Self-Organized Nanoparticles of Random and Block Copolymers of Sodium 2-(Acrylamido)-2-methyl-1-propanesulfonate and Sodium 11-(Acrylamido)undecanoate as Safe and Effective Zika Virus Inhibitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Virus
2.2. XTT Assay
2.3. RNA Isolation and RT-qPCR
2.4. Virus Inhibition Assay
2.5. In Vitro Mechanism of Action
2.6. Fate of Nanoparticles after Interaction with Vero Cells
2.7. Fluorescence Microscopy
2.8. Statistics
3. Results
3.1. Polymers
3.2. Cytotoxicity and Cellular Localization of PAMPS-b-PAaU
3.3. Antiviral Tests
3.4. Mechanism of Action
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knipe, D.M.; Howley, P. Fields Virology, 6th ed.; Lippincott Williams & Wilkins (LWW): Philadelphia, PA, USA, 2013; p. 2664. [Google Scholar]
- Dick, G.W.A. Zika Virus (I). Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Marchette, N.J.; Garcia, R.; Rudnick, A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am. J. Trop. Med. Hyg. 1969, 18, 411–441. [Google Scholar] [CrossRef] [PubMed]
- MacNamara, F.N. Zika virus: A report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1954, 48, 139–145. [Google Scholar] [CrossRef]
- Duffy, M.R.; Chen, T.H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Bossin, H.; Mallet, H.P.; Besnard, M.; Broult, J.; Baudouin, L.; Levi, J.E.; Sabino, E.C.; Ghawche, F.; Lanteri, M.C.; et al. Zika virus in French Polynesia 2013–14: Anatomy of a completed outbreak. Lancet Infect. Dis. 2018, 18, e172–e182. [Google Scholar] [CrossRef]
- Besnard, M.; Lastère, S.; Teissier, A.; Cao-Lormeau, V.M.; Musso, D. Evidence of perinatal transmission of zika virus, French Polynesia, December 2013 and February 2014. Eurosurveillance 2014, 19, 20751. [Google Scholar] [CrossRef] [Green Version]
- Brasil, P.; Pereira, J.P.; Moreira, M.E.; Nogueira, R.M.R.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.A.; Salles, T.S.; et al. Zika virus infection in pregnant women in Rio de Janeiro. N. Engl. J. Med. 2016, 375, 2321–2334. [Google Scholar] [CrossRef]
- Osorio-De-Castro, C.G.S.; Miranda, E.S.; De Freitas, C.M.; De Camargo, K.R.; Cranmer, H.H. The Zika Virus outbreak in Brazil: Knowledge gaps and challenges for risk reduction. Am. J. Public Health 2017, 107, 960–965. [Google Scholar] [CrossRef]
- Petersen, E.; Wilson, M.E.; Touch, S.; McCloskey, B.; Mwaba, P.; Bates, M.; Dar, O.; Mattes, F.; Kidd, M.; Ippolito, G.; et al. Rapid Spread of Zika Virus in The Americas—Implications for Public Health Preparedness for Mass Gatherings at the 2016 Brazil Olympic Games. Int. J. Infect. Dis. 2016, 44, 11–15. [Google Scholar] [CrossRef] [Green Version]
- PAHO. WHO Zika Cumulative Cases. Available online: https://www.paho.org/hq/index.php?option=com_content&view=article&id=12390:zika-cumulative-cases&Itemid=42090&lang=en (accessed on 1 December 2021).
- Hughes, R.A.; Cornblath, D.R. Guillain-Barré syndrome. Lancet 2005, 366, 1653–1666. [Google Scholar] [CrossRef]
- Moore, C.A.; Staples, J.E.; Dobyns, W.B.; Pessoa, A.; Ventura, C.V.; Da Fonseca, E.B.; Ribeiro, E.M.; Ventura, L.O.; Neto, N.N.; Arena, J.F.; et al. Characterizing the pattern of anomalies in congenital zika syndrome for pediatric clinicians. JAMA Pediatr. 2017, 171, 288–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honein, M.A.; Dawson, A.L.; Petersen, E.E.; Jones, A.M.; Lee, E.H.; Yazdy, M.M.; Ahmad, N.; Macdonald, J.; Evert, N.; Bingham, A.; et al. Birth defects among fetuses and infants of US women with evidence of possible zika virus infection during pregnancy. J. Am. Med. Assoc. 2017, 317, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.C. Development of infants with congenital zika syndrome: What do we know and what can we expect? Pediatrics 2018, 141, S154–S160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adachi, K.; Nielsen-Saines, K. Zika clinical updates: Implications for pediatrics. Curr. Opin. Pediatr. 2018, 30, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Parra, B.; Lizarazo, J.; Jiménez-Arango, J.A.; Zea-Vera, A.F.; González-Manrique, G.; Vargas, J.; Angarita, J.A.; Zuñiga, G.; Lopez-Gonzalez, R.; Beltran, C.L.; et al. Guillain–Barré Syndrome Associated with Zika Virus Infection in Colombia. N. Engl. J. Med. 2016, 375, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Zmonarski, S.Z.; Stojanowski, J.; Zmonarska, J. Polymers with antiviral properties: A brief review. Polym. Med. 2021, 50, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Bianculli, R.H.; Mase, J.D.; Schulz, M.D. Antiviral Polymers: Past Approaches and Future Possibilities. Macromol. 2020, 53, 9158–9186. [Google Scholar] [CrossRef]
- Li, J.; Yu, F.; Chen, Y.; Oupický, D. Polymeric drugs: Advances in the development of pharmacologically active polymers. J. Control. Release 2015, 219, 369–382. [Google Scholar] [CrossRef] [Green Version]
- Pinho, A.C.; Piedade, A.P. Polymeric Coatings with Antimicrobial Activity: A Short Review. Polymers 2020, 12, 2469. [Google Scholar] [CrossRef]
- Milewska, A.; Ciejka, J.; Kaminski, K.; Karewicz, A.; Bielska, D.; Zeglen, S.; Karolak, W.; Nowakowska, M.; Potempa, J.; Bosch, B.J.; et al. Novel polymeric inhibitors of HCoV-NL63. Antivir. Res. 2013, 97, 112–121. [Google Scholar] [CrossRef] [Green Version]
- Milewska, A.; Chi, Y.; Szczepanski, A.; Barreto-Duran, E.; Dabrowska, A.; Botwina, P.; Obloza, M.; Liu, K.; Liu, D.; Guo, X.; et al. HTCC as a Polymeric Inhibitor of SARS-CoV-2 and MERS-CoV. J. Virol. 2021, 95, e01622-20. [Google Scholar] [CrossRef] [PubMed]
- Botwina, P.; Obłoza, M.; Szczepański, A.; Szczubiałka, K.; Nowakowska, M.; Pyrć, K. In Vitro Inhibition of Zika Virus Replication with Poly(Sodium 4-Styrenesulfonate). Viruses 2020, 12, 926. [Google Scholar] [CrossRef] [PubMed]
- Barron, B.A.; Edwards, K.; Massare, S.J.; McGovern, M.S.; Williams, D.; Capps, S.J.; Dragon, D.M.; Fitzmorris, C.T.; Graul, E.E.; Insler, M.S.; et al. Predictors of recurrent herpes simplex virus keratitis. Cornea 2001, 20, 123–128. [Google Scholar]
- Mizusaki, M.; Shimada, Y.; Morishima, Y.; Yusa, S.-I. pH-Responsive Intra- and Inter-Molecularly Micelle Formation of Anionic Diblock Copolymer in Water. Polymers 2016, 8, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos-Soriano, J.; Reina, J.J.; Illescas, B.M.; de la Cruz, N.; Rodríguez-Pérez, L.; Lasala, F.; Rojo, J.; Delgado, R.; Martín, N. Synthesis of Highly Efficient Multivalent Disaccharide/[60]Fullerene Nanoballs for Emergent Viruses. J. Am. Chem. Soc. 2019, 141, 15403–15412. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Soriano, J.; Rojo, J. Glycodendritic structures as DC-SIGN binders to inhibit viral infections. Chem. Commun. 2021, 57, 5111–5126. [Google Scholar] [CrossRef] [PubMed]
- Yusa, S.-I.; Sakakibara, A.; Yamamoto, T.; Morishima, Y. Reversible pH-Induced Formation and Disruption of Unimolecular Micelles of an Amphiphilic Polyelectrolyte. Macromolecules 2002, 35, 5243–5249. [Google Scholar] [CrossRef]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef] [Green Version]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Ciejka, J.; Botwina, P.; Nowakowska, M.; Szczubiałka, K.; Pyrc, K. Synthetic sulfonated derivatives of poly(allylamine hydrochloride) as inhibitors of human metapneumovirus. PLoS ONE 2019, 14, e0214646. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamel, R.; Dejarnac, O.; Wichit, S.; Ekchariyawat, P.; Neyret, A.; Luplertlop, N.; Perera-Lecoin, M.; Surasombatpattana, P.; Talignani, L.; Thomas, F.; et al. Biology of Zika Virus Infection in Human Skin Cells. J. Virol. 2015, 89, 8880–8896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabata, T.; Petitt, M.; Puerta-Guardo, H.; Michlmayr, D.; Wang, C.; Fang-Hoover, J.; Harris, E.; Pereira, L. Zika Virus Targets Different Primary Human Placental Cells, Suggesting Two Routes for Vertical Transmission. Cell Host Microbe 2016, 20, 155–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meertens, L.; Labeau, A.; Dejarnac, O.; Cipriani, S.; Sinigaglia, L.; Bonnet-Madin, L.; Le Charpentier, T.; Hafirassou, M.L.; Zamborlini, A.; Cao-Lormeau, V.-M.; et al. Axl Mediates ZIKA Virus Entry in Human Glial Cells and Modulates Innate Immune Responses. Cell Rep. 2017, 18, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Zhang, Y.; Chen, J.; Sirohi, D.; Miller, A.; Chen, Z.; Lu, H.; Xu, J.; Kuhn, R.J.; Tao, W.A. Chemical proteomics tracks virus entry and uncovers NCAM1 as Zika virus receptor. Nat. Commun. 2020, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Zhao, J.; Liu, X.; Fraser, K.; Lin, L.; Zhang, X.; Zhang, F.; Dordick, J.S.; Linhardt, R.J. Interaction of Zika Virus Envelope Protein with Glycosaminoglycans. Biochemistry 2017, 56, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Albulescu, I.C.; Kovacikova, K.; Tas, A.; Snijder, E.J.; van Hemert, M.J. Suramin inhibits Zika virus replication by interfering with virus attachment and release of infectious particles. Antivir. Res. 2017, 143, 230–236. [Google Scholar] [CrossRef] [Green Version]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Zhao, J.; Stenzel, M.H. Entry of nanoparticles into cells: The importance of nanoparticle properties. Polym. Chem. 2018, 9, 259–272. [Google Scholar] [CrossRef]
- Unciti-Broceta, J.D.; Cortes, V.C.; Altea-Manzano, P.; Pernagallo, S.; Díaz-Mochón, J.J.; Sánchez-Martín, R.M. Number of Nanoparticles per Cell through a Spectrophotometric Method—A key parameter to Assess Nanoparticle-based Cellular Assays. Sci. Rep. 2015, 5, srep10091. [Google Scholar] [CrossRef] [Green Version]
- Poon, W.; Zhang, Y.-N.; Ouyang, B.; Kingston, B.R.; Wu, J.L.Y.; Wilhelm, S.; Chan, W.C.W. Elimination Pathways of Nanoparticles. ACS Nano 2019, 13, 5785–5798. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, D.; Chan, W.C.W. Elucidating the Mechanism of Cellular Uptake and Removal of Protein-Coated Gold Nanoparticles of Different Sizes and Shapes. Nano Lett. 2007, 7, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
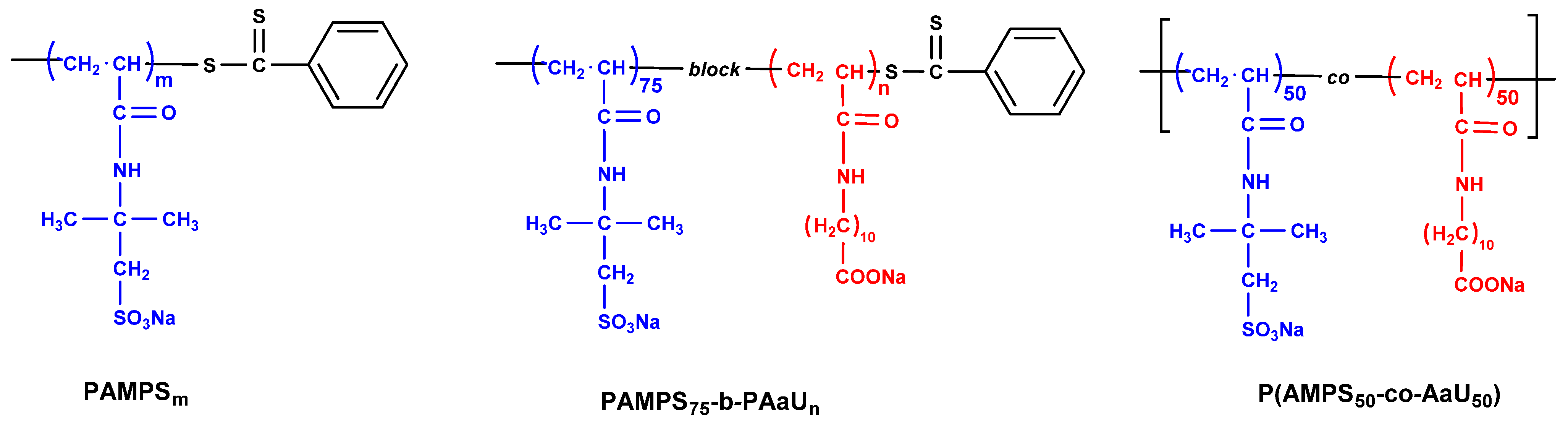




| Polymer | Mna (×104) | Ɖ a (×104) | Mw/Mna | D.P. (AMPS) b | D.P. (AaU) c |
|---|---|---|---|---|---|
| PAMPS40 | 0.94 | 1.21 | 1.28 | 40 | 0 |
| PAMPS75 | 1.72 | 2.18 | 1.26 | 75 | 0 |
| PAMPS170 | 3.93 | 4.65 | 1.18 | 170 | 0 |
| PAMPS75-b-PAaU3 | 1.85 | 2.61 | 1.41 | 75 | 3 |
| PAMPS75-b-PAaU12 | 3.20 | 4.53 | 1.42 | 75 | 12 |
| PAMPS75-b-PAaU28 | 5.05 | 6.72 | 1.33 | 75 | 28 |
| PAMPS75-b-PAaU39 | 7.63 | 9.36 | 1.23 | 75 | 39 |
| P(AMPS50-co-AaU50) | 4.38 d | 9.90 d | 2.26 d | 50 e | 50 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botwina, P.; Obłoza, M.; Zatorska-Płachta, M.; Kamiński, K.; Mizusaki, M.; Yusa, S.-I.; Szczubiałka, K.; Pyrc, K.; Nowakowska, M. Self-Organized Nanoparticles of Random and Block Copolymers of Sodium 2-(Acrylamido)-2-methyl-1-propanesulfonate and Sodium 11-(Acrylamido)undecanoate as Safe and Effective Zika Virus Inhibitors. Pharmaceutics 2022, 14, 309. https://doi.org/10.3390/pharmaceutics14020309
Botwina P, Obłoza M, Zatorska-Płachta M, Kamiński K, Mizusaki M, Yusa S-I, Szczubiałka K, Pyrc K, Nowakowska M. Self-Organized Nanoparticles of Random and Block Copolymers of Sodium 2-(Acrylamido)-2-methyl-1-propanesulfonate and Sodium 11-(Acrylamido)undecanoate as Safe and Effective Zika Virus Inhibitors. Pharmaceutics. 2022; 14(2):309. https://doi.org/10.3390/pharmaceutics14020309
Chicago/Turabian StyleBotwina, Pawel, Magdalena Obłoza, Maria Zatorska-Płachta, Kamil Kamiński, Masanobu Mizusaki, Shin-Ichi Yusa, Krzysztof Szczubiałka, Krzysztof Pyrc, and Maria Nowakowska. 2022. "Self-Organized Nanoparticles of Random and Block Copolymers of Sodium 2-(Acrylamido)-2-methyl-1-propanesulfonate and Sodium 11-(Acrylamido)undecanoate as Safe and Effective Zika Virus Inhibitors" Pharmaceutics 14, no. 2: 309. https://doi.org/10.3390/pharmaceutics14020309
APA StyleBotwina, P., Obłoza, M., Zatorska-Płachta, M., Kamiński, K., Mizusaki, M., Yusa, S.-I., Szczubiałka, K., Pyrc, K., & Nowakowska, M. (2022). Self-Organized Nanoparticles of Random and Block Copolymers of Sodium 2-(Acrylamido)-2-methyl-1-propanesulfonate and Sodium 11-(Acrylamido)undecanoate as Safe and Effective Zika Virus Inhibitors. Pharmaceutics, 14(2), 309. https://doi.org/10.3390/pharmaceutics14020309








