Oxidation of Drugs during Drug Product Development: Problems and Solutions
Abstract
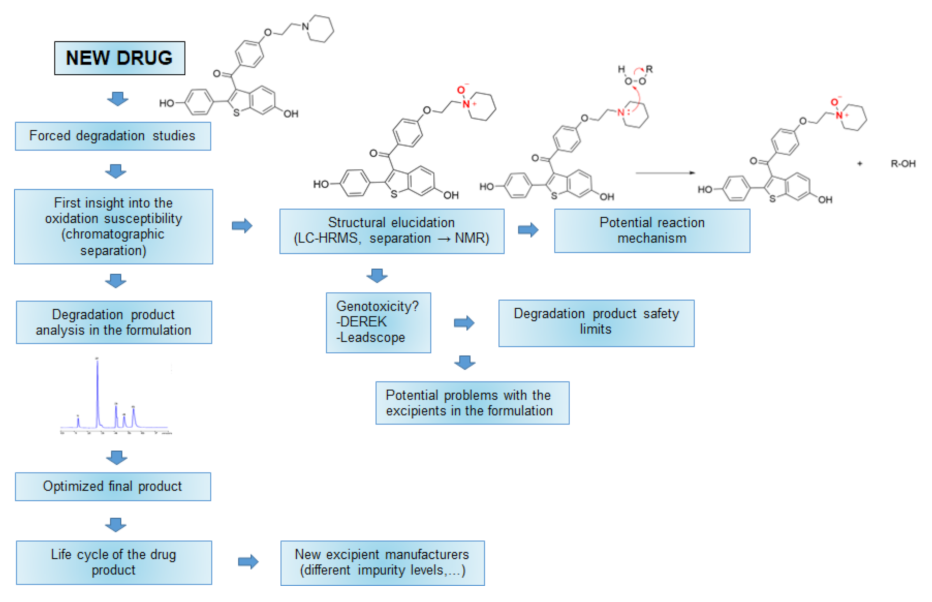
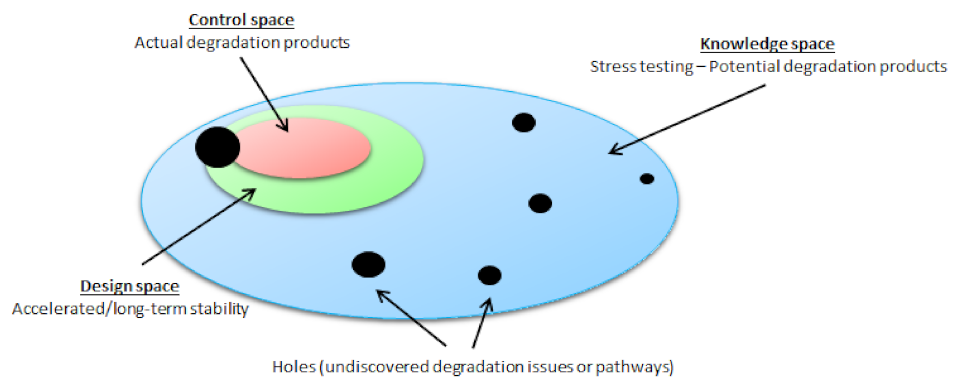
:1. Introduction
2. Oxidation Reactions
- Autoxidation (radical mediated);
- Nucleophilic/electrophilic (peroxide mediated);
- Oxidation that is mediated by single electron to dioxygen.
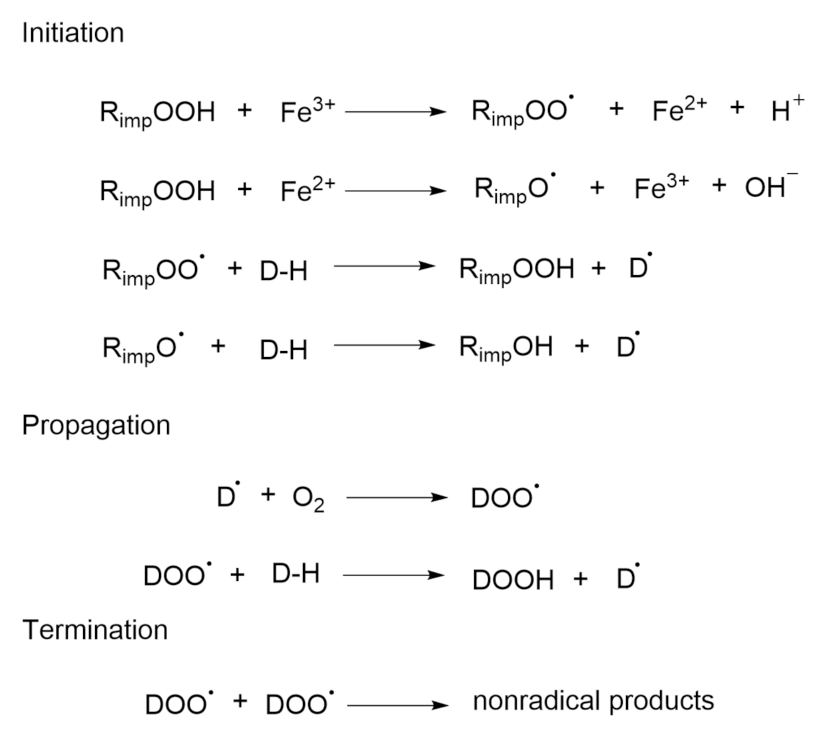
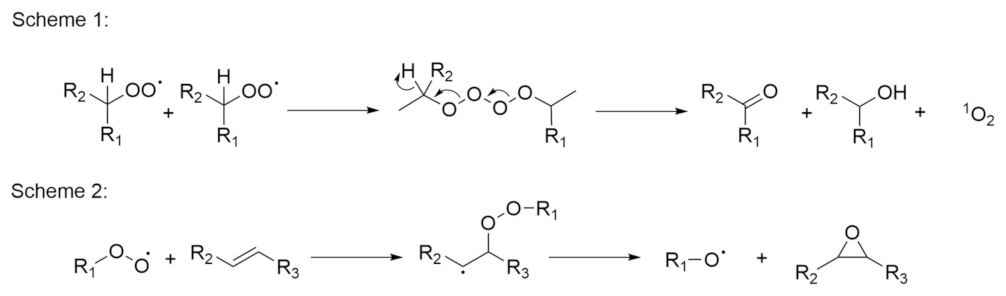
2.1. Autoxidation
2.2. Nucleophilic/Electrophilic (Peroxide Mediated)
2.3. Oxidation Mediated by Single Electron Transfer to Dioxygen
3. Drug Interactions with Excipient Impurities
3.1. Peroxides
3.2. Metals
4. Prevention
4.1. Peroxide Control Strategies
4.2. Storage Conditions
4.3. Antioxidants
- (a)
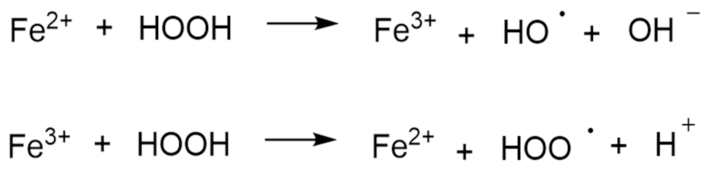
- Initiation inhibitors. Antioxidants can prevent radical chain reaction as they react with initiators of radical chain reactions. The most common representative of this group is ethylenediaminetetraacetic acid (EDTA), which can also acts as a heavy-metal chelating agent [81]. It is also necessary to pay attention to the type of metal that is complexed with EDTA. If iron ions are present, the addition of EDTA produces an iron–EDTA complex that, counterintuitively, accelerates the formation of hydroxyl radicals. On the other hand, an iron complex with diethylenetriaminepentaacetic acid does not accelerate the Fenton reaction. However, all of the studies that are described here were performed in aqueous solutions, so without additional testing it is difficult to assume the same applies to solid dosage forms [82].
- (b)
- (c)
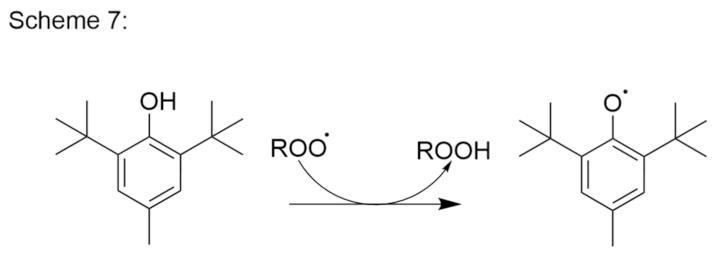
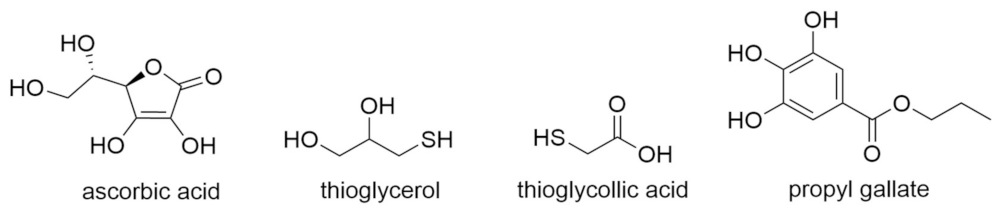
- Antioxidants as reducing agents. Antioxidants can act as reducing agents by being selectively oxidized, to thus protect the substrate by competitive reactivity. Ascorbic acid, thiols (e.g., thioglycerol, thioglycollic acid), and polyphenols (e.g., propyl gallate) can act as such reducing agents (Figure 11).
4.4. Metal Control Strategies
4.5. Packaging
5. Oxidative Susceptibility Testing
- Prediction of whether or not a drug is sensitive to oxidation;
- Determination which specific oxidative degradation mechanism is involved and consequently how to prevent it;
- Obtaining the oxidative impurities that are formed under accelerated and long-term storage conditions as information that can be used to develop appropriate stability-indicative chromatographic methods [29];
- Controlling genotoxic impurities;
- A better, more detailed understanding of a drug.
5.1. Autoxidation
5.2. Nucleophilic/Electrophilic (Peroxide Mediated)
5.3. Transition Metal Ions
- Reactions with hydroperoxides;
- Activation of molecular oxygen;
- Direct reaction of metal complexes with the substrate.
6. New Stressors for Accelerated Oxidation Studies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Guo, J.J.; Pandey, S.; Doyle, J.; Bian, B.; Lis, Y.; Raisch, D.W. A Review of Quantitative Risk–Benefit Methodologies for Assessing Drug Safety and Efficacy—Report of the ISPOR Risk–Benefit Management Working Group. Value Health 2010, 13, 657–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinclair, W.; Leane, M.; Clarke, G.; Dennis, A.; Tobyn, M.; Timmins, P. Physical Stability and Recrystallization Kinetics of Amorphous Ibipinabant Drug Product by Fourier Transform Raman Spectroscopy. J. Pharm. Sci. 2011, 100, 4687–4699. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. ICH Q1A (R2) Stability Testing of New Drug Substances and Products. Available online: https://www.ema.europa.eu/en/ich-q1a-r2-stability-testing-new-drug-substances-drug-products (accessed on 24 April 2021).
- Zumdahl, S.S.; Zumdahl, S.A. Chemistry, 9th ed.; Brooks/Cole, Cengage Learning: Belmont, CA, USA, 2014; ISBN 978-1-133-61109-7. [Google Scholar]
- Rams-Baron, M.; Jachowicz, R.; Boldyreva, E.; Zhou, D.; Jamroz, W.; Paluch, M. Physical Instability: A Key Problem of Amorphous Drugs. In Amorphous Drugs: Benefits and Challenges; Rams-Baron, M., Jachowicz, R., Boldyreva, E., Zhou, D., Jamroz, W., Paluch, M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 107–157. ISBN 978-3-319-72002-9. [Google Scholar]
- Ali, M.A.; Hemingway, R.; Ott, M.A. In Silico Drug Degradation Prediction. In Methods for Stability Testing of Pharmaceuticals; Methods in Pharmacology and Toxicology; Bajaj, S., Singh, S., Eds.; Springer: New York, NY, USA, 2018; pp. 53–73. ISBN 978-1-4939-7686-7. [Google Scholar]
- Parenty, A.D.C.; Button, W.G.; Ott, M.A. An Expert System To Predict the Forced Degradation of Organic Molecules. Mol. Pharm. 2013, 10, 2962–2974. [Google Scholar] [CrossRef] [PubMed]
- Rakibe, U.; Tiwari, R.; Mahajan, A.; Rane, V.; Wakte, P. LC and LC–MS/MS Studies for the Identification and Characterization of Degradation Products of Acebutolol. J. Pharm. Anal. 2018, 8, 357–365. [Google Scholar] [CrossRef]
- Anonymous. ICH M7 Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk. Available online: https://www.ema.europa.eu/en/ich-m7-assessment-control-dna-reactive-mutagenic-impurities-pharmaceuticals-limit-potential (accessed on 13 January 2022).
- Bhhatarai, B.; Wilson, D.M.; Parks, A.K.; Carney, E.W.; Spencer, P.J. Evaluation of TOPKAT, Toxtree, and Derek Nexus in Silico Models for Ocular Irritation and Development of a Knowledge-Based Framework To Improve the Prediction of Severe Irritation. Chem. Res. Toxicol. 2016, 29, 810–822. [Google Scholar] [CrossRef]
- Ahlberg, E.; Amberg, A.; Beilke, L.D.; Bower, D.; Cross, K.P.; Custer, L.; Ford, K.A.; Van Gompel, J.; Harvey, J.; Honma, M.; et al. Extending (Q)SARs to Incorporate Proprietary Knowledge for Regulatory Purposes: A Case Study Using Aromatic Amine Mutagenicity. Regul. Toxicol. Pharmacol. 2016, 77, 1–12. [Google Scholar] [CrossRef]
- Munro, I.C.; Renwick, A.G.; Danielewska-Nikiel, B. The Threshold of Toxicological Concern (TTC) in Risk Assessment. Toxicol. Lett. 2008, 180, 151–156. [Google Scholar] [CrossRef]
- Thresher, A.; Gosling, J.P.; Williams, R. Generation of TD50 Values for Carcinogenicity Study Data. Toxicol. Res. 2019, 8, 696–703. [Google Scholar] [CrossRef]
- Secretan, P.-H.; Sadou Yayé, H.; Sogaldi, A.; Antignac, M.; Tortolano, L.; Thirion, O.; Vieillard, V.; Yagoubi, N.; Do, B. Intrinsic Stability of the Antiviral Drug Umifenovir by Stress Testing and DFT Studies. J. Pharm. Biomed. Anal. 2021, 196, 113934. [Google Scholar] [CrossRef]
- Bellur Atici, E.; Yazar, Y.; Ağtaş, Ç.; Ridvanoğlu, N.; Karlığa, B. Development and Validation of Stability Indicating HPLC Methods for Related Substances and Assay Analyses of Amoxicillin and Potassium Clavulanate Mixtures. J. Pharm. Biomed. Anal. 2017, 136, 1–9. [Google Scholar] [CrossRef]
- Wang, D.; Cheow, W.S.; Amalina, N.; Faiezin, M.; Hadinoto, K. Selecting Optimal Pharmaceutical Excipient Formulation from Life Cycle Assessment Perspectives: A Case Study on Ibuprofen Tablet Formulations. J. Clean. Prod. 2021, 292, 126074. [Google Scholar] [CrossRef]
- Adam, S.; Suzzi, D.; Radeke, C.; Khinast, J.G. An Integrated Quality by Design (QbD) Approach towards Design Space Definition of a Blending Unit Operation by Discrete Element Method (DEM) Simulation. Eur. J. Pharm. Sci. 2011, 42, 106–115. [Google Scholar] [CrossRef]
- Design Space Development—How (And When) To Get Started. Available online: https://www.pharmaceuticalonline.com/doc/design-space-development-how-and-when-to-get-started-0001 (accessed on 26 August 2021).
- Beg, S.; Rahman, M.; Panda, S.S. Pharmaceutical QbD: Omnipresence in the Product Development Lifecycle. Eur. Pharm. Rev. 2017, 22, 58–64. [Google Scholar]
- Connors, K.A.; Amidon, G.L.; Stella, V.J. Chemical Stability of Pharmaceuticals: A Handbook for Pharmacists, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1986; ISBN 978-0-471-87955-8. [Google Scholar]
- Swarbrick, J.; Boylan, J.C. Encyclopedia of Pharmaceutical Technology; Marcel Dekker: New York, NY, USA, 2002; Volume 2002, ISBN 978-0-8247-2825-0. [Google Scholar]
- Simic, M.G. Free Radical Mechanisms in Autoxidation Processes. J. Chem. Educ. 1981, 58, 125. [Google Scholar] [CrossRef] [Green Version]
- Lienard, P.; Gavartin, J.; Boccardi, G.; Meunier, M. Predicting Drug Substances Autoxidation. Pharm. Res. 2015, 32, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Hammond, E.G.; White, P.J. A Brief History of Lipid Oxidation. J. Am. Oil Chem. Soc. 2011, 88, 891–897. [Google Scholar] [CrossRef]
- Chemistry (IUPAC), T.I.U. of P. and A. IUPAC—Radical (Free Radical) (R05066). Available online: https://goldbook.iupac.org/terms/view/R05066 (accessed on 12 October 2021).
- Ingold, K.U. Inhibition of the Autoxidation of Organic Substances in the Liquid Phase. Chem. Rev. 1961, 61, 563–589. [Google Scholar] [CrossRef]
- Gerard, M. Atmospheric Oxidation and Antioxidants, Volume I, 1st ed.; Elsevier: Berlin/Heidelberg, Germany, 1993; ISBN 978-0-444-89615-5. [Google Scholar]
- Choe, E.; Min, D.B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Baertschi, S.W.; Alsante, K.M.; Reed, R.A. Pharmaceutical Stress Testing: Predicting Drug Degradation; Taylor & Francis: London, UK, 2005; ISBN 978-0-8247-4021-4. [Google Scholar]
- Russell, G.A. Deuterium-Isotope Effects in the Autoxidation of Aralkyl Hydrocarbons. Mechanism of the Interaction of PEroxy Radicals1. J. Am. Chem. Soc. 1957, 79, 3871–3877. [Google Scholar] [CrossRef]
- Mayo, F.R. Free Radical Autoxidations of Hydrocarbons. Acc. Chem. Res. 1968, 1, 193–201. [Google Scholar] [CrossRef]
- Boccardi, G. Autoxidation of Drugs: Prediction of Degradation Impurities from Results of Reaction with Radical Chain Initiators. Farm. Soc. Chim. Ital. 1989 1994, 49, 431–435. [Google Scholar]
- Boccardi, G.; Deleuze, C.; Gachon, M.; Palmisano, G.; Vergnaud, J.P. Autoxidation of Tetrazepam in Tablets: Prediction of Degradation Impurities from the Oxidative Behavior in Solution. J. Pharm. Sci. 1992, 81, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.N.; Bonde, C.G. LC, LC–MS/TOF and MSn Studies for the Identification and Characterization of Degradation Products of Nelfinavir Mesylate. J. Pharm. Biomed. Anal. 2011, 55, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Beckwith, A.L.J.; Eichinger, P.H.; Mooney, B.A.; Prager, R.H. Amine Autoxidation in Aqueous Solution. Aust. J. Chem. 1983, 36, 719–739. [Google Scholar] [CrossRef]
- Nanda, K.K.; Ginnetti, A.; Wuelfing, W.P. Base-Mediated Oxidative Degradation of Secondary Amides Derived from p-Amino Phenol to Primary Amides in Drug Molecules. J. Pharm. Sci. 2020, 109, 3394–3403. [Google Scholar] [CrossRef] [PubMed]
- Guerrieri, P.; Taylor, L.S. Role of Salt and Excipient Properties on Disproportionation in the Solid-State. Pharm. Res. 2009, 26, 2015–2026. [Google Scholar] [CrossRef]
- Thakral, N.K.; Kelly, R.C. Salt Disproportionation: A Material Science Perspective. Int. J. Pharm. 2017, 520, 228–240. [Google Scholar] [CrossRef]
- Christensen, N.P.A.; Rantanen, J.; Cornett, C.; Taylor, L.S. Disproportionation of the calcium salt of atorvastatin in the presence of acidic excipients. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. EV 2012, 82, 410–416. [Google Scholar] [CrossRef]
- Qu, H.; Savolainen, M.; Christensen, L.P.; Rantanen, J. Process-Induced Phase Transformations in a Pharmaceutically Relevant Salt-Free Form System. Chem. Eng. Sci. 2012, 77, 65–70. [Google Scholar] [CrossRef]
- Satheesh, B.; Ganesh, K.K.S.; Saravanan, D. Simultaneous Determination of Tadalafil and Its Related Compounds in Pharmaceutical Dosage Forms by Uplc. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 1451–1465. [Google Scholar] [CrossRef]
- Reddy, P.S.; Raju, T.V.R.; Raju, P.S.; Varma, N.S.; Babu, K.S. Development and Validation of a Stability-Indicating RP-UPLC Method for the Estimation of Impurities in Cinacalcet Hydrochloride API and Its Formulation. Sci. Pharm. 2015, 83, 583–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankar, G.; Borkar, R.M.; Udutha, S.; Kanakaraju, M.; Charan, G.S.; Misra, S.; Srinivas, R. Identification and Structural Characterization of the Stress Degradation Products of Omeprazole Using Q-TOF-LC-ESI-MS/MS and NMR Experiments: Evaluation of the Toxicity of the Degradation Products. New J. Chem. 2019, 43, 7294–7306. [Google Scholar] [CrossRef]
- Harmon, P.A.; Biffar, S.; Pitzenberger, S.M.; Reed, R.A. Mechanism of the Solution Oxidation of Rofecoxib under Alkaline Conditions. Pharm. Res. 2005, 22, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Russell, G.A. The Autoxidation of 2-Nitropropane in Basic Solution1. J. Am. Chem. Soc. 1954, 76, 1595–1600. [Google Scholar] [CrossRef]
- Russell, G.A.; Bemis, A.G. The Oxidation of Carbanions. I. Oxidation of Triaryl Carbanions and Other Tertiary Carbanions1. J. Am. Chem. Soc. 1966, 88, 5491–5497. [Google Scholar] [CrossRef]
- Gersmann, H.R.; Bickel, A.F. Autoxidation of Ketones and Esters in Basic Solution. J. Chem. Soc. B Phys. Org. 1971, 2230–2237. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Jones, D.W. 653. Autoxidation in Basic Media. Part IV. Hydrocarbon Autoxidation. J. Chem. Soc. Resumed 1965, 3563–3570. [Google Scholar] [CrossRef]
- Kračun, M.; Kocijan, A.; Bastarda, A.; Grahek, R.; Plavec, J.; Kocjan, D. Isolation and Structure Determination of Oxidative Degradation Products of Atorvastatin. J. Pharm. Biomed. Anal. 2009, 50, 729–736. [Google Scholar] [CrossRef]
- Hovorka, S.W.; Schöneich, C. Oxidative Degradation of Pharmaceuticals: Theory, Mechanisms and Inhibition. J. Pharm. Sci. 2001, 90, 253–269. [Google Scholar] [CrossRef]
- Wasylaschuk, W.R.; Harmon, P.A.; Wagner, G.; Harman, A.B.; Templeton, A.C.; Xu, H.; Reed, R.A. Evaluation of Hydroperoxides in Common Pharmaceutical Excipients. J. Pharm. Sci. 2007, 96, 106–116. [Google Scholar] [CrossRef]
- Wu, Y.; Levons, J.; Narang, A.S.; Raghavan, K.; Rao, V.M. Reactive Impurities in Excipients: Profiling, Identification and Mitigation of Drug-Excipient Incompatibility. AAPS PharmSciTech 2011, 12, 1248–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tallon, M.A.; Malawer, E.G.; Machnicki, N.I.; Brush, P.J.; Wu, C.S.; Cullen, J.P. Effect of Crosslinker Structure upon the Rate of Hydroperoxide Formation in Dried, Crosslinked Poly(Vinylpyrrolidone). J. Appl. Polym. Sci. 2008, 107, 2776–2785. [Google Scholar] [CrossRef]
- Pérez, F.J.; Rubio, S. An Improved Chemiluminescence Method for Hydrogen Peroxide Determination in Plant Tissues. Plant Growth Regul. 2006, 48, 89–95. [Google Scholar] [CrossRef]
- Kulys, J.; Tetianec, L. Highly Sensitive Biosensor for the Hydrogen Peroxide Determination by Enzymatic Triggering and Amplification. Sens. Actuators B Chem. 2006, 113, 755–759. [Google Scholar] [CrossRef]
- Ivanova, A.S.; Merkuleva, A.D.; Andreev, S.V.; Sakharov, K.A. Method for Determination of Hydrogen Peroxide in Adulterated Milk Using High Performance Liquid Chromatography. Food Chem. 2019, 283, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Huang, C.-H. Simultaneous Quantification of Peracetic Acid and Hydrogen Peroxide in Different Water Matrices Using HPLC-UV. Chemosphere 2020, 257, 127229. [Google Scholar] [CrossRef] [PubMed]
- Nourooz-Zadeh, J.; Tajaddini-Sarmadi, J.; Wolff, S.P. Measurement of Plasma Hydroperoxide Concentrations by the Ferrous Oxidation-Xylenol Orange Assay in Conjunction with Triphenylphosphine. Anal. Biochem. 1994, 220, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Gay, C.; Collins, J.; Gebicki, J.M. Hydroperoxide Assay with the Ferric-Xylenol Orange Complex. Anal. Biochem. 1999, 273, 149–155. [Google Scholar] [CrossRef]
- Huang, T.; Garceau, M.E.; Gao, P. Liquid Chromatographic Determination of Residual Hydrogen Peroxide in Pharmaceutical Excipients Using Platinum and Wired Enzyme Electrodes. J. Pharm. Biomed. Anal. 2003, 31, 1203–1210. [Google Scholar] [CrossRef]
- Yue, H.; Bu, X.; Huang, M.-H.; Young, J.; Raglione, T. Quantitative Determination of Trace Levels of Hydrogen Peroxide in Crospovidone and a Pharmaceutical Product Using High Performance Liquid Chromatography with Coulometric Detection. Int. J. Pharm. 2009, 375, 33–40. [Google Scholar] [CrossRef]
- Dahl, T.; He, G.-X.; Samuels, G. Effect of Hydrogen Peroxide on the Viscosity of a Hydroxyethylcellulose-Based Gel. Pharm. Res. 1998, 15, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Kasraian, K.; Kuzniar, A.A.; Wilson, G.G.; Wood, J.A. Developing an Injectable Formula Containing an Oxygen-Sensitive Drug: A Case Study of Danofloxacin Injectable. Pharm. Dev. Technol. 1999, 4, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.M.; Taylor, W.F. Degradation of Fenprostalene in Polyethylene Glycol 400 Solution. J. Pharm. Sci. 1984, 73, 1414–1417. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.M.; Buettner, G.R.; Aust, S.D. Transition Metals as Catalysts of “Autoxidation” Reactions. Free Radic. Biol. Med. 1990, 8, 95–108. [Google Scholar] [CrossRef]
- Veprek-Siska, J. The Role of Metal Ions in Oxygen Activation. Acta Biol. Med. Ger. 1979, 38, 357–361. [Google Scholar]
- Kunz, T.; Brandt, N.; Seewald, T.; Methner, F.-J. Carbohydrates Addition during Brewing—Effects on Oxidative Processes and Formation of Specific Ageing Compounds. Brew. Sci. 2015, 68, 78. [Google Scholar]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [Green Version]
- Ohyashiki, T.; Kadoya, A.; Kushida, K. The Role of Fe3+ on Fe2+-Dependent Lipid Peroxidation in Phospholipid Liposomes. Chem. Pharm. Bull. 2002, 50, 203–207. [Google Scholar] [CrossRef] [Green Version]
- Eudralex Volume 3 Guideline on the Specification Limits for Residues of Metal Catalysts or Metal Reagents—ECA Academy. Available online: https://www.gmp-compliance.org/guidelines/gmp-guideline/eudralex-volume-3-guideline-on-the-specification-limits-for-residues-of-metal-catalysts-or-metal-reagents (accessed on 24 January 2022).
- Narang, A.S.; Rao, V.M.; Desai, D.S. Effect of Antioxidants and Silicates on Peroxides in Povidone. J. Pharm. Sci. 2012, 101, 127–139. [Google Scholar] [CrossRef]
- Buhler, V.; Filges, U.; Schneider, T. Stabilized Polyvinylpyrrolidone Formulation. Off. Gaz. U. S. Pat. Trademark Off. Pat. 2003. [Google Scholar]
- Obaidat, R.; Alnaief, M.; Jaeger, P. Significant Solubility of Carbon Dioxide in Soluplus® Facilitates Impregnation of Ibuprofen Using Supercritical Fluid Technology. Pharm. Dev. Technol. 2018, 23, 697–705. [Google Scholar] [CrossRef]
- Rahman, Z.; Kohli, K.; Khar, R.K.; Ali, M.; Charoo, N.A.; Shamsher, A.A.A. Characterization of 5-Fluorouracil Microspheres for Colonic Delivery. AAPS PharmSciTech 2006, 7, E47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bühler, V. Polyvinylpyrrolidone Excipients for Pharmaceuticals: Povidone, Crospovidone and Copovidone; Springer: Berlin/Heidelberg, 2005; ISBN 978-3-540-23412-8. [Google Scholar]
- Fitzpatrick, S.; McCabe, J.F.; Petts, C.R.; Booth, S.W. Effect of Moisture on Polyvinylpyrrolidone in Accelerated Stability Testing. Int. J. Pharm. 2002, 246, 143–151. [Google Scholar] [CrossRef]
- Buljubasich, L.; Bluemich, B.; Stapf, S. Reaction Monitoring of Hydrogen Peroxide Decomposition by NMR Relaxometry. Chem. Eng. Sci. 2010, 65, 1394–1399. [Google Scholar] [CrossRef]
- Kanofsky, J.R. Quenching of Singlet Oxygen by Human Plasma. Photochem. Photobiol. 1990, 51, 299–303. [Google Scholar] [CrossRef]
- Tx, X.; Bd, A. Distribution and Effect of Water Content on Molecular Mobility in Poly(Vinylpyrrolidone) Glasses: A Molecular Dynamics Simulation. Pharm. Res. 2005, 22, 1205–1214. [Google Scholar] [CrossRef]
- Akers, M.J. Antioxidants in Pharmaceutical Products. J. Parenter. Sci. Technol. Publ. Parenter. Drug Assoc. 1982, 36, 222–228. [Google Scholar]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Brovč, E.V.; Pajk, S.; Šink, R.; Mravljak, J. Protein Formulations Containing Polysorbates: Are Metal Chelators Needed at All? Antioxidants 2020, 9, 441. [Google Scholar] [CrossRef]
- Shahidi, F.; Janitha, P.K.; Wanasundara, P.D. Phenolic Antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef]
- Schillaci, C.; Nepravishta, R.; Bellomaria, A. Antioxidants In Food and Pharmaceutical Research. Albanian J. Pharm. Sci. 2014, 1, 9–15. [Google Scholar]
- Kaufman, M.J. Applications of Oxygen Polarography to Drug Stability Testing and Formulation Development: Solution-Phase Oxidation of Hydroxymethylglutaryl Coenzyme A (HMG-CoA) Reductase Inhibitors. Pharm. Res. 1990, 7, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Narang, A.S.; Desai, D.; Badawy, S. Impact of Excipient Interactions on Solid Dosage Form Stability. Pharm. Res. 2012, 29, 2660–2683. [Google Scholar] [CrossRef]
- Kahl, R.; Weinke, S.; Kappus, H. Comparison of Antioxidant and Prooxidant Activity of Various Synthetic Antioxidants. Adv. Exp. Med. Biol. 1990, 264, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, E.G.; Lyubimov, Y.I.; Malinina, T.G.; Lyubimova, E.Y.; Alexandrushkina, N.I.; Vanyushin, B.F.; Kolesova, G.M.; Yaguzhinsky, L.S. Ionol (BHT) Produces Superoxide Anion. Biochem. Biokhimiia 2002, 67, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, L.R.; Mazza, G. Assessing Antioxidant and Prooxidant Activities of Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Yarkala, S.; Amaravadi, S.; Rao, V.U.; Vijaykumar, V.; Navalgund, S.G.; Jagdish, B. Role of Excipients on N-Oxide Raloxifene Generation from Raloxifene-Excipients Binary Mixtures. Chem. Pharm. Bull. 2009, 57, 1174–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartauer, K.J.; Arbuthnot, G.N.; Baertschi, S.W.; Johnson, R.A.; Luke, W.D.; Pearson, N.G.; Rickard, E.C.; Tingle, C.A.; Tsang, P.K.; Wiens, R.E. Influence of Peroxide Impurities in Povidone and Crospovidone on the Stability of Raloxifene Hydrochloride in Tablets: Identification and Control of an Oxidative Degradation Product. Pharm. Dev. Technol. 2000, 5, 303–310. [Google Scholar] [CrossRef]
- Hong, J.; Lee, E.; Carter, J.C.; Masse, J.A.; Oksanen, D.A. Antioxidant-Accelerated Oxidative Degradation: A Case Study of Transition Metal Ion Catalyzed Oxidation in Formulation. Pharm. Dev. Technol. 2004, 9, 171–179. [Google Scholar] [CrossRef]
- Mahajan, R.; Templeton, A.; Harman, A.; Reed, R.A.; Chern, R.T. The Effect of Inert Atmospheric Packaging on Oxidative Degradation in Formulated Granules. Pharm. Res. 2005, 22, 128–140. [Google Scholar] [CrossRef]
- Waterman, K.C.; Adami, R.C.; Alsante, K.M.; Hong, J.; Landis, M.S.; Lombardo, F.; Roberts, C.J. Stabilization of Pharmaceuticals to Oxidative Degradation. Pharm. Dev. Technol. 2002, 7, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Felton, L.A.; Yang, J.; Shah, K.; Omidian, H.; Rocca, J.G. A Rapid Technique to Evaluate the Oxidative Stability of a Model Drug. Drug Dev. Ind. Pharm. 2007, 33, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Chatterjee, B.; Tekade, R.K. Current Regulatory Requirements and Practical Approaches for Stability Analysis of Pharmaceutical Products: A Comprehensive Review. Int. J. Pharm. 2018, 543, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, M.; Singh, S. Development of Validated Stability-Indicating Assay Methods—Critical Review. J. Pharm. Biomed. Anal. 2002, 28, 1011–1040. [Google Scholar] [CrossRef]
- Lei, Y.; Jin, B.; Ma, C.; Zhang, T.; Li, T. Identification of Forced Degradation Products of Tedizolid Phosphate by Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 2017, 139, 221–231. [Google Scholar] [CrossRef]
- Tsai, R.; Carrupt, P.; Testa, B.; Caldwell, J. Structure-Genotoxicity Relationships of Allylbenzenes and Propenylbenzenes: A Quantum Chemical Study. Chem. Res. Toxicol. 2002, 7, 73–76. [Google Scholar] [CrossRef]
- Baertschi, S.W.; Alsante, K.M.; Reed, R.A. Pharmaceutical Stress Testing: Predicting Drug Degradation, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4398-0180-2. [Google Scholar]
- Harmon, P.A.; Kosuda, K.; Nelson, E.; Mowery, M.; Reed, R.A. A Novel Peroxy Radical Based Oxidative Stressing System for Ranking the Oxidizability of Drug Substances. J. Pharm. Sci. 2006, 95, 2014–2028. [Google Scholar] [CrossRef]
- Nelson, E.D.; Thompson, G.M.; Yao, Y.; Flanagan, H.M.; Harmon, P.A. Solvent Effects on the AIBN Forced Degradation of Cumene: Implications for Forced Degradation Practices. J. Pharm. Sci. 2009, 98, 959–969. [Google Scholar] [CrossRef]
- Liubymova, A.K.; Bezbozhnaya, T.V.; Lobachev, V.L. Activation of Hydrogen Peroxide by Acetonitrile in the Oxidation of Thioethers: Reaction Kinetics and Mechanism. Kinet. Catal. 2021, 62, 342–349. [Google Scholar] [CrossRef]
- Hotha, K.K.; Roychowdhury, S.; Subramanian, V. Drug-Excipient Interactions: Case Studies and Overview of Drug Degradation Pathways. Am. J. Anal. Chem. 2016, 7, 107–140. [Google Scholar] [CrossRef] [Green Version]
- Vya, A.J.; Visana, N.M.; Patel, A.I.; Patel, A.B.; Patel, N.K.; Shah, S.R. Analytical Quality by Design in Stress Testing or Stability-Indicating Method. Asian J. Pharm. Anal. 2021, 11. [Google Scholar]
- Nanda, K.K.; Blincoe, W.D.; Allain, L.R.; Wuelfing, W.P.; Harmon, P.A. Iron(III)-Mediated Oxidative Degradation on the Benzylic Carbon of Drug Molecules in the Absence of Initiating Peroxides. J. Pharm. Sci. 2017, 106, 1347–1354. [Google Scholar] [CrossRef]
- Ghayas, S.; Qadeer, K.; Anwar, Z. Role of Catalysis and Catalytic Agents in Drug Stability. In Drug Stability and Chemical Kinetics; Akash, M.S.H., Rehman, K., Eds.; Springer: Singapore, 2020; pp. 95–119. ISBN 9789811564260. [Google Scholar]
- Sheldon, R.A.; Kochi, J.K. Metal-Catalyzed Oxidations of Organic Compounds in the Liquid Phase: A Mechanistic Approach. In Advances in Catalysis; Eley, D.D., Pines, H., Weisz, P.B., Eds.; Academic Press: Cambridge, MA, USA, 1976; Volume 25, pp. 272–413. [Google Scholar]
- Yoshioka, S.; Uchiyama, M. Kinetics and Mechanism of the Solid-State Decomposition of Propantheline Bromide. J. Pharm. Sci. 1986, 75, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Eyjolfsson, R. Diclofenac Sodium: Oxidative Degradation in Solution and Solid State. Drug Dev. Ind. Pharm. 2000, 26, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Modhave, D.; Barrios, B.; Paudel, A. PVP-H2O2 Complex as a New Stressor for the Accelerated Oxidation Study of Pharmaceutical Solids. Pharmaceutics 2019, 11, 457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, D.A.; Zhang, G.G.Z.; George, K.L.S.T.; Zhou, D. A Novel Accelerated Oxidative Stability Screening Method for Pharmaceutical Solids. J. Pharm. Sci. 2011, 100, 3529–3538. [Google Scholar] [CrossRef]
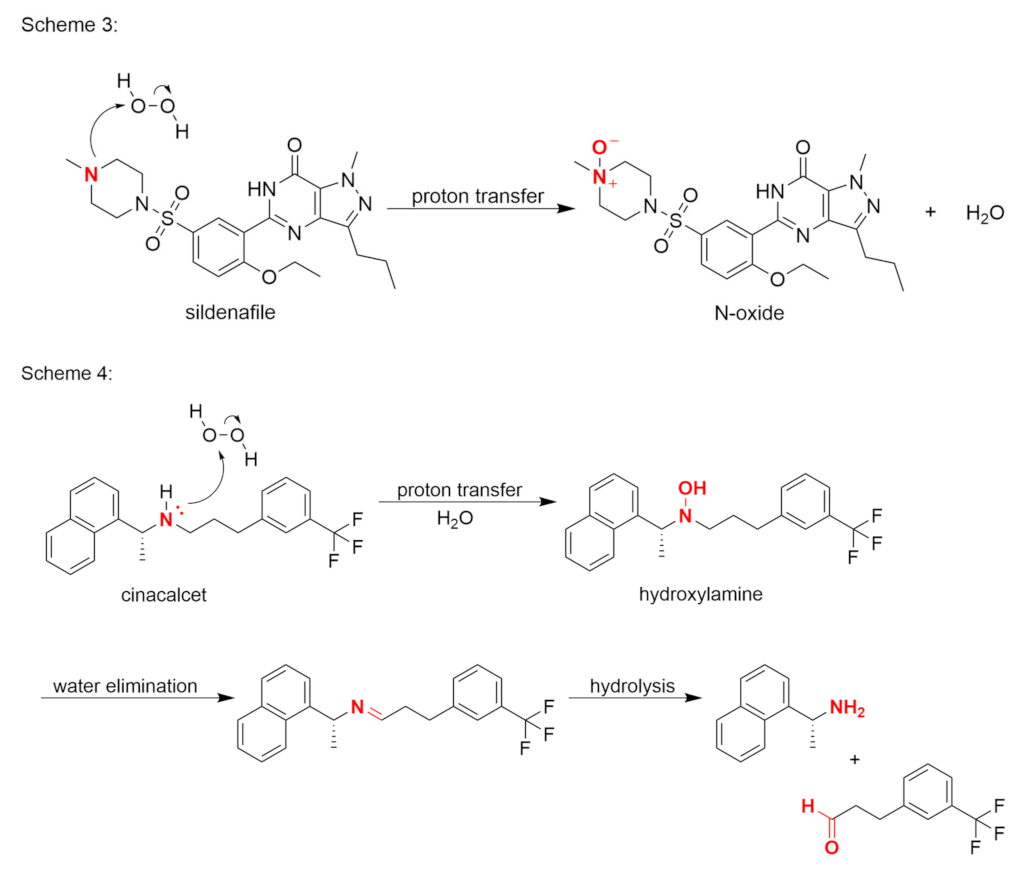
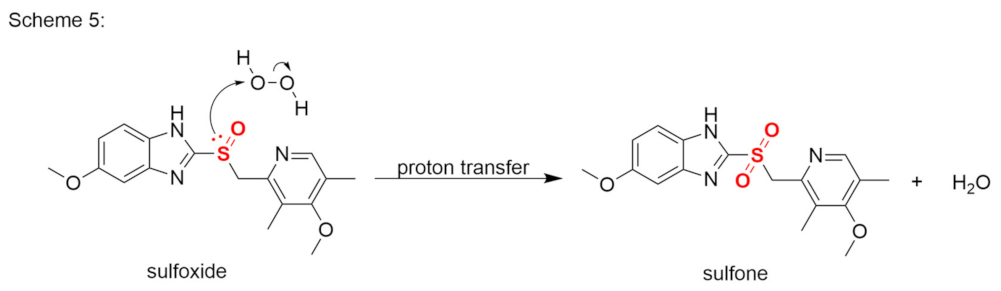
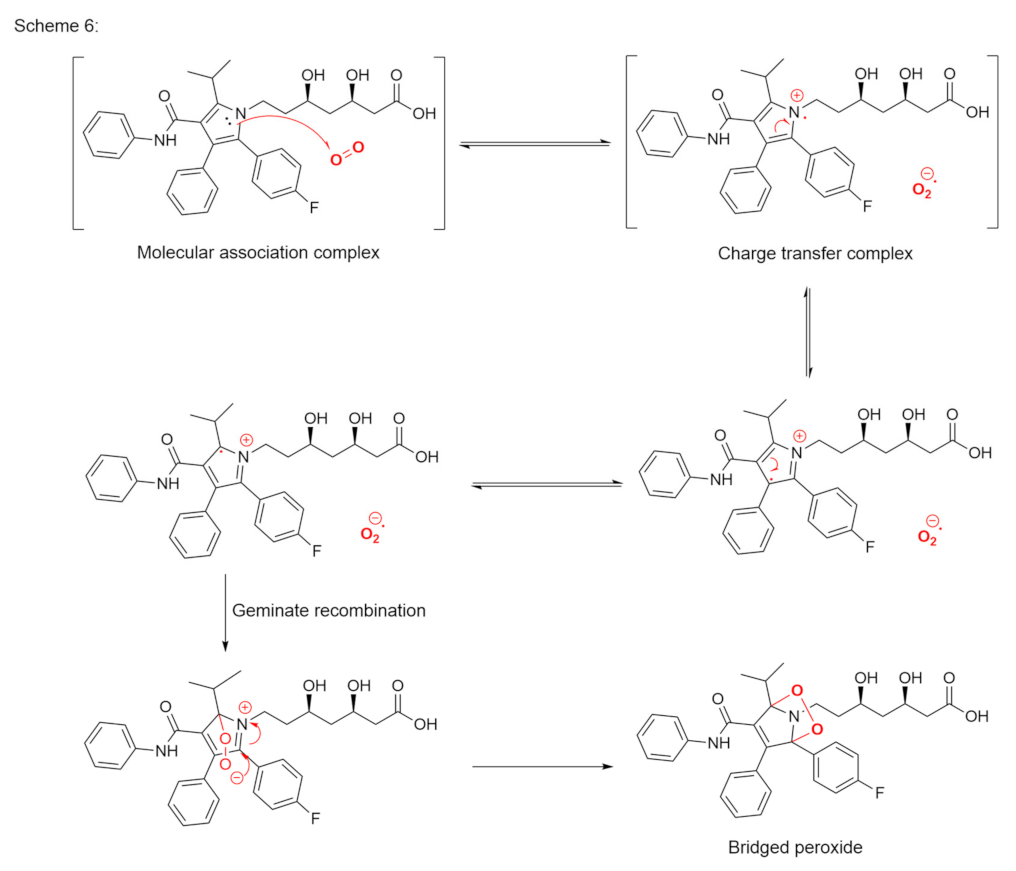
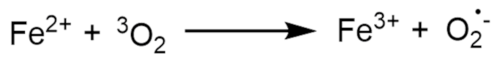
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabrič, A.; Hodnik, Ž.; Pajk, S. Oxidation of Drugs during Drug Product Development: Problems and Solutions. Pharmaceutics 2022, 14, 325. https://doi.org/10.3390/pharmaceutics14020325
Gabrič A, Hodnik Ž, Pajk S. Oxidation of Drugs during Drug Product Development: Problems and Solutions. Pharmaceutics. 2022; 14(2):325. https://doi.org/10.3390/pharmaceutics14020325
Chicago/Turabian StyleGabrič, Alen, Žiga Hodnik, and Stane Pajk. 2022. "Oxidation of Drugs during Drug Product Development: Problems and Solutions" Pharmaceutics 14, no. 2: 325. https://doi.org/10.3390/pharmaceutics14020325
APA StyleGabrič, A., Hodnik, Ž., & Pajk, S. (2022). Oxidation of Drugs during Drug Product Development: Problems and Solutions. Pharmaceutics, 14(2), 325. https://doi.org/10.3390/pharmaceutics14020325





