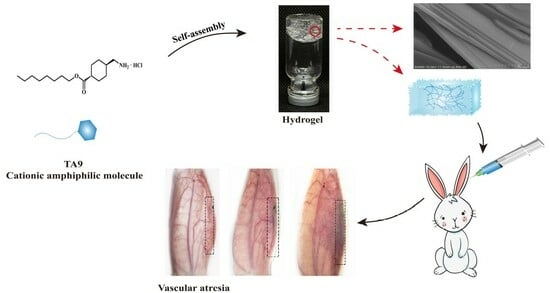
RETRACTED: A Novel Drug Self-Delivery System from Fatty Alcohol Esters of Tranexamic Acid for Venous Malformation Sclerotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
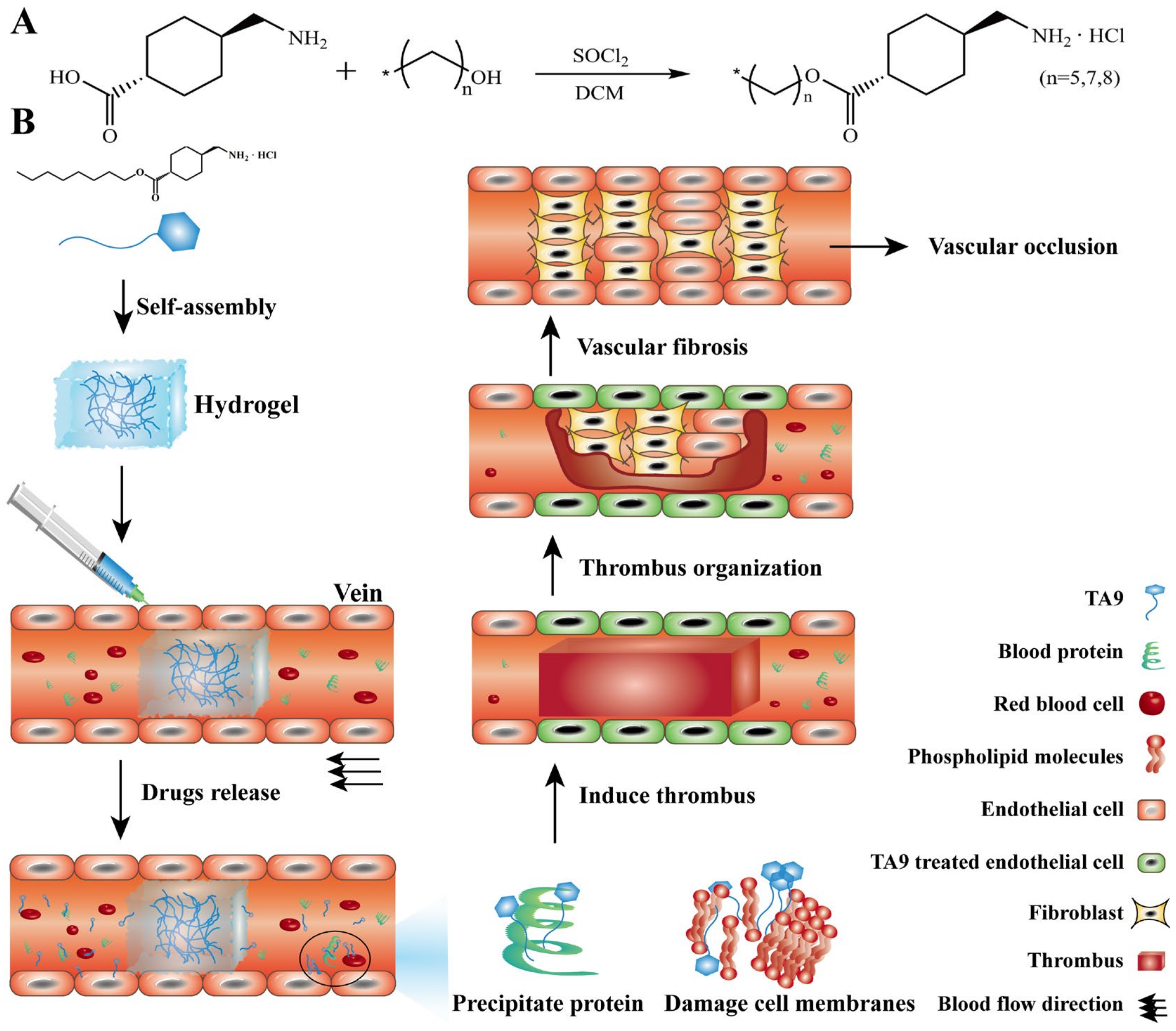
2.2. Synthesis and Characterization of TA6, TA8, and TA9
2.3. Metabolism Studies of TA6, TA8, and TA9 In Vitro
2.4. Preparation of TA6, TA8, and TA9 Hydrogel
2.5. Study of the Self-Assembly Behaviors of TA6, TA8, and TA9 Hydrogels
2.5.1. Gelation Mechanism
2.5.2. Microscopic Study
2.6. Characterization of TA6, TA8, and TA9 Hydrogel
2.6.1. Determination of CGC
2.6.2. Determination of tgel and TG-S
2.6.3. Rheology Studies
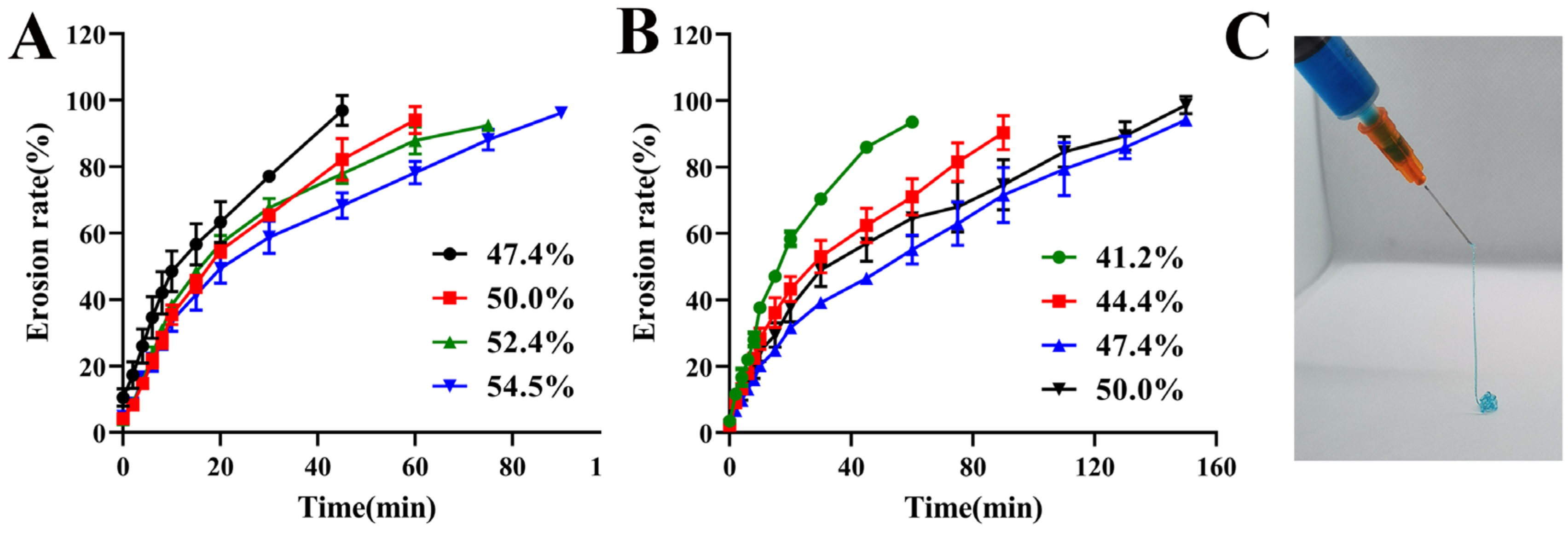
2.6.4. Erosion Behavior and Syringeability Studies
2.7. Study on the Mechanism of TA9 in Treating VM In Vitro
2.7.1. Cytotoxicity Assay
2.7.2. Cell Membrane Integrity Assay
2.7.3. Hemolysis Assay
2.7.4. Effect of TA9 on Fibrinogen
2.7.5. Evaluation of TA Inhibition on Plasmin
2.8. Pharmacodynamic Evaluation In Vivo
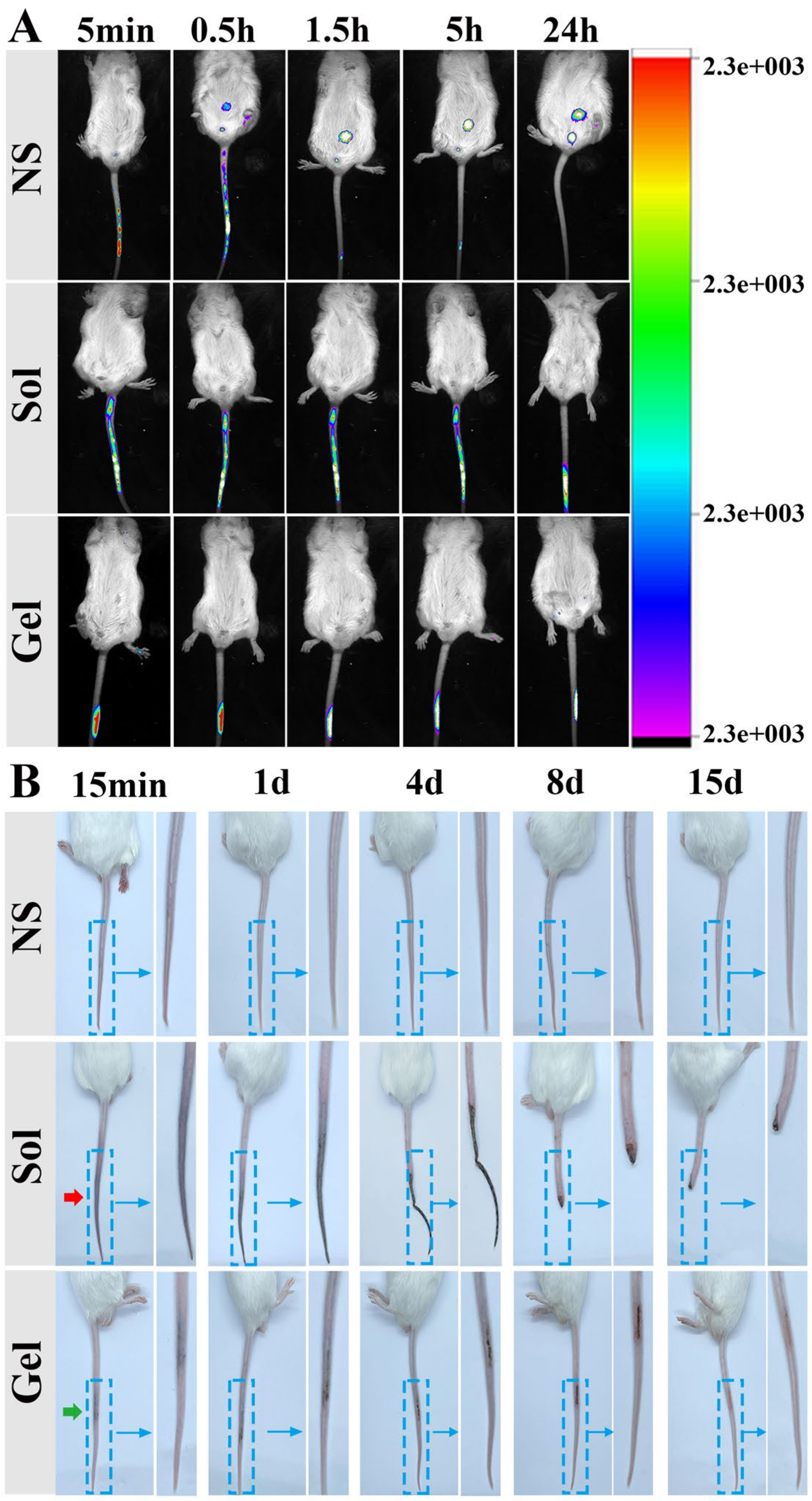
2.8.1. Study on Retention of TA9 Hydrogel In Vivo
2.8.2. Evaluation of Embolus in Mouse Tail Vein
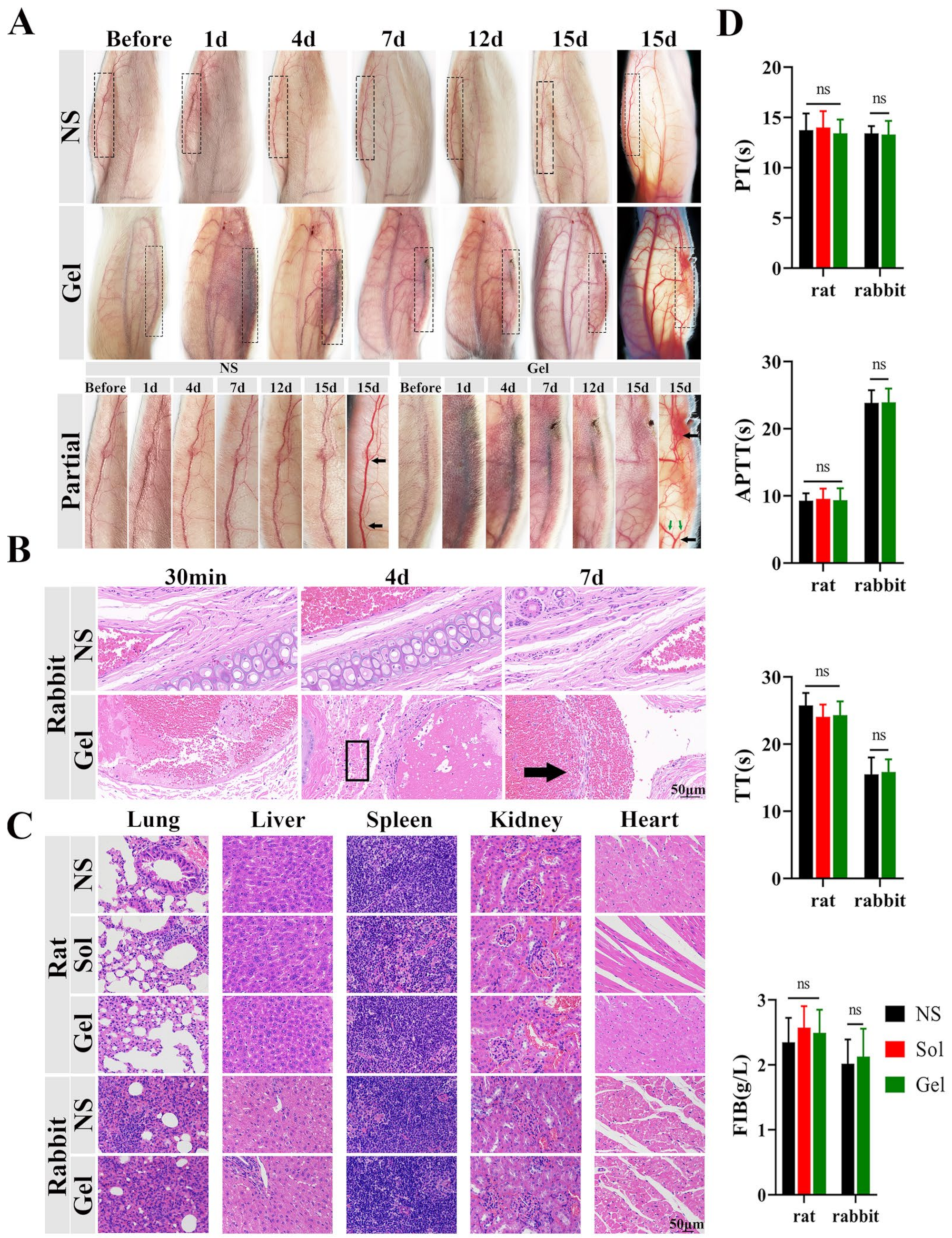
2.8.3. Evaluation of Embolus in Rabbit Ear Vein
2.8.4. Evaluation of the Systemic Safety Profiles of TA Hydrogels
2.9. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of TA6, TA8, and TA9
3.2. Metabolism Study of TA6, TA8, and TA9 In Vitro
3.3. Study on Self-Assembly Behaviors of TA6, TA8, and TA9 Hydrogels
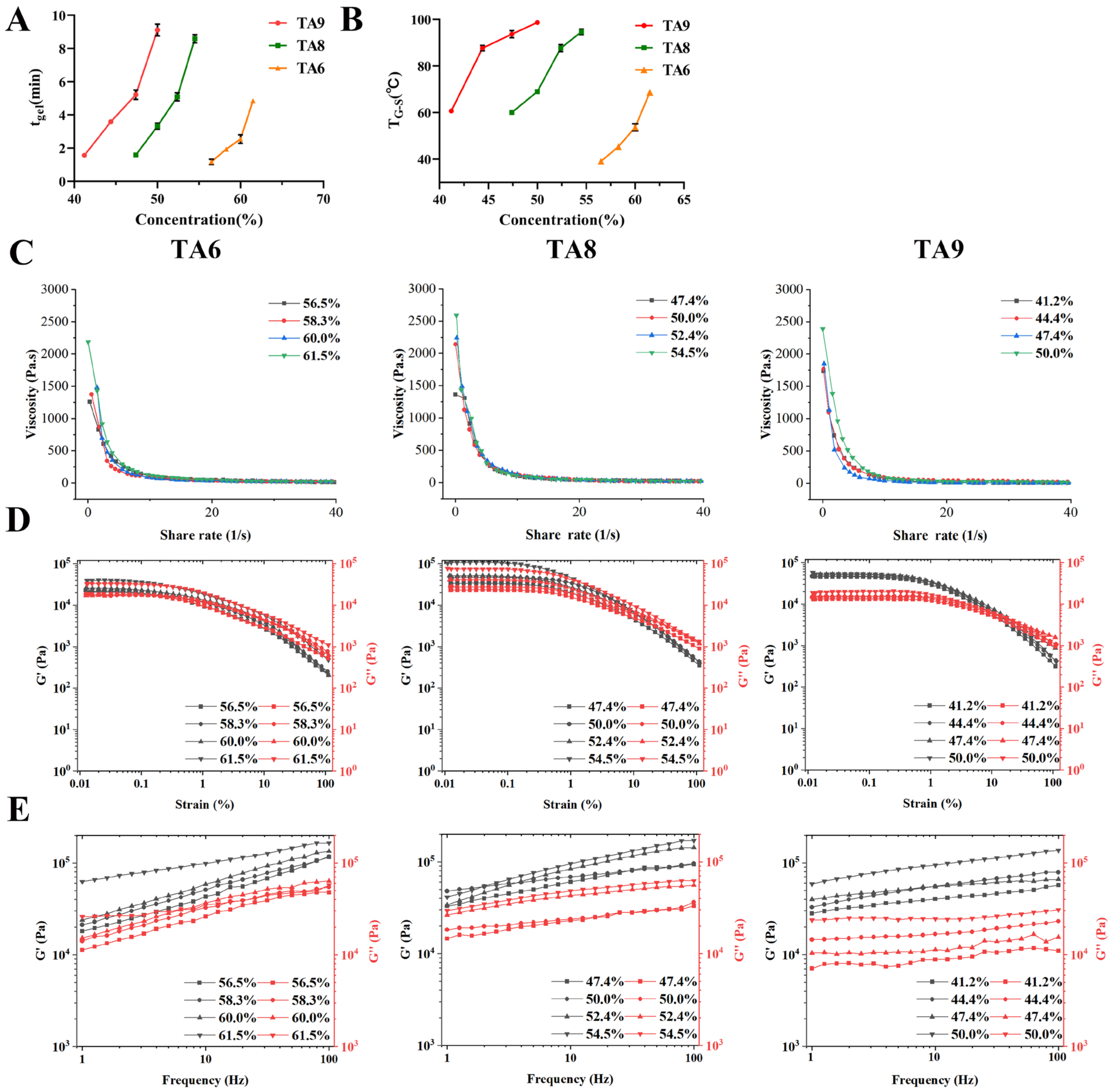
3.4. Characterization of TA6, TA8, and TA9 Hydrogels
3.4.1. Determination of CGC, tgel, and TG-S
3.4.2. Rheology Studies
3.4.3. Erosion Behavior and Syringeability Studies
3.5. Study on the Mechanism of TA9 in Treating VM In Vitro
3.5.1. Cytotoxicity Study and Cell Membrane Integrity Assay
3.5.2. Hemolysis Assay and Effect of TA9 on Fibrinogen
3.5.3. Evaluation of TA Inhibition on Plasmin
3.6. Pharmacodynamic Evaluation In Vivo
3.6.1. Study on the Retention of TA9 Hydrogel In Vivo
3.6.2. Evaluation of Embolus in Mouse Tail Vein
3.6.3. Evaluation of Embolus in Rabbit Ear Vein
3.6.4. Evaluation of Systemic Safety Profiles of TA Hydrogels
4. Discussion
5. Conclusions
6. Patent
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mulliken, J.; Glowacki, J. Hemangiomas and vascular malformations in infants and children: A classification based on endothelial characteristics. Plast. Reconstr. Surg. 1982, 69, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Baumgartner, I.; Berlien, P.; Bianchini, G.; Burrows, P.; Gloviczki, P.; Huang, Y.; Laredo, J.; Loose, D.; Markovic, J.; et al. Guideline. Diagnosis and treatment of venous malformations. consensus document of the international union of phlebology (iup): Updated-2013. Int. Angiol. J. Int. Union Angiol. 2014, 34, 97–149. [Google Scholar]
- Wetzel-Strong, S.; Detter, M.; Marchuk, D. The pathobiology of vascular malformations: Insights from human and model organism genetics. J. Pathol. 2017, 241, 281–293. [Google Scholar] [CrossRef] [PubMed]
- El-Hakim, I.; Alyamani, A. Management of palatal vascular malformation using absolute ethanol sclerotherapy. Clin. Pract. 2011, 1, e86. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, D. Polidocanol for endovenous microfoam sclerosant therapy. Expert Opin. Investig. Drugs 2009, 18, 1919–1927. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lin, X.; Chen, H.; Hu, X.; Fan, X.; Li, W.; Ma, G.; Yang, C.; Wang, W. Auricular arteriovenous malformations: Potential success of superselective ethanol embolotherapy. J. Vasc. Interv. Radiol. JVIR 2009, 20, 736–743. [Google Scholar] [CrossRef]
- Hayden, M.R. Developmental Biology: Frontiers for Clinical Genetics. Clin. Genet. 2014, 86. [Google Scholar] [CrossRef]
- Horbach, S.; Lokhorst, M.; Saeed, P.; de Goüyon Matignon de Pontouraude, C.; Rothová, A.; van der Horst, C. Sclerotherapy for low-flow vascular malformations of the head and neck: A systematic review of sclerosing agents. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 295–304. [Google Scholar] [CrossRef]
- Chen, F.; Song, S.; Wang, H.; Zhang, W.; Lin, C.; Ma, S.; Ye, T.; Zhang, L.; Yang, X.; Qin, X.; et al. Injectable chitosan thermogels for sustained and localized delivery of pingyangmycin in vascular malformations. Int. J. Pharm. 2014, 476, 232–240. [Google Scholar] [CrossRef]
- Van Damme, A.; Seront, E.; Dekeuleneer, V.; Boon, L.M.; Vikkula, M. New and Emerging Targeted Therapies for Vascular Malformations. Am. J. Clin. Dermatol. 2020, 21, 657–668. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, F.; Zheng, J.; Wang, H.; Qin, X.; Pan, W. Chitosan-based liposomal thermogels for the controlled delivery of pingyangmycin: Design, optimization and in vitro and in vivo studies. Drug Deliv. 2018, 25, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Del Castillo-Santaella, T.; Yang, Y.; Martínez-González, I.; Gálvez-Ruiz, M.; Cabrerizo-Vílchez, M.; Holgado-Terriza, J.; Selles-Galiana, F.; Maldonado-Valderrama, J. Effect of Hyaluronic Acid and Pluronic-F68 on the Surface Properties of Foam as a Delivery System for Polidocanol in Sclerotherapy. Pharmaceutics 2020, 12, 1039. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Yue, W.; Ibrahim, K.; Shen, J. A Long-Acting Curcumin Nanoparticle/In Situ Hydrogel Composite for the Treatment of Uveal Melanoma. Pharmaceutics 2021, 13, 1335. [Google Scholar] [CrossRef] [PubMed]
- Pierre, T.; Weiss, R.G. Low Molecular Mass Gelators of Organic Liquids and the Properties of Their Gels. Chem. Rev. 1997, 97, 3133–3160. [Google Scholar]
- Majumder, J.; Dastidar, P. An Easy Access to Organic Salt-Based Stimuli-Responsive and Multifunctional Supramolecular Hydrogels. Chem. Eur. J. 2016, 22, 9267–9276. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bian, S.; Zhao, M.; Han, X.; Liang, J.; Wang, K.; Jiang, Q.; Sun, Y.; Fan, Y.; Zhang, X. Stimuli-responsive biphenyl-tripeptide supramolecular hydrogels as biomimetic extracellular matrix scaffolds for cartilage tissue engineering. Acta Biomater. 2021, 131, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Mulvee, M.T.; Damodaran, K.K. Tuning Gel State Properties of Supramolecular Gels by Functional Group Modification. Molecules 2019, 24, 3472. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Dasgupta, A.; Das, P. Alkyl chain length dependent hydrogelation of L-tryptophan-based amphiphile. Langmuir ACS J. Surf. Colloids 2007, 23, 11769–11776. [Google Scholar] [CrossRef]
- Cinar, G.; Ozdemir, A.; Hamsici, S.; Gunay, G.; Dana, A.; Tekinay, A.B.; Guler, M.O. Local delivery of doxorubicin through supramolecular peptide amphiphile nanofiber gels. Biomater. Sci. 2016, 5, 67–76. [Google Scholar] [CrossRef]
- Wu, C.; Wang, X.; Shi, Y.; Wang, B.; Xue, W.; Zhang, Y. Transforming sustained release into on-demand release: Self-healing guanosine-borate supramolecular hydrogels with multiple responsiveness for Acyclovir delivery. Biomater. Sci. 2020, 8, 6190–6203. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, Y.; Mu, G.; Yang, L.; Wang, W.; Liu, J.; Liu, J. A peptide–drug hydrogel to enhance the anti-cancer activity of chlorambucil. Biomater. Sci. 2020, 8, 5638–5646. [Google Scholar] [CrossRef] [PubMed]
- Zhi, K.; Wang, J. Retraction: A self-assembled supramolecular natural product gel from liquidambaric acid in traditional Chinese medicine with inherent anti-inflammatory activity for drug delivery. J. Mater. Chem. B 2020, 8, 11109. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lei, L.; Zhang, Z.; Zhang, R.; Song, Q.; Li, X. Cation instructed steroidal prodrug supramolecular hydrogel. J. Colloid Interface Sci. 2018, 528, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Vemula, P.K.; Wiradharma, N.; Ankrum, J.; Miranda, O.R.; John, G.; Karp, J.M. Prodrugs as self-assembled hydrogels: A new paradigm for biomaterials. Curr. Opin. Biotechnol. 2013, 24, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Hanley, C.; Callum, J.; Jerath, A. Tranexamic acid and trauma coagulopathy: Where are we now? Br. J. Anaesth. 2021, 126, 12–17. [Google Scholar] [CrossRef]
- Delbecq, F.; Tsujimoto, K.; Ogue, Y.; Endo, H.; Kawai, T. N-stearoyl amino acid derivatives: Potent biomimetic hydro/organogelators as templates for preparation of gold nanoparticles. J. Colloid Interface Sci. 2013, 390, 17–24. [Google Scholar] [CrossRef]
- Komba, S.; Iwaura, R. NDevelopment of Low-Molecular-Mass Organogelators: Synthesis and Physical Properties of Linear Saturated Fatty Acyl-GABAs and Their Ester Derivatives. ACS omega 2021, 6, 20912–20923. [Google Scholar] [CrossRef]
- Miller, B.; Hansrisuk, A.; Highley, C.B.; Caliari, S.R. Guest–Host Supramolecular Assembly of Injectable Hydrogel Nanofibers for Cell Encapsulation. ACS Biomater. Sci. Eng. 2021, 7, 4164–4174. [Google Scholar] [CrossRef]
- Fan, J.; Zhong, H.; Zhang, X.; Yuan, T.; Chen, H.; Peng, H. Preparation and Characterization of Oleanolic Acid-Based Low-Molecular-Weight Supramolecular Hydrogels Induced by Heating. ACS Appl. Mater. Interfaces 2021, 13, 29130–29136. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Y.; Lang, L.; Yang, T.; Zhao, X.; Cai, C.; Liu, Z.; Ding, P. Nuclear localization signal peptide enhances transfection efficiency and decreases cytotoxicity of poly(agmatine/N,N′-cystamine-bis-acrylamide)/pDNA complexes. J. Cell. Biochem. 2019, 120, 16967–16977. [Google Scholar] [CrossRef]
- Millar, W.T.; Smith, J.F.B. The comparison of solid phase and fibrin plate methods for the measurement of plasminogen activators. Thromb. Res. 1983, 30, 431–439. [Google Scholar] [CrossRef]
- Gao, Z.; Ding, P.; Zhang, L.; Shi, J.; Yuan, S.; Wei, J.; Chen, D. Study of a Pingyangmycin delivery system: Zein/Zein–SAIB in situ gels. Int. J. Pharm. 2007, 328, 57–64. [Google Scholar] [CrossRef]
- Xu, L.; Liang, Y.; Sun, C.; Hao, N.; Yan, J.; Gao, W.; He, B. Substitution of Percutaneous Ethanol Injection with a Low Molecular Weight Peptide Gel Mimicking Chemoembolization for Cancer Therapy. Nanotheranostics 2017, 1, 313. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Skilling, K.J.; Citossi, F.; Bradshaw, T.D.; Ashford, M.; Kellam, B.; Marlow, M. Insights into low molecular mass organic gelators: A focus on drug delivery and tissue engineering applications. Soft Matter 2014, 10, 237–256. [Google Scholar] [CrossRef] [PubMed]
- Alonci, G.; Mocchi, R.; Sommatis, S.; Capillo, M.; Liga, E.; Janowska, A.; Nachbaur, L.; Zerbinati, N. Physico-Chemical Characterization and In Vitro Biological Evaluation of a Bionic Hydrogel Based on Hyaluronic Acid and l-Lysine for Medical Applications. Pharmaceutics 2021, 13, 1194. [Google Scholar] [CrossRef] [PubMed]
- Brouillard, P.; Vikkula, M. Vascular malformations: Localized defects in vascular morphogenesis. Clin. Genet. 2003, 63, 340–351. [Google Scholar] [CrossRef]
- Prasetyono, T.; Kreshanti, P. Efficacy of intra-lesional alcohol injection as alternative and/or complementary treatment of vascular malformations: A systematic review. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2010, 63, 1071–1079. [Google Scholar] [CrossRef]
- Heit, J.; Do, H.; Prestigiacomo, C.; Delgado-Almandoz, J.; English, J.; Gandhi, C.; Albuquerque, F.; Narayanan, S.; Blackham, K.; Abruzzo, T.; et al. Guidelines and parameters: Percutaneous sclerotherapy for the treatment of head and neck venous and lymphatic malformations. J. Neurointerv. Surg. 2017, 9, 611–617. [Google Scholar] [CrossRef]
- Bansode, N.D.; Sindhu, K.R.; Morel, C.; Rémy, M.; Verget, J.; Boiziau, C.; Barthélémy, P. A disulfide based low molecular weight gel for the selective sustained release of biomolecules. Biomater. Sci. 2020, 8, 3186–3192. [Google Scholar] [CrossRef]
- Estroff, L.; Hamilton, A. Water gelation by small organic molecules. Chem. Rev. 2004, 104, 1201–1218. [Google Scholar] [CrossRef]
- Khan, A.B.; Khan, J.M.; Ali, M.S.; Khan, R.H.; Din, K.U. Interaction of amphiphilic drugs with human and bovine serum albumins. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2012, 97, 119–124. [Google Scholar] [CrossRef]
- Torres-Luna, C.; Hu, N.; Koolivand, A.; Fan, X.; Zhu, Y.; Domszy, R.; Yang, J.; Yang, A.; Wang, N.S. Effect of a Cationic Surfactant on Microemulsion Globules and Drug Release from Hydrogel Contact Lenses. Pharmaceutics 2019, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- Schreier, S.; Malheiros, S.V.; de Paula, E. Surface active drugs: Self-association and interaction with membranes and surfactants. Physicochemical and biological aspects. Biochim. Biophys. Acta (BBA) Biomembr. 2000, 1508, 210–234. [Google Scholar] [CrossRef]
- Parsi, K.; Exner, T.; Connor, D.; Herbert, A.; Ma, D.; Joseph, J. The lytic effects of detergent sclerosants on erythrocytes, platelets, endothelial cells and microparticles are attenuated by albumin and other plasma components in vitro. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2008, 36, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Parsi, K.; Exner, T.; Low, J.; Fung Ma, D.; Joseph, J. In vitro effects of detergent sclerosants on clot formation and fibrinolysis. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2011, 41, 267–277. [Google Scholar] [CrossRef][Green Version]
- Franchini, M.; Mannucci, P. The never ending success story of tranexamic acid in acquired bleeding. Haematologica 2020, 105, 1201–1205. [Google Scholar] [CrossRef]
- Renckens, R.; Weijer, S.; de Vos, A.; Pater, J.; Meijers, J.; Hack, C.; Levi, M.; van der Poll, T. Inhibition of plasmin activity by tranexamic acid does not influence inflammatory pathways during human endotoxemia. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 483–488. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Song, D.; Hou, Q.; Ma, M.; Zhao, X.; Yang, T.; Xie, H.; Ding, P. RETRACTED: A Novel Drug Self-Delivery System from Fatty Alcohol Esters of Tranexamic Acid for Venous Malformation Sclerotherapy. Pharmaceutics 2022, 14, 343. https://doi.org/10.3390/pharmaceutics14020343
Chen Y, Song D, Hou Q, Ma M, Zhao X, Yang T, Xie H, Ding P. RETRACTED: A Novel Drug Self-Delivery System from Fatty Alcohol Esters of Tranexamic Acid for Venous Malformation Sclerotherapy. Pharmaceutics. 2022; 14(2):343. https://doi.org/10.3390/pharmaceutics14020343
Chicago/Turabian StyleChen, Yongfeng, Di Song, Qianqian Hou, Mengrui Ma, Xiaoyun Zhao, Tianzhi Yang, Huichao Xie, and Pingtian Ding. 2022. "RETRACTED: A Novel Drug Self-Delivery System from Fatty Alcohol Esters of Tranexamic Acid for Venous Malformation Sclerotherapy" Pharmaceutics 14, no. 2: 343. https://doi.org/10.3390/pharmaceutics14020343
APA StyleChen, Y., Song, D., Hou, Q., Ma, M., Zhao, X., Yang, T., Xie, H., & Ding, P. (2022). RETRACTED: A Novel Drug Self-Delivery System from Fatty Alcohol Esters of Tranexamic Acid for Venous Malformation Sclerotherapy. Pharmaceutics, 14(2), 343. https://doi.org/10.3390/pharmaceutics14020343