Lidosomes: Innovative Vesicular Systems Prepared from Lidocaine Surfadrug
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Lidosomes Preparation
2.3. Niosomal Characterization
Determination of the Amount of LID in Vesicular Systems
2.4. Ex-Vivo Permeation Study
2.5. Skin LID Retention Studies
2.6. Statistical Analysis
3. Results and Discussion
LD Stability
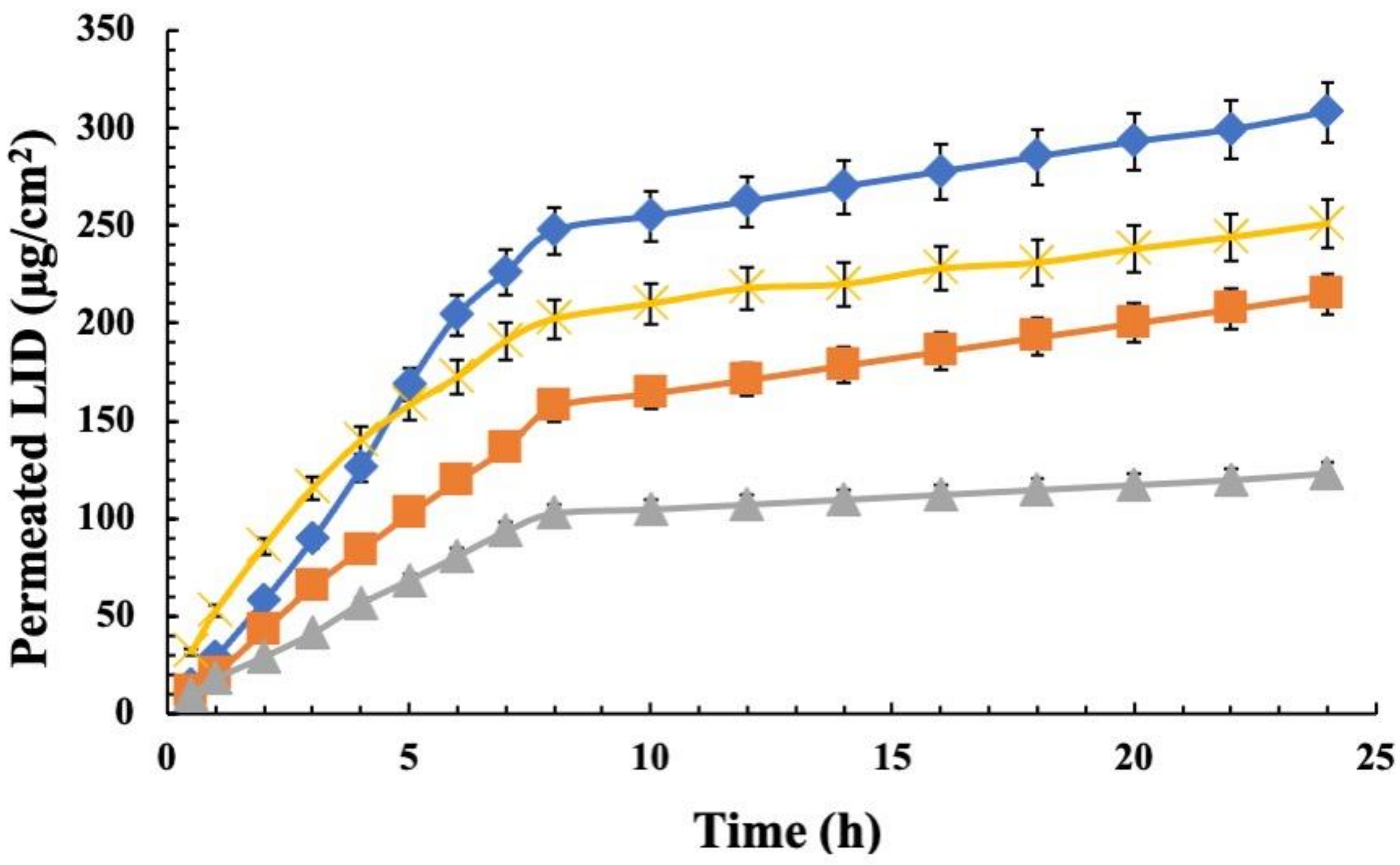
4. Ex-Vivo Permeation Studies
4.1. Lidosomes
4.2. Gel Formulations and Combined Drug Therapy
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarheed, O.; Dibi, M.; Ramesh, K.; Drechsler, M. Fabrication of Alginate-Based O/W Nanoemulsions for Transdermal Drug Delivery of Lidocaine: Influence of the Oil Phase and Surfactant. Molecules 2021, 26, 2556. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Shi, P. Transcriptional transactivator peptide modified lidocaine-loaded nanoparticulate drug delivery system for topical anesthetic therapy. Drug Deliv. 2016, 23, 3193–3199. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, M.; Zhao, J.; Zhang, X.; Huang, Z.; Zang, Y.; Ding, Y.; Zhang, J.; Ding, Z. Transdermal Delivery of Lidocaine-Loaded Elastic Nano-Liposomes with Microneedle Array Pretreatment. Biomedicines 2021, 9, 592. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Q.; Zhang, B.L.; Chu, H.Q.; Liang, L.; Chen, B.Z.; Zheng, H.; Guo, X.D. A high-dosage microneedle for programmable lidocaine delivery and enhanced local long-lasting analgesia. Biomater. Adv. 2022, 133, 112620. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liao, X.; Zheng, B. Studies on local anesthetic lidocaine hydrochloride delivery via photo-triggered implantable polymeric microneedles as a patient-controlled transdermal analgesia system. J. Biomater. Sci. Polym. Ed. 2022, 33, 155–173. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B. Editorial (thematic issue: “Nanosize drug delivery system”). Curr. Pharm. Biotechnol. 2013, 14, 1221. [Google Scholar] [CrossRef]
- Yasamineh, S.; Yasamineh, P.; Kalajahi, H.G.; Gholizadeh, O.; Yekanipour, Z.; Afkhami, H.; Eslami, M.; Kheirkhah, A.H.; Taghizadeh, M.; Yazdani, Y.; et al. A state-of-the-art review on the recent advances of niosomes as a targeted drug delivery system. Int. J. Pharm. 2022, 624, 121878. [Google Scholar] [CrossRef] [PubMed]
- Shakiba-Maram, N.; Avarvand, O.K.; Mohtasham, N.; Ahmady, A.Z. Lidocaine Hydrochloride Nanoparticles Preparation using Multiple Emulsions and its Physicochemical Evaluation. Int. J. Nanosci. 2021, 20, 2150022. [Google Scholar] [CrossRef]
- Vigato, A.A.; Machado, I.P.; del Valle, M.; da Ana, P.A.; Sepulveda, A.F.; Yokaichiya, F.; Franco, M.K.K.D.; Loiola, M.C.; Tófoli, G.R.; Cereda, C.M.S.; et al. Monoketonic Curcuminoid-Lidocaine Co-Deliver Using Thermosensitive Organogels: From Drug Synthesis to Epidermis Structural Studies. Pharmaceutics 2022, 14, 293. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Yao, X.; Du, X.; An, S. Local anesthetic lidocaine-encapsulated polymyxin–chitosan nanoparticles delivery for wound healing: In vitro and in vivo tissue regeneration. Drug Deliv. 2021, 28, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Daryab, M.; Faizi, M.; Mahboubi, A.; Aboofazeli, R. Preparation and Characterization of Lidocaine-Loaded, Microemulsion-Based Topical Gels. Iran. J. Pharm. Sci. 2022, 21, e123787. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Y.; Han, S.; Qu, Z.; Zhao, J.; Chen, Y.; Chen, Z.; Duan, J.; Pan, Y.; Tang, X. Penetration enhancement of lidocaine hydrochlorid by a novel chitosan coated elastic liposome for transdermal drug delivery. J. Biomed. Nanotechnol. 2011, 7, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.M.; Hasan, O.A.; El Sisi, A.M. Preparation and optimization of lidocaine transferosomal gel containing permeation enhancers: A promising approach for enhancement of skin permeation. Int. J. Nanomed. 2019, 14, 1551–1562. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, V.R.; Dagade, D.; Hundiwale, D.G.; Patil, K.J. Volumetric studies of aqueous solutions of local anesthetical drug compounds [hydrochlorides of procaine (PC HCl), lidocaine (LC HCl) and tetracaine (TC HCl)] at 298.15 K. J. Mol. Liq. 2011, 164, 239–242. [Google Scholar] [CrossRef]
- Tavano, L.; Nicoletta, F.P.; Picci, N.; Muzzalupo, R. Cromolyn as surface active drug (surfadrug): Effect of the self-association on diffusion and percutaneous permeation. Colloids Surf. B. Biointerfaces 2016, 139, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Tavano, L.; Mazzotta, E.; Muzzalupo, R. Innovative topical formulations from diclofenac sodium used as surfadrug: The birth of Diclosomes. Colloids Surf. B Biointerfaces 2018, 164, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, M.; Jain, N.K. Niosomes: A Controlled and Novel Drug Delivery System. Indian Drugs 1994, 31, 81–86. [Google Scholar]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252, IN26–IN27. [Google Scholar] [CrossRef]
- Fenton, R.R.; Easdale, W.J.; Er, H.M.; O’Mara, S.M.; McKeage, M.J.; Russell, P.J.; Hambley, T.W. Preparation, DNA binding, and in vitro cytotoxicity of a pair of enantiomeric platinum (II) complexes, [(R)-and (S)-3-aminohexahydroazepine] dichloro-platinum (II). Crystal structure of the S enantiomer. J. Med. Chem. 1997, 40, 1090–1098. [Google Scholar]
- Antunes, F.E.; Gentile, L.; Rossi, C.O.; Tavano, L.; Ranieri, G.A. Gels of Pluronic F127 and nonionic surfactants from rheological characterization to controlled drug permeation. Colloids Surf. B Biointerfaces 2011, 87, 42–48. [Google Scholar] [CrossRef]
- Golzari, S.E.; Soleimanpour, H.; Mahmoodpoor, A.; Safari, S.; Ala, A. Lidocaine and Pain Management in the Emergency Department: A Review Article. Anesthesiol. Pain Med. 2014, 3, e15444. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, V.R.; Dagade, D.; Terdale, S.S.; Hundiwale, D.G.; Patil, K.J. Activity and Activity Coefficient Studies of Aqueous Binary Solutions of Procaine, Lidocaine, and Tetracaine Hydrochloride at 298.15 K. J. Chem. Eng. Data 2012, 57, 3114–3122. [Google Scholar] [CrossRef]
- Hata, T.; Matsuki, H.; Kaneshina, S. Effect of local anesthetics on the bilayer membrane of dipalmitoylphosphatidylcholine: Interdigitation of lipid bilayer and vesicle–micelle transition. Biophys. Chem. 2000, 87, 25–36. [Google Scholar] [CrossRef]
- Arora, P.; Mukherjee, B. Design, development, physicochemical, and in vitro and in vivo evaluation of transdermal patches containing diclofenac diethylammonium salt. J. Pharm. Sci. 2002, 91, 2076–2089. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.A.; Bialik, W.; Brain, K.R. The effects of surfactants on penetration across the skin. Int. J. Cosmet. Sci. 1993, 15, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Som, I.; Bhatia, K. Status of surfactants as penetration enhancers in transdermal drug delivery. J. Pharm. Bioallied Sci. 2012, 4, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Tewes, F.; Corrigan, O.I.; Healy, A.M. Surfactants in Pharmaceutical Products and Systems. In Encyclopedia of Pharmaceutical Science and Technology, 4th ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 3464–3476. [Google Scholar]
- Nascimento, M.H.M.; Franco, M.K.K.D.; Yokaichyia, F.; de Paula, E.; Lombello, C.B.; de Araujo, D.R. Hyaluronic acid in Pluronic F-127/F-108 hydrogels for postoperative pain in arthroplasties: Influence on physico-chemical properties and structural requirements for sustained drug-release. Int. J. Biol. Macromol. 2018, 111, 1245–1254. [Google Scholar] [CrossRef]
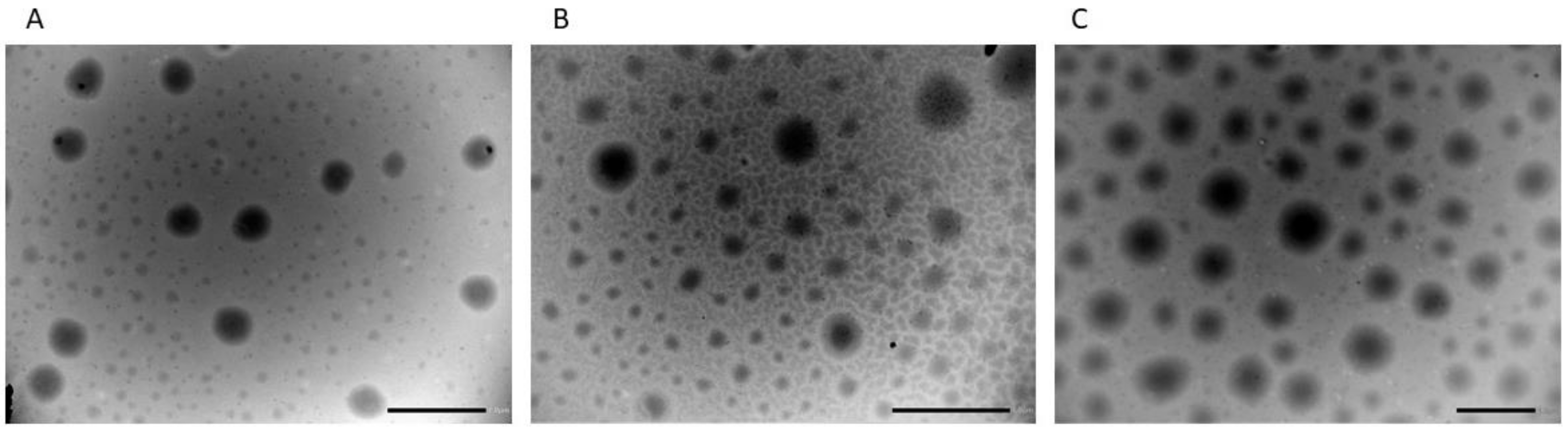


| Formulation | LID (mg) | CA (mg) | Hydration Medium |
|---|---|---|---|
| LD HCl A | 14 | H2O | |
| LD HCl B | 27 | H2O | |
| LD 5.5 A | 27 | H2O pH 5.5 | |
| LD 5.5 B | 30 | H2O pH 5.5 | |
| LD | 30 | H2O | |
| LD:CA | 27 | 4 | H2O |
| LD 7.9 | 30 | H2O pH 7.9 | |
| LD HCl 7.9 | 30 | H2O pH 7.9 |
| Formulation | Diameter (nm) | I.P. | ζ-Potential (mV) | DL(%) LID | E(%) CA |
|---|---|---|---|---|---|
| LD HCl A | 707 ± 15 | 0.249 | −13.0 ± 0.709 | 2.13% ± 0.2 | - |
| LD HCl B | 506 ± 12 | 0.288 | −14.3 ± 1.190 | 1.19% ± 0.7 | - |
| LD 5.5 A | 430 ± 10 | 0.264 | −26.1 ± 0.900 | 19.5% ± 0.5 | - |
| LD 5.5 B | 574 ± 11 | 0.271 | −27.6 ±0.833 | 35.6% ± 0.3 | - |
| LD | 437 ± 10 | 0.229 | −23.5 ± 0.208 | 37.1% ± 0.2 | - |
| LD:CA | 519 ± 14 | 0.277 | −23.1 ±0.493 | 67.5% ± 0.2 | 87.75% ± 0.8 |
| LD 7.9 | 512 ± 11 | 0.287 | −31.2 ±0.666 | 61.2% ± 0.3 | - |
| LD HCl 7.9 | 612 ± 13 | 0.277 | −30.5 ±0.351 | 64.8% ± 0.6 | - |
| Formulations | Time (day) | Diameter (nm) | P.I. | ζ-potential (mV) | DL% |
|---|---|---|---|---|---|
| LD | 0 | 437 ± 10 | 0.229 | −23.5 ± 0.208 | 37.1 ± 0.2 |
| 15 | 321 ± 9 | 0.235 | −23.2 ± 0.907 | 36.9 ± 0.3 | |
| 30 | 305± 11 | 0.295 | −22.3 ± 0.819 | 37.3 ± 0.2 | |
| 60 | 253± 9 | 0.180 | −21.4 ± 0.896 | 36.7 ± 0.5 | |
| 90 | 175 ± 9 | 0.294 | −19.3 ± 0.451 | 36.8 ± 0.4 | |
| LD 5.5 B | 0 | 574 ± 15 | 0.271 | −27.6 ±0.833 | 35.6 ± 0.3 |
| 15 | 570 ± 17 | 0.204 | −27.5 ± 0.173 | 34.3 ± 0.2 | |
| 30 | 520 ± 19 | 0.235 | −27.4 ± 0.864 | 34.4 ± 0.3 | |
| 60 | 506 ± 19 | 0.215 | −25.7 ± 0.366 | 33.4 ± 0.2 | |
| 90 | 501± 18 | 0.276 | −26.5 ± 0.456 | 33.3 ± 0.2 |
| Formulation | LID Retained into Skin (μg/cm2) |
|---|---|
| LD | 387.02 |
| LD 5.5 B | 352.16 |
| LD 7.9 | 545.67 |
| LID SOL | 165.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romeo, M.; Mazzotta, E.; Perrotta, I.D.; Muzzalupo, R. Lidosomes: Innovative Vesicular Systems Prepared from Lidocaine Surfadrug. Pharmaceutics 2022, 14, 2190. https://doi.org/10.3390/pharmaceutics14102190
Romeo M, Mazzotta E, Perrotta ID, Muzzalupo R. Lidosomes: Innovative Vesicular Systems Prepared from Lidocaine Surfadrug. Pharmaceutics. 2022; 14(10):2190. https://doi.org/10.3390/pharmaceutics14102190
Chicago/Turabian StyleRomeo, Martina, Elisabetta Mazzotta, Ida Daniela Perrotta, and Rita Muzzalupo. 2022. "Lidosomes: Innovative Vesicular Systems Prepared from Lidocaine Surfadrug" Pharmaceutics 14, no. 10: 2190. https://doi.org/10.3390/pharmaceutics14102190
APA StyleRomeo, M., Mazzotta, E., Perrotta, I. D., & Muzzalupo, R. (2022). Lidosomes: Innovative Vesicular Systems Prepared from Lidocaine Surfadrug. Pharmaceutics, 14(10), 2190. https://doi.org/10.3390/pharmaceutics14102190








