Smartcrystals for Efficient Dissolution of Poorly Water-Soluble Meloxicam
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Production of the Nanocrystal (Smartcrystal)
2.2.2. Production of Dry Nanocrystal
2.2.3. Particle Size and Zeta Potential Measurement of the Nanosuspensions
2.2.4. Analysis of Dry Nanocrystals
Scanning Electron Microscopy (SEM)
X-Ray Powder Diffraction (XRPD)
Differential Scanning Calorimetry (DSC)
Saturation Solubility
In Vitro Studies
3. Results
3.1. Particle Size of the Prepared Smartcrystals
3.2. Zeta Potential of the Prepared Nanocrystals
3.3. Particle Size of the Dry Nanocrystals
3.4. Morphology
3.5. Structural Analyses (DSC and XRPD)
3.6. Solubility Studies
3.7. Dissolution Behaviors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lipinski, C. Poor aqueous solubility—An industry wide problem in drug discovery. Am. Pharm. Rev. 2002, 5, 82–85. [Google Scholar]
- Kawabataa, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications—ScienceDirect. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef]
- Zhang, X.; Xing, H.; Zhao, Y.; Ma, Z. Pharmaceutical dispersion techniques for dissolution and bioavailability enhancement of poorly water-soluble drugs. Pharmaceutics 2018, 10, 74. [Google Scholar] [CrossRef]
- Sareen, S.; Mathew, G.; Joseph, L. Improvement in solubility of poor water-soluble drugs by solid dispersion. Int. J. Pharm. Investig. 2012, 2, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, e195727. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Vazifehasl, Z.; Salatin, S.; Adibkia, K.; Javadzadeh, Y. Nanosizing of drugs: Effect on dissolution rate. Res. Pharm. Sci. 2015, 10, 95–108. [Google Scholar] [PubMed]
- Junghanns, J.-U.A.H.; Müller, R.H. Nanocrystal technology, drug delivery and clinical applications. Int. J. Nanomed. 2008, 3, 295–310. [Google Scholar]
- Kipp, J.; Wong, J.; Doty, M.; Rebbeck, C. Microprecipitation Method for Preparing Submicron Suspensions. U.S. Patent US-7037528-B2, 2 May 2006. [Google Scholar]
- Müller, R.H.; Möschwitzer, J.P. Method and apparatus for the production of ultrafine particles and coating of such particles. U.S. Patent US-9168498-B2, 27 October 2015. [Google Scholar]
- Rolf, P. Nanocrystals for Use in Topical Cosmetic Formulations and Method of Production Thereof. U.S. Patent US-9114077-B2, 25 August 2015. [Google Scholar]
- Al Shaal, L.; Müller, R.H.; Shegokar, R. SmartCrystal combination technology—Scale up from lab to pilot scale and long term stability. Int. J. Pharm. Sci. 2010, 65, 877–884. [Google Scholar]
- Lee, J. Drug nano- and microparticles processed into solid dosage forms: Physical properties. J. Pharm. Sci. 2003, 92, 2057–2068. [Google Scholar] [CrossRef]
- Na, G.C.; Stevens, H.J.; Yuan, B.O.; Rajagopalan, N. Physical stability of ethyl diatrizoate nanocrystalline suspension in steam sterilization. Pharm. Res. 1999, 16, 569–574. [Google Scholar] [CrossRef]
- Möschwitzer, J.; Müller, R.H. New method for the effective production of ultrafine drug nanocrystals. J. Nanosci. Nanotechnol. 2006, 6, 3145–3153. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Xu, S.; Li, S. Understanding a relaxation behavior in a nanoparticle suspension for drug delivery applications. Int. J. Pharm. 2008, 351, 236–243. [Google Scholar] [CrossRef]
- Keck, C.; Kobierski, S.; Mauludin, R.; Müller, R.H. Second generation of drug nanocrystals for delivery of poorly soluble drugs: SmartCrystal technology. Dosis 2008, 24, 124–128. [Google Scholar]
- Pyo, S.M.; Meinke, M.; Keck, C.M.; Müller, R.H. Rutin-increased antioxidant activity and skin penetration by nanocrystal technology (SmartCrystals). Cosmetics 2016, 3, 9. [Google Scholar] [CrossRef]
- Al Shaal, L.; Shegokar, R.; Müller, R.H. Production and characterization of antioxidant apigenin nanocrystals as a novel UV skin protective formulation. Int. J. Pharm. Sci. 2011, 420, 133–140. [Google Scholar] [CrossRef]
- Mishra, P.R.; Al Shaal, L.; Müller, R.H.; Keck, C.M. Production and characterization of hesperetin nanosuspensions for dermal delivery. Int. J. Pharm. Sci. 2009, 371, 182–189. [Google Scholar] [CrossRef]
- Mitri, K.; Shegokar, R.; Gohla, S.; Anselmi, C.; Müller, R.H. Lutein nanocrystals as antioxidant formulation for oral and dermal delivery. Int. J. Pharm. Sci. 2011, 420, 141–146. [Google Scholar] [CrossRef]
- Kalvakuntla, S.; Deshpande, M.; Attari, Z.; Kunnatur, B.K. Preparation and characterization of nanosuspension of aprepitant by H96 process. Adv. Pharm. Bull. 2016, 6, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.; Müller, R.H.; Möschwitzer, J.P. Application of the combinative particle size reduction technology H 42 to produce fast dissolving glibenclamide tablets. Eur. J. Pharm. Sci. 2013, 49, 565–577. [Google Scholar] [CrossRef]
- Gülsün, T.; Gürsoy, R.N.; Öner, L. Nanocrystal technology for oral delivery of poorly water-soluble drugs. FABAD J. Pharm. Sci 2009, 34, 55–65. [Google Scholar]
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316. [Google Scholar] [CrossRef]
- Alshweiat, A.; Katona, G.; Csóka, I.; Ambrus, R. Design and characterization of loratadine nanosuspension prepared by ultrasonic-assisted precipitation. Eur. J. Pharm. Sci. 2018, 122, 94–104. [Google Scholar] [CrossRef]
- Abdelwahed, W.; Degobert, G.; Fessi, H. Freeze-drying of nanocapsules: Impact of annealing on the drying process. Int. J. Pharm. 2006, 324, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Sverdlov Arzi, R.; Sosnik, A. Electrohydrodynamic atomization and spray-drying for the production of pure drug nanocrystals and co-crystals. Adv. Drug Deliv. Rev. 2018, 131, 79–100. [Google Scholar] [CrossRef] [PubMed]
- Weyna, D.R.; Cheney, M.L.; Shan, N.; Hanna, M.; Zaworotko, M.J.; Sava, V.; Song, S.; Sanchez-Ramos, J.R. Improving solubility and pharmacokinetics of meloxicam via multiple-component crystal formation. Mol. Pharm. 2012, 9, 2094–2102. [Google Scholar] [CrossRef] [PubMed]
- Luger, P.; Daneck, K.; Engel, W.; Trummlitz, G.; Wagner, K. Structure and physicochemical properties of meloxicam, a new NSAID. Eur. J. Pharm. Sci. 1996, 4, 175–187. [Google Scholar] [CrossRef]
- Ochi, M.; Kawachi, T.; Toita, E.; Hashimoto, I.; Yuminoki, K.; Onoue, S.; Hashimoto, N. Development of nanocrystal formulation of meloxicam with improved dissolution and pharmacokinetic behaviors. Int. J. Pharm. 2014, 474, 151–156. [Google Scholar] [CrossRef]
- Lee, J.; Choi, J.-Y.; Park, C.H. Characteristics of polymers enabling nano-comminution of water-insoluble drugs. Int. J. Pharm. 2008, 355, 328–336. [Google Scholar] [CrossRef]
- Lindfors, L.; Skantze, P.; Skantze, U.; Rasmusson, M.; Zackrisson, A.; Olsson, U. Amorphous drug nanosuspensions. 1. Inhibition of ostwald ripening. Langmuir 2006, 22, 906–910. [Google Scholar] [CrossRef]
- Kürti, L.; Kukovecz, Á.; Kozma, G.; Ambrus, R.; Deli, M.A.; Szabó-Révész, P. Study of the Parameters Influencing the Co-Grinding Process for the Production of Meloxicam Nanoparticles. Powder Technol. 2011, 212, 210–217. [Google Scholar] [CrossRef]
- Bartos, C.; Jójárt-Laczkovich, O.; Katona, G.; Budai-Szűcs, M.; Ambrus, R.; Bocsik, A.; Gróf, I.; Deli, M.A.; Szabó-Révész, P. Optimization of a combined wet milling process in order to produce poly(vinyl alcohol) stabilized nanosuspension. Drug Des. Devel. Ther 2018, 12, 1567–1580. [Google Scholar] [CrossRef] [PubMed]
- Bartos, C.; Ambrus, R.; Kovács, A.; Gáspár, R.; Sztojkov-Ivanov, A.; Márki, Á.; Janáky, T.; Tömösi, F.; Kecskeméti, G.; Szabó-Révész, P. Investigation of absorption routes of meloxicam and its salt form from intranasal delivery systems. Molecules 2018, 23, 784. [Google Scholar] [CrossRef] [PubMed]
- Bartos, C.; Ambrus, R.; Sipos, P.; Budai-Szűcs, M.; Csányi, E.; Gáspár, R.; Márki, Á.; Adrienn, B.S.; Sztojkov-Ivanov, A.; Horváth, T.; et al. Study of sodium hyaluronate-based intranasal formulations containing micro- or nanosized meloxicam particles. Int. J. Pharm. 2015, 491, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.; Müller, R.H. Production and characterization of a budesonide nanosuspension for pulmonary administration. Pharm. Res. 2002, 19, 189–194. [Google Scholar] [CrossRef]
- Bozdag, S.; Dillen, K.; Vandervoort, J.; Ludwig, A. The effect of freeze-drying with different cryoprotectants and gamma-irradiation sterilization on the characteristics of ciprofloxacin HCl-loaded poly(D,L-lactide-glycolide) nanoparticles. J. Pharm. Pharmacol. 2010, 57, 699–707. [Google Scholar] [CrossRef]
- Kumar, K.N.; Mallik, S.; Sarkar, K. Role of freeze-drying in the presence of mannitol on the echogenicity of echogenic liposomes. J. Acoust. Soc. Am. 2017, 142, 3670–3676. [Google Scholar] [CrossRef]
- Iurian, S.; Tomuta, I.; Bogdan, C.; Rus, L.; Tokes, T.; Barbu-Tudoran, L.; Achim, M.; Moldovan, M.; Leucuta, S. Defining the design space for freeze-dried orodispersible tablets with meloxicam. Drug Dev. Ind. Pharm. 2016, 42, 1977–1989. [Google Scholar] [CrossRef]
- Nassab, P.R.; Rajkó, R.; Szabó-Révész, P. Physicochemical characterization of meloxicam-mannitol binary systems. J. Pharm. Biomed. Anal. 2006, 41, 1191–1197. [Google Scholar] [CrossRef]
- Verhoeven, N.; Neoh, T.L.; Furuta, T.; Yamamoto, C.; Ohashi, T.; Yoshii, H. Characteristics of dehydration kinetics of dihydrate trehalose to its anhydrous form in ethanol by DSC. Food Chem. 2012, 132, 1638–1643. [Google Scholar] [CrossRef]
- Jacon Freitas, J.T.; Santos Viana, O.M.M.; Bonfilio, R.; Doriguetto, A.C.; de Araújo, M.B. Analysis of polymorphic contamination in meloxicam raw materials and its effects on the physicochemical quality of drug product. Eur. J. Pharm. Sci. 2017, 109, 347–358. [Google Scholar] [CrossRef]
- Kaialy, W.; Khan, U.; Mawlud, S. Influence of mannitol concentration on the physicochemical, mechanical and pharmaceutical properties of lyophilised mannitol. Int. J. Pharm. 2016, 510, 73–85. [Google Scholar] [CrossRef]
- Torrado, S.; Torrado, S. Characterization of physical state of mannitol after freeze-drying: Effect of acetylsalicylic acid as a second crystalline cosolute. Chem. Pharm. Bull. 2002, 50, 567–570. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sundaramurthi, P.; Suryanarayanan, R. Trehalose crystallization during freeze-drying: Implications on lyoprotection. J. Phys. Chem. Lett. 2010, 1, 510–514. [Google Scholar] [CrossRef]
- Colombo, M.; Orthmann, S.; Bellini, M.; Staufenbiel, S.; Bodmeier, R. Influence of drug brittleness, nanomilling time, and freeze-drying on the crystallinity of poorly water-soluble drugs and its implications for solubility enhancement. AAPS PharmSciTech 2017, 18, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.J.; Pygall, S.R.; Cooper, V.B.; Mann, J.C. Overcoming sink limitations in dissolution testing: A review of traditional methods and the potential utility of biphasic systems. J. Pharm. Pharm. 2012, 64, 1549–1559. [Google Scholar] [CrossRef]
- Noyes, A.; Whitney, W. The Rate of Solution of Solid Substances in Their Own Solutions. J. Am. Chem. Soc. 1897, 19, 930–934. [Google Scholar] [CrossRef]
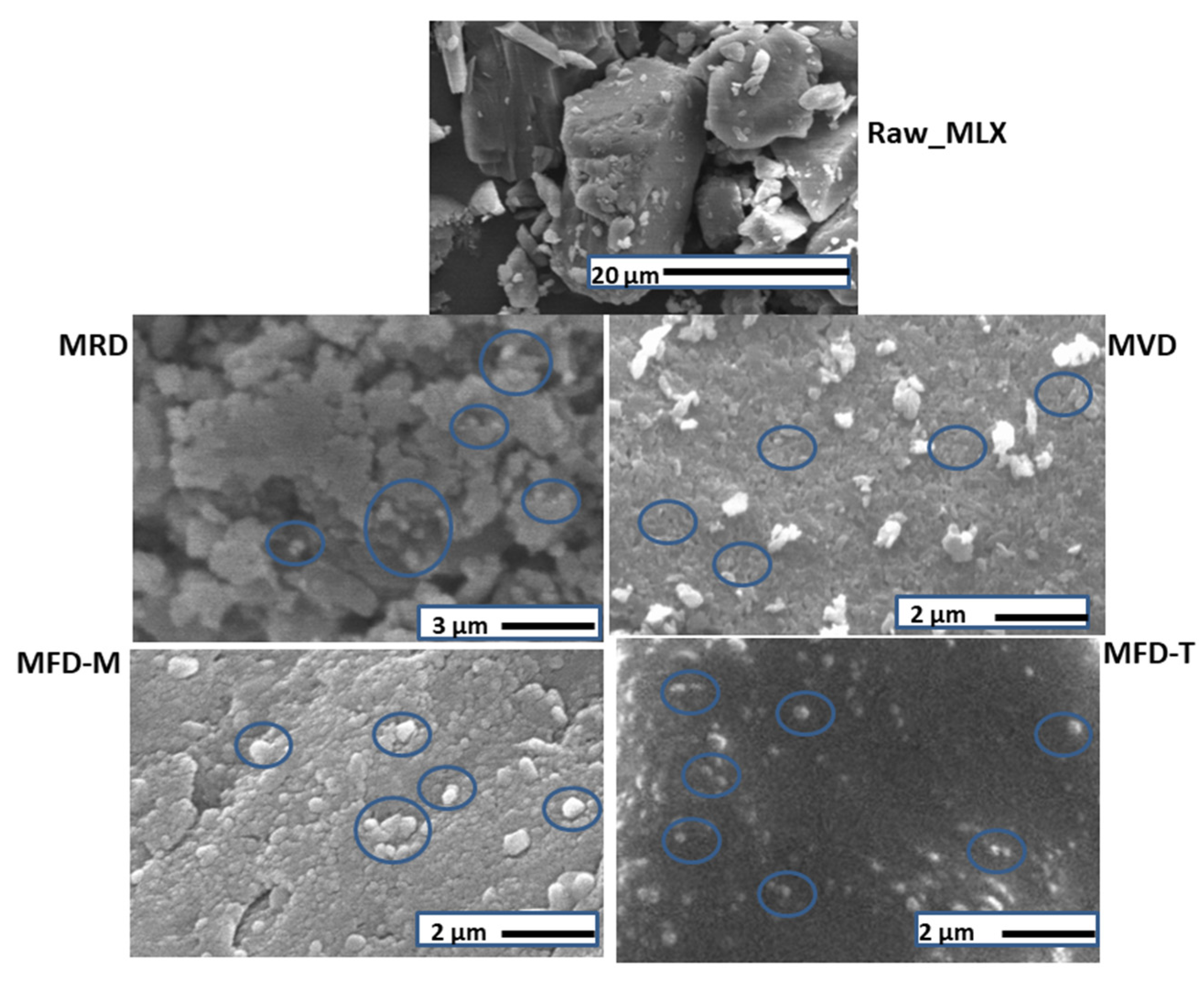
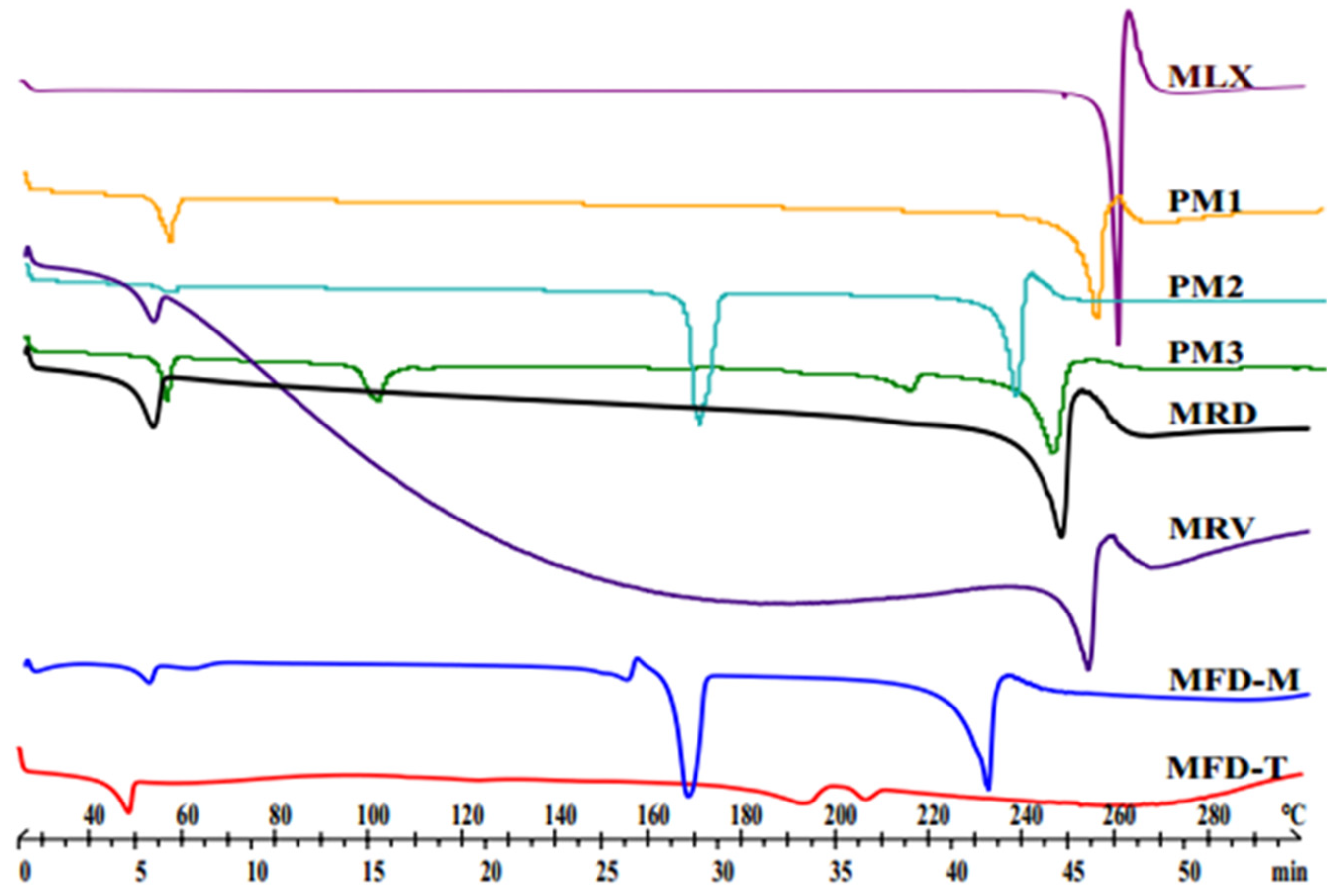
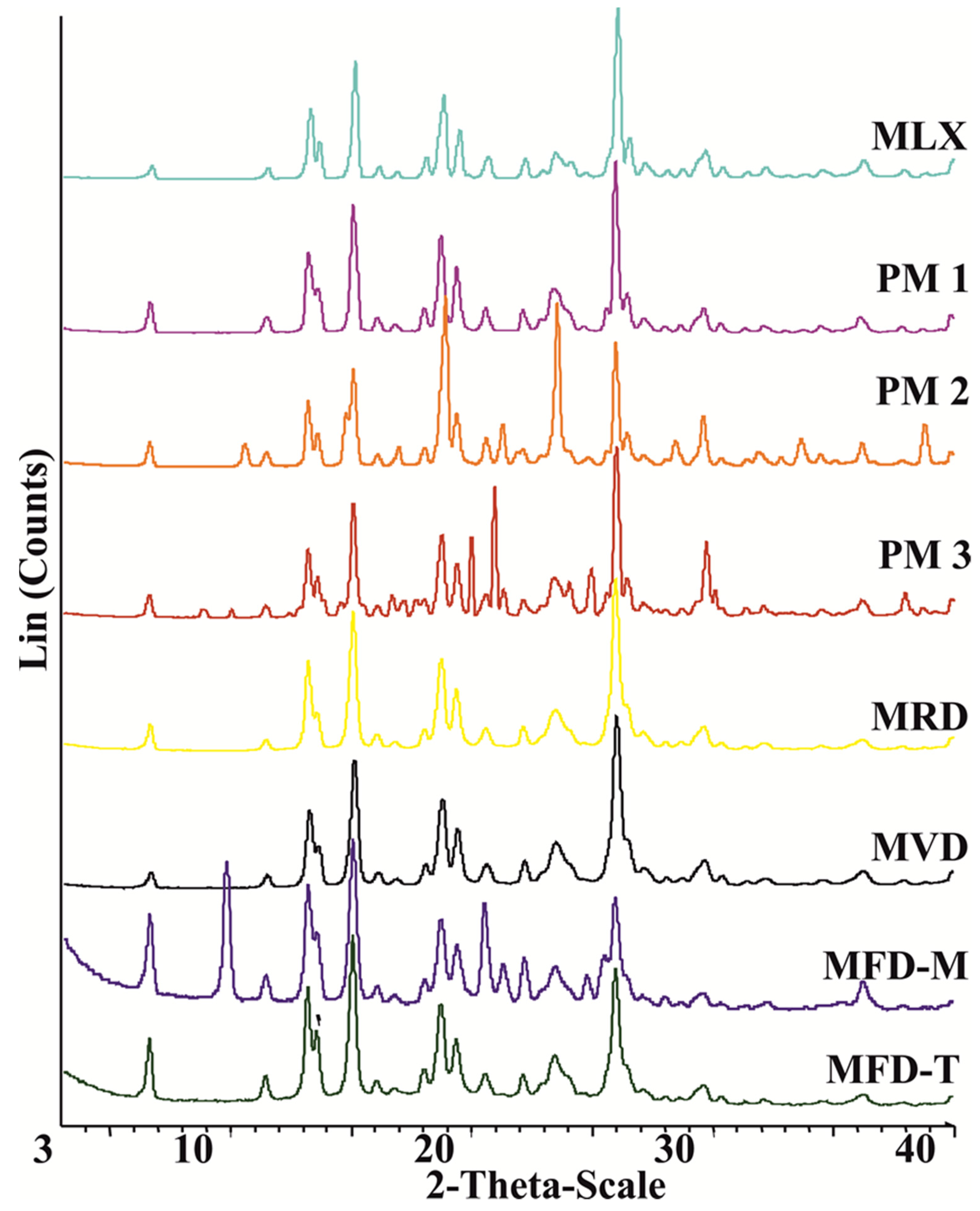
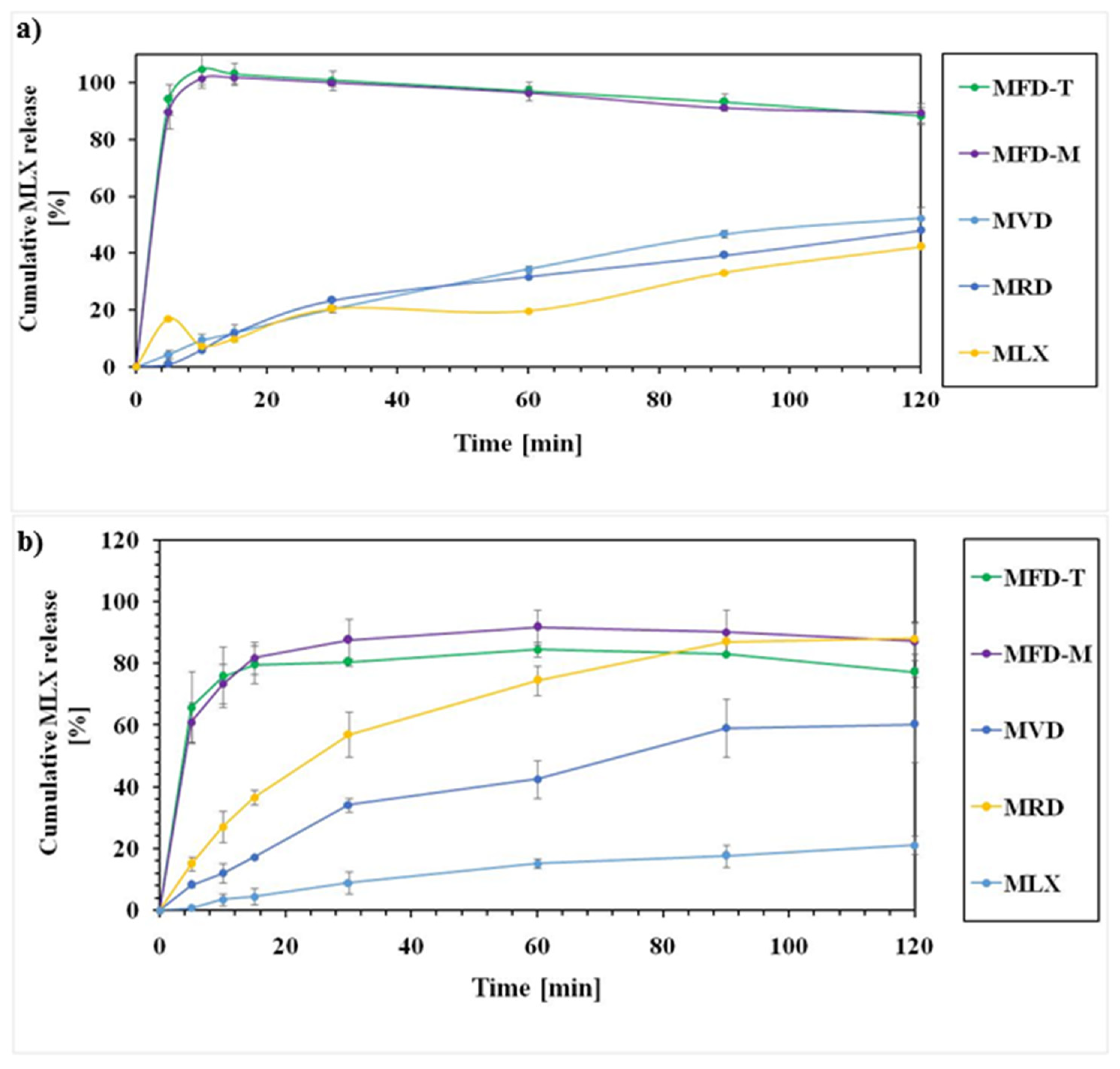
| Sample Notation | Description | Content | w:w Ratio |
|---|---|---|---|
| MRD | MLX nanosuspension dried at room temperature | MLX: F68 | 5:1 |
| MVD | MLX nanosuspension dried by vacuum oven | MLX: F68 | 5:1 |
| MFD-M | MLX nanosuspension dried by freeze-drying with 3%, w/v mannitol (M) as a cryoprotectant | MLX: F68: M | 5:1:3 |
| MFD-T | MLX nanosuspension dried by freeze-drying with 3%, w/v trehalose (T) as a cryoprotectant | MLX: F68: T | 5:1:3 |
| Sample | MPS (nm) | PDI | ZP (mV) |
|---|---|---|---|
| Raw_MLX | 36,540 | 0.77 ± 0.43 | −2.3 |
| MRD | 729.3 ± 25.12 | 0.53 ± 0.12 | −18.7 |
| MVD | 601.4 ± 15.23 | 0.49 ± 0.09 | −18.0 |
| MFD-M | 374.75 ± 12.56 | 0.25 ± 0.02 | −20.9 |
| MFD-T | 325.75 ± 2.47 | 0.26 ± 0.01 | −33.1 |
| Sample | % XCI |
|---|---|
| MRD | 70.69 |
| MVD | 66.63 |
| MFD-M | 77.80 |
| MFD-T | 59.24 |
| Sample | PBS | |
|---|---|---|
| pH, 5.6 | pH, 7.4 | |
| MLX | 22.08 ± 1.16 | 477.55 ± 92.60 |
| MRD | 45.09 ± 1.18 | 492.74 ± 1.46 |
| MVD | 48.04 ± 6.70 | 478.93 ± 3.46 |
| MFD-M | 37.88 ± 2.81 | 1166.48 ± 7.57 |
| MFD-T | 37.88 + 2.81 | 588.21 ± 23.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrus, R.; Alshweiat, A.; Szabó-Révész, P.; Bartos, C.; Csóka, I. Smartcrystals for Efficient Dissolution of Poorly Water-Soluble Meloxicam. Pharmaceutics 2022, 14, 245. https://doi.org/10.3390/pharmaceutics14020245
Ambrus R, Alshweiat A, Szabó-Révész P, Bartos C, Csóka I. Smartcrystals for Efficient Dissolution of Poorly Water-Soluble Meloxicam. Pharmaceutics. 2022; 14(2):245. https://doi.org/10.3390/pharmaceutics14020245
Chicago/Turabian StyleAmbrus, Rita, Areen Alshweiat, Piroska Szabó-Révész, Csilla Bartos, and Ildikó Csóka. 2022. "Smartcrystals for Efficient Dissolution of Poorly Water-Soluble Meloxicam" Pharmaceutics 14, no. 2: 245. https://doi.org/10.3390/pharmaceutics14020245
APA StyleAmbrus, R., Alshweiat, A., Szabó-Révész, P., Bartos, C., & Csóka, I. (2022). Smartcrystals for Efficient Dissolution of Poorly Water-Soluble Meloxicam. Pharmaceutics, 14(2), 245. https://doi.org/10.3390/pharmaceutics14020245








