Using Copper-Doped Mesoporous Bioactive Glass Nanospheres to Impart Anti-Bacterial Properties to Dental Composites
Abstract
1. Introduction
- Binary filler blend consisting of Ba-glass microfillers as a base and 10 wt% of either Cu-MBGN, silica nanofillers, or 45S5 BG with a total filler amount of 65 wt%.
- Ternary filler blend consisting of Ba-glass microfillers as a base and a combination of 1, 5 or 10 wt% Cu-MBGN with silica filler up to 15 wt%, making a total filler amount of 70 wt%. As a bioactive control, 15 wt% 45S5 was added to the Ba-glass microfillers (15-BG) and as an inert control, 15 wt% silica fillers were added to the filler base of Ba-glass microfillers.
2. Materials and Methods
2.1. Sample Preparation
2.2. Light Curing
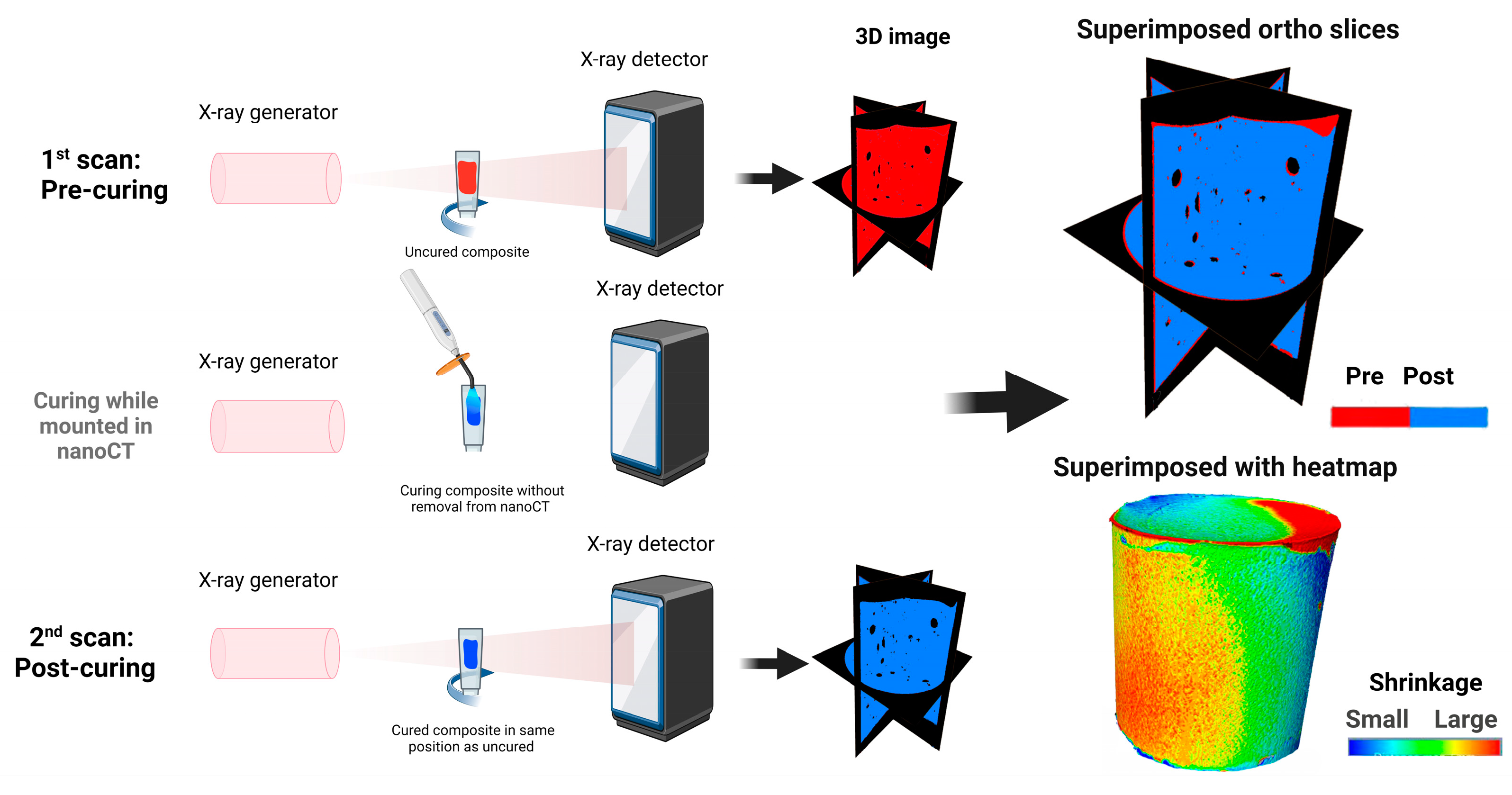
2.3. Micro-Computed Tomography (Micro-CT) Imaging and Polymerization Shrinkage Analysis
2.4. Degree of Conversion
2.5. Nano-CT Imaging and Porosity Analysis
2.6. Ion Release Profile
2.7. Surface Morphology and Particle Distribution of Fillers with SEM and EDX
2.8. Biofilm Formation and Anti-Bacterial Activity
2.9. Statistical Analysis
3. Results
3.1. Characterization with SEM-EDX
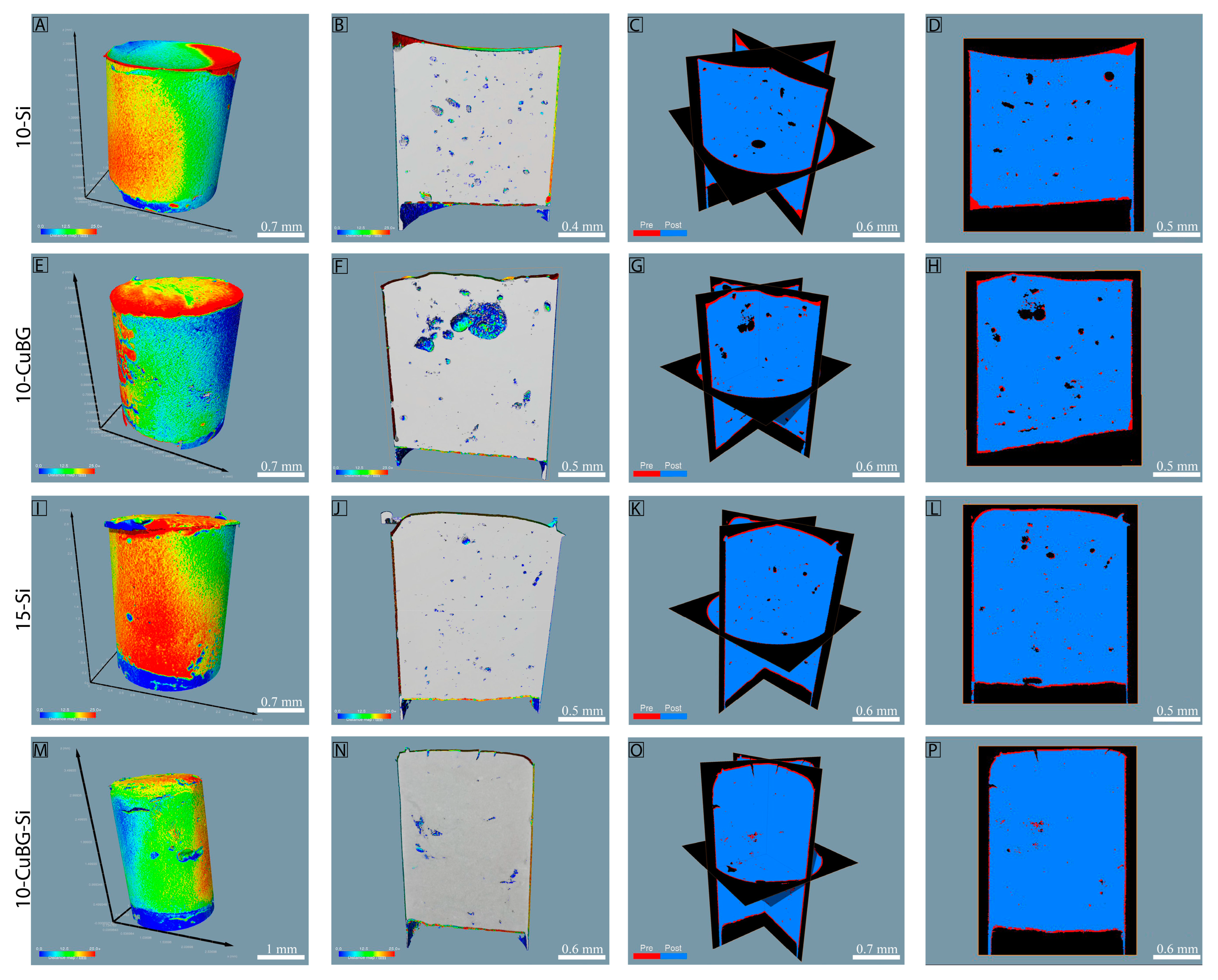
3.2. Polymerization Shrinkage using Micro-CT and Nano-CT
3.3. Porosity by Nano-CT
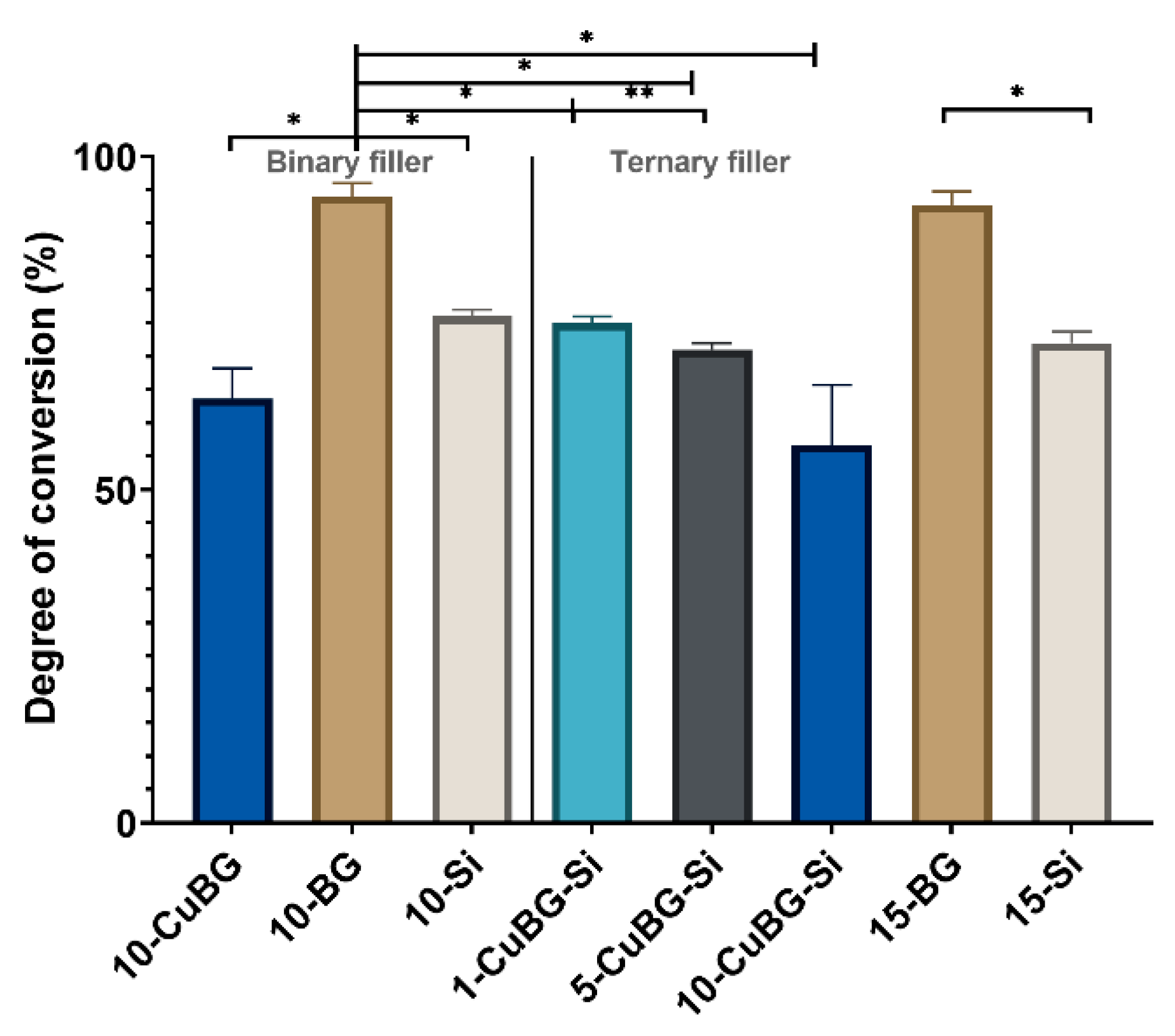
3.4. Degree of Conversion
3.5. Ion Release Profile with ICP-MS
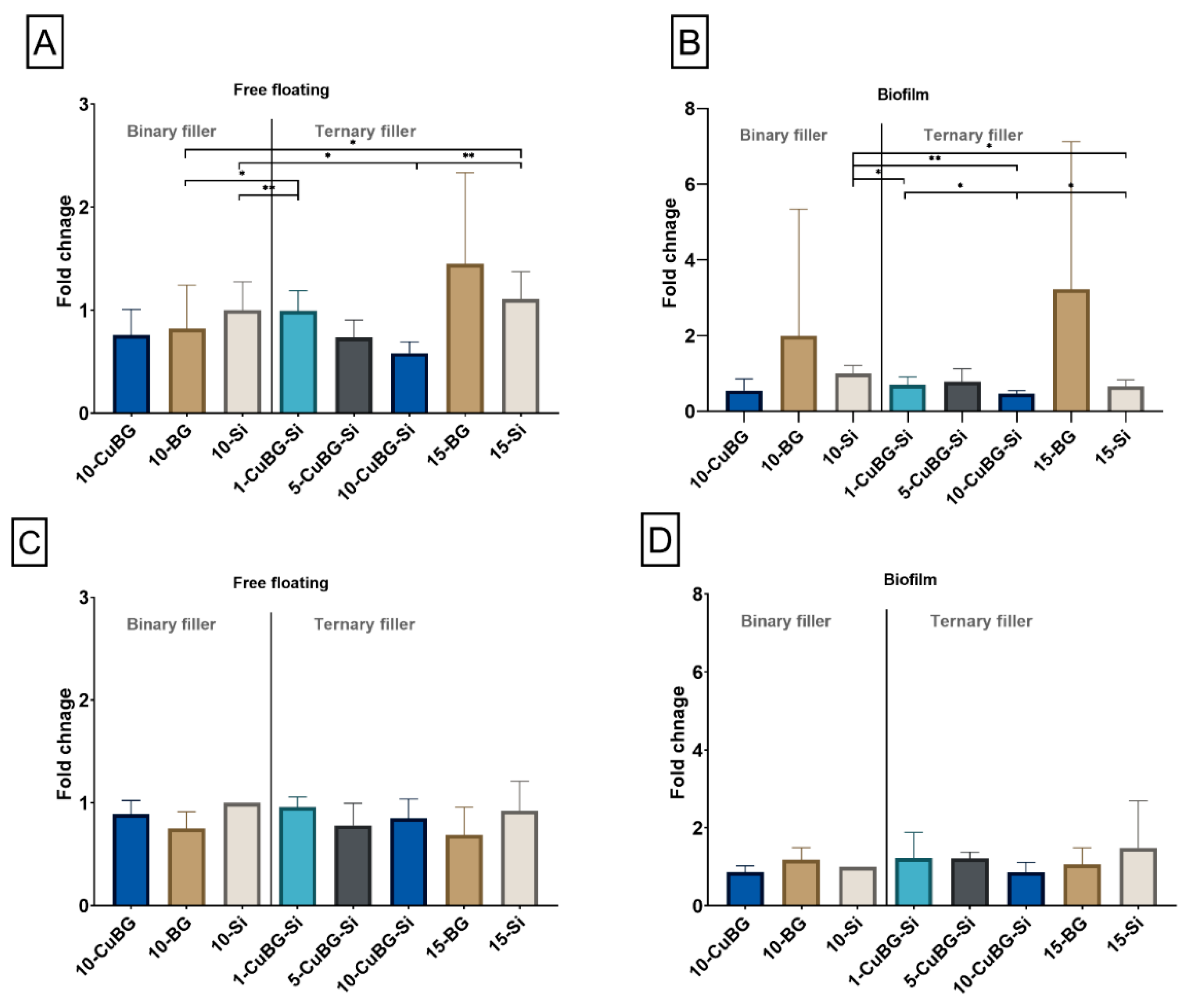
3.6. Anti-Bacterial Effect and Biofilm Formation on the Surface of the Composite Specimens
4. Discussion
4.1. Binary Filler Group
4.2. Ternary Filler Group
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kassebaum, N.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.; Marcenes, W. Global burden of untreated caries: A systematic review and metaregression. J. Dent. Res. 2015, 94, 650–658. [Google Scholar] [CrossRef]
- Gauthier, R.; Aboulleil, H.; Chenal, J.-M.; Chevalier, J.; Colon, P.; Grosgogeat, B. Consideration of Dental Tissues and Composite Mechanical Properties in Secondary Caries Development: A Critical Review. J. Adhes. Dent. 2021, 23, 297–308. [Google Scholar] [PubMed]
- Hussain, B.; Le Thieu, M.K.; Johnsen, G.F.; Reseland, J.E.; Haugen, H.J. Can CAD/CAM resin blocks be considered as substitute for conventional resins? Dent. Mater. 2017, 33, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Opdam, N.; Van De Sande, F.; Bronkhorst, E.; Cenci, M.; Bottenberg, P.; Pallesen, U.; Gaengler, P.; Lindberg, A.; Huysmans, M.; Van Dijken, J. Longevity of posterior composite restorations: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 943–949. [Google Scholar] [CrossRef]
- Kidd, E.A. Diagnosis of secondary caries. J. Dent. Educ. 2001, 65, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Marovic, D.; Tarle, Z.; Hiller, K.-A.; Müller, R.; Rosentritt, M.; Skrtic, D.; Schmalz, G. Reinforcement of experimental composite materials based on amorphous calcium phosphate with inert fillers. Dent. Mater. 2014, 30, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Marovic, D.; Tarle, Z.; Hiller, K.A.; Müller, R.; Ristic, M.; Rosentritt, M.; Skrtic, D.; Schmalz, G. Effect of silanized nanosilica addition on remineralizing and mechanical properties of experimental composite materials with amorphous calcium phosphate. Clin. Oral Investig. 2014, 18, 783–792. [Google Scholar] [CrossRef]
- Marović, D.; Šariri, K.; Demoli, N.; Ristić, M.; Hiller, K.-A.; Škrtić, D.; Rosentritt, M. Remineralizing amorphous calcium phosphate based composite resins: The influence of inert fillers on monomer conversion, polymerization shrinkage, and microhardness. Croat. Med. J. 2016, 57, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Šantić, A.; Gamulin, O.; Marovic, D.; Moguš-Milanković, A.; Tarle, Z. Impedance changes during setting of amorphous calcium phosphate composites. Dent. Mater. 2016, 32, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Spanovic, N.; Bjelovucic, R.; Marovic, D.; Schmalz, G.; Gamulin, O.; Tarle, Z. Long-term water sorption and solubility of experimental bioactive composites based on amorphous calcium phosphate and bioactive glass. Dent. Mater. J. 2019, 38, 555–564. [Google Scholar] [CrossRef]
- Tarle, Z.; Hickel, R.; Ilie, N. Mechanical properties of experimental composites containing bioactive glass after artificial aging in water and ethanol. Clin. Oral Investig. 2019, 23, 2733–2741. [Google Scholar]
- Par, M.; Tarle, Z.; Hickel, R.; Ilie, N. Dentin bond strength of experimental composites containing bioactive glass: Changes during aging for up to 1 year. J. Adhes. Dent 2018, 20, 325–334. [Google Scholar] [PubMed]
- Par, M.; Tarle, Z.; Hickel, R.; Ilie, N. Polymerization kinetics of experimental bioactive composites containing bioactive glass. J. Dent. 2018, 76, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Spanovic, N.; Bjelovucic, R.; Skenderovic, H.; Gamulin, O.; Tarle, Z. Curing potential of experimental resin composites with systematically varying amount of bioactive glass: Degree of conversion, light transmittance and depth of cure. J. Dent. 2018, 75, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Abbassy, M.A.; Bakry, A.S.; Alshehri, N.I.; Alghamdi, T.M.; Rafiq, S.A.; Aljeddawi, D.H.; Nujaim, D.S.; Hassan, A.H. 45S5 Bioglass paste is capable of protecting the enamel surrounding orthodontic brackets against erosive challenge. J. Orthod. Sci. 2019, 8, 5. [Google Scholar] [PubMed]
- Bakry, A.S.; Abbassy, M.A. The efficacy of a bioglass (45S5) paste temporary filling used to remineralize enamel surfaces prior to bonding procedures. J. Dent. 2019, 85, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Abbassy, M.A.; Bakry, A.S.; Almoabady, E.H.; Almusally, S.M.; Hassan, A.H. Characterization of a novel enamel sealer for bioactive remineralization of white spot lesions. J. Dent. 2021, 109, 103663. [Google Scholar] [CrossRef] [PubMed]
- Langhorst, S.; O’donnell, J.; Skrtic, D. In vitro remineralization of enamel by polymeric amorphous calcium phosphate composite: Quantitative microradiographic study. Dent. Mater. 2009, 25, 884–891. [Google Scholar] [CrossRef]
- Skrtic, D.; Antonucci, J.M.; Eanes, E.; Eidelman, N. Dental composites based on hybrid and surface-modified amorphous calcium phosphates. Biomaterials 2004, 25, 1141–1150. [Google Scholar] [CrossRef]
- Tiskaya, M.; Al-Eesa, N.; Wong, F.; Hill, R. Characterization of the bioactivity of two commercial composites. Dent. Mater. 2019, 35, 1757–1768. [Google Scholar] [CrossRef]
- Porenczuk, A.; Jankiewicz, B.; Naurecka, M.; Bartosewicz, B.; Sierakowski, B.; Gozdowski, D.; Kostecki, J.; Nasiłowska, B.; Mielczarek, A. A comparison of the remineralizing potential of dental restorative materials by analyzing their fluoride release profiles. Adv. Clin. Exp. Med. 2019, 28, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Atai, M.; Pahlavan, A.; Moin, N. Nano-porous thermally sintered nano silica as novel fillers for dental composites. Dent. Mater. 2012, 28, 133–145. [Google Scholar] [CrossRef]
- Samuel, S.P.; Li, S.; Mukherjee, I.; Guo, Y.; Patel, A.C.; Baran, G.; Wei, Y. Mechanical properties of experimental dental composites containing a combination of mesoporous and nonporous spherical silica as fillers. Dent. Mater. 2009, 25, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Xavier, T.A.; FRÓES-SALGADO, N.R.d.G.; Meier, M.M.; Braga, R.R. Influence of silane content and filler distribution on chemical-mechanical properties of resin composites. Braz. Oral Res. 2015, 29, 1–8. [Google Scholar] [CrossRef]
- Bari, A.; Bloise, N.; Fiorilli, S.; Novajra, G.; Vallet-Regí, M.; Bruni, G.; Torres-Pardo, A.; González-Calbet, J.M.; Visai, L.; Vitale-Brovarone, C. Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration. Acta Biomater. 2017, 55, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Simila, H.O.; Boccaccini, A.R. Sol-gel bioactive glass containing biomaterials for restorative dentistry: A review. Dent. Mater. 2022, 38, 725–747. [Google Scholar] [CrossRef]
- Zheng, K.; Sui, B.; Ilyas, K.; Boccaccini, A.R. Porous bioactive glass micro-and nanospheres with controlled morphology: Developments, properties and emerging biomedical applications. Mater. Horiz. 2021, 8, 300–335. [Google Scholar] [CrossRef]
- Zheng, K.; Wu, J.; Li, W.; Dippold, D.; Wan, Y.; Boccaccini, A.R. Incorporation of Cu-containing bioactive glass nanoparticles in gelatin-coated scaffolds enhances bioactivity and osteogenic activity. ACS Biomater. Sci. Eng. 2018, 4, 1546–1557. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, K.; Liang, R.; Mainka, A.; Taccardi, N.; Roether, J.A.; Detsch, R.; Goldmann, W.H.; Virtanen, S.; Boccaccini, A.R. Cu-releasing bioactive glass/polycaprolactone coating on Mg with antibacterial and anticorrosive properties for bone tissue engineering. Biomed. Mater. 2017, 13, 015001. [Google Scholar] [CrossRef]
- Zheng, K.; Kang, J.; Rutkowski, B.; Gawȩda, M.; Zhang, J.; Wang, Y.; Founier, N.; Sitarz, M.; Taccardi, N.; Boccaccini, A.R. Toward highly dispersed mesoporous bioactive glass nanoparticles with high Cu concentration using Cu/ascorbic acid complex as precursor. Front. Chem. 2019, 7, 497. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.V.; Curcio, M.; Raucci, M.G.; Barbaro, K.; Fasolino, I.; Teghil, R.; Ambrosio, L.; De Bonis, A.; Boccaccini, A.R. Cu-releasing bioactive glass coatings and their in vitro properties. ACS Appl. Mater. Interfaces 2019, 11, 5812–5820. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Balasubramanian, P.; Paterson, T.E.; Stein, R.; MacNeil, S.; Fiorilli, S.; Vitale-Brovarone, C.; Shepherd, J.; Boccaccini, A.R. Ag modified mesoporous bioactive glass nanoparticles for enhanced antibacterial activity in 3D infected skin model. Mater. Sci. Eng. C 2019, 103, 109764. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhai, D.; Lv, F.; Yu, Q.; Ma, H.; Yin, J.; Yi, Z.; Liu, M.; Chang, J.; Wu, C. Preparation of copper-containing bioactive glass/eggshell membrane nanocomposites for improving angiogenesis, antibacterial activity and wound healing. Acta Biomater. 2016, 36, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Jandt, K.D.; Watts, D.C. Nanotechnology in dentistry: Present and future perspectives on dental nanomaterials. Dent. Mater. 2020, 36, 1365–1378. [Google Scholar] [CrossRef]
- Rivera, L.R.; Cochis, A.; Biser, S.; Canciani, E.; Ferraris, S.; Rimondini, L.; Boccaccini, A.R. Antibacterial, pro-angiogenic and pro-osteointegrative zein-bioactive glass/copper based coatings for implantable stainless steel aimed at bone healing. Bioact. Mater. 2021, 6, 1479–1490. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Xu, M.; Han, P.; Chen, L.; Chang, J.; Xiao, Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 2013, 34, 422–433. [Google Scholar] [CrossRef]
- Marovic, D.; Haugen, H.J.; Negovetic Mandic, V.; Par, M.; Zheng, K.; Tarle, Z.; Boccaccini, A.R. Incorporation of copper-doped mesoporous bioactive glass nanospheres in experimental dental composites: Chemical and mechanical characterization. Materials 2021, 14, 2611. [Google Scholar] [CrossRef]
- Marovic, D.; Tauböck, T.T.; Haugen, H.J.; Negovetic Mandic, V.; Wüthrich, D.; Burrer, P.; Zheng, K.; Attin, T.; Tarle, Z.; Boccaccini, A.R. Impact of Copper-Doped Mesoporous Bioactive Glass Nanospheres on the Polymerisation Kinetics and Shrinkage Stress of Dental Resin Composites. Int. J. Mol. Sci. 2022, 23, 8195. [Google Scholar] [CrossRef] [PubMed]
- Kopperud, H.M.; Johnsen, G.F.; Lamolle, S.; Kleven, I.S.; Wellendorf, H.; Haugen, H.J. Effect of short LED lamp exposure on wear resistance, residual monomer and degree of conversion for Filtek Z250 and Tetric EvoCeram composites. Dent. Mater. 2013, 29, 824–834. [Google Scholar] [CrossRef]
- Xiong, J.; Sun, X.; Li, Y.C.; Chen, J.H. Polymerization Shrinkage, Stress, and Degree of Conversion in Silorane- and Dimethacrylate-Based Dental Composites. J. Appl. Polym. Sci. 2011, 122, 1882–1888. [Google Scholar] [CrossRef]
- Gerdolle, D.A.; Mortier, E.; Droz, D. Microleakage and Polymerization Shrinkage of Various Polymer Restorative Materials. J. Dent. Child. 2008, 75, 125–133. [Google Scholar]
- de Gee, A.J.; Davidson, C.L.; Smith, A. A modified dilatometer for continuous recording of volumetric polymerization shrinkage of composite restorative materials. J. Dent. 1981, 9, 36–42. [Google Scholar] [CrossRef]
- Sun, J.; Lin-Gibson, S. X-ray microcomputed tomography for measuring polymerization shrinkage of polymeric dental composites. Dent. Mater. 2008, 24, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Marovic, D.; Posavec, K.; Marić, I.; Štajdohar, D.; Muradbegović, A.; Tauböck, T.T.; Attin, T.; Tarle, Z. Long-Term Assessment of Contemporary Ion-Releasing Restorative Dental Materials. Materials 2022, 15, 4042. [Google Scholar] [CrossRef]
- Marovic, D.; Par, M.; Macan, M.; Klarić, N.; Plazonić, I.; Tarle, Z. Aging-Dependent Changes in Mechanical Properties of the New Generation of Bulk-Fill Composites. Materials 2022, 15, 902. [Google Scholar] [CrossRef]
- Ferracane, J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Etemadifar, Z.; Daneshkazemi, A.; Nateghi, M. Antimicrobial effect of copper oxide nanoparticles on some oral bacteria and candida species. J. Dent. Biomater. 2017, 4, 347. [Google Scholar]
- Tiskaya, M.; Shahid, S.; Gillam, D.; Hill, R. The use of bioactive glass (BAG) in dental composites: A critical review. Dent. Mater. 2021, 37, 296–310. [Google Scholar] [CrossRef]
- Par, M.; Gubler, A.; Attin, T.; Tarle, Z.; Tarle, A.; Tauböck, T.T. Ion release and hydroxyapatite precipitation of resin composites functionalized with two types of bioactive glass. J. Dent. 2022, 118, 103950. [Google Scholar] [CrossRef]
- Par, M.; Gubler, A.; Attin, T.; Tarle, Z.; Tauböck, T.T. Anti-demineralizing protective effects on enamel identified in experimental and commercial restorative materials with functional fillers. Sci. Rep. 2021, 11, 11806. [Google Scholar] [CrossRef]
- Par, M.; Gubler, A.; Attin, T.; Tarle, Z.; Tarle, A.; Tauböck, T.T. Experimental Bioactive Glass-Containing Composites and Commercial Restorative Materials: Anti-Demineralizing Protection of Dentin. Biomedicines 2021, 9, 1616. [Google Scholar] [CrossRef]
- Xu, V.W.; Nizami, M.Z.I.; Yin, I.X.; Yu, O.Y.; Lung, C.Y.K.; Chu, C.H. Application of Copper Nanoparticles in Dentistry. Nanomaterials 2022, 12, 805. [Google Scholar] [CrossRef] [PubMed]
- Andreadis, G.; Topitsoglou, V.; Kalfas, S. Acidogenicity and acidurance of dental plaque and saliva sediment from adults in relation to caries activity and chlorhexidine exposure. J. Oral Microbiol. 2015, 7, 26197. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Li, M.; Gregory, R.L. Bacterial interactions in dental biofilm. Virulence 2011, 2, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Richards, V.P.; Alvarez, A.J.; Luce, A.R.; Bedenbaugh, M.; Mitchell, M.L.; Burne, R.A.; Nascimento, M.M. Microbiomes of site-specific dental plaques from children with different caries status. Infect. Immun. 2017, 85, e00106–e00117. [Google Scholar] [CrossRef] [PubMed]
- Lukic, D.; Karygianni, L.; Flury, M.; Attin, T.; Thurnheer, T. Endodontic-Like Oral Biofilms as Models for Multispecies Interactions in Endodontic Diseases. Microorganisms 2020, 8, 674. [Google Scholar] [CrossRef] [PubMed]
- Guggenheim, B.; Giertsen, E.; Schupbach, P.; Shapiro, S. Validation of an in vitro biofilm model of supragingival plaque. J. Dent. Res. 2001, 80, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Ammann, T.W.; Belibasakis, G.N.; Thurnheer, T. Impact of early colonizers on in vitro subgingival biofilm formation. PLoS ONE 2013, 8, e83090. [Google Scholar] [CrossRef]
- Braga, R.R.; Ballester, R.Y.; Ferracane, J.L. Factors involved in the development of polymerization shrinkage stress in resin-composites: A systematic review. Dent. Mater. 2005, 21, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Haugen, H.J.; Qasim, S.B.; Matinlinna, J.P.; Vallittu, P.; Nogueira, L.P. Nano-CT as tool for characterization of dental resin composites. Sci. Rep. 2020, 10, 15520. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Mei, L.; Busscher, H.J.; van der Mei, H.C.; Ren, Y. Influence of surface roughness on streptococcal adhesion forces to composite resins. Dent. Mater. 2011, 27, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Haugen, H.J.; Marovic, D.; Khai Le Thieu, M.; Reseland, J.E.; Johnsen, G.F. Bulk fill composites have similar performance to conventional dental composites. Int. J. Mol. Sci. 2020, 21, 5136. [Google Scholar] [CrossRef] [PubMed]
- Wanitwisutchai, T.; Monmaturapoj, N.; Srisatjaluk, R.; Subannajui, K.; Dechkunakorn, S.; Anuwongnukroh, N.; Pongprueksa, P. Buffering capacity and antibacterial properties among bioactive glass-containing orthodontic adhesives. Dent. Mater. J. 2021, 40, 1169–1176. [Google Scholar] [CrossRef]
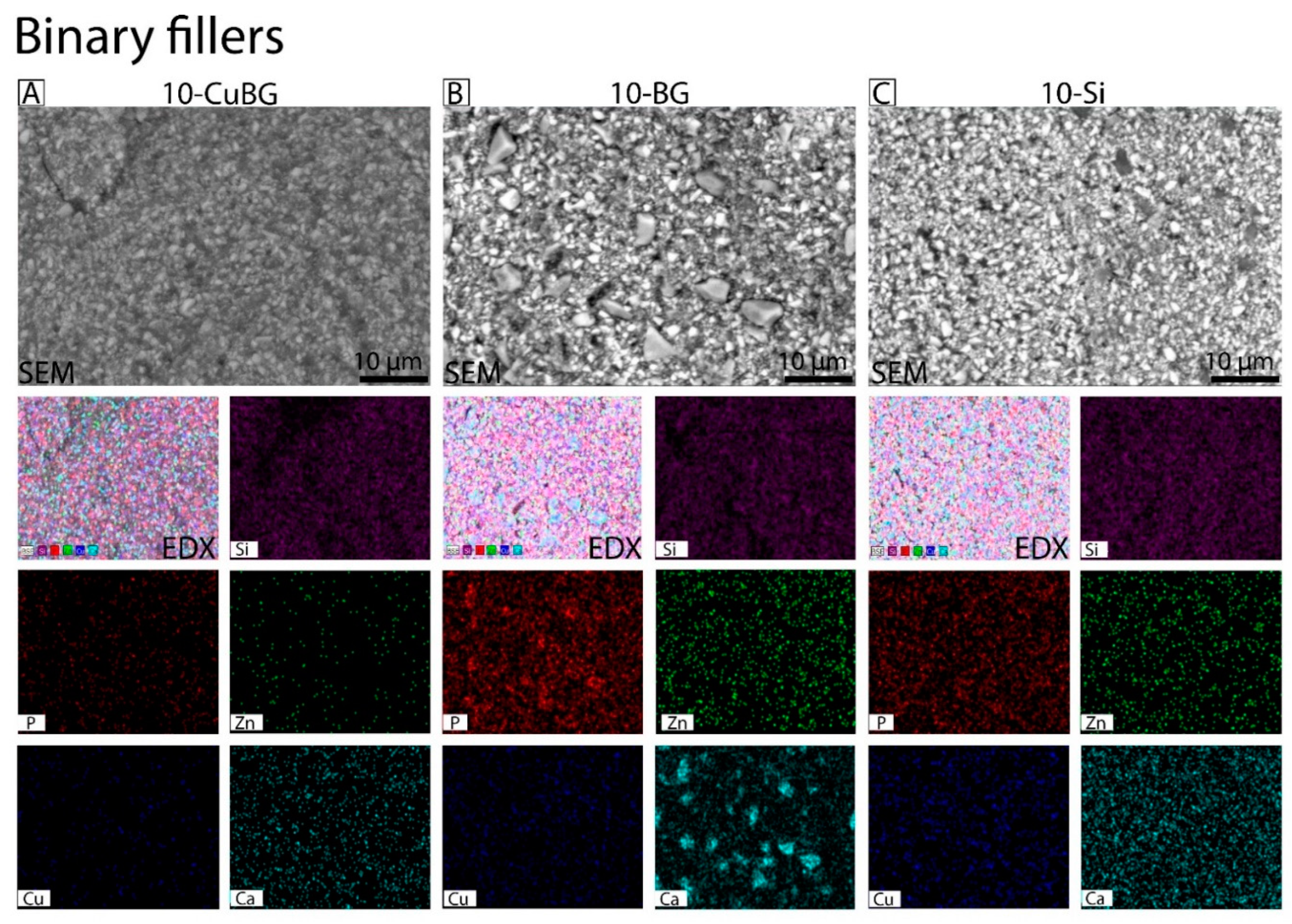
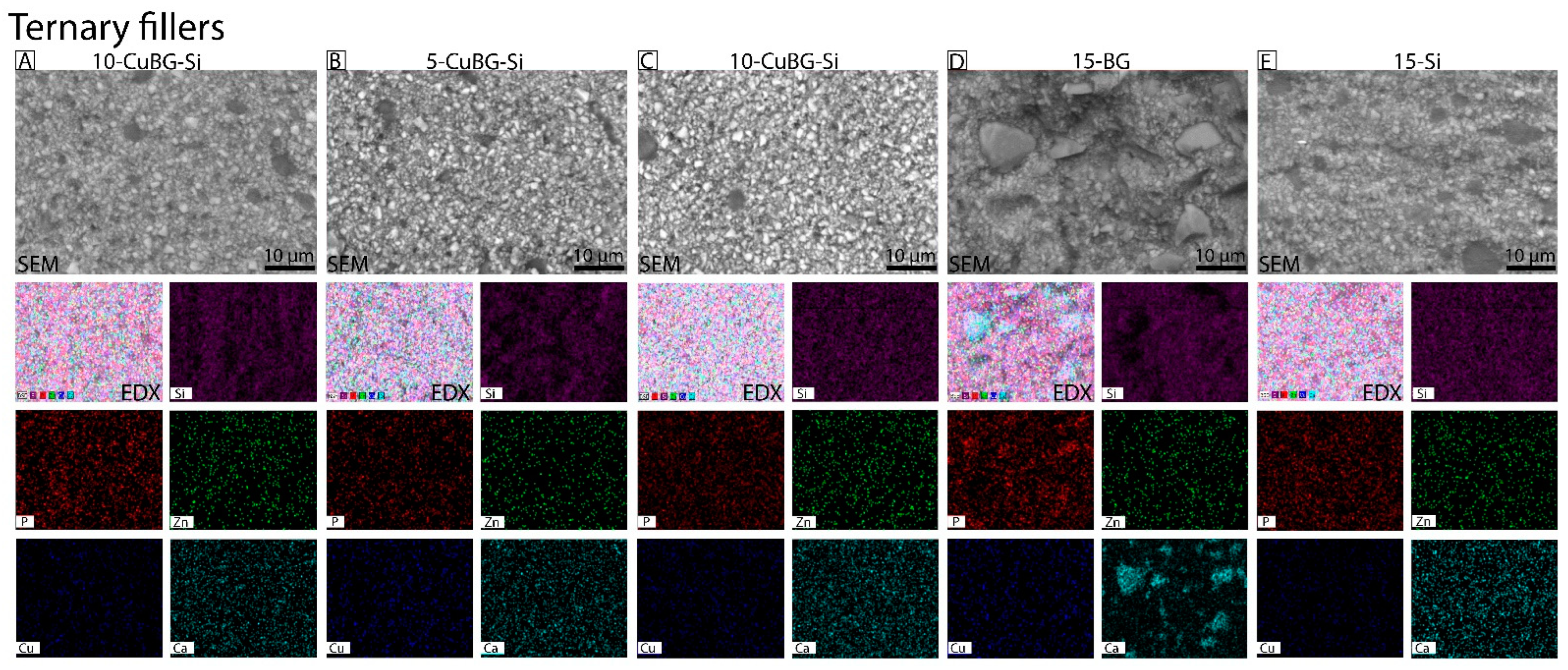
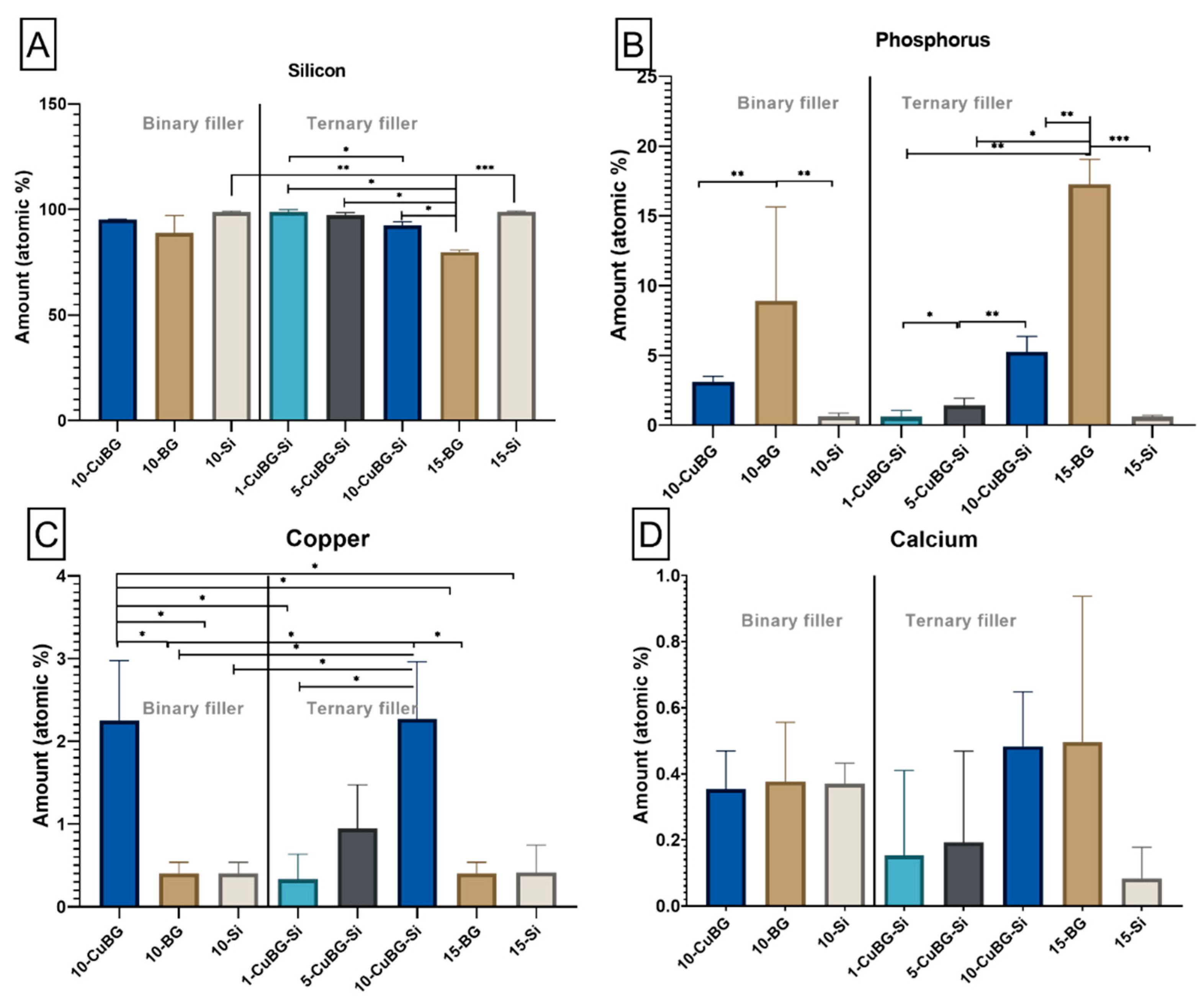
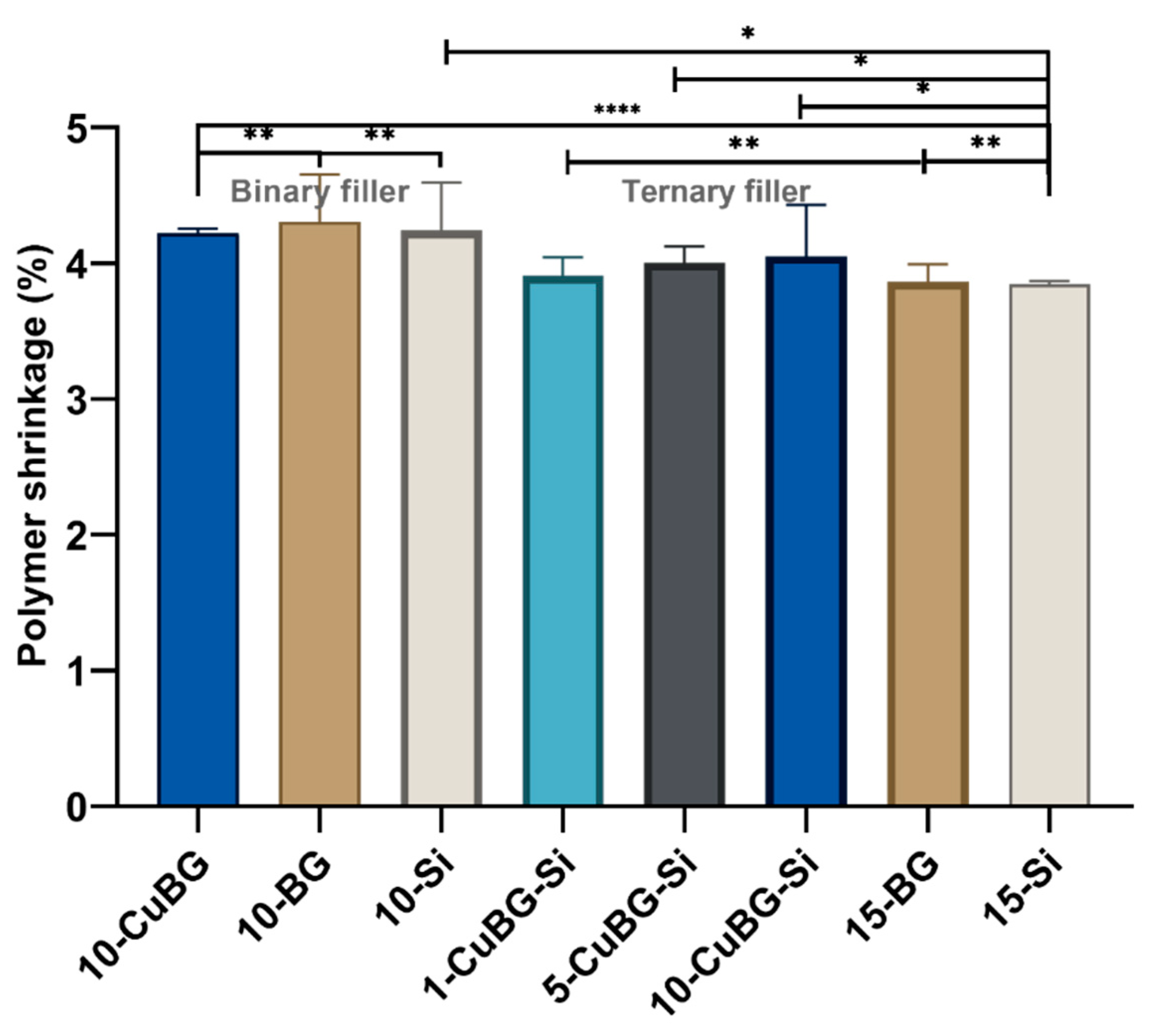


| Group | Material | Resin | Ba Glass Microfillers | Cu-MBGN | Silica Nanofillers | 45S5 BG |
|---|---|---|---|---|---|---|
| Binary filler group (65% filler load) | 10-CuBG | 35% | 55% | 10% | - | - |
| 10-BG | - | - | 10% | |||
| 10-Si | - | 10% | - | |||
| Ternary filler group (70% filler load) | 1-CuBG-Si | 30% | 55% | 1% | 14% | - |
| 5-CuBG-Si | 5% | 10% | - | |||
| 10-CuBG-Si | 10% | 5% | - | |||
| 15-BG | - | - | 15% | |||
| 15-Si | - | 15% | - |
| Name | Type | Manufacturer/ Product | Composition (wt%) | Size (d50) | Silanization |
|---|---|---|---|---|---|
| Cu-MBGN | Experimental/Bioactive | Produced in-house [31] | SiO2 84.8% CaO 9.4% CuO 5.8% * | ~100 nm | No |
| 45S5 BG | Commercial/Bioactive | Schott, Mainz, Germany G018-144 | SiO2 45% Na2O 24.5% CaO 24.5% P2O5 6% | 4.0 μm ** | No |
| Ba glass microfillers | Commercial/Inert | Schott, Mainz, Germany GM27884 | SiO2 55.0% BaO 25.0% B2O3 10.0% Al2O3 10.0% | 1.0 μm ** | Yes *** 3.20% |
| Silica nanofillers | Commercial/Inert | Evonik Degussa, Hanau, Germany Aerosil DT | SiO2 > 99.8% | 12 nm | Yes *** 4–6% |
| B (µg/L) | Ca (µg/L) | Cu (µg/L) |
|---|---|---|
| 150 ± 3 | 31,000 ± 310 | 20.0 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munir, A.; Marovic, D.; Nogueira, L.P.; Simm, R.; Naemi, A.-O.; Landrø, S.M.; Helgerud, M.; Zheng, K.; Par, M.; Tauböck, T.T.; et al. Using Copper-Doped Mesoporous Bioactive Glass Nanospheres to Impart Anti-Bacterial Properties to Dental Composites. Pharmaceutics 2022, 14, 2241. https://doi.org/10.3390/pharmaceutics14102241
Munir A, Marovic D, Nogueira LP, Simm R, Naemi A-O, Landrø SM, Helgerud M, Zheng K, Par M, Tauböck TT, et al. Using Copper-Doped Mesoporous Bioactive Glass Nanospheres to Impart Anti-Bacterial Properties to Dental Composites. Pharmaceutics. 2022; 14(10):2241. https://doi.org/10.3390/pharmaceutics14102241
Chicago/Turabian StyleMunir, Arooj, Danijela Marovic, Liebert Parreiras Nogueira, Roger Simm, Ali-Oddin Naemi, Sander Marius Landrø, Magnus Helgerud, Kai Zheng, Matej Par, Tobias T. Tauböck, and et al. 2022. "Using Copper-Doped Mesoporous Bioactive Glass Nanospheres to Impart Anti-Bacterial Properties to Dental Composites" Pharmaceutics 14, no. 10: 2241. https://doi.org/10.3390/pharmaceutics14102241
APA StyleMunir, A., Marovic, D., Nogueira, L. P., Simm, R., Naemi, A.-O., Landrø, S. M., Helgerud, M., Zheng, K., Par, M., Tauböck, T. T., Attin, T., Tarle, Z., Boccaccini, A. R., & Haugen, H. J. (2022). Using Copper-Doped Mesoporous Bioactive Glass Nanospheres to Impart Anti-Bacterial Properties to Dental Composites. Pharmaceutics, 14(10), 2241. https://doi.org/10.3390/pharmaceutics14102241











