Population Pharmacokinetic/Pharmacodynamic Modelling of Daptomycin for Schedule Optimization in Patients with Renal Impairment
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Population and Study Design
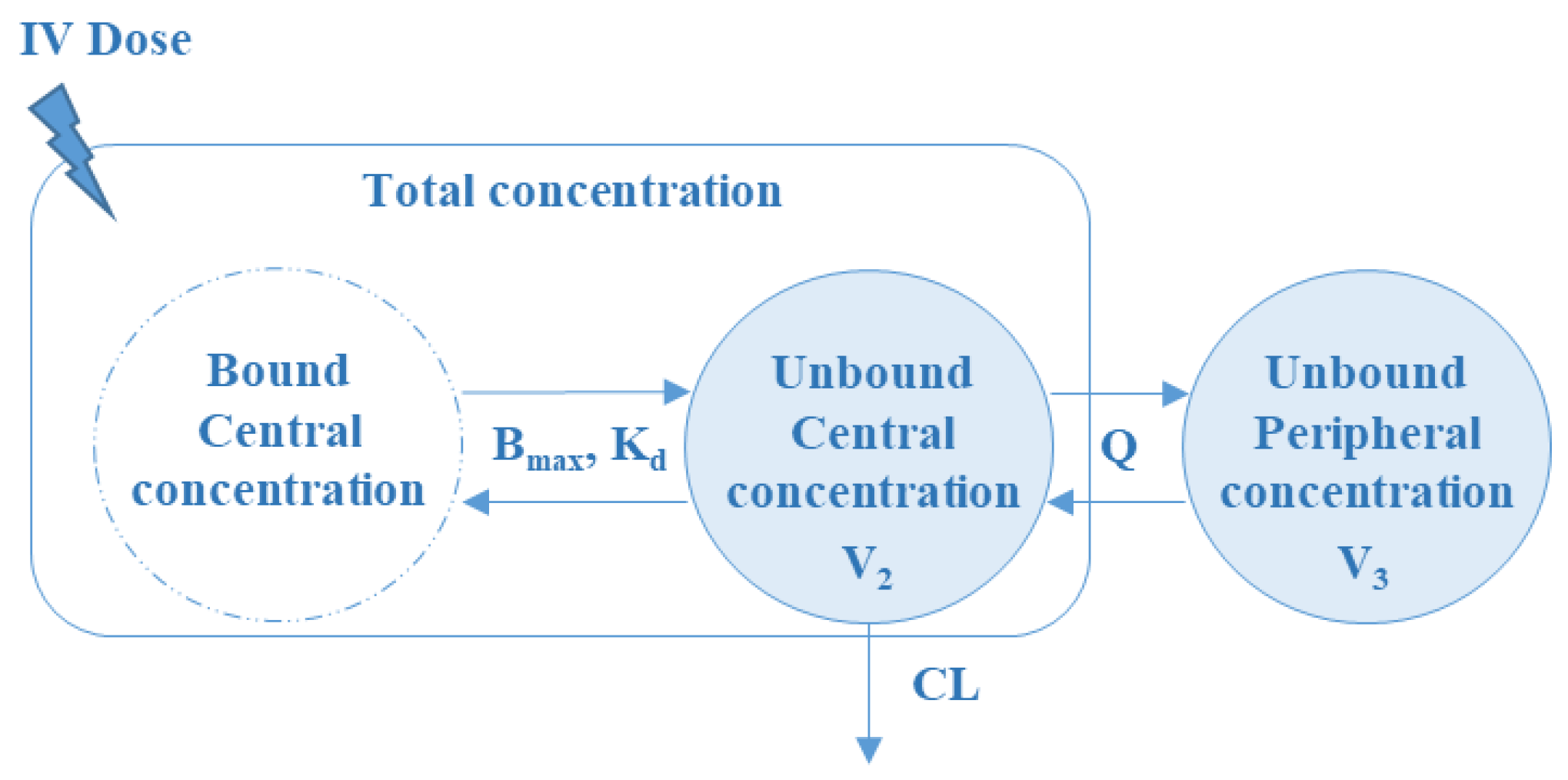
3.2. Population Pharmacokinetic Model
3.2.1. Base Population PK Model
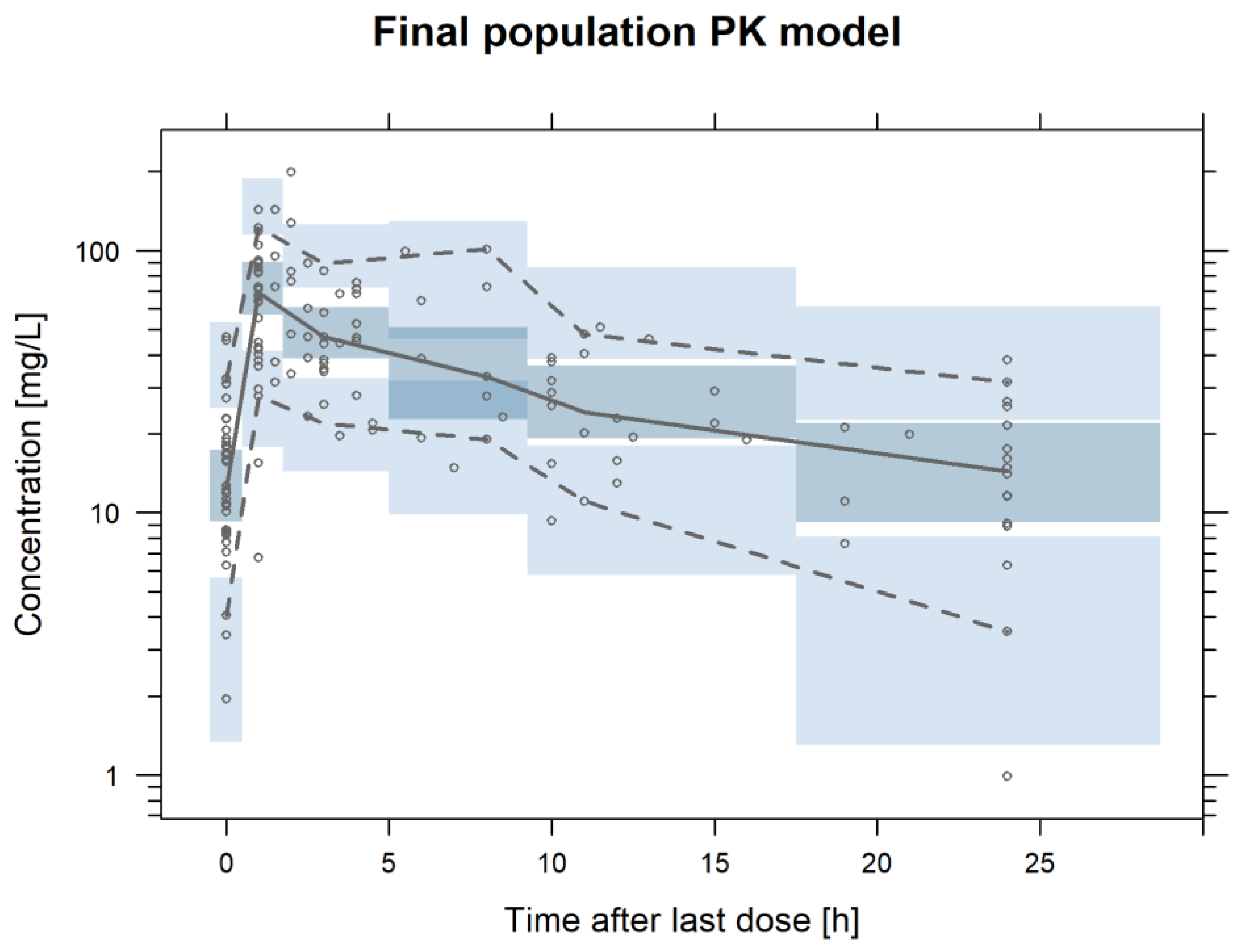
3.2.2. Final Population PK Model
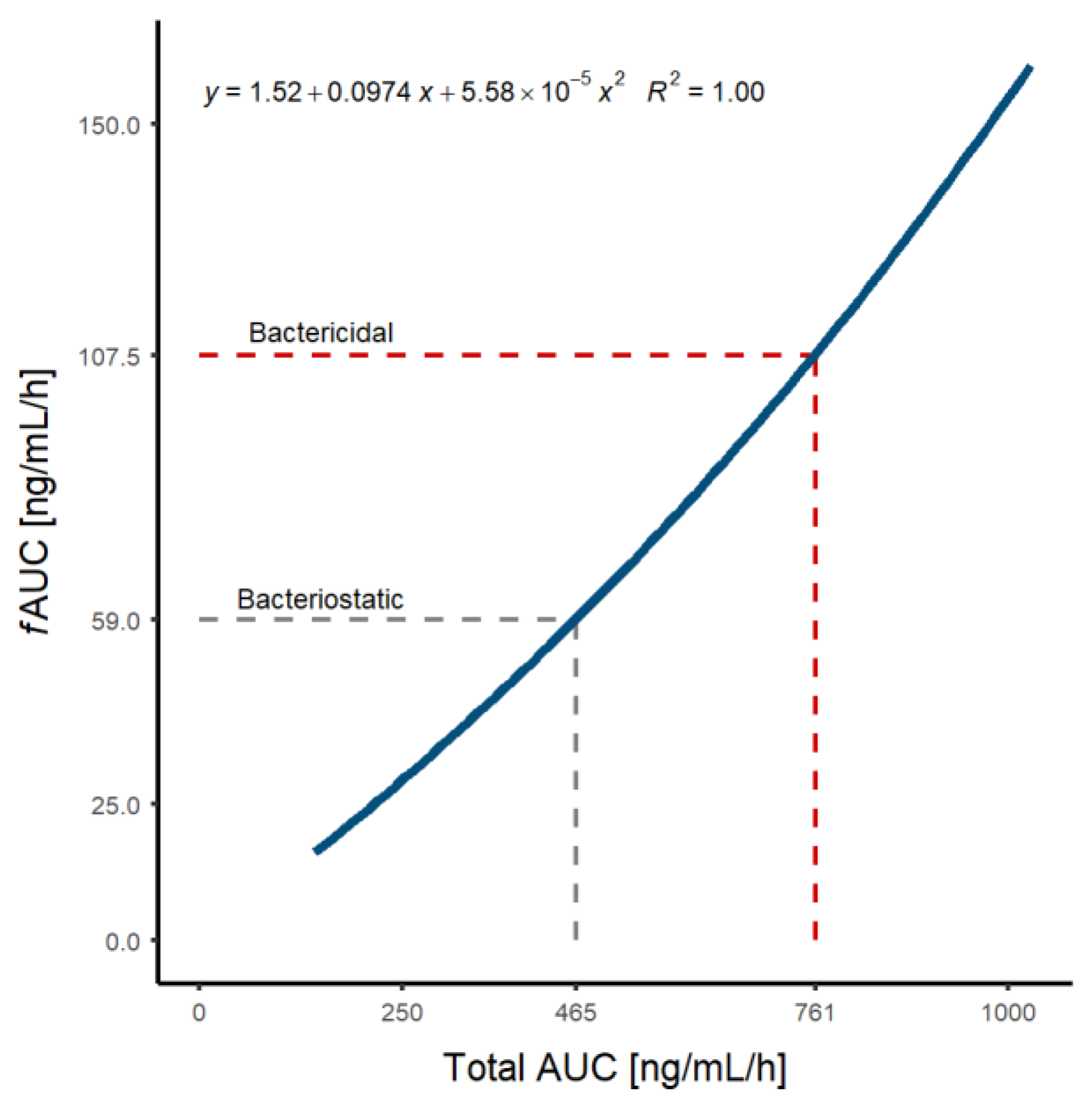
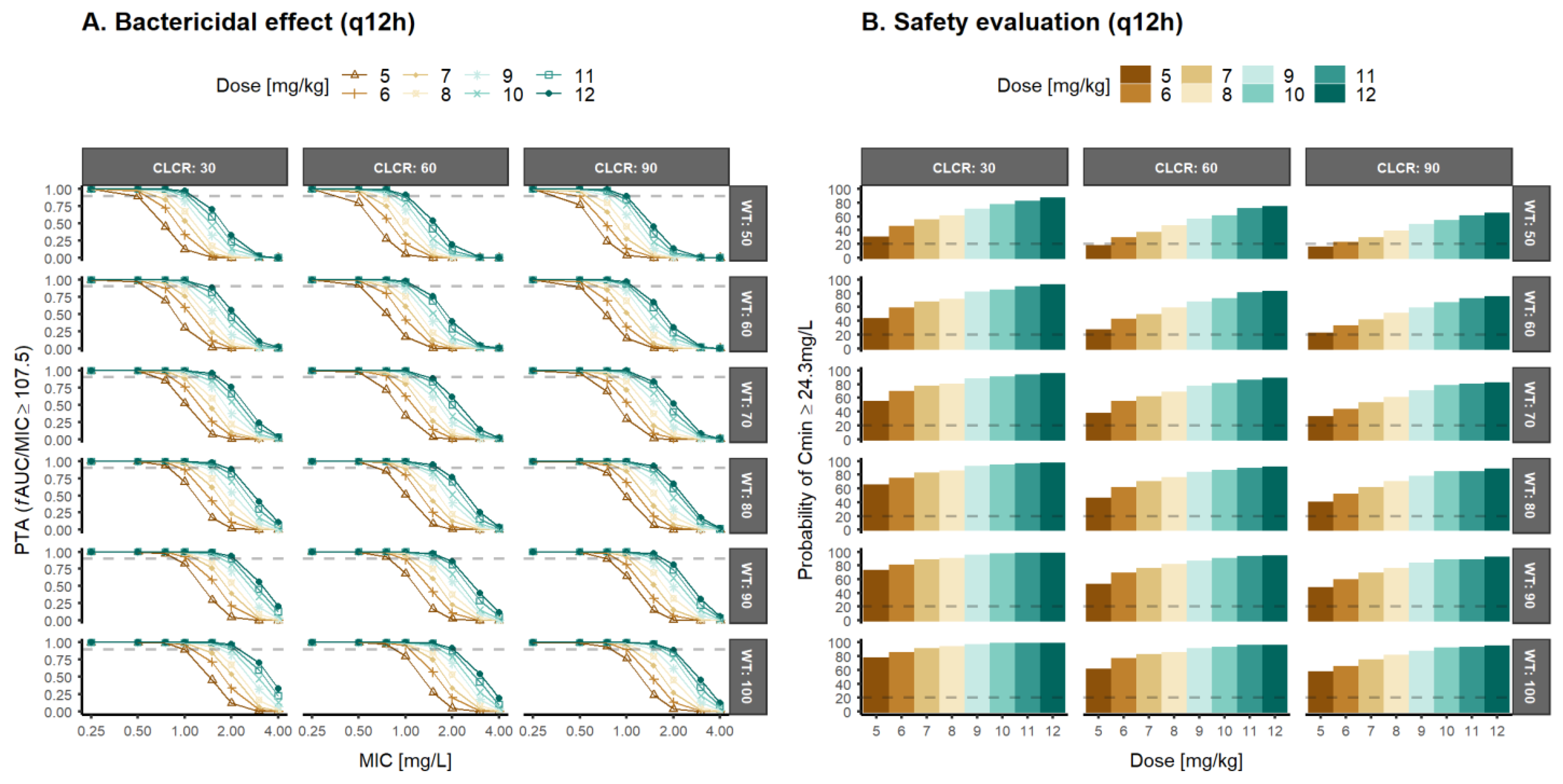
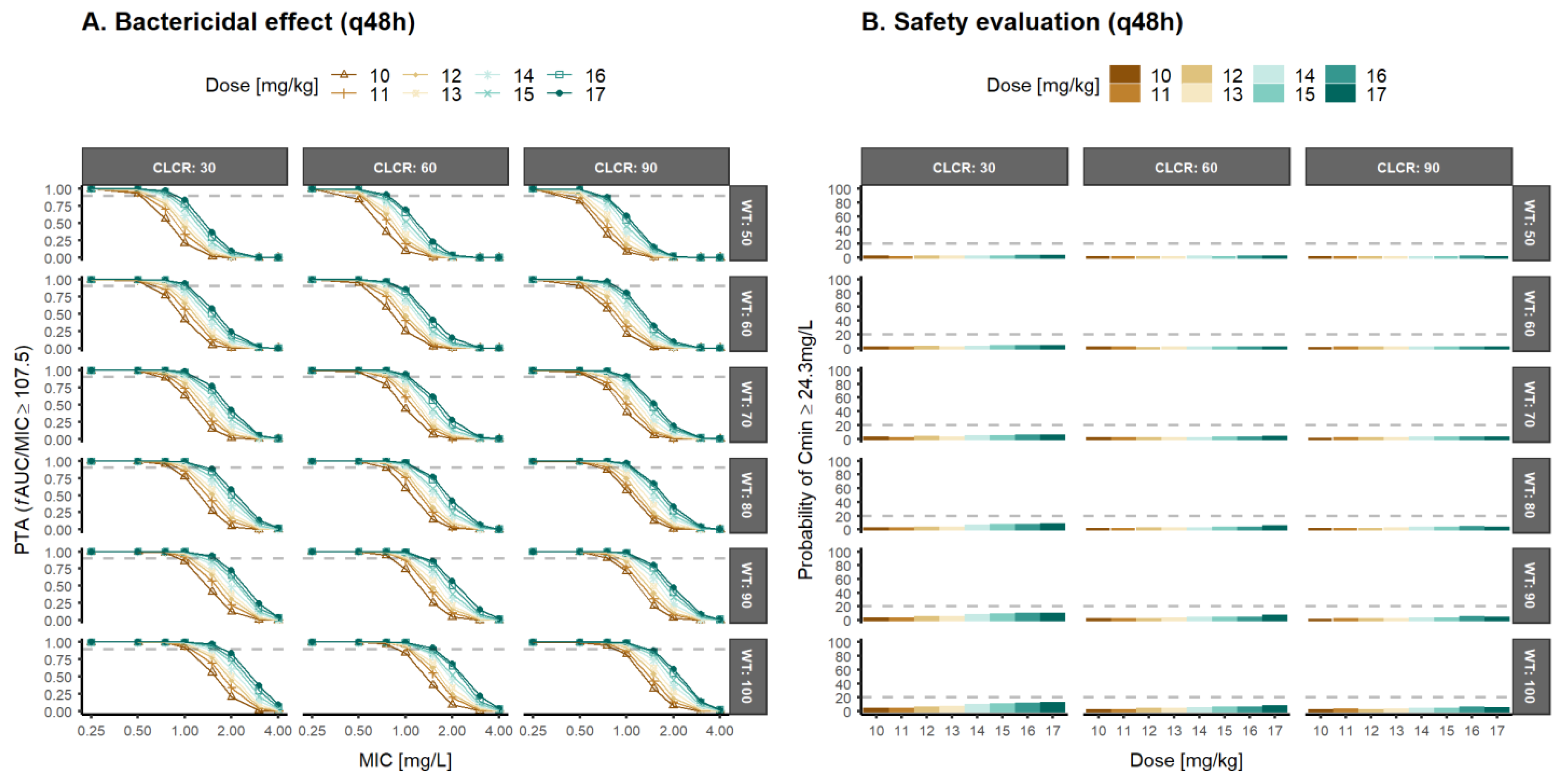
3.3. Population Pharmacokinetic/Pharmacodynamic Simulations and Optimal Dosage Selection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- US FDA Daptomycin (Marketed as Cubicin) Information. 2017. Available online: https://www.Accessdata.Fda.Gov/Drugsatfda_docs/Label/2017/021572s059lbl.Pdf (accessed on 10 April 2022).
- European Medicines Agency. Cubicin: EPAR—Product Information (WC500036049.Pdf); European Medicines Agency: London, UK, 2006.
- European Medicines Agency. Cubicin: EPAR—Scientific Discussion (WC500036046.Pdf); European Medicines Agency: London, UK, 2006.
- Vilhena, C.; Bettencourt, A. Daptomycin: A Review of Properties, Clinical Use, Drug Delivery and Resistance. Mini.-Rev. Med. Chem. 2012, 12, 202–209. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Cubicin (Daptomycin); EMEA/H/C/000637; European Medicines Agency: London, UK, 2006.
- Avolio, A.D.; Pensi, D.; Baietto, L.; Pacini, G.; Di Perri, G.; Francesco, P.; de Rosa, G. Daptomycin Pharmacokinetics and Pharmacodynamics in Septic and Critically Ill Patients. Drugs 2016, 76, 1161–1174. [Google Scholar] [CrossRef]
- Soraluce, A.; Asín-Prieto, E.; Rodríguez-Gascón, A.; Isla, A.; Barrasa, H.; Maynar, J.; Carcelero, E.; Soy, D. Population Pharmacokinetics of Daptomycin in Critically Ill Patients. Int. J. Antimicrob. Agents 2018, 52, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Nakafusa, J.; Misago, N.; Miura, Y.; Kayaba, M.; Tanaka, T.; Narisawa, Y. The Importance of Serum Creatine Phosphokinase Level in the Early Diagnosis, and as a Prognostic Factor, of Vibrio Vulnificus Infection. Br. J. Dermatol. 2001, 145, 280–284. [Google Scholar] [CrossRef] [PubMed]
- DN Gilbert. The Sanford Guide to Antimicrobial Therapy; BI Publications Pvt Ltd.: New Delhi, India, 2006. [Google Scholar]
- Canut, A.; Isla, A.; Betriu, C.; Gascón, A.R. Pharmacokinetic—Pharmacodynamic Evaluation of Daptomycin, Tigecycline, and Linezolid versus Vancomycin for the Treatment of MRSA Infections in Four Western European Countries. Eur. J. Clin. Microbiol. 2012, 31, 2227–2235. [Google Scholar] [CrossRef]
- Ukimura, A.; Oda, K.; Yoshida, M.; Nishihara, M.; Kawanishi, F.; Yamada, T.; Nakano, T.; Ooi, Y.; Uchida, T.; Shibata, Y.; et al. Observational Study to Determine the Optimal Dose of Daptomycin Based on Pharmacokinetic/Pharmacodynamic Analysis. J. Infect. Chemother. 2019, 26, 379–384. [Google Scholar] [CrossRef]
- Gray, D.A.; Wenzel, M. More Than a Pore: A Current Perspective on the In Vivo Mode of Action of the Lipopeptide Antibiotic Daptomycin. Antibiotics 2020, 9, 17. [Google Scholar] [CrossRef]
- EUCAST Daptomycin: Rational for the EUCAST Clinical Breakpoints, Version 2. Mayo 2021. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/Daptomycin_Rationale_Document_2.0_20210512.pdf (accessed on 31 May 2021).
- Trecarichi, E.M.; Pagano, L.; Candoni, A.; Pastore, D.; Cattaneo, C.; Fanci, R.; Nosari, A.; Caira, M.; Spadea, A.; Busca, A.; et al. Current Epidemiology and Antimicrobial Resistance Data for Bacterial Bloodstream Infections in Patients with Hematologic Malignancies: An Italian Multicentre Prospective Survey. Clin. Microbiol. Infect. 2015, 21, 337–343. [Google Scholar] [CrossRef]
- Rolston, K.V.I.; Yadegarynia, D.; Kontoyiannis, D.P.; Raad, I.I.; Ho, D.H. The Spectrum of Gram-Positive Bloodstream Infections in Patients with Hematologic Malignancies, and the in Vitro Activity of Various Quinolones against Gram-Positive Bacteria Isolated from Cancer Patients. Int. J. Infect. Dis. 2006, 10, 223–230. [Google Scholar] [CrossRef]
- Benvenuto, M.; Benziger, D.P.; Yankelev, S.; Vigliani, G. Pharmacokinetics and Tolerability of Daptomycin at Doses up to 12 Milligrams per Kilogram of Body Weight Once Daily in Healthy Volunteers. Antimicrob. Agents Chemother. 2006, 50, 3245–3249. [Google Scholar] [CrossRef]
- Dvorchik, B.; Arbeit, R.D.; Chung, J.; Liu, S.; Knebel, W.; Kastrissios, H. Population Pharmacokinetics of Daptomycin. Antimicrob. Agents Chemother. 2008, 48, 2799–2807. [Google Scholar] [CrossRef] [PubMed][Green Version]
- di Paolo, A.; Tascini, C.; Polillo, M.; Gemignani, G.; Nielsen, E.I.; Bocci, G.; Karlsson, M.O.; Menichetti, F.; Danesi, R. Population Pharmacokinetics of Daptomycin in Patients Affected by Severe Gram-Positive Infections. Int. J. Antimicrob. Agents 2013, 42, 250–255. [Google Scholar] [CrossRef]
- Chaves, R.L.; Chakraborty, A.; Benziger, D.; Tannenbaum, S. Clinical and Pharmacokinetic Considerations for the Use of Daptomycin in Patients with Staphylococcus Aureus Bacteraemia and Severe Renal Impairment. J. Antimicrob. Chemother. 2014, 69, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Soon, R.L.; Turner, S.J.; Forrest, A.; Tsuji, B.T.; Brown, J. Pharmacokinetic/Pharmacodynamic Evaluation of the Efficacy and Safety of Daptomycin against Staphylococcus Aureus. Int. J. Antimicrob. Agents 2013, 42, 53–58. [Google Scholar] [CrossRef]
- Xu, X.; Khadzhynov, D.; Peters, H.; Chaves, R.L.; Hamed, K.; Levi, M.; Corti, N. Population Pharmacokinetics of Daptomycin in Adult Patients Undergoing Continuous Renal Replacement Therapy. Br. J. Clin. Pharmacol. 2017, 83, 498–509. [Google Scholar] [CrossRef]
- Xie, F.; Li, S.; Cheng, Z. Population Pharmacokinetics and Dosing Considerations of Daptomycin in Critically Ill Patients Undergoing Continuous Renal Replacement Therapy. J. Antimicrob. Chemother. 2020, 75, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial Therapeutic Drug Monitoring in Critically Ill Adult Patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Liu, Y.; Wang, J.; Cai, L.; He, L.; Yang, X.; Xu, H.; He, X.; Yang, X.; Wei, C.; et al. Population Pharmacokinetics and Individual Analysis of Daptomycin in Kidney Transplant Recipients. Eur. J. Pharm. Sci. 2021, 162, 105818. [Google Scholar] [CrossRef]
- Grégoire, N.; Marchand, S.; Ferrandière, M.; Lasocki, S.; Seguin, P.; Vourc’h, M.; Barbaz, M.; Gaillard, T.; Launey, Y.; Asehnoune, K.; et al. Population Pharmacokinetics of Daptomycin in Critically Ill Patients with Various Degrees of Renal Impairment. J. Antimicrob. Chemother. 2018, 74, 117–125. [Google Scholar] [CrossRef]
- Geriak, M.; Haddad, F.; Rizvi, K.; Rose, W.; Kullar, R.; LaPlante, K.; Yu, M.; Vasina, L.; Ouellette, K.; Zervos, M.; et al. Clinical Data on Daptomycin plus Ceftaroline versus Standard of Care Monotherapy in the Treatment of Methicillin-Resistant Staphylococcus Aureus Bacteremia. Antimicrob. Agents Chemother. 2019, 63, e02483-18. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, M.; Li, X.; Xiao, X. Pharmacokinetic/Pharmacodynamic Analysis of Daptomycin Against Staphylococcus aureus and Enterococcus faecium in Pediatric Patients by Monte Carlo Simulation. J. Clin. Pharmacol. 2020, 60, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Satlin, M.J.; Nicolau, D.P.; Humphries, R.M.; Kuti, J.L.; Campeau, S.A.; Lewis II, J.S.; Weinstein, M.P.; Jorgensen, J.H. Development of Daptomycin Susceptibility Breakpoints for Enterococcus Faecium and Revision of the Breakpoints for Other Enterococcal Species by the Clinical and Laboratory Standards Institute. Clin. Infect. Dis. 2019, 70, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Ogami, C.; Tsuji, Y.; Kasai, H.; Hiraki, Y.; Yamamoto, Y. Evaluation of Pharmacokinetics and the Stability of Daptomycin in Serum at Various Temperatures. Int. J. Infect. Dis. 2017, 57, 38–43. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cockcroft, D.W.; Gault, H. Prediction of Creatinine Clearance from Serum Creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Tobin, C.M.; Darville, J.M.; Lovering, A.M.; MacGowan, A.P. An HPLC Assay for Daptomycin in Serum. J. Antimicrob. Chemother. 2008, 62, 1462–1463. [Google Scholar] [CrossRef]
- Tanaka, R.; Suzuki, Y.; Goto, K.; Yasuda, N.; Koga, H.; Kai, S.; Ohchi, Y.; Sato, Y.; Kitano, T.; Itoh, H. Development and Validation of Sensitive and Selective Quantification of Total and Free Daptomycin in Human Plasma Using Ultra-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 2019, 165, 56–64. [Google Scholar] [CrossRef]
- Lindbom, L.; Pihlgren, P.; Jonsson, N. PsN-Toolkit—A Collection of Computer Intensive Statistical Methods for Non-Linear Mixed Effect Modeling Using NONMEM. Comput. Methods Programs Biomed. 2005, 79, 241–257. [Google Scholar] [CrossRef]
- Jun, H.; Rong, Y.; Yih, C.; Ho, J.; Cheng, W.; Kiang, T.K.L. Comparisons of Four Protein-Binding Models Characterizing the Pharmacokinetics of Unbound Phenytoin in Adult Patients Using Non-Linear Mixed-Effects Modeling. Drugs R & D 2020, 20, 343–358. [Google Scholar] [CrossRef]
- Aulin, L.B.S.; de Paepe, P.; Dhont, E.; de Jaeger, A.; vande Walle, J.; Vandenberghe, W.; McWhinney, B.C.; Ungerer, J.P.J.; van Hasselt, J.G.C.; de Cock, P.A.J.G. Population Pharmacokinetics of Unbound and Total Teicoplanin in Critically Ill Pediatric Patients. Clin. Pharmacokinet. 2021, 60, 353–363. [Google Scholar] [CrossRef]
- Charles, B.; Norris, R.; Xiao, X.; Hague, W. Population Pharmacokinetics of Metformin in Late Pregnancy. Ther. Drug Monit. 2006, 28, 67–72. [Google Scholar] [CrossRef]
- Hennig, S.; Norris, R.; Tu, Q.; van Breda, K.; Riney, K.; Foster, K.; Lister, B.; Charles, B. Population Pharmacokinetics of Phenytoin in Critically Ill Children. J. Clin. Pharmacol. 2015, 55, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Toutain, P.L.; Bousquet-Melou, A. Free Drug Fraction vs. Free Drug Concentration: A Matter of Frequent Confusion. J. Vet. Pharmacol. Ther. 2002, 25, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Heine, R.; Kane, S.P.; Huitema, A.D.R.; Krasowski, M.D.; Maarseveen, E.M. Nonlinear Protein Binding of Phenytoin in Clinical Practice: Development and Validation of a Mechanistic Prediction Model. Br. J. Clin. Pharmacol. 2019, 85, 2360–2368. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ohnmacht, C.; Hage, D.S. Studies of Phenytoin Binding to Human Serum Albumin by High-Performance Affinity Chromatography. J. Chromatogr. B 2004, 809, 137–145. [Google Scholar] [CrossRef]
- Musteata, F.M. Calculation of Normalized Drug Concentrations in the Presence of Altered Plasma Protein Binding. Clin. Pharmacokinet. 2012, 51, 55–68. [Google Scholar] [CrossRef]
- Savic, R.M.; Karlsson, M.O. Importance of Shrinkage in Empirical Bayes Estimates for Diagnostics: Problems and Solutions. AAPS J. 2009, 11, 558–569. [Google Scholar] [CrossRef]
- Wade, J.R.; Beal, S.L.; Sambol, N.C. Interaction between Structural, Statistical, and Covariate Models in Population Pharmacokinetic Analysis. J. Pharmacokinet. Biopharm. 1994, 22, 165–177. [Google Scholar] [CrossRef]
- Hooker, A.C.; Staatz, C.E.; Karlsson, M.O. Conditional Weighted Residuals (CWRES): A Model Diagnostic for the FOCE Method. Pharm. Res. 2007, 24, 2187–2197. [Google Scholar] [CrossRef]
- Comets, E.; Brendel, K.; Mentré, F. Computing Normalised Prediction Distribution Errors to Evaluate Nonlinear Mixed-Effect Models: The Npde Add-on Package for R. Comput. Methods Programs Biomed. 2008, 90, 154–166. [Google Scholar] [CrossRef]
- Sherwin, C.M.T.; Kiang, T.K.L.; Spigarelli, M.G.; Ensom, M.H.H. Fundamentals of Population Pharmacokinetic Modelling. Clin. Pharmacokinet. 2012, 51, 573–590. [Google Scholar] [CrossRef]
- Kiang, T.K.L.; Sherwin, C.M.T.; Spigarelli, M.G.; Ensom, M.H.H. Fundamentals of Population Pharmacokinetic Modelling. Clin. Pharmacokinet. 2012, 51, 515–525. [Google Scholar] [CrossRef]
- Bergstrand, M.; Hooker, A.C.; Wallin, J.E.; Karlsson, M.O. Prediction-Corrected Visual Predictive Checks for Diagnosing Nonlinear Mixed-Effects Models. AAPS J. 2011, 13, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Bhavnani, S.M.; Rubino, C.M.; Ambrose, P.G.; Drusano, G.L. Daptomycin Exposure and the Probability of Elevations in the Creatine Phosphokinase Level: Data from a Randomized Trial of Patients with Bacteremia and Endocarditis. Clin. Infect. Dis. 2010, 12208, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Mouton, J.W.; Dudley, M.N.; Cars, O.; Derendorf, H.; Drusano, G.L. Standardization of Pharmacokinetic/Pharmacodynamic (PK/PD) Terminology for Anti-Infective Drugs: An Update. J. Antimicrob. Chemother. 2005, 55, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, H.; Tsuji, Y.; Yamashina, T.; Tsuruta, M.; Hiraki, Y.; Tsuruyama, M.; Ogami, C.; Kawasuji, H.; Sakamaki, I.; Yamamoto, Y. Pharmacokinetics and Pharmacodynamics of Daptomycin in a Clinical Setting. J. Infect. Chemother. 2020, 26, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Safdar, N.; Andes, D.; Craig, W.A. In Vivo Pharmacodynamic Activity of Daptomycin. Antimicrob. Agents Chemother. 2004, 48, 63–68. [Google Scholar] [CrossRef]
- Schneider, E.K.; Huang, J.X.; Carbone, V.; Han, M.; Zhu, Y.; Nang, S.; Khoo, K.K.; Mak, J.; Cooper, M.A.; Li, J.; et al. Plasma Protein Binding Structure-Activity Relationships Related to the N-Terminus of Daptomycin. ACS Infect. Dis. 2017, 3, 249–258. [Google Scholar] [CrossRef]
- Sellers, E.M.; Koch-Weser, J. Clinical Implications of Drug-Albumin Interaction. In Albumin: Structure, Function and Uses; Rosenoer, V.M., Oratz, M., Rothschild, M.A., Eds.; Pergamon Press: Oxford, UK, 1977; pp. 159–182. [Google Scholar]
- Lai, C.-C.; Sheng, W.-H.; Wang, J.-T.; Cheng, A.; Chuang, Y.-C.; Chen, Y.-C.; Chang, S.-C. Safety and Efficacy of High-Dose Daptomycin as Salvage Therapy for Severe Gram-Positive Bacterial Sepsis in Hospitalized Adult Patients. BMC Infect. Dis. 2013, 13, 66. [Google Scholar] [CrossRef]
- Durante-Mangoni, E.; Andini, R.; Parrella, A.; Mattucci, I.; Cavezza, G.; Senese, A.; Trojaniello, C.; Caprioli, R.; Diana, M.V.; Utili, R. Safety of Treatment with High-Dose Daptomycin in 102 Patients with Infective Endocarditis. Int. J. Antimicrob. Agents 2016, 48, 61–68. [Google Scholar] [CrossRef]
- Casapao, A.M.; Kullar, R.; Davis, S.L.; Levine, D.P.; Zhao, J.J.; Potoski, B.A.; Goff, D.A.; Crank, C.W.; Segreti, J.; Sakoulas, G.; et al. Multicenter Study of High-Dose Daptomycin for Treatment of Enterococcal Infections. Antimicrob. Agents Chemother. 2013, 57, 4190–4196. [Google Scholar] [CrossRef]
- Kullar, R.; Casapao, A.M.; Davis, S.L.; Levine, D.P.; Zhao, J.J.; Crank, C.W.; Segreti, J.; Sakoulas, G.; Cosgrove, S.E.; Rybak, M.J. A Multicentre Evaluation of the Effectiveness and Safety of High-Dose Daptomycin for the Treatment of Infective Endocarditis. J. Antimicrob. Chemother. 2013, 68, 2921–2926. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.E.; Lindfield, K.C.; Steenbergen, J.N.; Benziger, D.P.; Blackerby, K.J.; Knapp, A.G.; Martone, W.J. A Pilot Study of High-Dose Short Duration Daptomycin for the Treatment of Patients with Complicated Skin and Skin Structure Infections Caused by Gram-Positive Bacteria. Int. J. Clin. Pract. 2008, 62, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Moise, P.A.; Hershberger, E.; Amodio-Groton, M.I.; Lamp, K.C. Safety and Clinical Outcomes When Utilizing High-Dose (≥8 Mg/Kg) Daptomycin Therapy. Ann. Pharmacother. 2009, 43, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Nicco, E.; Ginocchio, F.; Ansaldi, F.; de Florentiis, D.; Viscoli, C. High-Dose Daptomycin in Documented Staphylococcus Aureus Infections. Int. J. Antimicrob. Agents 2010, 36, 459–461. [Google Scholar] [CrossRef]
- Parra-Ruiz, J.; Peña-Monje, A.; Tomás-Jiménez, C.; Pomares-Mora, J.; Hernández-Quero, J. Eficacia y Seguridad de Daptomicina En Dosis Elevadas (≥8 mg/Kg/Día). Enferm. Infecc. Microbiol. Clin. 2011, 29, 425–427. [Google Scholar] [CrossRef]
- Kullar, R.; Davis, S.L.; Levine, D.P.; Zhao, J.J.; Crank, C.W.; Segreti, J.; Sakoulas, G.; Cosgrove, S.E.; Rybak, M.J. High-Dose Daptomycin for Treatment of Complicated Gram-Positive Infections: A Large, Multicenter, Retrospective Study. Pharmacotherapy 2011, 31, 527–536. [Google Scholar] [CrossRef]
- Gregoire, N.; Chauzy, A.; Buyck, J.; Rammaert, B.; Couet, W.; Marchand, S. Clinical Pharmacokinetics of Daptomycin. Clin. Pharmacokinet. 2020, 60, 271–281. [Google Scholar] [CrossRef]
- Reiber, C.; Senn, O.; Müller, D.; Kullak-ublick, G.A. Therapeutic Drug Monitoring of Daptomycin: A Retrospective Monocentric Analysis. Ther. Drug Monit. 2015, 37, 634–640. [Google Scholar] [CrossRef]
- Cojutti, P.G.; Candoni, A.; Ramos-martin, V.; Lazzarotto, D.; Zannier, M.E.; Fanin, R.; Hope, W.; Pea, F.; Carlo, M. Population Pharmacokinetics and Dosing Considerations for the Use of Daptomycin in Adult Patients with Haematological Malignancies. J. Antimicrob. Chemother. 2017, 72, 2342–2350. [Google Scholar] [CrossRef]
- Campos Moreno, E.; Merino Sanjuán, M.; Merino, V.; Nácher, A.; Algarra, R.V.M.; Casabó, V.G. Population Modelling to Describe Pharmacokinetics of Amiodarone in Rats: Relevance of Plasma Protein and Tissue Depot Binding. Eur. J. Pharm. Sci. 2007, 30, 190–197. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, K.; Gras-Colomer, E.; Climente-Martí, M.; Mangas-Sanjuán, V.; Merino-Sanjuán, M. Pharmacometric Characterization of Entero-Hepatic Circulation Processes of Orally Administered Formulations of Amiodarone under Complex Binding Kinetics. Eur. J. Pharm. Sci. 2022, 174, 106198. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | 46 Patients Median (IQR)/n (%) | |
|---|---|---|
| Demographics | Sex (Male) (n, %) | 43 (93%) |
| Age (years) | 68 (59–81) | |
| Body weight (kg) | 75 (65–85) | |
| Height (m) | 1.7 (1.6–1.7) | |
| BMI (Kg/m2) | 25.9 (23.4–31.1) | |
| Treatment | Dose (mg) | 675 (500–765) |
| Dose per kilogram (mg/kg) | 9.1 (7.5–10.0) | |
| Treatment duration (days) | 11 (7–15) | |
| Clinical data | Serum albumin (g/dL) | 2.9 (2.4–3.4) |
| Serum protein (g/dL) | 6.0 (5.0–6.4) | |
| Serum cretinine (g/dL) | 0.9 (0.6–1.3) | |
| Creatinine clearance (mL/min/1.73 m2) | 93 (50–136) | |
| Renal function | ||
| >90 mL/min/1.73 m2 | 16 (34.9%) | |
| 60–89 mL/min/1.73 m2 | 13 (28.2%) | |
| 30–59 mL/min/1.73 m2 | 14 (30.4%) | |
| 15–29 mL/min/1.73 m2 | 3 (6.5%) | |
| <15 mL/min/1.73 m2 | 0 (0%) | |
| Pathogenic micro-organism (36/46) | S. aureus | 17 (47.2%) |
| S. epidermidis | 12 (33.3%) | |
| S. hominis | 2 (5.6%) | |
| E. fecalis | 2 (5.6%) | |
| S. sacrophyticus | 2 (5.6%) | |
| S. lugdunensis | 1 (2.7%) | |
| MIC micro-organism | 0.5 (0.25, 0.5) | |
| Population PK Model Estimates | Bootstrap Results | |||||
|---|---|---|---|---|---|---|
| Value | RSE (%) | Shrinkage (%) | Median | RSE * (%) | 95%CI | |
| Fixed-Effect | ||||||
| CL (L/h) | 6.98 | 14 | 7.01 | 15 | [6.63–7.44] | |
| V1 (L) | 0.95 | 9 | 0.97 | 10 | [0.92–1.09] | |
| Q (L/h) | 1.96 | 21 | 1.93 | 19 | [1.43–2.48] | |
| V2 (L/h) | 21 | 19 | 20.5 | 21 | [19.3–22.1] | |
| Bmax (mg/L) | 160 | 26 | 157 | 24 | [129–183] | |
| KD (mg/L) | 3.56 | 15 | 3.61 | 12 | [3.17–3.93] | |
| CrCl on CL | 0.19 | 12 | 0.19 | 13 | [0.18–0.22] | |
| Inter-individual variability | ||||||
| CL (%) | 32 | 11 | 12 | 33 | 10 | [21–42] |
| V2 (%) | 47 | 23 | 17 | 46 | 24 | [52–94] |
| Residual unexplained variability | ||||||
| Additive on Log-scale (%) | 22 | 8 | 5 | 21 | 8 | [18–24] |
| Moderate Renal Impairment (CLCR = 30 mL/min/1.73 m2) | Mild Renal Impairment (CLCR = 60 mL/min/1.73 m2) | Normal Renal Function (CLCR = 90 mL/min/1.73 m2) | Body Weight | |
|---|---|---|---|---|
| MIC ≤ 0.5 mg/L | 10 mg/kg q24h | 11 mg/kg q24h | 12 mg/kg q24h | 50 kg |
| 9 mg/kg q24h | 10 mg/kg q24h | 10 mg/kg q24h | 60 kg | |
| 8 mg/kg q24h | 9 mg/kg q24h | 9 mg/kg q24h | 70 kg | |
| 7 mg/kg q24h | 7 mg/kg q24h | 8 mg/kg q24h | 80 kg | |
| 6 mg/kg q24h | 7 mg/kg q24h | 7 mg/kg q24h | 90 kg | |
| 5 mg/kg q24h | 6 mg/kg q24h | 6 mg/kg q24h | 100 kg | |
| MIC ≤ 1 mg/L | 17 * mg/kg q48h | 17 * mg/kg q48h | 17 * mg/kg q48h | 50 kg |
| 16 mg/kg q48h | 17 * mg/kg q48h | 17 * mg/kg q48h | 60 kg | |
| 14 mg/kg q48h | 16 mg/kg q48h | 17 mg/kg q48h | 70 kg | |
| 12 mg/kg q48h | 14 mg/kg q48h | 15 mg/kg q48h | 80 kg | |
| 11 mg/kg q48h | 12 mg/kg q48h | 13 mg/kg q48h | 90 kg | |
| 10 mg/kg q48h | 11 mg/kg q48h | 12 mg/kg q48h | 100 kg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Martínez, T.; Bellés-Medall, M.D.; García-Cremades, M.; Ferrando-Piqueres, R.; Mangas-Sanjuán, V.; Merino-Sanjuan, M. Population Pharmacokinetic/Pharmacodynamic Modelling of Daptomycin for Schedule Optimization in Patients with Renal Impairment. Pharmaceutics 2022, 14, 2226. https://doi.org/10.3390/pharmaceutics14102226
García-Martínez T, Bellés-Medall MD, García-Cremades M, Ferrando-Piqueres R, Mangas-Sanjuán V, Merino-Sanjuan M. Population Pharmacokinetic/Pharmacodynamic Modelling of Daptomycin for Schedule Optimization in Patients with Renal Impairment. Pharmaceutics. 2022; 14(10):2226. https://doi.org/10.3390/pharmaceutics14102226
Chicago/Turabian StyleGarcía-Martínez, Teresa, María Dolores Bellés-Medall, Maria García-Cremades, Raúl Ferrando-Piqueres, Victor Mangas-Sanjuán, and Matilde Merino-Sanjuan. 2022. "Population Pharmacokinetic/Pharmacodynamic Modelling of Daptomycin for Schedule Optimization in Patients with Renal Impairment" Pharmaceutics 14, no. 10: 2226. https://doi.org/10.3390/pharmaceutics14102226
APA StyleGarcía-Martínez, T., Bellés-Medall, M. D., García-Cremades, M., Ferrando-Piqueres, R., Mangas-Sanjuán, V., & Merino-Sanjuan, M. (2022). Population Pharmacokinetic/Pharmacodynamic Modelling of Daptomycin for Schedule Optimization in Patients with Renal Impairment. Pharmaceutics, 14(10), 2226. https://doi.org/10.3390/pharmaceutics14102226








