A ‘Relay’-Type Drug-Eluting Nerve Guide Conduit: Computational Fluid Dynamics Modeling of the Drug Eluting Efficiency of Various Drug Release Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. 2D Multichannel NGC Model
2.2. Drug Release Systems
2.3. CFD Modeling
2.4. GDNF Volume Fraction
3. Results
3.1. A ‘Relay’-Type NGC Design
3.2. GDNF Volume Fraction under the Assumption of Constant Simulation Time
3.3. GDNF Volume Fraction under the Assumption of Constant Growth Factor Mass
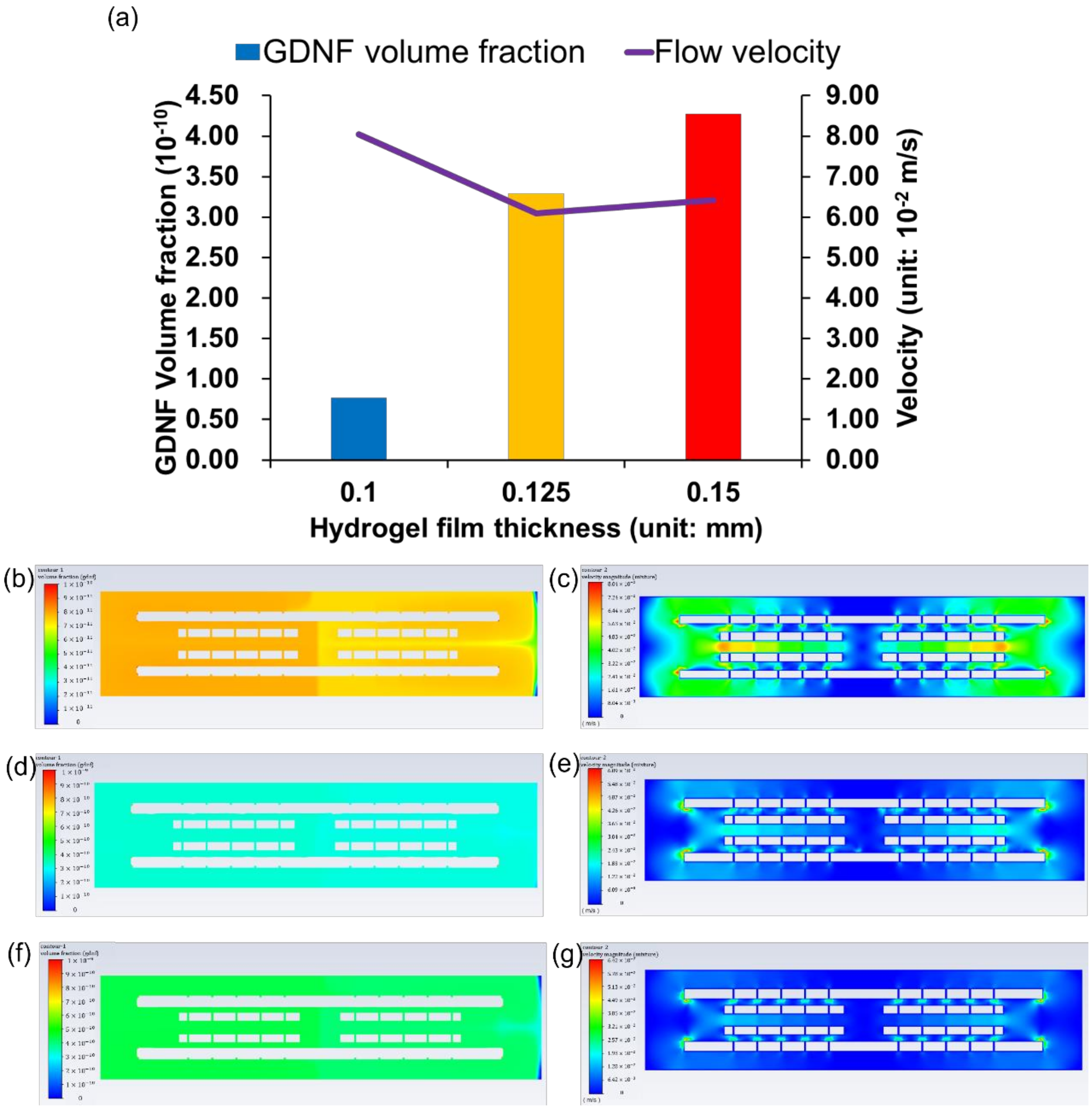
3.4. GDNF Volume Fraction among Different Hydrogel Films
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cinteza, D.; Persinaru, I.; Maciuceanu Zarnescu, B.M.; Ionescu, D.; Lascar, I. Peripheral Nerve Regeneration—An Appraisal of the Current Treatment Options. Maedica 2015, 10, 65–68. [Google Scholar] [PubMed]
- Sedaghati, T.; Jell, G.; Seifalian, A.M. Chapter 57—Nerve Regeneration and Bioengineering. In Regenerative Medicine Applications in Organ Transplantation; Orlando, G., Lerut, J., Soker, S., Stratta, R.J., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 799–810. [Google Scholar] [CrossRef]
- Dietzmeyer, N.; Förthmann, M.; Grothe, C.; Haastert-Talini, K. Modification of tubular chitosan-based peripheral nerve implants: Applications for simple or more complex approaches. Neural Regen. Res. 2020, 15, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Hood, B.; Levene, H.B.; Levi, A.D. Transplantation of autologous Schwann cells for the repair of segmental peripheral nerve defects. Neurosurg. Focus 2009, 26, E4. [Google Scholar] [CrossRef] [PubMed]
- Cinal, H.; Barin, E.Z.; Kara, M.; Karaduman, H.; Cengiz, I.Z.; Tan, O.; Demirci, E. A new method to harvest the sural nerve graft. Eurasian J. Med. 2020, 52, 12–15. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Zangene, E.; Manouchehri, S.; Amirabad, L.M.; Baheiraei, N.; Hadjighasem, M.R.; Farokhi, M.; Ganjali, M.R.; Walker, B.W.; Saeb, M.R.; et al. Conductive biomaterials as nerve conduits: Recent advances and future challenges. Appl. Mater. Today 2020, 20, 100784. [Google Scholar] [CrossRef]
- Gupta, S.; Mohapatra, D.; Chittoria, R.; Subbarayan, E.; Reddy, S.; Chavan, V.; Aggarwal, A.; Reddy, L. Human skin allograft: Is it a viable option in management of burn patients? J. Cutan. Aesthet. Surg. 2019, 12, 132–135. [Google Scholar] [CrossRef]
- Zhang, S.; Vijayavenkataraman, S.; Chong, G.L.; Fuh, J.Y.H.; Lu, W.F. Computational Design and Optimization of Nerve Guidance Conduits for Improved Mechanical Properties and Permeability. J. Biomech. Eng. 2019, 141, 051007. [Google Scholar] [CrossRef]
- Spencer, A.R.; Shirzaei Sani, E.; Soucy, J.R.; Corbet, C.C.; Primbetova, A.; Koppes, R.A.; Annabi, N. Bioprinting of a Cell-Laden Conductive Hydrogel Composite. ACS Appl. Mater. Interfaces 2019, 11, 30518–30533. [Google Scholar] [CrossRef]
- Fadia, N.B.; Bliley, J.M.; DiBernardo, G.A.; Crammond, D.J.; Schilling, B.K.; Sivak, W.N.; Spiess, A.M.; Washington, K.M.; Waldner, M.; Liao, H.T.; et al. Long-gap peripheral nerve repair through sustained release of a neurotrophic factor in nonhuman primates. Sci. Transl. Med. 2020, 12, 527. [Google Scholar] [CrossRef]
- Xu, P.; Rosen, K.M.; Hedstrom, K.; Rey, O.; Guha, S.; Hart, C.; Corfas, G. Nerve injury induces glial cell line-derived neurotrophic factor (GDNF) expression in schwann cells through purinergic signaling and the PKC-PKD pathway. GLIA 2013, 61, 1029–1040. [Google Scholar] [CrossRef]
- Höke, A.; Gordon, T.; Zochodne, D.W.; Sulaiman, O.A.R. A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Exp. Neurol. 2002, 173, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Troullinaki, M.; Alexaki, V.-I.; Mitroulis, I.; Witt, A.; Klotzsche–von Ameln, A.; Chung, K.-J.; Chavakis, T.; Economopoulou, M. Nerve growth factor regulates endothelial cell survival and pathological retinal angiogenesis. J. Cell. Mol. Med. 2019, 23, 2362–2371. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020, 106, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Arslantunali, D.; Dursun, T.; Yucel, D.; Hasirci, N.; Hasirci, V. Peripheral nerve conduits: Technology update. Med. Devices Evid. Res. 2014, 7, 405–424. [Google Scholar] [CrossRef]
- Zhou, X.; Quann, E.; Gallicano, G.I. Differentiation of nonbeating embryonic stem cells into beating cardiomyocytes is dependent on downregulation of PKCβ and ζ in concert with upregulation of PKCε. Dev. Biol. 2003, 255, 407–422. [Google Scholar] [CrossRef]
- Kokai, L.E.; Bourbeau, D.; Weber, D.; McAtee, J.; Marra, K.G. Sustained growth factor delivery promotes axonal regeneration in long gap peripheral nerve repair. Tissue Eng. Part A 2011, 17, 1263–1275. [Google Scholar] [CrossRef]
- Silva, A.K.A.; Richard, C.; Bessodes, M.; Scherman, D.; Merten, O.W. Growth factor delivery approaches in hydrogels. Biomacromolecules 2009, 10, 9–18. [Google Scholar] [CrossRef]
- Kokai, L.E.; Ghaznavi, A.M.; Marra, K.G. Incorporation of double-walled microspheres into polymer nerve guides for the sustained delivery of glial cell line-derived neurotrophic factor. Biomaterials 2010, 31, 2313–2322. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Sheth, S.; Barnard, E.; Hyatt, B.; Rathinam, M.; Zustiak, S.P. Predicting Drug Release From Degradable Hydrogels Using Fluorescence Correlation Spectroscopy and Mathematical Modeling. Front. Bioeng. Biotechnol. 2019, 7, 410. [Google Scholar] [CrossRef]
- Carbinatto, F.M.; De Castro, A.D.; Evangelista, R.C.; Cury, B.S.F. Insights into the swelling process and drug release mechanisms from cross-linked pectin/high amylose starch matrices. Asian J. Pharm. Sci. 2014, 9, 27–34. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Yan, W.-C.; Lu, W.F.; Wang, C.-H.; Fuh, J.Y.H. 3D bioprinting of tissues and organs for regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 296–332. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Vijayavenkataraman, S. 3D-printable conductive materials for tissue engineering and biomedical applications. Bioprinting 2021, 24, e00166. [Google Scholar] [CrossRef]
- Soman, S.S.; Vijayavenkataraman, S. Perspectives on 3d bioprinting of peripheral nerve conduits. Int. J. Mol. Sci. 2020, 21, 5792. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]
- Murphy, W.L.; Peters, M.C.; Kohn, D.H.; Mooney, D.J. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials 2000, 21, 2521–2527. [Google Scholar] [CrossRef]
- Cho, H.J.; Madhurakkat Perikamana, S.K.; Lee, J.H.; Lee, J.; Lee, K.M.; Shin, C.S.; Shin, H. Effective immobilization of BMP-2 mediated by polydopamine coating on biodegradable nanofibers for enhanced in vivo bone formation. ACS Appl. Mater. Interfaces 2014, 6, 11225–11235. [Google Scholar] [CrossRef]
- Koffler, J.; Zhu, W.; Qu, X.; Platoshyn, O.; Dulin, J.N.; Brock, J.; Graham, L.; Lu, P.; Sakamoto, J.; Marsala, M.; et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 2019, 25, 263–269. [Google Scholar] [CrossRef]
- Yao, L.; Daly, W.; Newland, B.; Yao, S.; Wang, W.; Chen, B.K.K.; Madigan, N.; Windebank, A.; Pandit, A. Improved axonal regeneration of transected spinal cord mediated by multichannel collagen conduits functionalized with neurotrophin-3 gene. Gene Ther. 2013, 20, 1149–1157. [Google Scholar] [CrossRef]
- Pawelec, K.M.; Koffler, J.; Shahriari, D.; Galvan, A.; Tuszynski, M.H.; Sakamoto, J. Microstructure and in vivo characterization of multi-channel nerve guidance scaffolds. Biomed. Mater. 2018, 13, 044104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Fan, C.; Wang, J.; Xiong, H.; Zhu, T.; Liu, Y.; Pan, H.; Weijia Lu, W. Bioinspired multichannel nerve guidance conduit based on shape memory nanofibers for potential application in peripheral nerve repair. ACS Nano 2020, 14, 12579–12595. [Google Scholar] [CrossRef]
- Yao, L.; Billiar, K.L.; Windebank, A.J.; Pandit, A. Multichanneled collagen conduits for peripheral nerve regeneration: Design, fabrication, and characterization. Tissue Eng. Part C Methods 2010, 16, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, K.J.; Grinberg, Y.; Joseph, S.; Triolo, R.J. Human distal sciatic nerve fascicular anatomy: Implications for ankle control using nerve-cuff electrodes. J. Rehabil. Res. Dev. 2012, 49, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, L.; Bao, Y.; Yan, X.; Yin, Y.; Li, Y.; Wang, X.; Huang, Z.; Xu, P. Preparation and characterization of injectable chitosan-hyaluronic acid hydrogels for nerve growth factor sustained release. J. Bioact. Compat. Polym. 2017, 32, 146–162. [Google Scholar] [CrossRef]
- Wang, P.; Berry, D.; Moran, A.; He, F.; Tam, T.; Chen, L.; Chen, S. Controlled Growth Factor Release in 3D-Printed Hydrogels. Adv. Healthc. Mater. 2020, 9, 1900977. [Google Scholar] [CrossRef]
- Trujillo, S.; Gonzalez-Garcia, C.; Rico, P.; Reid, A.; Windmill, J.; Dalby, M.J.; Salmeron-Sanchez, M. Engineered 3D hydrogels with full-length fibronectin that sequester and present growth factors. Biomaterials 2020, 252, 120104. [Google Scholar] [CrossRef]
- Kim, H.; Kong, W.H.; Seong, K.Y.; Sung, D.K.; Jeong, H.; Kim, J.K.; Yang, S.Y.; Hahn, S.K. Hyaluronate—Epidermal Growth Factor Conjugate for Skin Wound Healing and Regeneration. Biomacromolecules 2016, 17, 3694–3705. [Google Scholar] [CrossRef]
- Hong, J.P.; Kim, Y.W.; Lee, S.K.; Kim, S.H.; Min, K.H. The effect of continuous release of recombinant human epidermal growth factor (rh-EGF) in chitosan film on full thickness excisional porcine wounds. Ann. Plast. Surg. 2008, 61, 457–462. [Google Scholar] [CrossRef]
- Gil, E.S.; Panilaitis, B.; Bellas, E.; Kaplan, D.L. Functionalized Silk Biomaterials for Wound Healing. Adv. Healthc. Mater. 2013, 2, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ji, R.; Fu, Y.; Qi, H.; Kong, F.; Li, H.; Tangwarodomnukun, V. Investigation on particle motions and resultant impact erosion on quartz crystals by the micro-particle laden waterjet and airjet. Powder Technol. 2020, 360, 452–461. [Google Scholar] [CrossRef]
- Lackington, W.A.; Kočí, Z.; Alekseeva, T.; Hibbitts, A.J.; Kneafsey, S.L.; Chen, G.; O’Brien, F.J. Controlling the dose-dependent, synergistic and temporal effects of NGF and GDNF by encapsulation in PLGA microparticles for use in nerve guidance conduits for the repair of large peripheral nerve defects. J. Control. Release 2019, 304, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Shen, Z.; Ding, G. Simulation of interstitial fluid flow in ligaments: Comparison among Stokes, Brinkman and Darcy models. Int. J. Biol. Sci. 2013, 9, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Arora, J.; Hickey, J.M.; Majumdar, R.; Esfandiary, R.; Bishop, S.M.; Samra, H.S.; Middaugh, C.R.; Weis, D.D.; Volkin, D.B. Hydrogen exchange mass spectrometry reveals protein interfaces and distant dynamic coupling effects during the reversible self-association of an IgG1 monoclonal antibody. mAbs 2015, 7, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Li, Y.; Ding, G. Interstitial fluid flow: The mechanical environment of cells and foundation of meridians. Evid.-Based Complement. Altern. Med. 2012, 2012, 853516. [Google Scholar] [CrossRef]
- Fischer, H.; Polikarpov, I.; Craievich, A.F. Average protein density is a molecular-weight-dependent function. Protein Sci. 2004, 13, 2825–2828. [Google Scholar] [CrossRef]
- Rodríguez, F.J.; Verdú, E.; Ceballos, D.; Navarro, X. Nerve guides seeded with autologous Schwann cells improve nerve regeneration. Exp. Neurol. 2000, 161, 571–584. [Google Scholar] [CrossRef]
- Giordano, C.; Albani, D.; Gloria, A.; Tunesi, M.; Rodilossi, S.; Russo, T.; Forloni, G.; Ambrosio, L.; Cigada, A. Nanocomposites for Neurodegenerative Diseases: Hydrogel-Nanoparticle Combinations for a Challenging Drug Delivery. Int. J. Artif. Organs 2011, 34, 1115–1127. [Google Scholar] [CrossRef]






| Total Release Time (s) | Burst Release Mass (ng) | Burst Release Time (s) | Burst Release Velocity (ng/s) | Continuous Release Mass (ng) | Continuous Release Time (s) | Continuous Release Velocity (ng/s) | |
|---|---|---|---|---|---|---|---|
| Original release profile | 5,184,000 | 5 | 86,400 | 5.79 × 10−5 | 1.5 | 5,097,600 | 2.94 × 10−7 |
| Modified release profile | 519 | 0.0005 | 9 | 5.56 × 10−5 | 0.00015 | 510 | 2.94 × 10−7 |
| Drug Release Systems | Microsphere Diameter (mm) | Microsphere Quantity | Microsphere Placement | Surface Area (mm2) | Burst Release Time (s) | Continuous Release Time (s) | Total Simulation Time (s) |
|---|---|---|---|---|---|---|---|
| Single-layer microsphere | 0.1 | 8 | Front/middle/back | 0.251 | 9 | 510 | 519 |
| Single-layer microsphere | 0.1 | 16 | Front+middle/front+back/middle+back | 0.502 | 4.5 | 255 | 259.5 |
| Single-layer microsphere | 0.1 | 24 | Front+middle+back | 0.753 | 3 | 170 | 173 |
| Single-layer microsphere | 0.125 | 8 | Middle | 0.393 | 6 | 326.5 | 332.5 |
| Single-layer microsphere | 0.15 | 8 | Middle | 0.565 | 4 | 226.5 | 230.5 |
| Single-layer microsphere | 0.1 | 6 | Middle | 0.188 | 12 | 680 | 692 |
| Single-layer microsphere | 0.1 | 4 | Middle | 0.126 | 18 | 1020 | 1038 |
| Double-layer microsphere | 0.1 | 16 | Middle | 0.502 | 4.5 | 255 | 259.5 |
| Bulk hydrogel | 0.5 | 1 | Middle | 0.785 | 3 | 163 | 166 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Vijayavenkataraman, S. A ‘Relay’-Type Drug-Eluting Nerve Guide Conduit: Computational Fluid Dynamics Modeling of the Drug Eluting Efficiency of Various Drug Release Systems. Pharmaceutics 2022, 14, 230. https://doi.org/10.3390/pharmaceutics14020230
Zhou J, Vijayavenkataraman S. A ‘Relay’-Type Drug-Eluting Nerve Guide Conduit: Computational Fluid Dynamics Modeling of the Drug Eluting Efficiency of Various Drug Release Systems. Pharmaceutics. 2022; 14(2):230. https://doi.org/10.3390/pharmaceutics14020230
Chicago/Turabian StyleZhou, Jiarui, and Sanjairaj Vijayavenkataraman. 2022. "A ‘Relay’-Type Drug-Eluting Nerve Guide Conduit: Computational Fluid Dynamics Modeling of the Drug Eluting Efficiency of Various Drug Release Systems" Pharmaceutics 14, no. 2: 230. https://doi.org/10.3390/pharmaceutics14020230
APA StyleZhou, J., & Vijayavenkataraman, S. (2022). A ‘Relay’-Type Drug-Eluting Nerve Guide Conduit: Computational Fluid Dynamics Modeling of the Drug Eluting Efficiency of Various Drug Release Systems. Pharmaceutics, 14(2), 230. https://doi.org/10.3390/pharmaceutics14020230






