Microfluidic Synthesis and Purification of Magnetoliposomes for Potential Applications in the Gastrointestinal Delivery of Difficult-to-Transport Drugs
Abstract
:1. Introduction
2. Materials and Methods
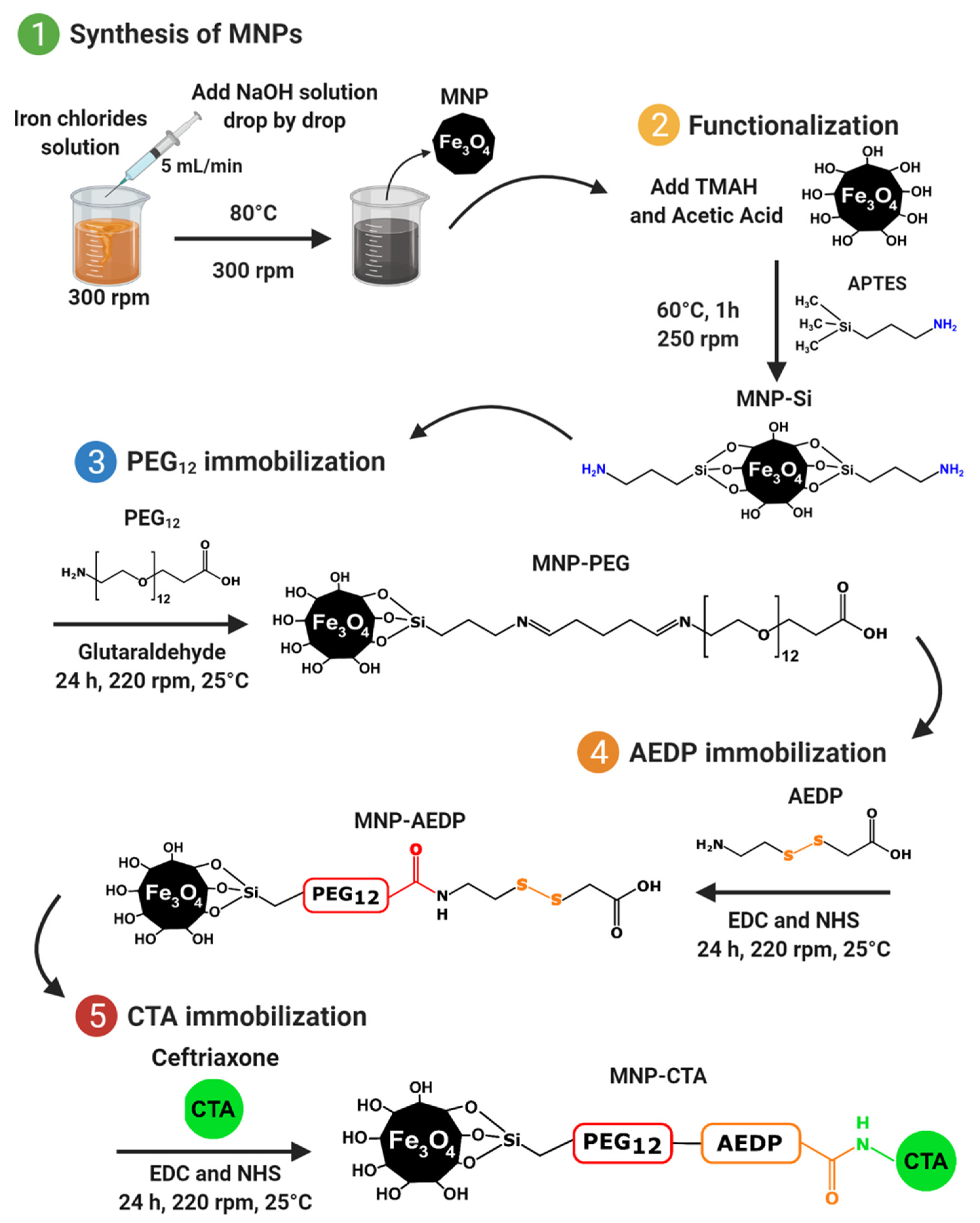
2.1. Magnetite Nanoparticles Synthesis and Functionalization
2.2. Magnetoliposomes Synthesis Using the Microfluidic Approach
2.2.1. Lipidic-MNPs Phase Preparation
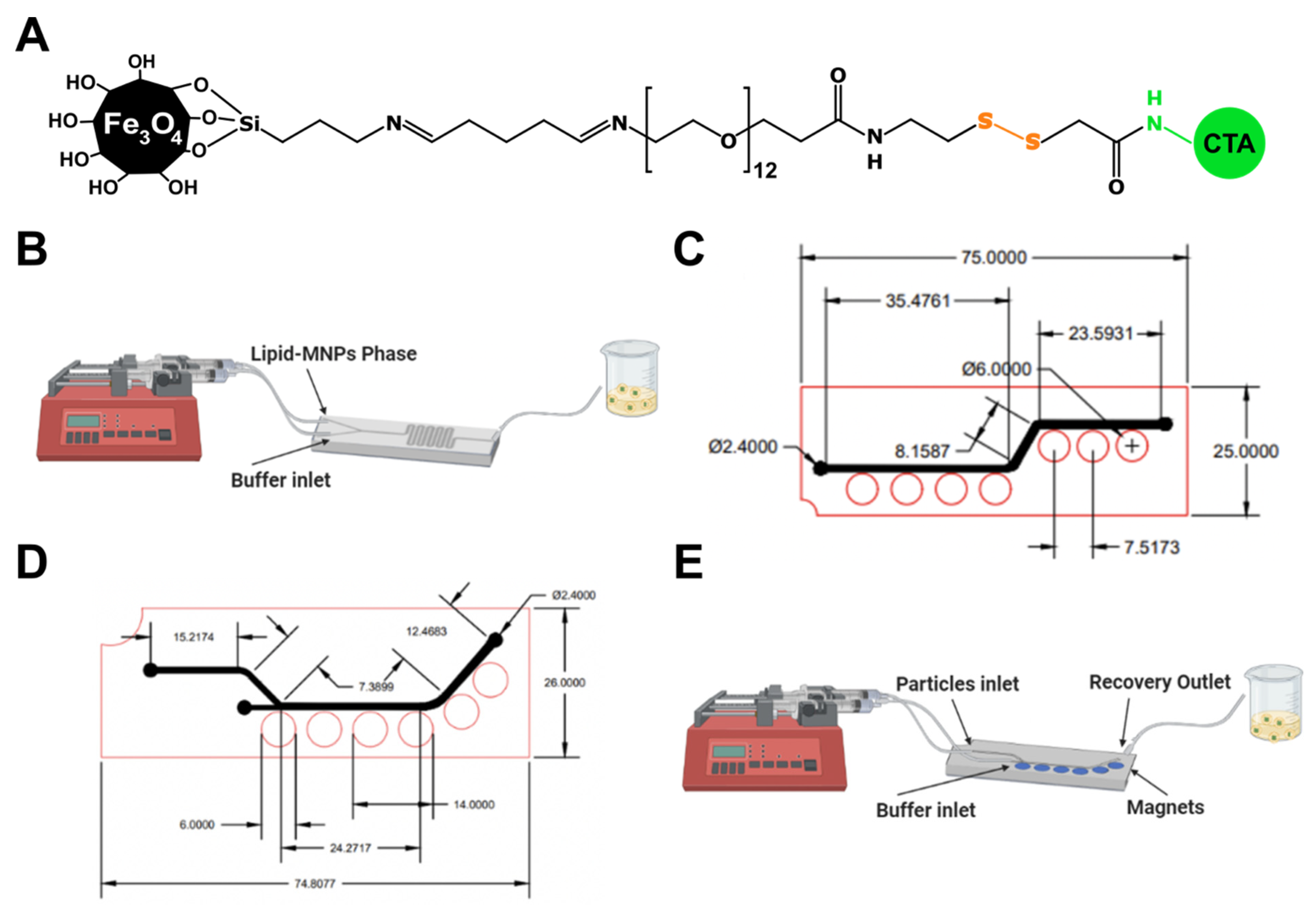
2.2.2. Microfluidic System Manufacture and Experimental Setup
2.3. Magnetoliposomes Characterization
2.4. Magnetolipsomes Encapsulation Efficiency (EE%)
2.5. Magnetoliposomes Purification
2.5.1. Lipidic-Nanoconjugates Phase Preparation
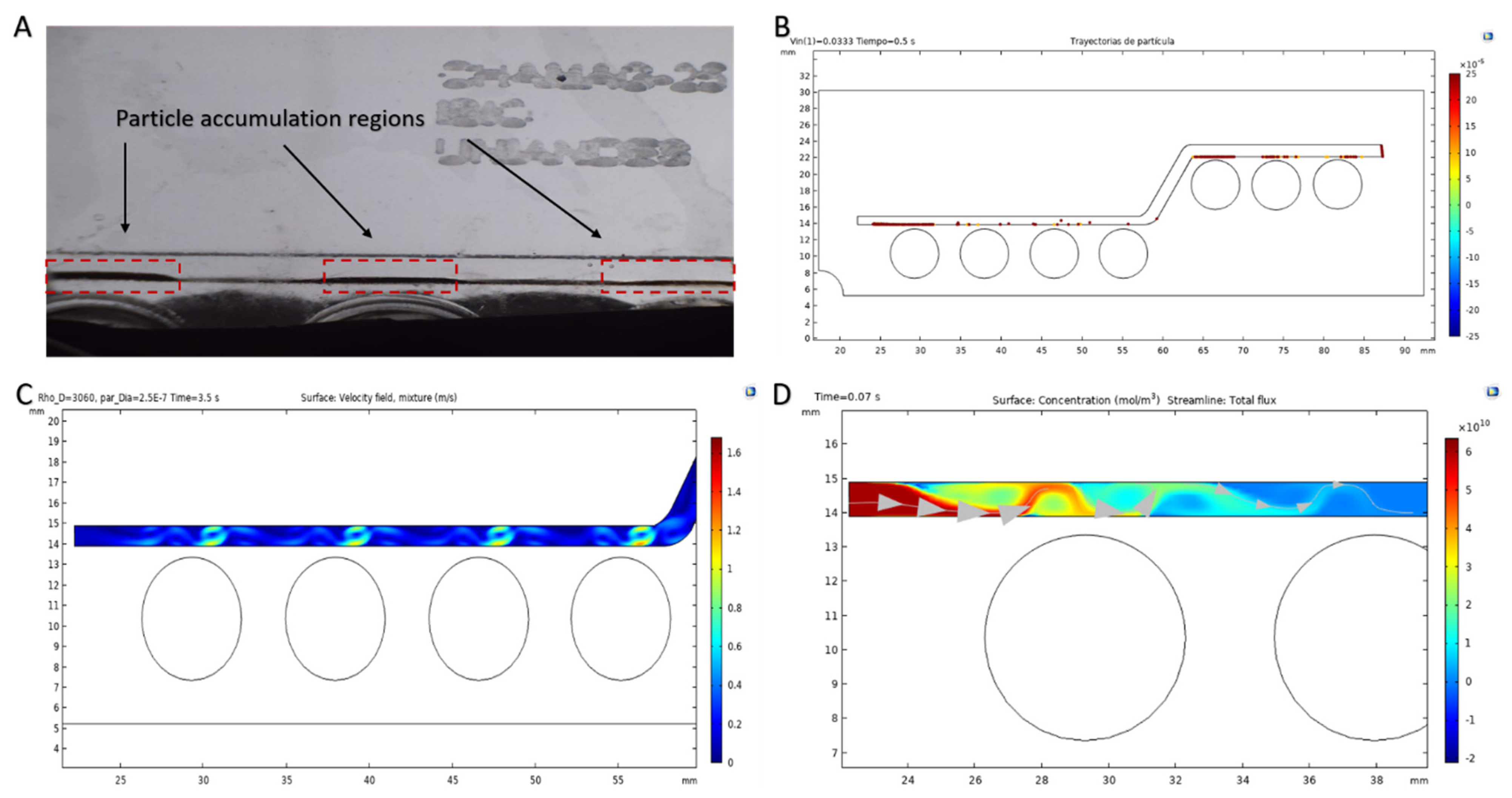
2.5.2. Multiphysics Simulations of Magnetophoretic Separation via the Particle Tracing Module
2.5.3. Multiphysics Simulations of Magnetophoretic Separation via the Mixture Model
2.5.4. Microfluidic System Manufacture and Experimental Setup
2.6. In Vitro Testing of MLPs
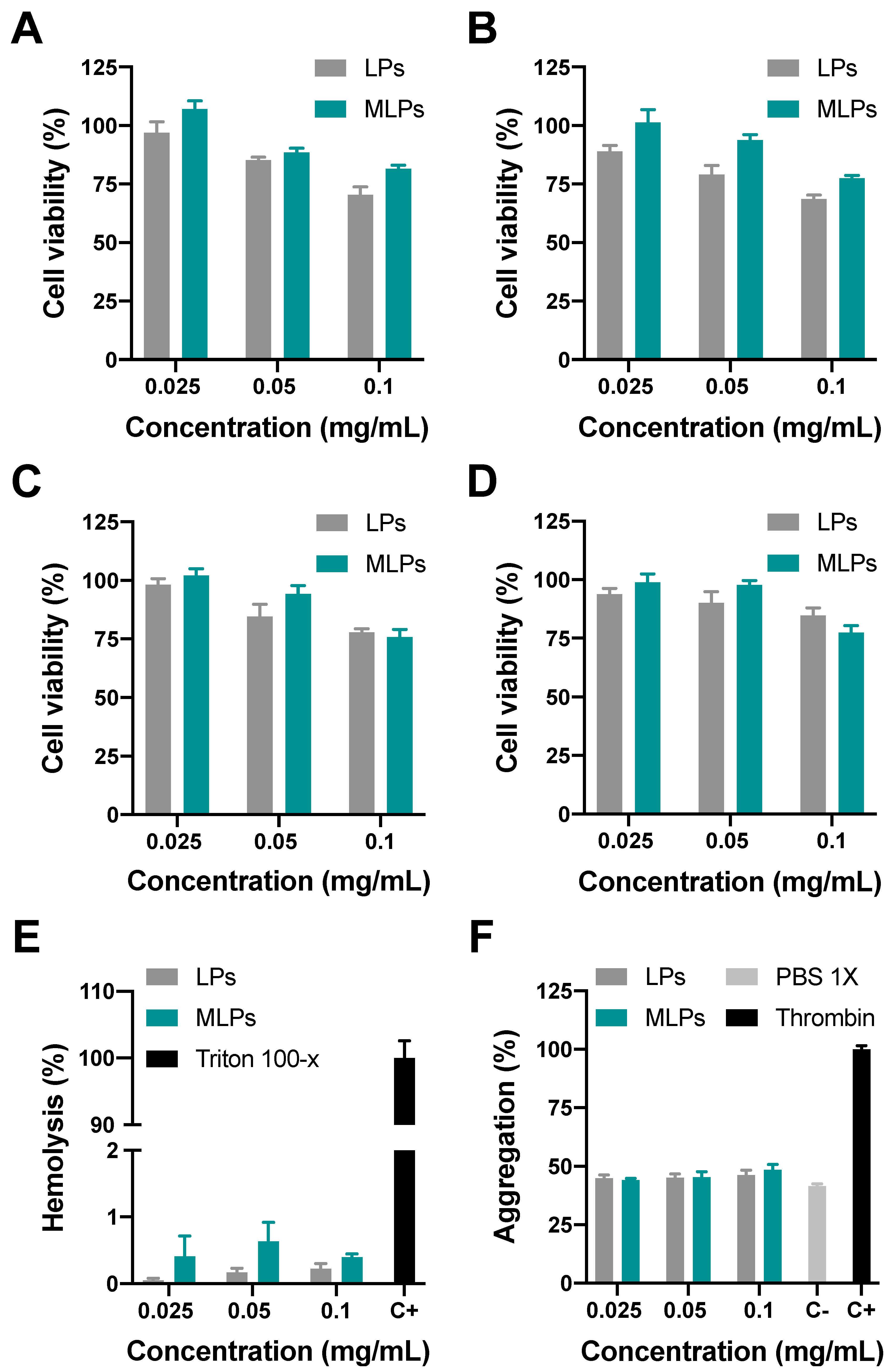
2.6.1. Hemocompatibility
2.6.2. Platelet Aggregation
2.6.3. Cytotoxicity
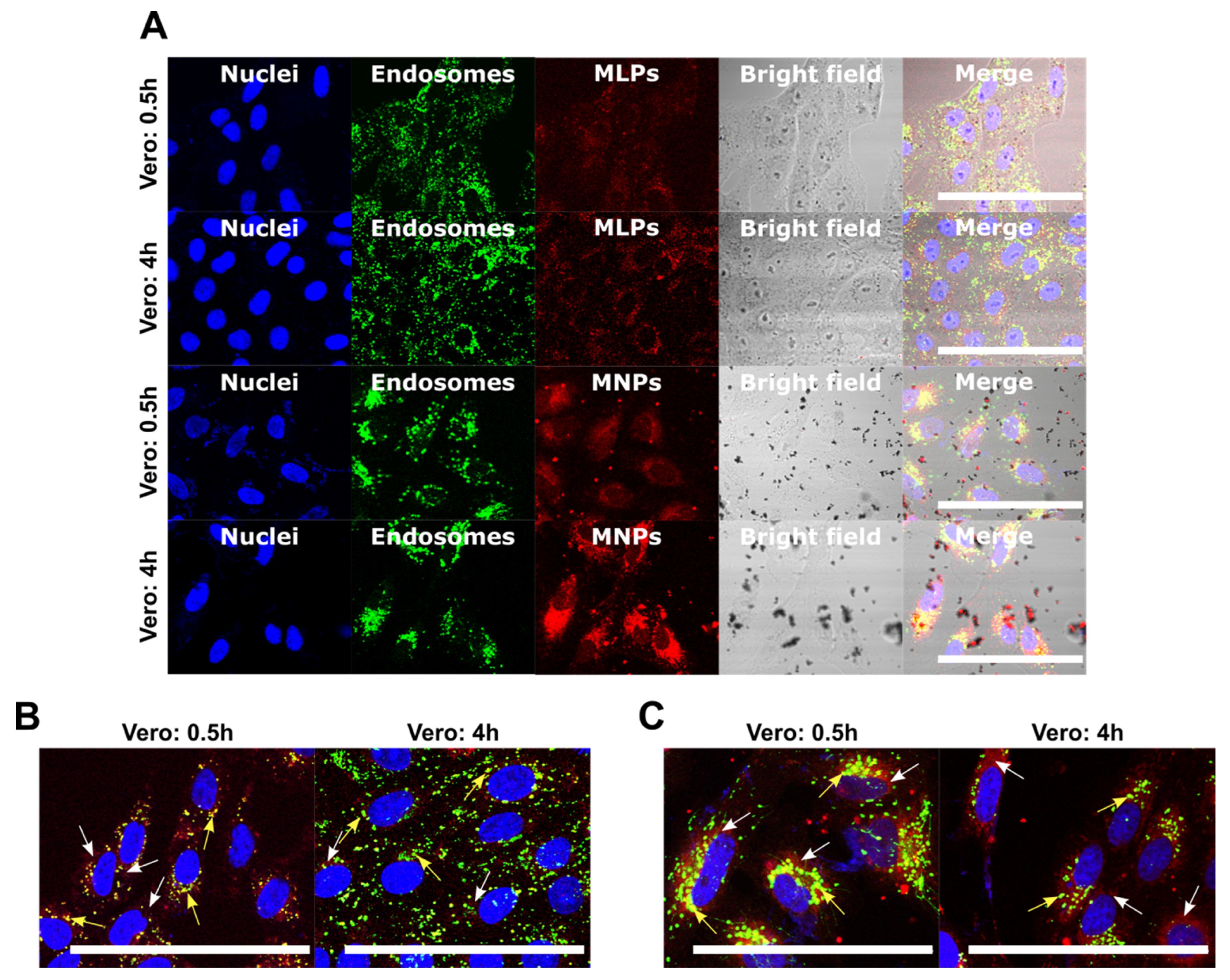
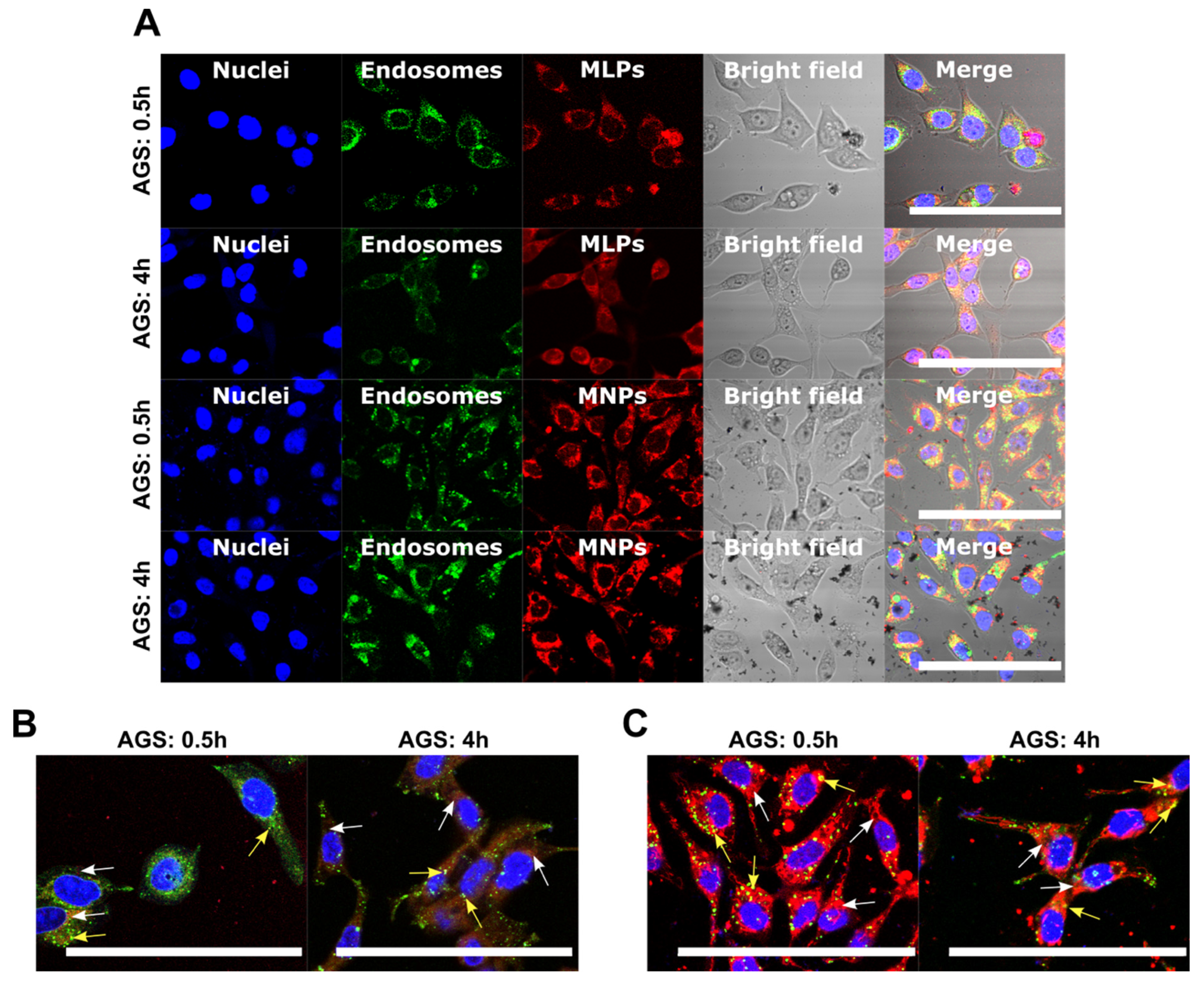
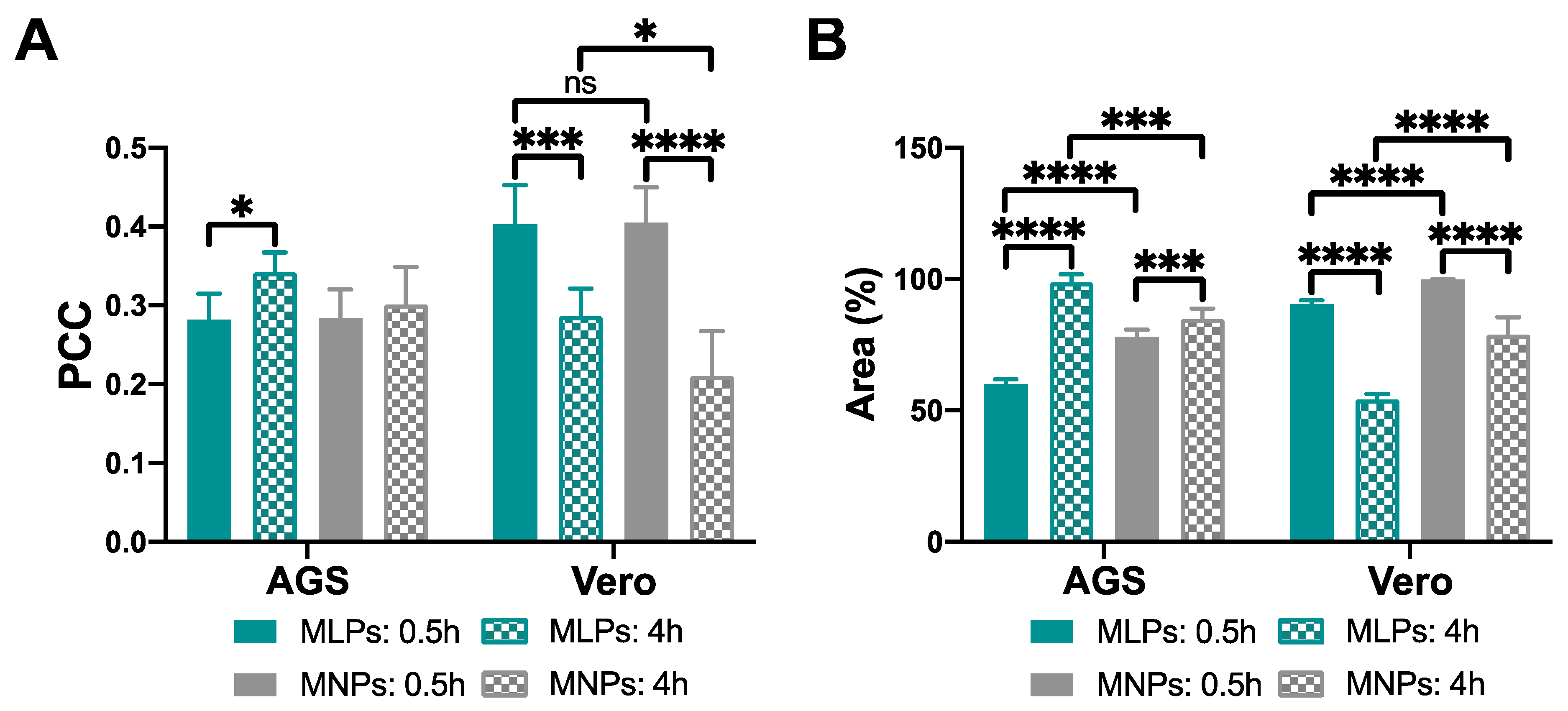
2.6.4. Cell Internalization and Endosomal Escape Analysis
2.7. Statistical Analyses
3. Results and Discussion
3.1. Characterization of Magnetoliposomes Using the Microfluidic Approach
3.2. Magnetolipsomes Encapsulation Efficiency
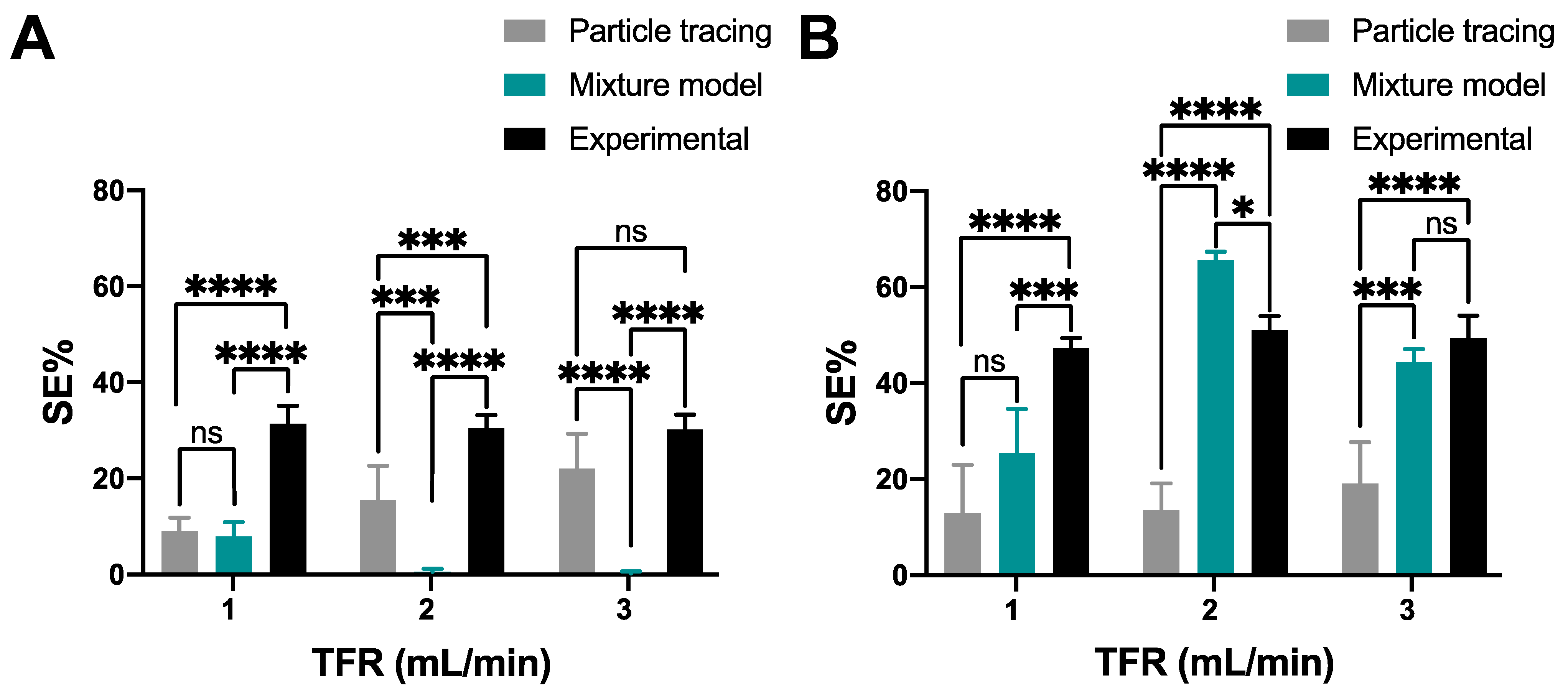
3.3. Magnetoliposomes Purification
3.4. In Vitro Testing of MLPs
3.4.1. Biocompatibility
3.4.2. Cell Internalization and Endosomal Escape Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Homayun, B.; Lin, X.; Choi, H.-J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [Green Version]
- Youhanna, S.; Lauschke, V.M. The Past, Present and Future of Intestinal in Vitro Cell Systems for Drug Absorption Studies. J. Pharm. Sci. 2021, 110, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.P.; Gupta, S. Optimizing Efficacy of Amphotericin B through Nanomodification. Int. J. Nanomed. 2006, 1, 417. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, J.; McClements, D.J.; Zou, L. Encapsulation of β-Carotene-Loaded Oil Droplets in Caseinate/Alginate Microparticles: Enhancement of Carotenoid Stability and Bioaccessibility. J. Funct. Foods 2018, 40, 527–535. [Google Scholar] [CrossRef]
- Feng, J.; Wu, Y.; Zhang, L.; Li, Y.; Liu, S.; Wang, H.; Li, C. Enhanced Chemical Stability, Intestinal Absorption, and Intracellular Antioxidant Activity of Cyanidin-3-O-Glucoside by Composite Nanogel Encapsulation. J. Agric. Food Chem. 2019, 67, 10432–10447. [Google Scholar] [CrossRef]
- Kouhi, M.; Prabhakaran, M.P.; Ramakrishna, S. Edible Polymers: An Insight into Its Application in Food, Biomedicine and Cosmetics. Trends Food Sci. Technol. 2020, 103, 248–263. [Google Scholar] [CrossRef]
- Cheng, Y.; Ji, Y. RGD-Modified Polymer and Liposome Nanovehicles: Recent Research Progress for Drug Delivery in Cancer Therapeutics. Eur. J. Pharm. Sci. 2019, 128, 8–17. [Google Scholar] [CrossRef]
- Czuba, E.; Diop, M.; Mura, C.; Schaschkow, A.; Langlois, A.; Bietiger, W.; Neidl, R.; Virciglio, A.; Auberval, N.; Julien-David, D.; et al. Oral Insulin Delivery, the Challenge to Increase Insulin Bioavailability: Influence of Surface Charge in Nanoparticle System. Int. J. Pharm. 2018, 542, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Moura, R.P.; Pacheco, C.; Pêgo, A.P.; des Rieux, A.; Sarmento, B. Lipid Nanocapsules to Enhance Drug Bioavailability to the Central Nervous System. J. Control. Release 2020, 322, 390–400. [Google Scholar] [CrossRef]
- Ho, B.X.; Loh, S.J.H.; Chan, W.K.; Soh, B.S. In Vivo Genome Editing as a Therapeutic Approach. Int. J. Mol. Sci. 2018, 19, 2721. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Acosta, C.M.; Cifuentes, J.; Castellanos, M.C.; Moreno, R.J.; Muñoz-Camargo, C.; Cruz, J.C.; Reyes, L.H. Ph-Responsive, Cell-Penetrating, Core/Shell Magnetite/Silver Nanoparticles for the Delivery of Plasmids: Preparation, Characterization, and Preliminary in Vitro Evaluation. Pharmaceutics 2020, 12, 561. [Google Scholar] [CrossRef]
- Hidai, C.; Kitano, H. Nonviral Gene Therapy for Cancer: A Review. Diseases 2018, 6, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, J.; Cifuentes, J.; Cuellar, M.; Suarez-Arnedo, A.; Cruz, J.C.; Muñoz-Camargo, C. Cell-Penetrating and Antibacterial BUF-II Nanobioconjugates: Enhanced Potency via Immobilization on Polyetheramine-Modified Magnetite Nanoparticles. Int. J. Nanomed. 2019, 14, 8483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuellar, M.; Cifuentes, J.; Perez, J.; Suarez-Arnedo, A.; Serna, J.A.; Groot, H.; Muñoz-Camargo, C.; Cruz, J.C. Novel BUF2-Magnetite Nanobioconjugates with Cell-Penetrating Abilities. Int. J. Nanomed. 2018, 13, 8087. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Barbosa, N.; Suárez-Arnedo, A.; Cifuentes, J.; Gonzalez Barrios, A.F.; Silvera Batista, C.A.; Osma, J.F.; Muñoz-Camargo, C.; Cruz, J.C. Magnetite–OmpA Nanobioconjugates as Cell-Penetrating Vehicles with Endosomal Escape Abilities. ACS Biomater. Sci. Eng. 2019, 6, 415–424. [Google Scholar] [CrossRef]
- Choi, W.I.; Sahu, A.; Wurm, F.R.; Jo, S.-M. Magnetoliposomes with Size Controllable Insertion of Magnetic Nanoparticles for Efficient Targeting of Cancer Cells. RSC Adv. 2019, 9, 15053–15060. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Pinel, B.; Jabalera, Y.; Ortiz, R.; Cabeza, L.; Jimenez-Lopez, C.; Melguizo, C.; Prados, J. Biomimetic Magnetoliposomes as Oxaliplatin Nanocarriers: In Vitro Study for Potential Application in Colon Cancer. Pharmaceutics 2020, 12, 589. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.D.; Rodrigues, A.R.O.; Bañobre-López, M.; Almeida, B.G.; Amorim, C.O.; Amaral, V.S.; Coutinho, P.J.; Castanheira, E. Magnetoliposomes Based on Shape Anisotropic Calcium/Magnesium Ferrite Nanoparticles as Nanocarriers for Doxorubicin. Pharmaceutics 2021, 13, 1248. [Google Scholar] [CrossRef]
- Karavolos, M.; Holban, A. Nanosized Drug Delivery Systems in Gastrointestinal Targeting: Interactions with Microbiota. Pharmaceuticals 2016, 9, 62. [Google Scholar] [CrossRef]
- Vamanu, E.; Gatea, F. Correlations between Microbiota Bioactivity and Bioavailability of Functional Compounds: A Mini-Review. Biomedicines 2020, 8, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabulici, C.M.; Sârbu, I.; Vamanu, E. The Bioactive Potential of Functional Products and Bioavailability of Phenolic Compounds. Foods 2020, 9, 953. [Google Scholar] [CrossRef]
- Vamanu, E.; Rai, S.N. The Link between Obesity, Microbiota Dysbiosis, and Neurodegenerative Pathogenesis. Diseases 2021, 9, 45. [Google Scholar] [CrossRef]
- Pereira, D.S.; Cardoso, B.D.; Rodrigues, A.R.O.; Amorim, C.O.; Amaral, V.S.; Almeida, B.G.; Queiroz, M.-J.R.; Martinho, O.; Baltazar, F.; Calhelha, R.C.; et al. Magnetoliposomes Containing Calcium Ferrite Nanoparticles for Applications in Breast Cancer Therapy. Pharmaceutics 2019, 11, 477. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Lu, Z.; Li, Y.; Yang, J.; Zhang, X. Surface Modification of Iron Oxide-Based Magnetic Nanoparticles for Cerebral Theranostics: Application and Prospection. Nanomaterials 2020, 10, 1441. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, P.D.; Pellosi, D.S.; Tedesco, A.C. Magnetic Nanoparticles: Applications in Biomedical Processes as Synergic Drug-Delivery Systems. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 371–396. [Google Scholar]
- Rodrigues, A.R.O.; Almeida, B.G.; Araújo, J.P.; Queiroz, M.-J.R.; Coutinho, P.J.; Castanheira, E.M. Magnetoliposomes for Dual Cancer Therapy. In Inorganic Frameworks as Smart Nanomedicines; Elsevier: Amsterdam, The Netherlands, 2018; pp. 489–527. [Google Scholar]
- Rodrigues, A.R.O.; Gomes, I.; Almeida, B.G.; Araújo, J.P.; Castanheira, E.M.; Coutinho, P.J. Magnetoliposomes Based on Nickel/Silica Core/Shell Nanoparticles: Synthesis and Characterization. Mater. Chem. Phys. 2014, 148, 978–987. [Google Scholar] [CrossRef] [Green Version]
- Bonnaud, C.; Monnier, C.A.; Demurtas, D.; Jud, C.; Vanhecke, D.; Montet, X.; Hovius, R.; Lattuada, M.; Rothen-Rutishauser, B.; Petri-Fink, A. Insertion of Nanoparticle Clusters into Vesicle Bilayers. ACS Nano 2014, 8, 3451–3460. [Google Scholar] [CrossRef] [PubMed]
- Floris, A.; Ardu, A.; Musinu, A.; Piccaluga, G.; Fadda, A.M.; Sinico, C.; Cannas, C. SPION@ Liposomes Hybrid Nanoarchitectures with High Density SPION Association. Soft Matter 2011, 7, 6239–6247. [Google Scholar] [CrossRef]
- Carugo, D.; Bottaro, E.; Owen, J.; Stride, E.; Nastruzzi, C. Liposome Production by Microfluidics: Potential and Limiting Factors. Sci. Rep. 2016, 6, 25876. [Google Scholar]
- Mota-Cobián, A.; Velasco, C.; Mateo, J.; España, S. Optimization of Purification Techniques for Lumen-Loaded Magnetoliposomes. Nanotechnology 2020, 31, 145102. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Hussain, M.T.; Roces, C.B.; Anderluzzi, G.; Kastner, E.; Salmaso, S.; Kirby, D.J.; Perrie, Y. Microfluidics Based Manufacture of Liposomes Simultaneously Entrapping Hydrophilic and Lipophilic Drugs. Int. J. Pharm. 2016, 514, 160–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Ahmady, Z.S.; Donno, R.; Gennari, A.; Prestat, E.; Marotta, R.; Mironov, A.; Newman, L.; Lawrence, M.J.; Tirelli, N.; Ashford, M.; et al. Enhanced Intraliposomal Metallic Nanoparticle Payload Capacity Using Microfluidic-Assisted Self-Assembly. Langmuir 2019, 35, 13318–13331. [Google Scholar] [CrossRef]
- Conde, A.J.; Batalla, M.; Cerda, B.; Mykhaylyk, O.; Plank, C.; Podhajcer, O.; Cabaleiro, J.M.; Madrid, R.E.; Policastro, L. Continuous Flow Generation of Magnetoliposomes in a Low-Cost Portable Microfluidic Platform. Lab. Chip 2014, 14, 4506–4512. [Google Scholar] [CrossRef]
- Takagi, J.; Yamada, M.; Yasuda, M.; Seki, M. Continuous Particle Separation in a Microchannel Having Asymmetrically Arranged Multiple Branches. Lab. Chip 2005, 5, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Salafi, T.; Zeming, K.K.; Zhang, Y. Advancements in Microfluidics for Nanoparticle Separation. Lab. Chip 2017, 17, 11–33. [Google Scholar] [CrossRef] [Green Version]
- Kye, H.G.; Park, B.S.; Lee, J.M.; Song, M.G.; Song, H.G.; Ahrberg, C.D.; Chung, B.G. Dual-Neodymium Magnet-Based Microfluidic Separation Device. Sci. Rep. 2019, 9, 9502. [Google Scholar] [CrossRef]
- Khashan, S.A.; Dagher, S.; Alazzam, A.; Mathew, B.; Hilal-Alnaqbi, A. Microdevice for Continuous Flow Magnetic Separation for Bioengineering Applications. J. Micromechan. Microeng. 2017, 27, 55016. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Lin, L.; Zhao, K.; Song, Y.; Huang, M.; Zhu, Z.; Zhou, L.; Yang, C. Auto-Affitech: An Automated Ligand Binding Affinity Evaluation Platform Using Digital Microfluidics with a Bidirectional Magnetic Separation Method. Lab. Chip 2020, 20, 1577–1585. [Google Scholar] [CrossRef]
- Aranguren, A.; Torres, C.E.; Muñoz-Camargo, C.; Osma, J.F.; Cruz, J.C. Synthesis of Nanoscale Liposomes via Low-Cost Microfluidic Systems. Micromachines 2020, 11, 1050. [Google Scholar] [CrossRef]
- Garg, H.; Kharola, A.S.; Karar, V. Numerical Analysis of Different Magnet Shapes on Heat Transfer Application Using Ferrofluid. In Proceedings of the 2015 COMSOL Conference, Pune, India, October 29–30 2015; Volume 51, p. 160030. [Google Scholar]
- Bruno, N.M.; Ciocanel, C.; Kipple, A. Modeling Flow of Magnetorheological Fluid through a Micro-Channel. In Proceedings of the 2009 COMSOL Conference, Boston, MA, USA, October 8–10 2009; pp. 1–7. [Google Scholar]
- Yang, M.; O’Handley, R.; Fang, Z. Modeling of Ferrofluid Passive Cooling System. In Proceedings of the 2010 COMSOL Conference, Boston, MA, USA, October 7–9 2010; pp. 1–6. [Google Scholar]
- Torres-Díaz, I.; Rinaldi, C. Recent Progress in Ferrofluids Research: Novel Applications of Magnetically Controllable and Tunable Fluids. Soft Matter 2014, 10, 8584–8602. [Google Scholar] [CrossRef]
- Kumar, C.; Hejazian, M.; From, C.; Saha, S.C.; Sauret, E.; Gu, Y.; Nguyen, N.-T. Modeling of Mass Transfer Enhancement in a Magnetofluidic Micromixer. Phys. Fluids 2019, 31, 63603. [Google Scholar] [CrossRef]
- García-Jimeno, S.; Escribano, E.; Queralt, J.; Estelrich, J. Magnetoliposomes Prepared by Reverse-Phase Followed by Sequential Extrusion: Characterization and Possibilities in the Treatment of Inflammation. Int. J. Pharm. 2011, 405, 181–187. [Google Scholar] [CrossRef]
- Multiphysics, C. Comsol Multiphysics 5.3 CFD Module User’s Guide. 2014, pp. 274–289. Available online: https://doc.comsol.com/5.3/doc/com.comsol.help.cfd/CFDModuleUsersGuide.pdf (accessed on 28 November 2021).
- Campaña, A.L.; Sotelo, D.C.; Oliva, H.A.; Aranguren, A.; Ornelas-Soto, N.; Cruz, J.C.; Osma, J.F. Fabrication and Characterization of a Low-Cost Microfluidic System for the Manufacture of Alginate–Lacasse Microcapsules. Polymers 2020, 12, 1158. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal Drug Delivery Systems: From Concept to Clinical Applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, Q.; Xuan, S.; Gong, X. Size-Selective Separation of Magnetic Nanospheres in a Microfluidic Channel. Microfluid. Nanofluid. 2017, 21, 47. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Xue, Z.; Xia, H.; Yang, N.; Zhang, R. Numerical Analysis of Capture and Isolation of Magnetic Nanoparticles in Microfluidic System. Mod. Phys. Lett. B 2018, 32, 1840075. [Google Scholar] [CrossRef]
- Lorente, C.; Cabeza, L.; Clares, B.; Ortiz, R.; Halbaut, L.; Delgado, Á.V.; Perazzoli, G.; Prados, J.; Arias, J.L.; Melguizo, C. Formulation and in Vitro Evaluation of Magnetoliposomes as a Potential Nanotool in Colorectal Cancer Therapy. Colloids Surf. B Biointerfaces 2018, 171, 553–565. [Google Scholar] [CrossRef]
- Ye, H.; Tong, J.; Wu, J.; Xu, X.; Wu, S.; Tan, B.; Shi, M.; Wang, J.; Zhao, W.; Jiang, H.; et al. Preclinical Evaluation of Recombinant Human IFNα2b-Containing Magnetoliposomes for Treating Hepatocellular Carcinoma. Int. J. Nanomed. 2014, 9, 4533. [Google Scholar]
- Crowley, L.C.; Marfell, B.J.; Waterhouse, N.J. Analyzing Cell Death by Nuclear Staining with Hoechst 33342. Cold Spring Harb. Protoc. 2016, 2016, pdb-prot087205. [Google Scholar] [CrossRef]
- Rueda-Gensini, L.; Cifuentes, J.; Castellanos, M.C.; Puentes, P.R.; Serna, J.A.; Muñoz-Camargo, C.; Cruz, J.C. Tailoring Iron Oxide Nanoparticles for Efficient Cellular Internalization and Endosomal Escape. Nanomaterials 2020, 10, 1816. [Google Scholar] [CrossRef]
- Kawish, M.; Elhissi, A.; Jabri, T.; Muhammad Iqbal, K.; Zahid, H.; Shah, M.R. Enhancement in Oral Absorption of Ceftriaxone by Highly Functionalized Magnetic Iron Oxide Nanoparticles. Pharmaceutics 2020, 12, 492. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Zendejas, V.E.; Torres-Martinez, A.C.; Salas-Morales, B.; Fortoul, T.I.; Montaño, L.F.; Rendon-Huerta, E.P. Claudin-6, 7, or 9 Overexpression in the Human Gastric Adenocarcinoma Cell Line AGS Increases Its Invasiveness, Migration, and Proliferation Rate. Cancer Investig. 2011, 29, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Oyama, J.; Node, K. Gut Microbiota and Hypertension. Hypertens. Res. 2019, 42, 741–743. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Value | Units |
|---|---|---|
| 1.00 | T | |
| 1.00 × 10−3 | Pa.s | |
| 12.57 × 10−7 | H/m | |
| 2.50 | DV | |
| 1.50 | DV | |
| 5180 | kg/m3 | |
| 3063 | kg/m3 | |
| 1.00 × 10−7 | m | |
| 2.50 × 10−7 | m |
| Parameter | Value | Units |
|---|---|---|
| 1.00 | T | |
| 1.00 × 10−3 | Pa.s | |
| 2.50 | DV | |
| 1.50 | DV | |
| 12.57 × 10−7 | H/m | |
| 1000 | kg/m3 | |
| 5180 | kg/m3 | |
| 3063 | kg/m3 | |
| 1.00 × 10−7 | m | |
| 2.50 × 10−7 | m | |
| 4.83 × 10−12 | m2/s | |
| 1.93 × 10−12 | m2/s | |
| 0.20 | DV | |
| 4.00 × 10−4 | m3/kg | |
| 1.33 × 10−4 | m3/kg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, C.E.; Cifuentes, J.; Gómez, S.C.; Quezada, V.; Giraldo, K.A.; Puentes, P.R.; Rueda-Gensini, L.; Serna, J.A.; Muñoz-Camargo, C.; Reyes, L.H.; et al. Microfluidic Synthesis and Purification of Magnetoliposomes for Potential Applications in the Gastrointestinal Delivery of Difficult-to-Transport Drugs. Pharmaceutics 2022, 14, 315. https://doi.org/10.3390/pharmaceutics14020315
Torres CE, Cifuentes J, Gómez SC, Quezada V, Giraldo KA, Puentes PR, Rueda-Gensini L, Serna JA, Muñoz-Camargo C, Reyes LH, et al. Microfluidic Synthesis and Purification of Magnetoliposomes for Potential Applications in the Gastrointestinal Delivery of Difficult-to-Transport Drugs. Pharmaceutics. 2022; 14(2):315. https://doi.org/10.3390/pharmaceutics14020315
Chicago/Turabian StyleTorres, Carlos E., Javier Cifuentes, Saúl C. Gómez, Valentina Quezada, Kevin A. Giraldo, Paola Ruiz Puentes, Laura Rueda-Gensini, Julian A. Serna, Carolina Muñoz-Camargo, Luis H. Reyes, and et al. 2022. "Microfluidic Synthesis and Purification of Magnetoliposomes for Potential Applications in the Gastrointestinal Delivery of Difficult-to-Transport Drugs" Pharmaceutics 14, no. 2: 315. https://doi.org/10.3390/pharmaceutics14020315
APA StyleTorres, C. E., Cifuentes, J., Gómez, S. C., Quezada, V., Giraldo, K. A., Puentes, P. R., Rueda-Gensini, L., Serna, J. A., Muñoz-Camargo, C., Reyes, L. H., Osma, J. F., & Cruz, J. C. (2022). Microfluidic Synthesis and Purification of Magnetoliposomes for Potential Applications in the Gastrointestinal Delivery of Difficult-to-Transport Drugs. Pharmaceutics, 14(2), 315. https://doi.org/10.3390/pharmaceutics14020315













