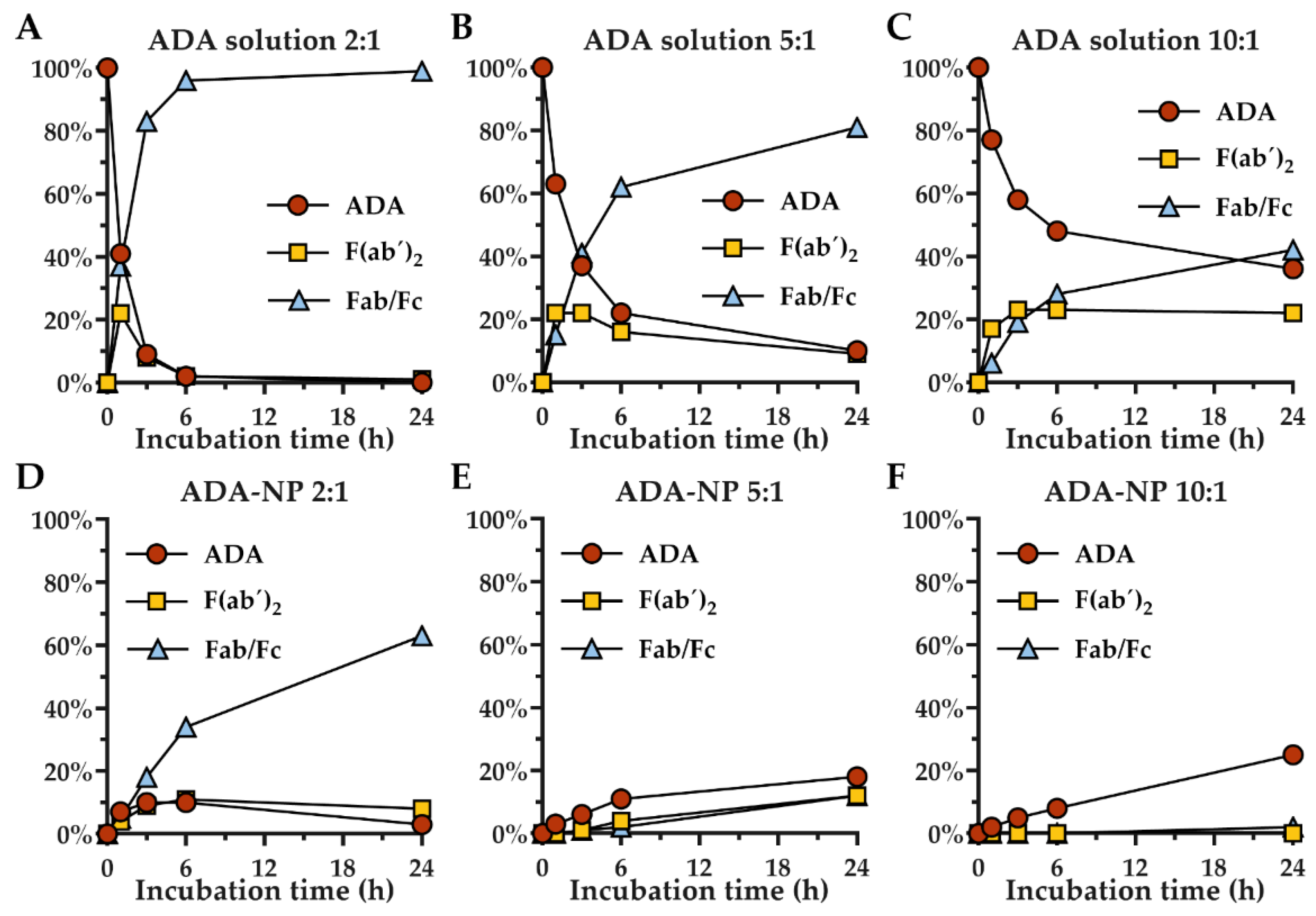
3.4. Stability of Adalimumab against Proteolytic Degradation
3.5. TNF-α Neutralization in Cell Models
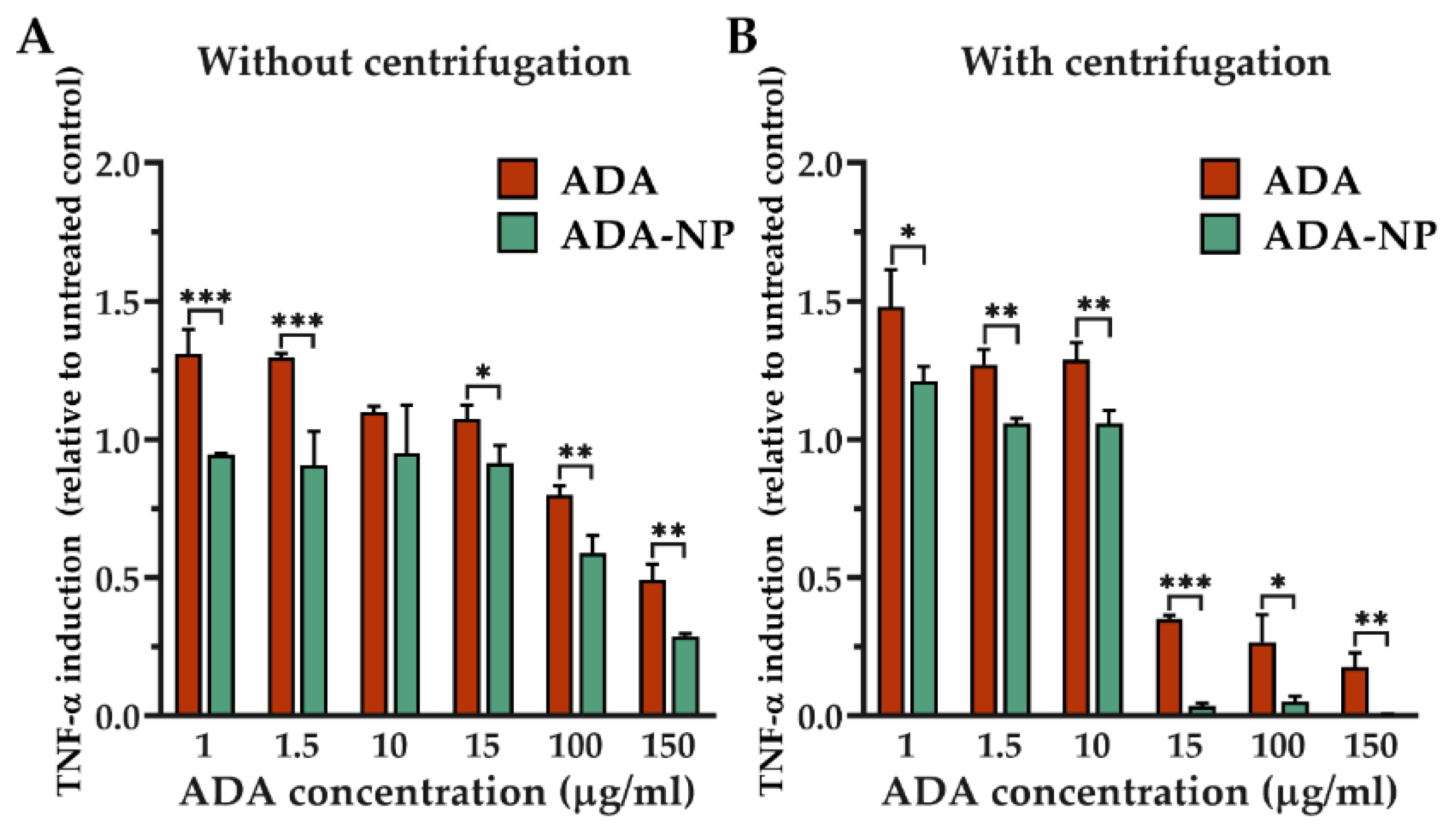
3.6. Cell–Nanoparticle Interactions
3.7. Potential Immunogenicity of ADA and ADA-NP
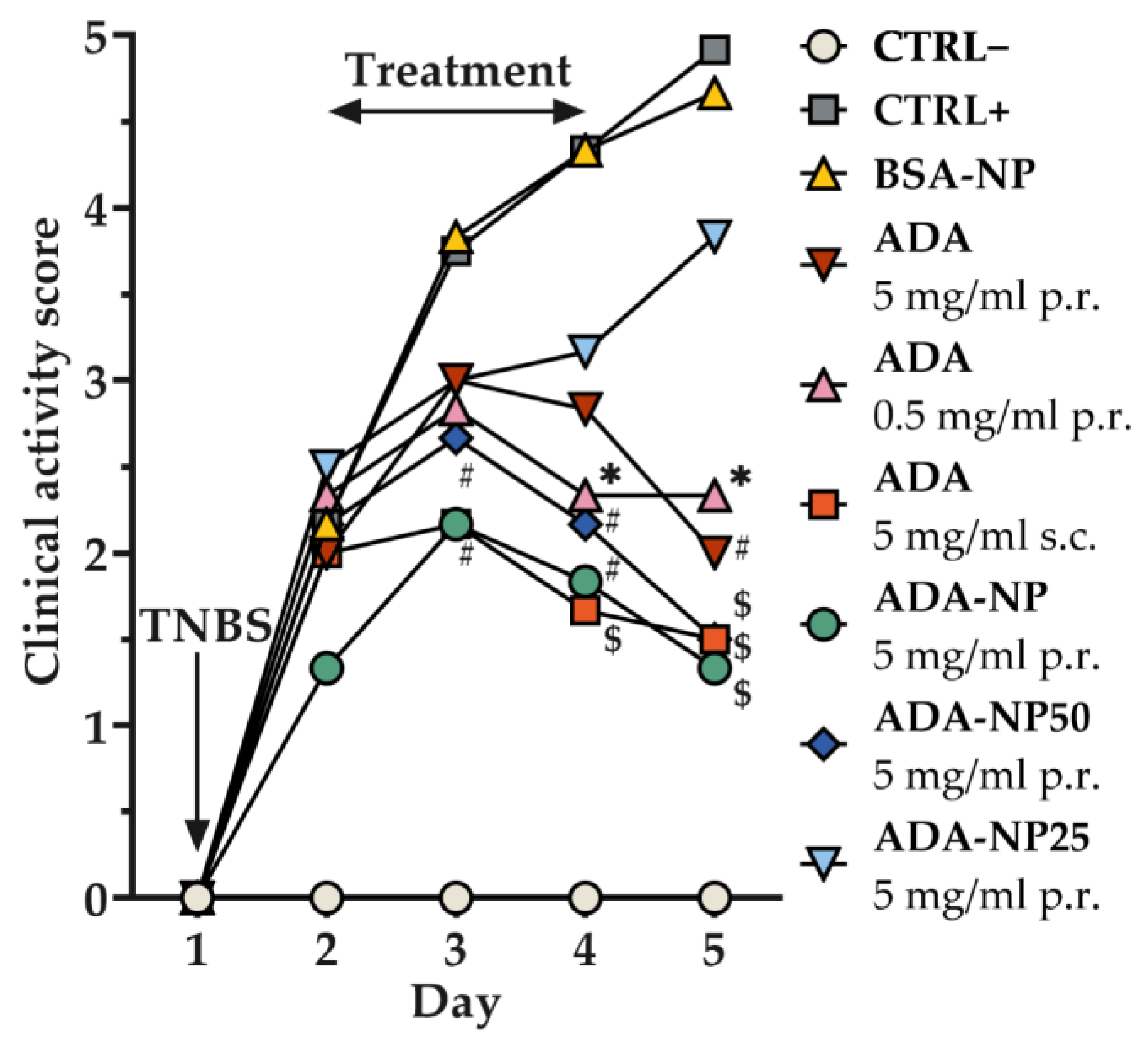
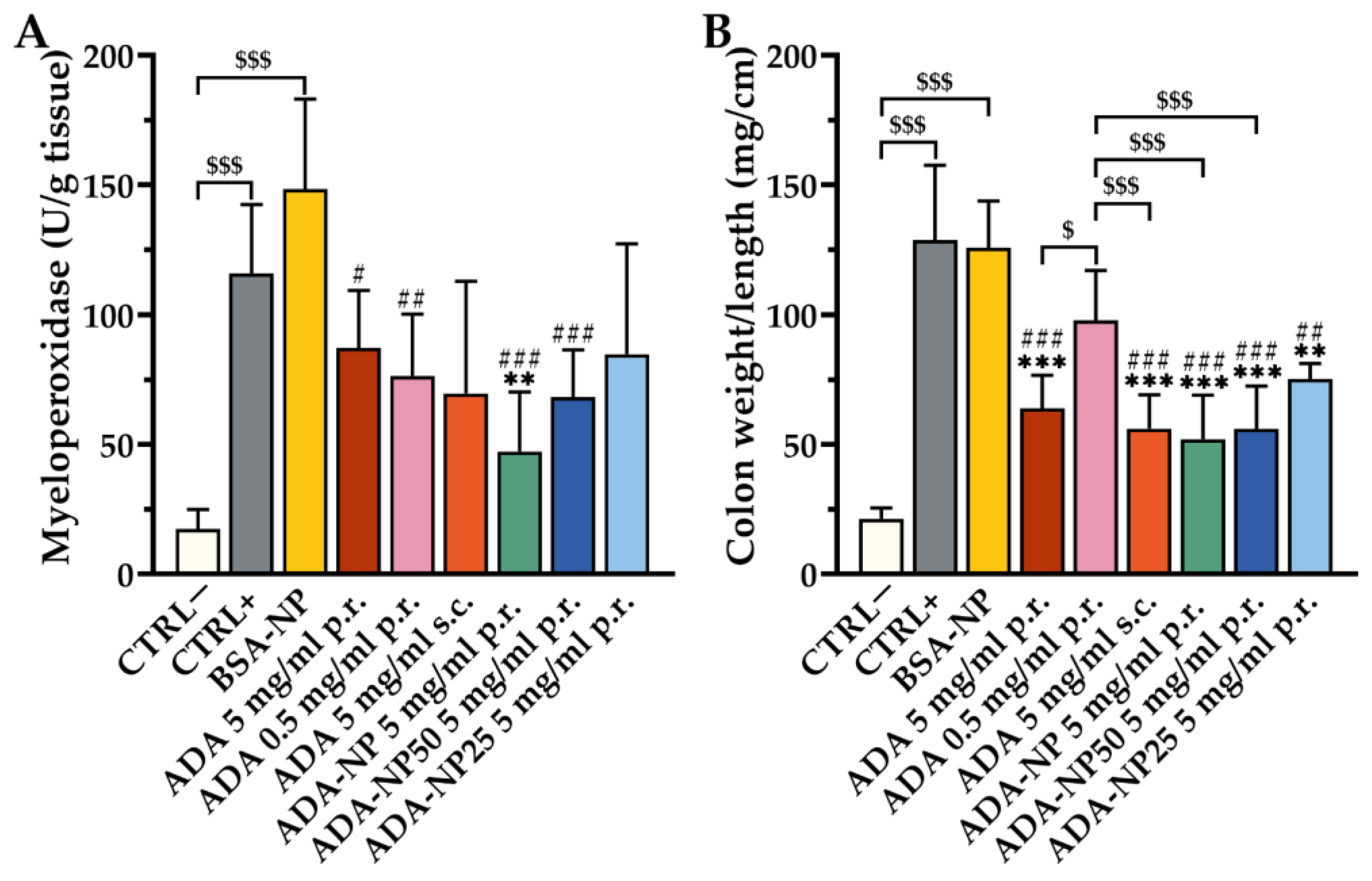
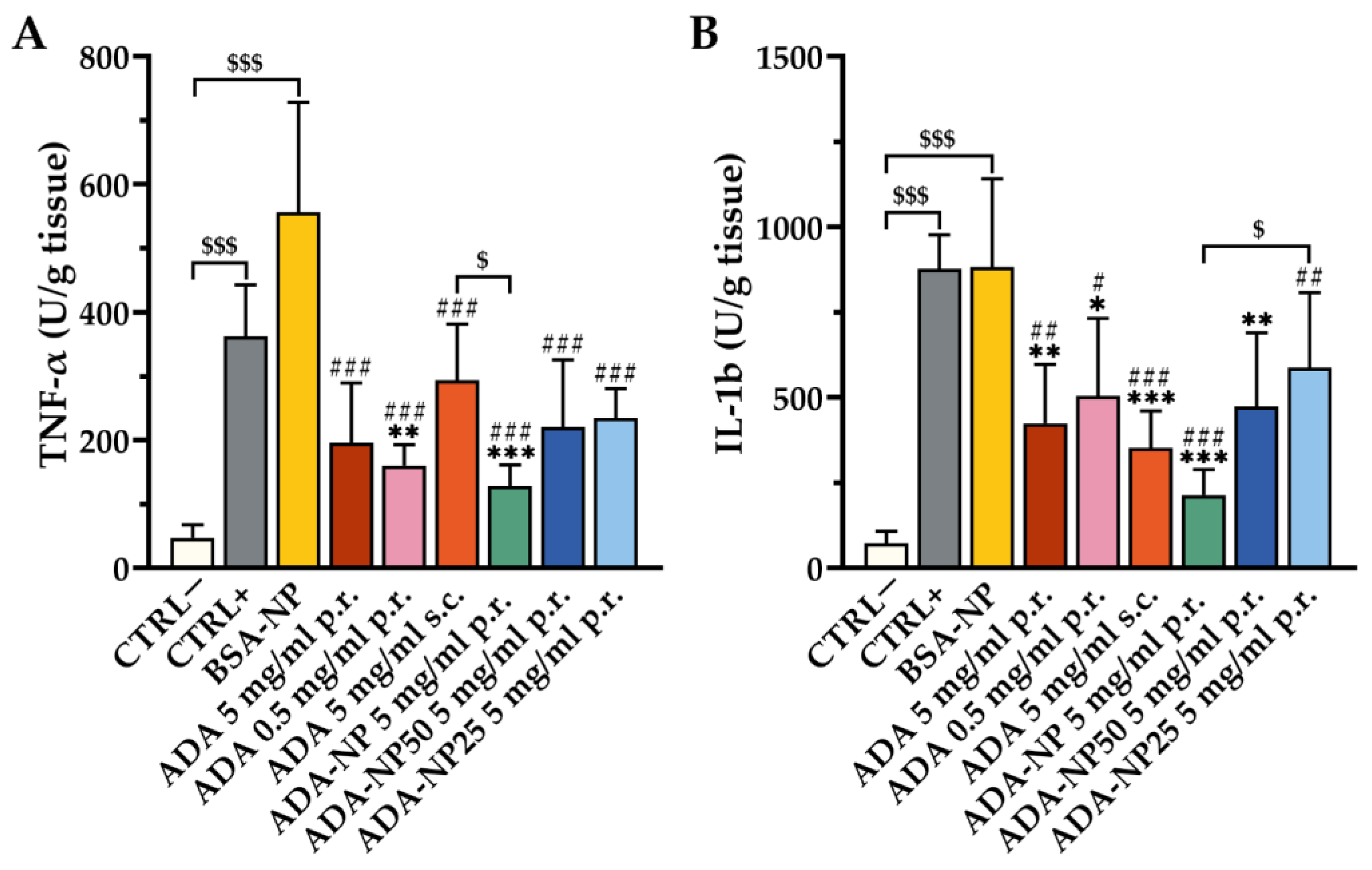
3.8. In Vivo Therapeutic Efficacy
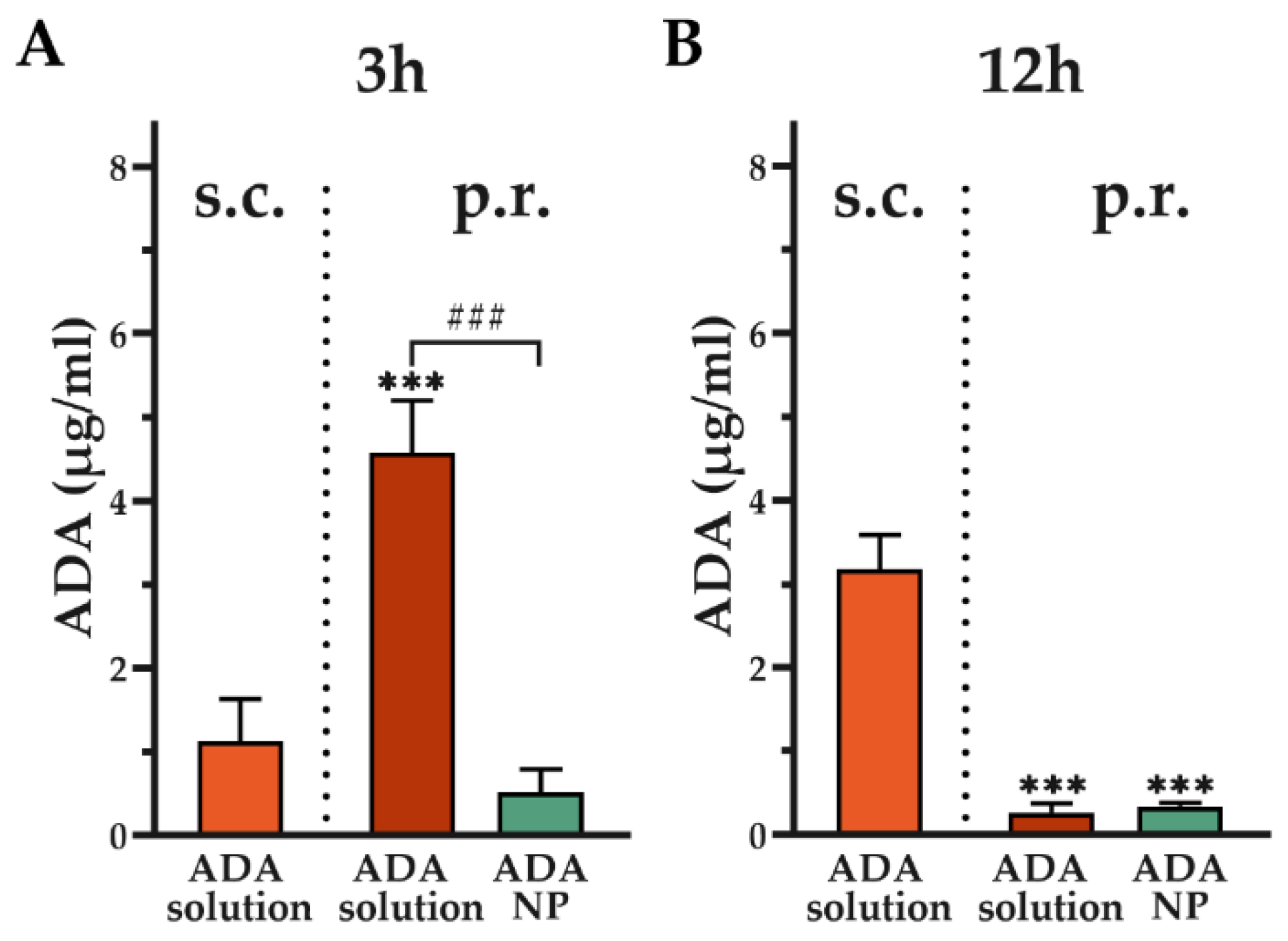
3.9. Assessment of Systemic Adalimumab Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Primers 2020, 6, 74. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Hibi, T.; Ogata, H. Novel pathophysiological concepts of inflammatory bowel disease. J. Gastroenterol. 2006, 41, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Strober, W.; Fuss, I.J.; Blumberg, R.S. The Immunology of Mucosal Models of Inflammation. Annu. Rev. Immunol. 2002, 20, 495–549. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Fiocchi, C. Ulcerative colitis. N. Engl. J. Med. 2011, 365, 1713–1725. [Google Scholar] [CrossRef] [Green Version]
- Chudy-Onwugaje, K.O.; Christian, K.E.; Farraye, F.A.; Cross, R.K. A State-of-the-Art Review of New and Emerging Therapies for the Treatment of IBD. Inflamm. Bowel Dis. 2019, 25, 820–830. [Google Scholar] [CrossRef]
- Pineton de Chambrun, G.; Peyrin-Biroulet, L.; Lémann, M.; Colombel, J.-F. Clinical implications of mucosal healing for the management of IBD. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Billmeier, U.; Dieterich, W.; Neurath, M.F.; Atreya, R. Molecular mechanism of action of anti-tumor necrosis factor antibodies in inflammatory bowel diseases. World J. Gastroenterol. 2016, 22, 9300. [Google Scholar] [CrossRef]
- Eriksson, C.; Cao, Y.; Rundquist, S.; Zhulina, Y.; Henriksson, I.; Montgomery, S.; Halfvarson, J. Changes in medical management and colectomy rates: A population-based cohort study on the epidemiology and natural history of ulcerative colitis in Örebro, Sweden, 1963–2010. Aliment Pharm. Ther. 2017, 46, 748–757. [Google Scholar] [CrossRef] [Green Version]
- Harbord, M.; Eliakim, R.; Bettenworth, D.; Karmiris, K.; Katsanos, K.; Kopylov, U.; Kucharzik, T.; Molnár, T.; Raine, T.; Sebastian, S.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J. Crohn’s Colitis 2017, 11, 769–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuel, S.; Ingle, S.B.; Dhillon, S.; Yadav, S.; Harmsen, W.S.; Zinsmeister, A.R.; Tremaine, W.J.; Sandborn, W.J.; Loftus, E.V. Cumulative Incidence and Risk Factors for Hospitalization and Surgery in a Population-based Cohort of Ulcerative Colitis. Inflamm. Bowel Dis. 2013, 19, 1858–1866. [Google Scholar] [CrossRef] [Green Version]
- Holmer, A.; Singh, S. Overall and comparative safety of biologic and immunosuppressive therapy in inflammatory bowel diseases. Expert Rev. Clin. Immunol. 2019, 15, 969–979. [Google Scholar] [CrossRef]
- Li, X.; Lu, C.; Yang, Y.; Yu, C.; Rao, Y. Site-specific targeted drug delivery systems for the treatment of inflammatory bowel disease. Biomed. Pharmacother. 2020, 129, 110486. [Google Scholar] [CrossRef]
- Laukoetter, M.G.; Nava, P.; Nusrat, A. Role of the intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 401. [Google Scholar] [CrossRef] [PubMed]
- McGuckin, M.A.; Eri, R.; Simms, L.A.; Florin, T.H.J.; Radford-Smith, G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm. Bowel Dis. 2009, 15, 100–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Lamprecht, A. Selective nanoparticle adhesion can enhance colitis therapy: IBD. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 311–312. [Google Scholar] [CrossRef]
- Greish, K.; Nehoff, H.; Parayath, N.; Domanovitch, L.; Taurin, S. Nanomedicine for drug targeting: Strategies beyond the enhanced permeability and retention effect. Int. J. Nanomed. 2014, 9, 2539. [Google Scholar] [CrossRef] [Green Version]
- Lamprecht, A. Nanomedicines in gastroenterology and hepatology. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 195–204. [Google Scholar] [CrossRef]
- Takedatsu, H.; Mitsuyama, K.; Torimura, T. Nanomedicine and drug delivery strategies for treatment of inflammatory bowel disease. World J. Gastroenterol. 2015, 21, 11343–11352. [Google Scholar] [CrossRef]
- Lamprecht, A.; Ubrich, N.; Yamamoto, H.; Maincent, P.; Kawashima, Y.; Lehr, C.-M. Biodegradable Nanoparticles for Targeted Drug Delivery in Treatment of Inflammatory Bowel Disease. J. Pharmacol. Exp. Ther. 2001, 299, 775–781. [Google Scholar]
- Lamprecht, A.; Yamamoto, H.; Takeuchi, H.; Kawashima, Y. A pH-sensitive microsphere system for the colon delivery of tacrolimus containing nanoparticles. J. Control. Release 2005, 104, 337–346. [Google Scholar] [CrossRef]
- Laroui, H.; Dalmasso, G.; Nguyen, H.T.T.; Yan, Y.; Sitaraman, S.V.; Merlin, D. Drug-Loaded Nanoparticles Targeted to the Colon With Polysaccharide Hydrogel Reduce Colitis in a Mouse Model. Gastroenterology 2010, 138, 843–853.e2. [Google Scholar] [CrossRef]
- Nakase, H.; Okazaki, K.; Tabata, Y.; Uose, S.; Ohana, M.; Uchida, K.; Nishi, T.; Debreceni, A.; Itoh, T.; Kawanami, C.; et al. An Oral Drug Delivery System Targeting Immune-Regulating Cells Ameliorates Mucosal Injury in Trinitrobenzene Sulfonic Acid-Induced Colitis. J. Pharmacol. Exp. Ther. 2001, 297, 1122–1128. [Google Scholar]
- Yazeji, T.; Moulari, B.; Beduneau, A.; Stein, V.; Dietrich, D.; Pellequer, Y.; Lamprecht, A. Nanoparticle-based delivery enhances anti-inflammatory effect of low molecular weight heparin in experimental ulcerative colitis. Drug Deliv. 2017, 24, 811–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, D.; Mouftah, S.; Steffen, R.; Beduneau, A.; Pellequer, Y.; Lamprecht, A. Ion milling coupled field emission scanning electron microscopy reveals current misunderstanding of morphology of polymeric nanoparticles. Eur. J. Pharm. Biopharm. 2015, 89, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Moulari, B.; Pertuit, D.; Pellequer, Y.; Lamprecht, A. The targeting of surface modified silica nanoparticles to inflamed tissue in experimental colitis. Biomaterials 2008, 29, 4554–4560. [Google Scholar] [CrossRef] [PubMed]
- Pertuit, D.; Moulari, B.; Betz, T.; Nadaradjane, A.; Neumann, D.; Ismaïli, L.; Refouvelet, B.; Pellequer, Y.; Lamprecht, A. 5-amino salicylic acid bound nanoparticles for the therapy of inflammatory bowel disease. J. Control. Release 2007, 123, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Pellequer, Y.; Meissner, Y.; Ubrich, N.; Lamprecht, A. Epithelial Heparin Delivery via Microspheres Mitigates Experimental Colitis in Mice. J. Pharmacol. Exp. Ther. 2007, 321, 726–733. [Google Scholar] [CrossRef] [Green Version]
- Vauthier, C.; Bouchemal, K. Methods for the Preparation and Manufacture of Polymeric Nanoparticles. Pharm. Res. 2009, 26, 1025–1058. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Fuss, I.; Kelsall, B.L.; Stüber, E.; Strober, W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J. Exp. Med. 1995, 182, 1281–1290. [Google Scholar] [CrossRef] [Green Version]
- Cooper, H.S.; Murthy, S.N.; Shah, R.S.; Sedergran, D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Investig. 1993, 69, 238–249. [Google Scholar] [PubMed]
- Krawisz, E.; Sharon, P.; Stenson, W.F. Quantitative Assay for Acute Intestinal Inflammation Based on Myeloperoxidase Activity: Assessment of inflammation in rat and hamster models. Gastroenterology 1984, 87, 1344–1350. [Google Scholar] [CrossRef]
- Muthas, D.; Reznichenko, A.; Balendran, C.A.; Böttcher, G.; Clausen, I.G.; Kärrman Mårdh, C.; Ottosson, T.; Uddin, M.; MacDonald, T.T.; Danese, S.; et al. Neutrophils in ulcerative colitis: A review of selected biomarkers and their potential therapeutic implications. Scand. J. Gastroenterol. 2017, 52, 125–135. [Google Scholar] [CrossRef]
- Arruebo, M.; Valladares, M.; González-Fernández, Á. Antibody-Conjugated Nanoparticles for Biomedical Applications. J. Nanomater. 2009, 2009, 439389. [Google Scholar] [CrossRef] [Green Version]
- M Cardoso, M.; N Peca, I.; CA Roque, A. Antibody-Conjugated Nanoparticles for Therapeutic Applications. Curr. Med. Chem. 2012, 19, 3103–3127. [Google Scholar] [CrossRef]
- Mundargi, R.C.; Babu, V.R.; Rangaswamy, V.; Patel, P.; Aminabhavi, T.M. Nano/micro technologies for delivering macromolecular therapeutics using poly(d,l-lactide-co-glycolide) and its derivatives. J. Control. Release 2008, 125, 193–209. [Google Scholar] [CrossRef]
- Day, E.S.; Bickford, L.R.; Slater, J.H.; Riggall, N.S.; Drezek, R.A.; West, J.L. Antibody-conjugated gold-gold sulfide nanoparticles as multifunctional agents for imaging and therapy of breast cancer. Int. J. Nanomed. 2010, 5, 445. [Google Scholar] [CrossRef] [Green Version]
- Khashayar, P.; Amoabediny, G.; Larijani, B.; Hosseini, M.; Vanfleteren, J. Fabrication and Verification of Conjugated AuNP-Antibody Nanoprobe for Sensitivity Improvement in Electrochemical Biosensors. Sci. Rep. 2017, 7, 16070. [Google Scholar] [CrossRef] [Green Version]
- Kocbek, P.; Obermajer, N.; Cegnar, M.; Kos, J.; Kristl, J. Targeting cancer cells using PLGA nanoparticles surface modified with monoclonal antibody. J. Control. Release 2007, 120, 18–26. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Prado, A.R.; Keijok, W.J.; Antunes, P.W.P.; Yapuchura, E.R.; Guimarães, M.C.C. Impact of conjugation strategies for targeting of antibodies in gold nanoparticles for ultrasensitive detection of 17β-estradiol. Sci. Rep. 2019, 9, 13859. [Google Scholar] [CrossRef] [Green Version]
- Puertas, S.; Batalla, P.; Moros, M.; Polo, E.; del Pino, P.; Guisán, J.M.; Grazú, V.; de la Fuente, J.M. Taking Advantage of Unspecific Interactions to Produce Highly Active Magnetic Nanoparticle−Antibody Conjugates. ACS Nano 2011, 5, 4521–4528. [Google Scholar] [CrossRef]
- Yu, M.K.; Park, J.; Jon, S. Targeting Strategies for Multifunctional Nanoparticles in Cancer Imaging and Therapy. Theranostics 2012, 2, 3–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, B.; Evers, T.H.; Prins, M.W.J. How Antibody Surface Coverage on Nanoparticles Determines the Activity and Kinetics of Antigen Capturing for Biosensing. Anal. Chem. 2014, 86, 8158–8166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, B.; Songe, P.; Evers, T.H.; Prins, M.W.J. The influence of covalent immobilization conditions on antibody accessibility on nanoparticles. Analyst 2017, 142, 4247–4256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambeir, A.-M.; Meester, I.D. Regulation of intestinal permeability: The role of proteases. World J. Gastroenterol. 2017, 23, 2106. [Google Scholar]
- Perrier, C.; de Hertogh, G.; Cremer, J.; Vermeire, S.; Rutgeerts, P.; Van Assche, G.; Szymkowski, D.E.; Ceuppens, J.L. Neutralization of Membrane TNF, but Not Soluble TNF, Is Crucial for the Treatment of Experimental Colitis. Inflamm. Bowel Dis. 2013, 19, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Deora, A.; Hegde, S.; Lee, J.; Choi, C.-H.; Chang, Q.; Lee, C.; Eaton, L.; Tang, H.; Wang, D.; Lee, D.; et al. Transmembrane TNF-dependent uptake of anti-TNF antibodies. mAbs 2017, 9, 680–695. [Google Scholar] [CrossRef]
- Jafary, F.; Panjehpour, M.; Varshosaz, J.; Yaghmaei, P. Stability Improvement of Immobilized Alkaline Phosphatase Using Chitosan Nanoparticles. Braz. J. Chem. Eng. 2016, 33, 243–250. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Abraham, C.; Medzhitov, R. Interactions Between the Host Innate Immune System and Microbes in Inflammatory Bowel Disease. Gastroenterology 2011, 140, 1729–1737. [Google Scholar] [CrossRef] [Green Version]
- Műzes, G.; Molnár, B.; Tulassay, Z.; Sipos, F. Changes of the cytokine profile in inflammatory bowel diseases. World J. Gastroenterol. 2012, 18, 5848–5861. [Google Scholar] [CrossRef] [Green Version]
- Nenci, A.; Becker, C.; Wullaert, A.; Gareus, R.; van Loo, G.; Danese, S.; Huth, M.; Nikolaev, A.; Neufert, C.; Madison, B.; et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 2007, 446, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, A.; Lehr, C.-M. Size-Dependent Bioadhesion of Micro- and Nanoparticulate Carriers to the Inflamed Colonic Mucosa. Pharm. Res. 2001, 18, 788–793. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, X.; Fang, J.; Sun, X. Nanomedicines for the treatment of rheumatoid arthritis: State of art and potential therapeutic strategies. Acta Pharm. Sin. B 2021, 11, 1158–1174. [Google Scholar] [CrossRef] [PubMed]
- Targownik, L.E.; Bernstein, C.N. Infectious and Malignant Complications of TNF Inhibitor Therapy in IBD. Am. J. Gastroenterol. 2013, 108, 1835–1842. [Google Scholar] [CrossRef]
- Nestorov, I. Clinical pharmacokinetics of TNF antagonists: How do they differ? Semin. Arthritis Rheum. 2005, 34, 12–18. [Google Scholar] [CrossRef]
- Atiqi, S.; Hooijberg, F.; Loeff, F.C.; Rispens, T.; Wolbink, G.J. Immunogenicity of TNF-Inhibitors. Front. Immunol. 2020, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Vaisman-Mentesh, A.; Gutierrez-Gonzalez, M.; DeKosky, B.J.; Wine, Y. The Molecular Mechanisms That Underlie the Immune Biology of Anti-drug Antibody Formation Following Treatment with Monoclonal Antibodies. Front. Immunol. 2020, 11, 1951. [Google Scholar] [CrossRef]
- Silva, L.C.; Ortigosa, L.C.; Benard, G. Anti-TNF-α agents in the treatment of immune-mediated inflammatory diseases: Mechanisms of action and pitfalls. Immunotherapy 2010, 2, 817–833. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.-F.; Feagan, B.G.; Sandborn, W.J.; Van Assche, G.; Robinson, A.M. Therapeutic Drug Monitoring of Biologics for Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2012, 18, 349–358. [Google Scholar] [CrossRef]
- Nunes, R.; das Neves, J.; Sarmento, B. Nanoparticles for the regulation of intestinal inflammation: Opportunities and challenges. Nanomedicine 2019, 14, 2631–2644. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, A.; Ubrich, N.; Yamamoto, H.; Schäfer, U.; Takeuchi, H.; Lehr, C.M.; Maincent, P.; Kawashima, Y. Design of rolipram-loaded nanoparticles: Comparison of two preparation methods. J. Control. Release 2001, 71, 297–306. [Google Scholar] [CrossRef]
- Meissner, Y.; Pellequer, Y.; Lamprecht, A. Nanoparticles in inflammatory bowel disease: Particle targeting versus pH-sensitive delivery. Int. J. Pharm. 2006, 316, 138–143. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ries, M.; Moulari, B.; Shetab Boushehri, M.A.; Ali, M.E.; Molnar, D.; Béduneau, A.; Pellequer, Y.; Lamprecht, A. Adalimumab Decorated Nanoparticles Enhance Antibody Stability and Therapeutic Outcome in Epithelial Colitis Targeting. Pharmaceutics 2022, 14, 352. https://doi.org/10.3390/pharmaceutics14020352
Ries M, Moulari B, Shetab Boushehri MA, Ali ME, Molnar D, Béduneau A, Pellequer Y, Lamprecht A. Adalimumab Decorated Nanoparticles Enhance Antibody Stability and Therapeutic Outcome in Epithelial Colitis Targeting. Pharmaceutics. 2022; 14(2):352. https://doi.org/10.3390/pharmaceutics14020352
Chicago/Turabian StyleRies, Markus, Brice Moulari, Maryam A. Shetab Boushehri, Mohamed Ehab Ali, Daniel Molnar, Arnaud Béduneau, Yann Pellequer, and Alf Lamprecht. 2022. "Adalimumab Decorated Nanoparticles Enhance Antibody Stability and Therapeutic Outcome in Epithelial Colitis Targeting" Pharmaceutics 14, no. 2: 352. https://doi.org/10.3390/pharmaceutics14020352
APA StyleRies, M., Moulari, B., Shetab Boushehri, M. A., Ali, M. E., Molnar, D., Béduneau, A., Pellequer, Y., & Lamprecht, A. (2022). Adalimumab Decorated Nanoparticles Enhance Antibody Stability and Therapeutic Outcome in Epithelial Colitis Targeting. Pharmaceutics, 14(2), 352. https://doi.org/10.3390/pharmaceutics14020352








