Hydrophilic Scaffolds Containing Extracts of Stryphnodendron adstringens and Abarema cochliacarpa for Wound Healing: In Vivo Proofs of Concept
Abstract
1. Introduction
2. Methods
2.1. Plant Material
2.2. Extract Preparation
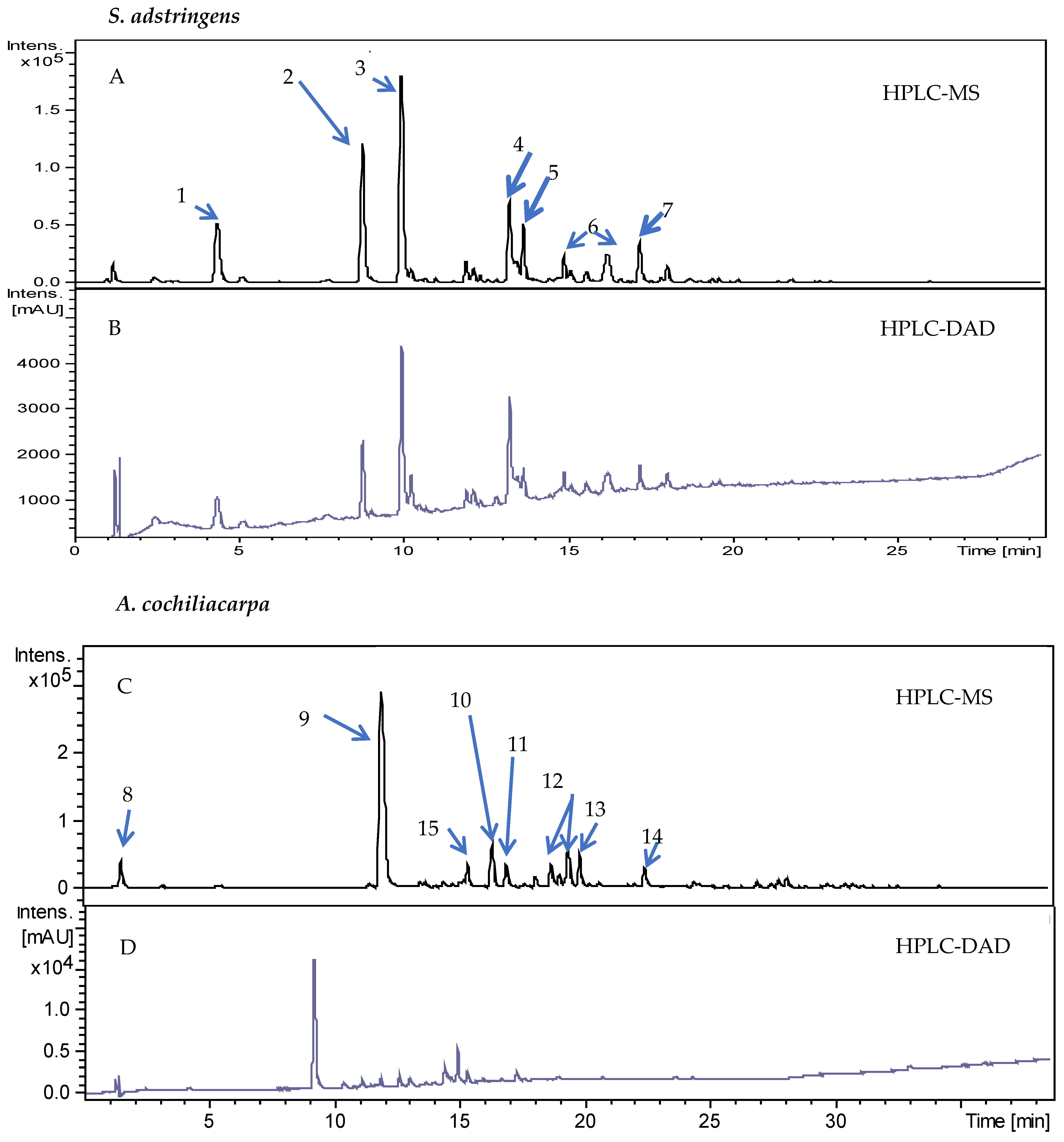
2.3. Chromatographic Analysis
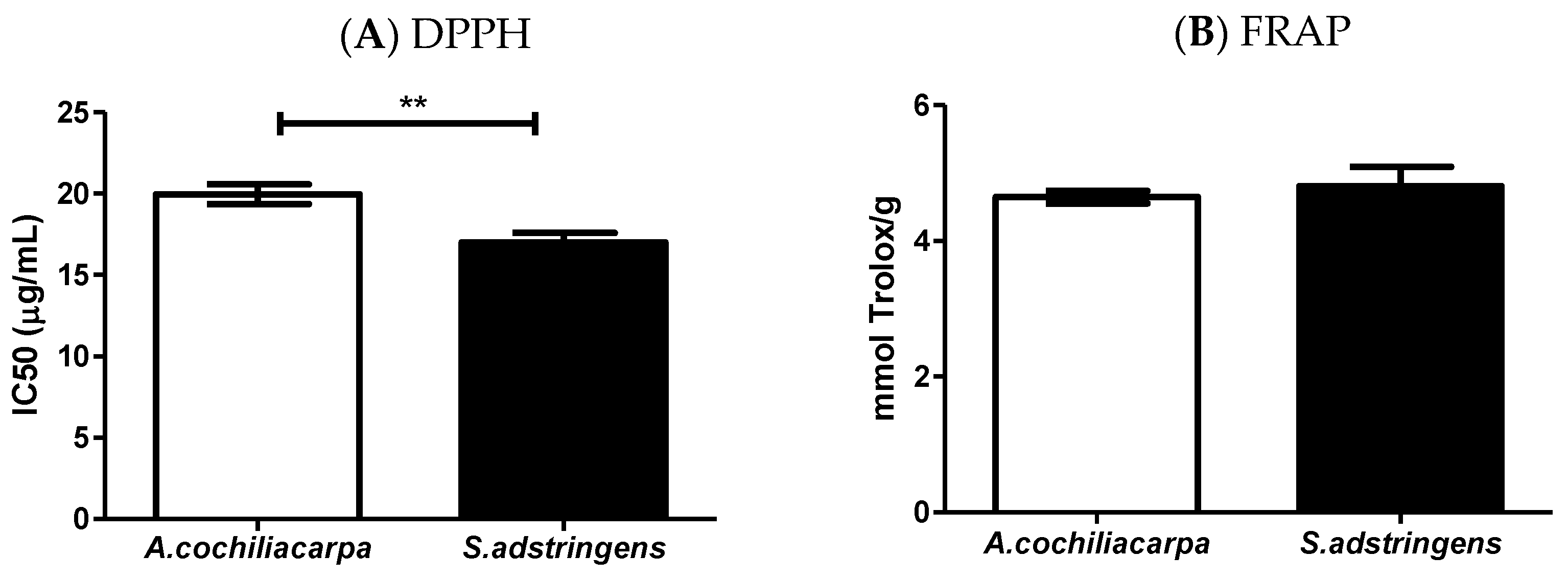
2.4. Extract Antioxidant Evaluation
2.5. Production of Polymeric Biomembranes
2.6. Mechanical Properties
2.7. Water Vapor Permeability
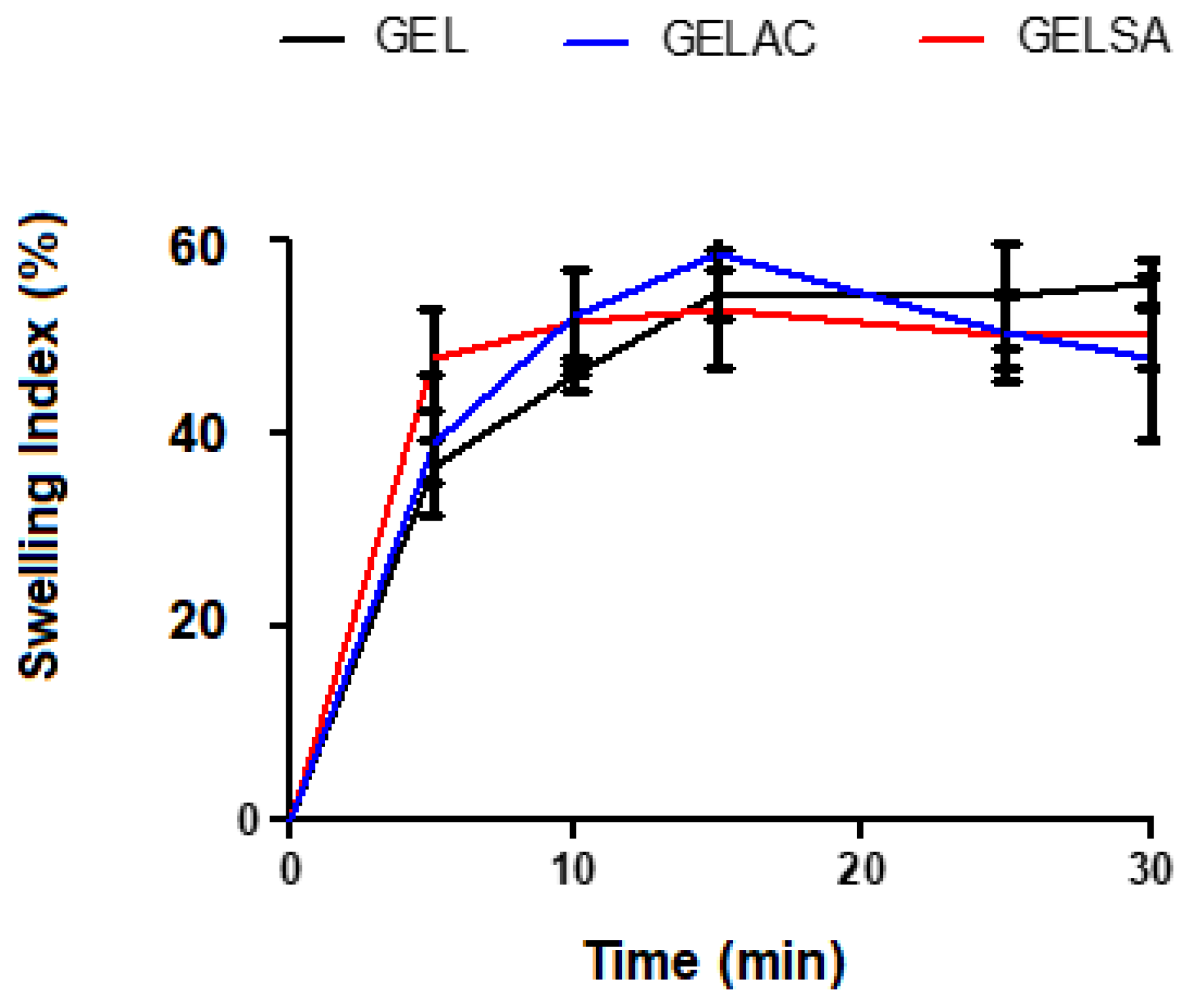
2.8. Swelling Index
2.9. Colorimetry
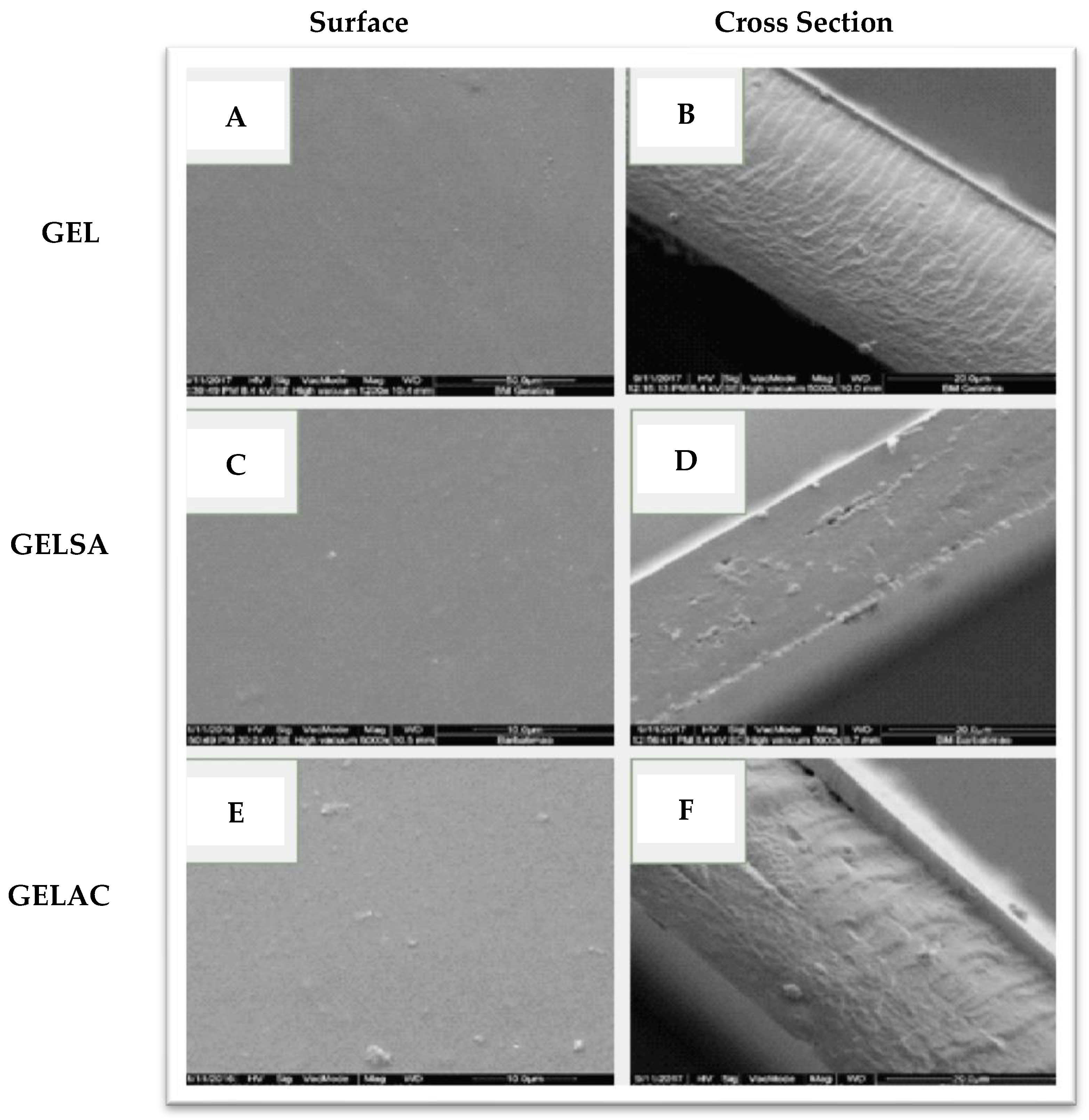
2.10. Scanning Electron Microscopy (SEM)
2.11. Fourier Transform Infrared Absorption Spectroscopy (FTIR)
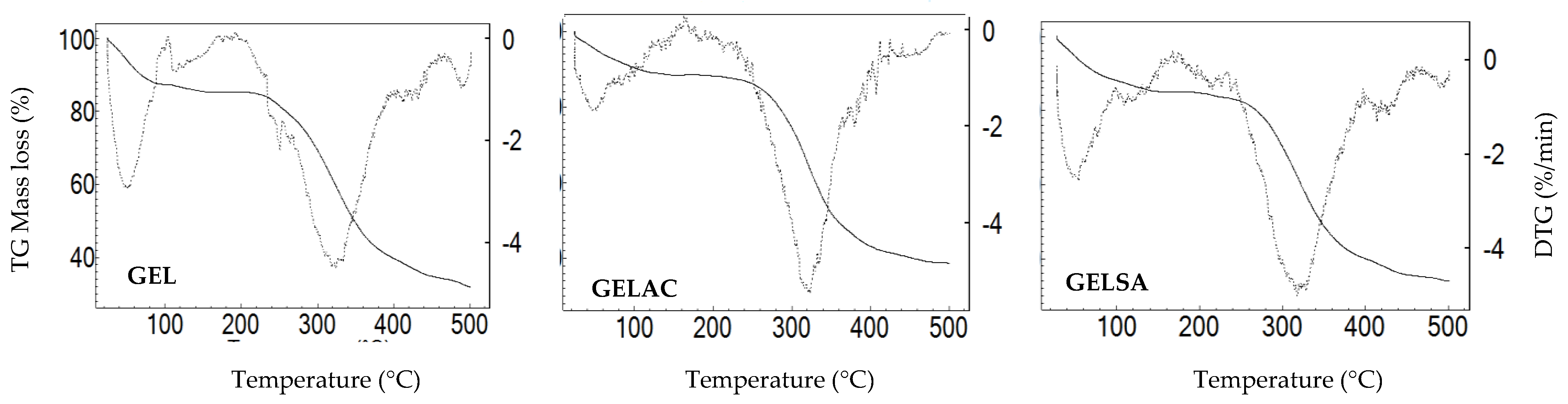
2.12. Thermogravimetric Analysis (TGA)
2.13. In Vivo Healing Biological Assay
2.13.1. Animals and Experimental Groups
2.13.2. Surgical Procedure
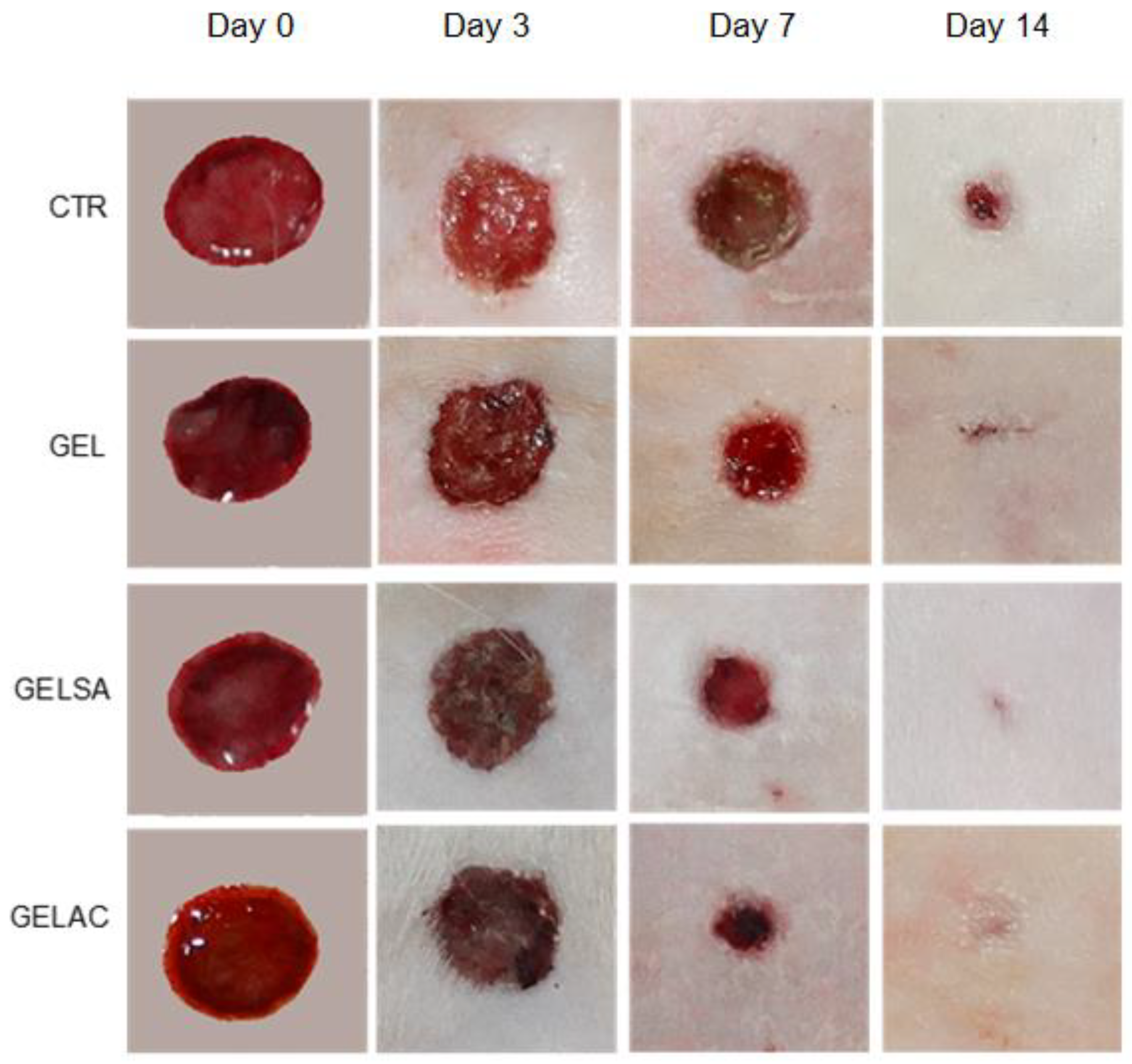
2.13.3. Determination of Wound Closure Index
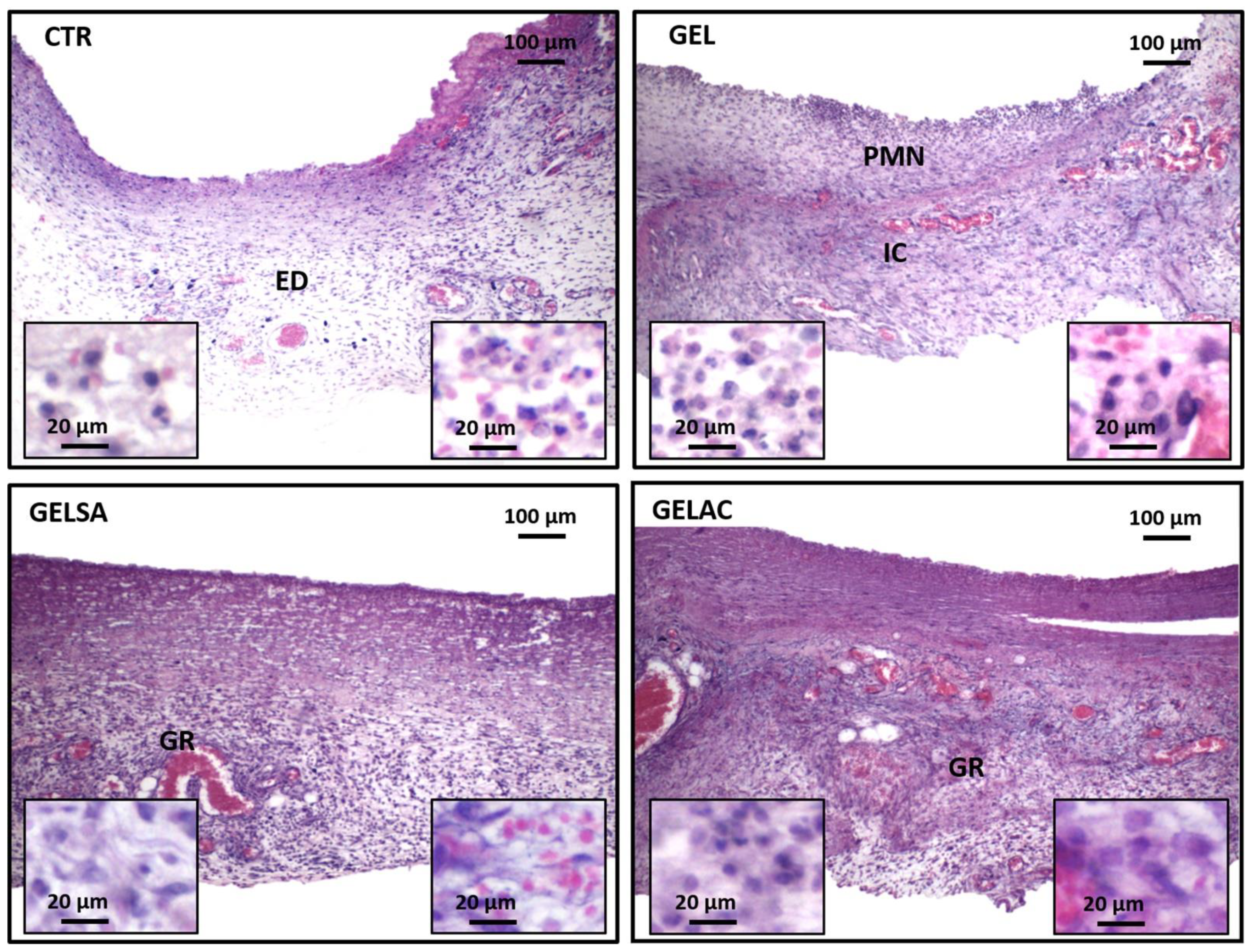
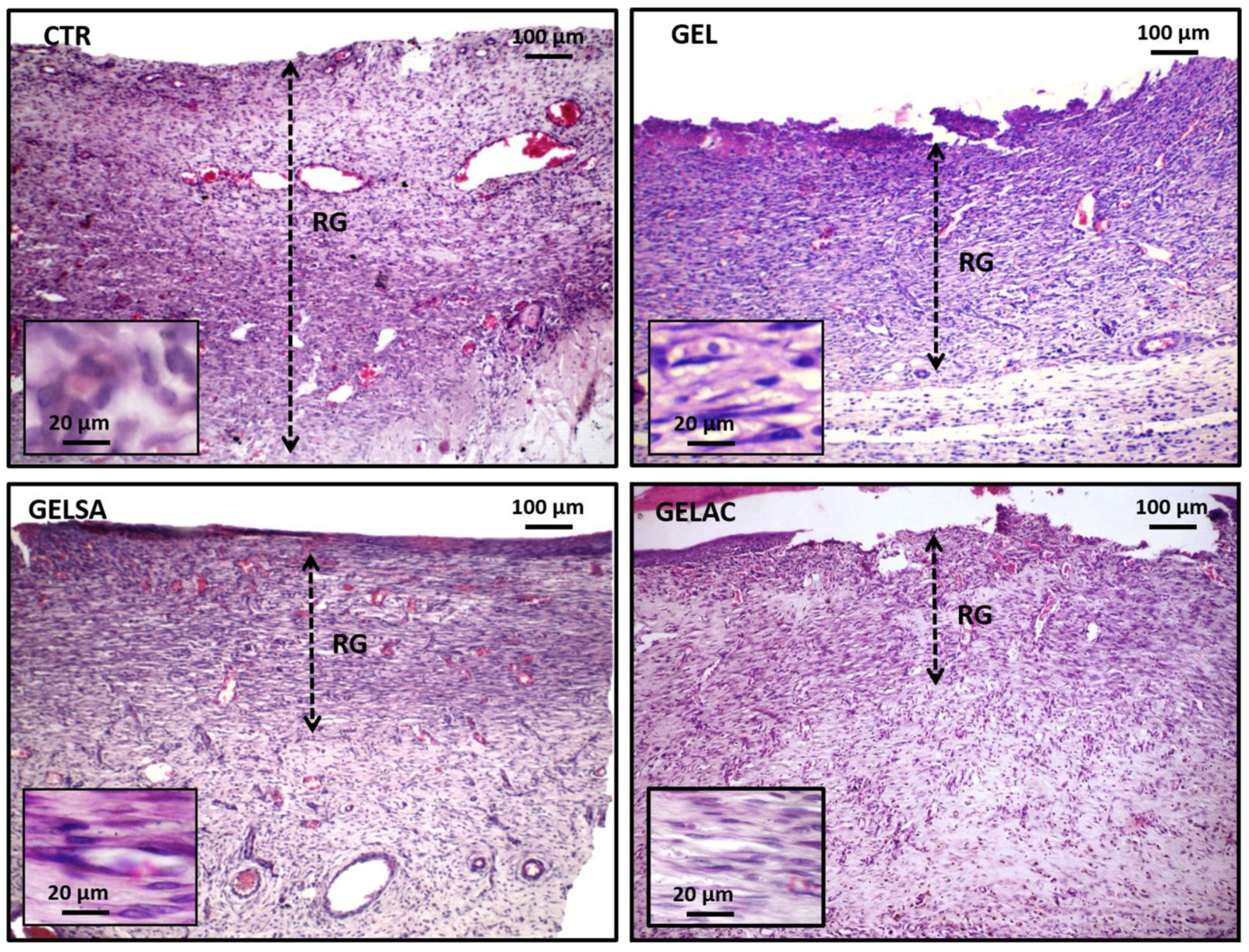
2.13.4. Removal of Specimens and Histological/Histochemical Procedures
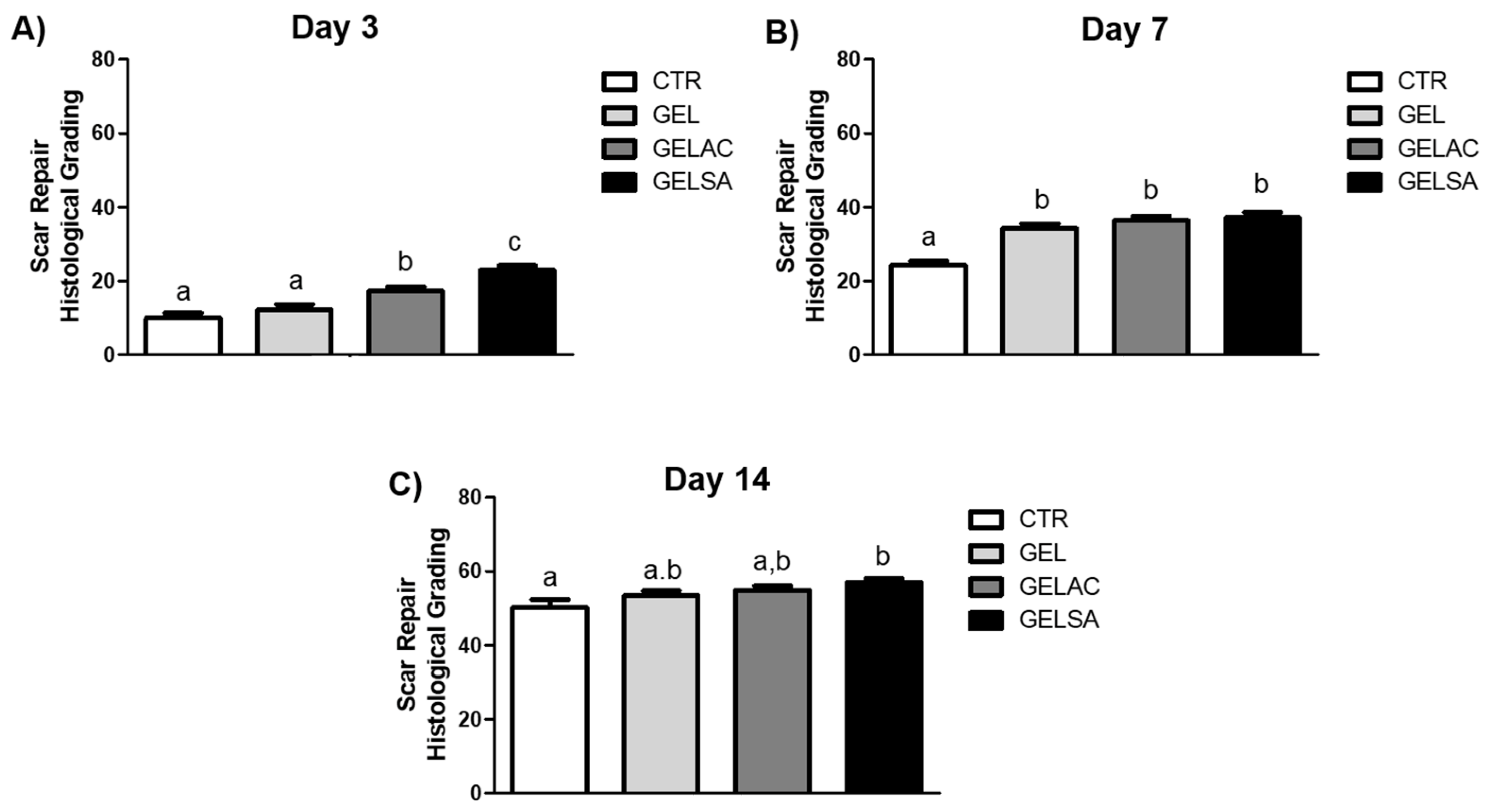
2.13.5. Assessment of the Histological Grading of Wound Repair
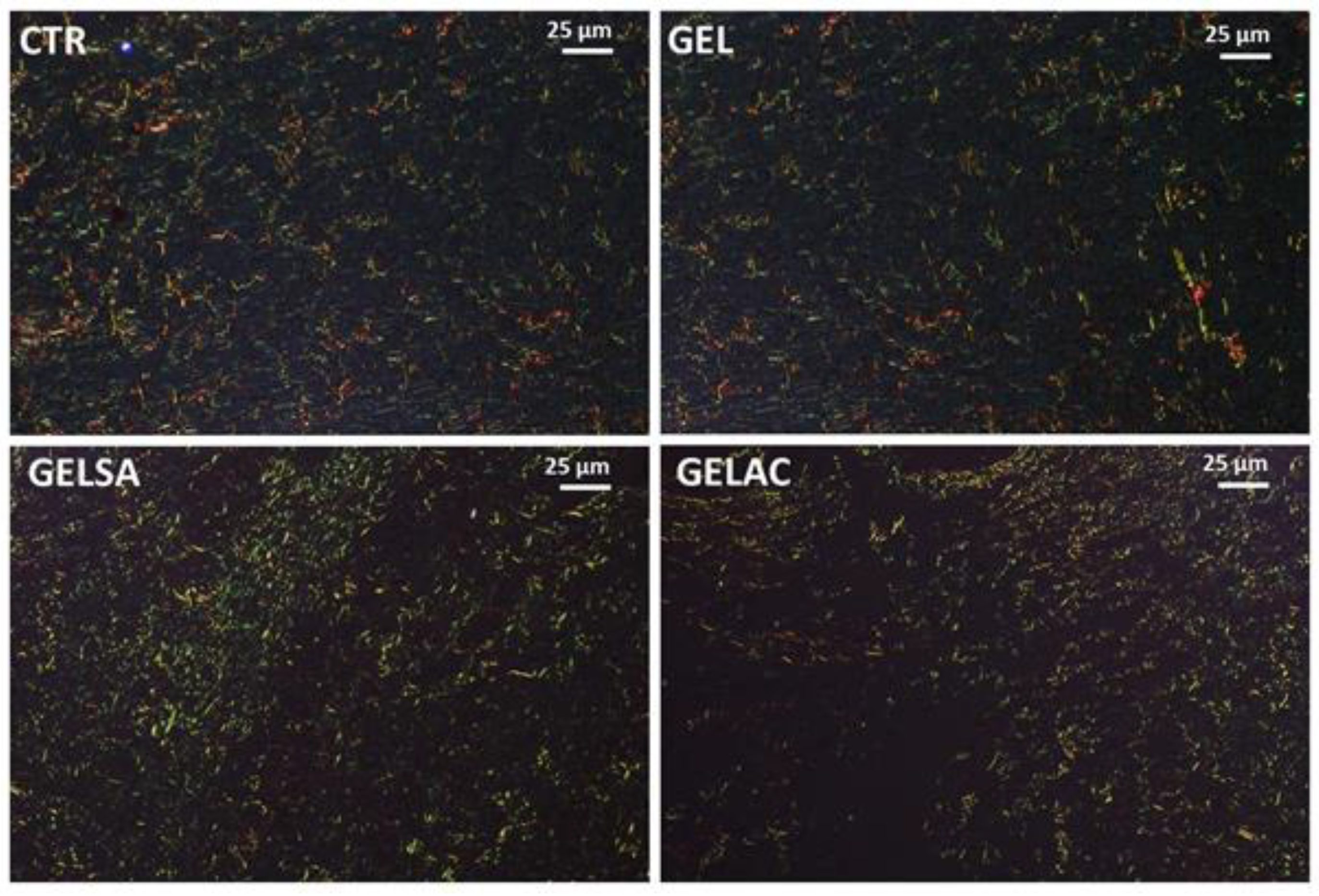
2.13.6. Analysis of Collagen Deposition
2.14. Statistical Analysis
3. Results and Discussion
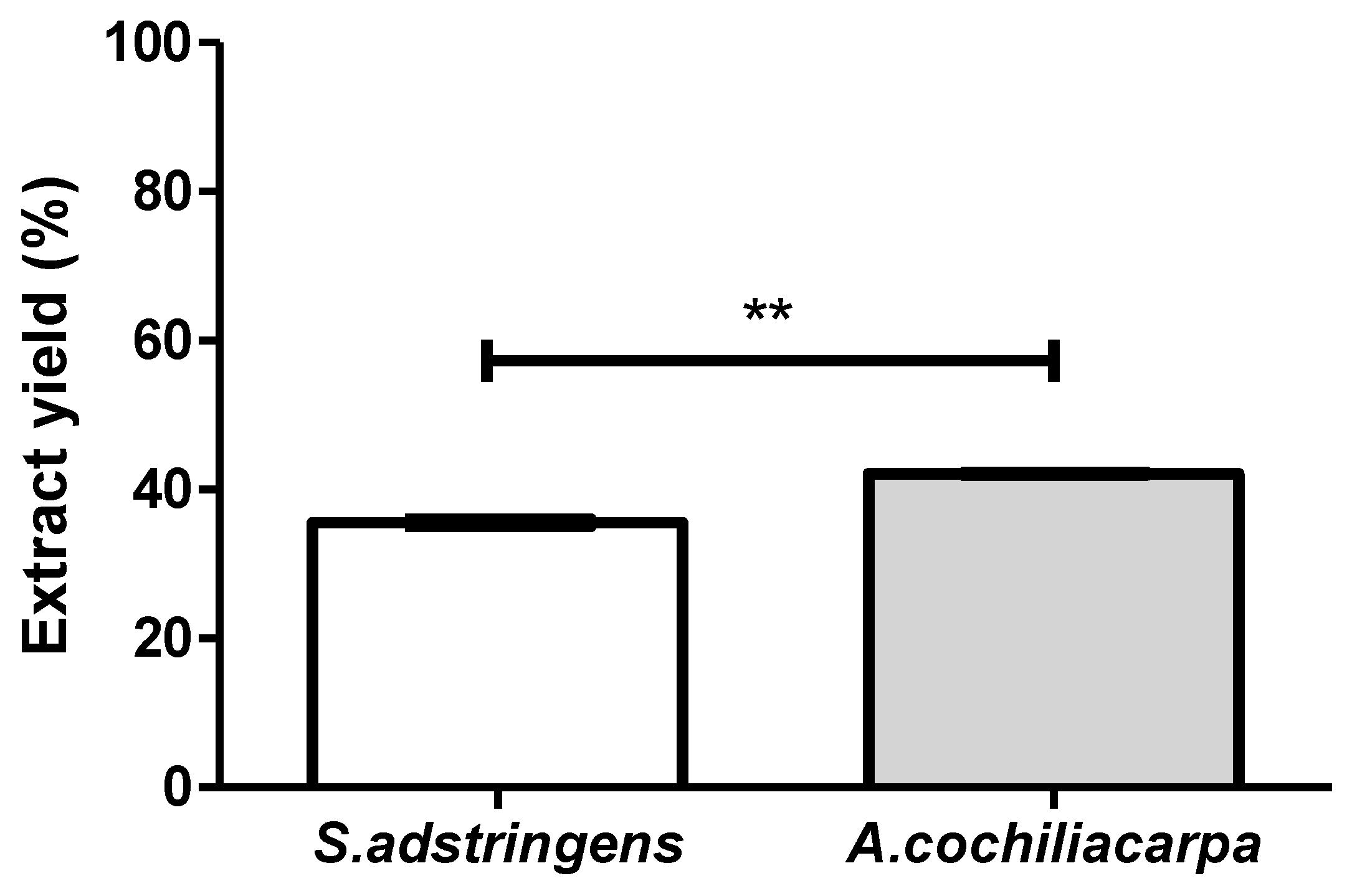
3.1. Extract Characterization
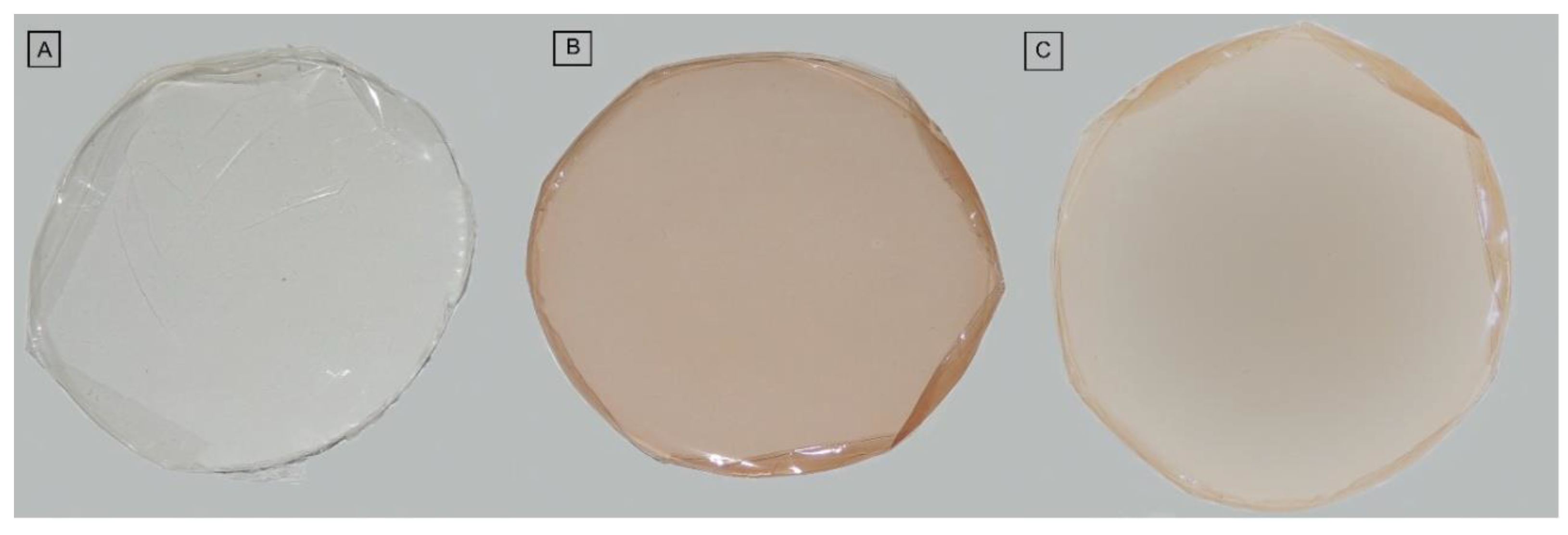
3.2. Characterization of Biomembranes
3.3. Biological Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef]
- Laurano, R.; Boffito, M.; Ciardelli, G.; Chiono, V. Wound dressing products: A translational investigation from the bench to the market. Eng. Regen. 2022, 3, 182–200. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Zou, F.; Sun, X.; Wang, X. Elastic, hydrophilic and biodegradable poly (1, 8-octanediol-co-citric acid)/polylactic acid nanofibrous membranes for potential wound dressing applications. Polym. Degrad. Stab. 2019, 166, 163–173. [Google Scholar] [CrossRef]
- Ndlovu, S.P.; Ngece, K.; Alven, S.; Aderibigbe, B.A. Gelatin-Based Hybrid Scaffolds: Promising Wound Dressings. Polymers 2021, 13, 2959. [Google Scholar] [CrossRef] [PubMed]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Jaul, E.; Barron, J.; Rosenzweig, J.P.; Menczel, J. An overview of co-morbidities and the development of pressure ulcers among older adults. BMC Geriatr. 2018, 18, 305. [Google Scholar] [CrossRef] [PubMed]
- Nunes, P.S.; Albuquerque, R.L., Jr.; Cavalcante, D.R.; Dantas, M.D.; Cardoso, J.C.; Bezerra, M.S.; Souza, J.C.; Serafini, M.R.; Quitans, L.J., Jr.; Bonjardim, L.R.; et al. Collagen-based films containing liposome-loaded usnic acid as dressing for dermal burn healing. J. Biomed. Biotechnol. 2011, 2011, 761593. [Google Scholar] [CrossRef] [PubMed]
- Nunes, P.S.; Rabelo, A.S.; Souza, J.C.; Santana, B.V.; da Silva, T.M.; Serafini, M.R.; Dos Passos Menezes, P.; Dos Santos Lima, B.; Cardoso, J.C.; Alves, J.C.; et al. Gelatin-based membrane containing usnic acid-loaded liposome improves dermal burn healing in a porcine model. Int. J. Pharm. 2016, 513, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.S.; Santos, I.; Pereira-Filho, R.N.; Gomes, S.V.F.; Lima-Verde, I.B.; Marques, M.N.; Cardoso, J.C.; Severino, P.; Souto, E.B.; Albuquerque-Junior, R.L.C. Histological Evidence of Wound Healing Improvement in Rats Treated with Oral Administration of Hydroalcoholic Extract of Vitis labrusca. Curr. Issues Mol. Biol. 2021, 43, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, C.M.P.; Pacheco, M.S.; de Moraes, M.A.; Lopes, P.S.; Severino, P.; Souto, E.B.; da Silva, C.F. Effect of Chitosan and Aloe Vera Extract Concentrations on the Physicochemical Properties of Chitosan Biofilms. Polymers 2021, 13, 1187. [Google Scholar] [CrossRef]
- do Nascimento, M.F.; Cardoso, J.C.; Santos, T.S.; Tavares, L.A.; Pashirova, T.N.; Severino, P.; Souto, E.B.; Albuquerque-Junior, R.L.C. Development and Characterization of Biointeractive Gelatin Wound Dressing Based on Extract of Punica granatum Linn. Pharmaceutics 2020, 12, 1204. [Google Scholar] [CrossRef]
- de Carvalho, F.M.d.A.; Schneider, J.K.; de Jesus, C.V.F.; de Andrade, L.N.; Amaral, R.G.; David, J.M.; Krause, L.C.; Severino, P.; Soares, C.M.F.; Caramão Bastos, E.; et al. Brazilian Red Propolis: Extracts Production, Physicochemical Characterization, and Cytotoxicity Profile for Antitumor Activity. Biomolecules 2020, 10, 726. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, K.C.; Barbosa, T.C.; Nery, M.; Chaud, M.V.; da Silva, C.F.; Andrade, L.N.; Correa, C.B.; Jaguer, A.; Padilha, F.F.; Cardoso, J.C.; et al. Antibacterial activity of chitosan/collagen membranes containing red propolis extract. Pharmazie 2020, 75, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Bene, K.; Sinan, K.I.; Zengin, G.; Diuzheva, A.; Jekő, J.; Cziáky, Z.; Aumeeruddy, M.Z.; Xiao, J.; Mahomoodally, M.F. A multidirectional investigation of stem bark extracts of four African plants: HPLC-MS/MS profiling and biological potentials. J. Pharm. Biomed. Anal. 2019, 168, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Nik Salleh, N.N.H.; Othman, F.A.; Kamarudin, N.A.; Tan, S.C. The Biological Activities and Therapeutic Potentials of Baicalein Extracted from Oroxylum indicum: A Systematic Review. Molecules 2020, 25, 5677. [Google Scholar] [CrossRef] [PubMed]
- Shamala, T.; Surendra, B.S.; Chethana, M.V.; Bolakatti, G.; Shanmukhappa, S. Extraction and isolation of Isoflavonoids from stem bark of Bauhinia purpurea (L): Its biological antipsychotic and analgesic activities. Smart Mater. Med. 2022, 3, 179–187. [Google Scholar] [CrossRef]
- Oliveira, R.F.; Ribeiro, P.R.; Santos, G.K.M.; Oliveira, C.S.; Silva, P.R.C.; Oliveira, H.A.; Trindade, R.d.C.; Fernandez, L.G. Evaluation of the hepatotoxicity of Abarema cochliacarpos extracts in mice Mus musculus. Rev. Bras. De Farmacogn. 2013, 23, 674–679. [Google Scholar] [CrossRef][Green Version]
- Pellenz, N.L.; Barbisan, F.; Azzolin, V.F.; Santos Marques, L.P.; Mastella, M.H.; Teixeira, C.F.; Ribeiro, E.E.; da Cruz, I.B.M. Healing activity of Stryphnodendron adstringens (Mart.), a Brazilian tannin-rich species: A review of the literature and a case series. Wound Med. 2019, 26, 100163. [Google Scholar] [CrossRef]
- Nascimento, C.A.; Santos, A.C.M.d.; Silva, D.M.d.; Barbosa, N.R.; Moura, E.L.d.; Balliano, T.L.; Figueiredo, E.V.M.d.S.; Farias, K.F.d.; Pitta, G.B.B. Evidence about properties of the extract of Stryphnodendron adstringens (Mart.) Coville (Barbatimão) for clinical practice. Res. Soc. Dev. 2021, 10, e3010111350. [Google Scholar] [CrossRef]
- Debone, H.S.; Lopes, P.S.; Severino, P.; Yoshida, C.M.P.; Souto, E.B.; da Silva, C.F. Chitosan/Copaiba oleoresin films for would dressing application. Int. J. Pharm. 2019, 555, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, P. Assessment of the histological state of the healing wound. Plast. Aesthetic Res. 2015, 2, 239–242. [Google Scholar] [CrossRef]
- Lopes, G.C.; Sanches, A.C.C.; Nakamura, C.V.; Dias Filho, B.P.; Hernandes, L.; Mello, J.C.P.d. Influence of extracts of Stryphnodendron polyphyllum Mart. and Stryphnodendron obovatum Benth. on the cicatrisation of cutaneous wounds in rats. J. Ethnopharmacol. 2005, 99, 265–272. [Google Scholar] [CrossRef]
- Bisbis, M.B.; Gruda, N.; Blanke, M. Potential impacts of climate change on vegetable production and product quality—A review. J. Clean. Prod. 2018, 170, 1602–1620. [Google Scholar] [CrossRef]
- Lopes, G.C.; Vieira Machado, F.A.; Mendes de Toledo, C.E.; Sakuragui, C.M.; Palazzo de Mello, J.C. Chemotaxonomic significance of 5-deoxyproanthocyanidins in Stryphnodendron species. Biochem. Syst. Ecol. 2008, 36, 925–931. [Google Scholar] [CrossRef]
- Audi, E.A.; Mendes De Toledo, C.E.; Solera Dos Santos, F.; Bellanda, P.R.; Alves-Do-Prado, W.; Ueda-Nakamura, T.; Nakamura, C.V.; Sakuragui, C.M.; Bersani-Amado, C.A.; Palazzo De Mello, J.C. Biological activity and quality control of extract and stem bark from Stryphnodendron adstringens. Acta Farm. Bonaer. 2004, 23, 328–333. [Google Scholar]
- Dias, A.S.; Lima, A.C.B.; Santos, A.L.M.L.; Rabelo, T.K.; Serafini, M.R.; Andrade, C.R.; Fernandes, X.A.; Moreira, J.C.F.; Gelain, D.P.; Estevam, C.S.; et al. Redox properties of Abarema cochliacarpos (Gomes) Barneby & Grime (Fabaceae) stem bark ethanol extract and fractions. Nat. Prod. Res. 2013, 27, 1479–1483. [Google Scholar] [CrossRef]
- da Silva, M.S.; Sánchez-Fidalgo, S.; Talero, E.; Cárdeno, A.; da Silva, M.A.; Villegas, W.; Souza Brito, A.R.; de La Lastra, C.A. Anti-inflammatory intestinal activity of Abarema cochliacarpos (Gomes) Barneby & Grimes in TNBS colitis model. J. Ethnopharmacol. 2010, 128, 467–475. [Google Scholar] [CrossRef]
- Skrzydlewska, E.; Ostrowska, J.; Farbiszewski, R.; Michalak, K. Protective effect of green tea against lipid peroxidation in the rat liver, blood serum and the brain. Phytomedicine Int. J. Phytother. Phytopharm. 2002, 9, 232–238. [Google Scholar] [CrossRef]
- Souza, T.M.; Severi, J.A.; Silva, V.Y.A.; Santos, E.; Pietro, R.C.L.R. Bioprospecção de atividade antioxidante e antimicrobiana da casca de Stryphnodendron adstringens (Mart.) Coville (Leguminosae-Mimosoidae). Rev. Ciências Farm. Básica E Apl. 2009, 28, 221–226. [Google Scholar]
- Uriarte-Montoya, M.H.; Arias-Moscoso, J.L.; Plascencia-Jatomea, M.; Santacruz-Ortega, H.; Rouzaud-Sández, O.; Cardenas-Lopez, J.L.; Marquez-Rios, E.; Ezquerra-Brauer, J.M. Jumbo squid (Dosidicus gigas) mantle collagen: Extraction, characterization, and potential application in the preparation of chitosan-collagen biofilms. Bioresour. Technol. 2010, 101, 4212–4219. [Google Scholar] [CrossRef] [PubMed]
- Drunkler, D.A.; Fallcão, L.D.; Bordignon-Luiz, M.T. Influence of the tannic and gallic acids on stability of betacyanins from red beetroot (Beta vulgaris L.) crude extract. Alim Nutr. 2004, 15, 35–41. [Google Scholar]
- Liang, C.; Ju, W.; Pei, S.; Tang, Y.; Xiao, Y. Pharmacological Activities and Synthesis of Esculetin and Its Derivatives: A Mini-Review. Molecules 2017, 22, 387. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, H.; Ren, L.; Talukder, M.E.; Chen, S.; Shao, J. Study on the Preparation of Cellulose Acetate Separation Membrane and New Adjusting Method of Pore Size. Membranes 2022, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Souza, I.C.L.; Do Nascimento, M.F.; De Souza Neta, R.G.; Dos Santos, J.E.C.; Costa, L.P.; Cardoso, J.C.; De Albuquerque-Junior, R.L.C. Effect of the maltodextrin-induced chemical reticulation on the physical properties and healing potential of collagen-based membranes containing Brazilian red propolis extract. Int. J. Med. Med. Sci. 2013, 5, 514–524. [Google Scholar]
- Walles, T.; Herden, T.; Haverich, A.; Mertsching, H. Influence of scaffold thickness and scaffold composition on bioartificial graft survival. Biomaterials 2003, 24, 1233–1239. [Google Scholar] [CrossRef]
- Pasini Cabello, S.D.; Takara, E.A.; Marchese, J.; Ochoa, N.A. Influence of plasticizers in pectin films: Microstructural changes. Mater. Chem. Phys. 2015, 162, 491–497. [Google Scholar] [CrossRef]
- Hoque, M.S.; Benjakul, S.; Prodpran, T. Properties of film from cuttlefish (Sepia pharaonis) skin gelatin incorporated with cinnamon, clove and star anise extracts. Food Hydrocoll. 2011, 25, 1085–1097. [Google Scholar] [CrossRef]
- Charulatha, V.; Rajaram, A. Influence of different crosslinking treatments on the physical properties of collagen membranes. Biomaterials 2003, 24, 759–767. [Google Scholar] [CrossRef]
- Laftah, W.A.; Hashim, S.; Ibrahim, A.N. Polymer Hydrogels: A Review. Polym. -Plast. Technol. Eng. 2011, 50, 1475–1486. [Google Scholar] [CrossRef]
- Batista, R.A.; Espitia, P.J.P.; Vergne, D.M.C.; Vicente, A.A.; Pereira, P.A.C.; Cerqueira, M.A.; Teixeira, J.A.; Jovanovic, J.; Severino, P.; Souto, E.B.; et al. Development and Evaluation of Superabsorbent Hydrogels Based on Natural Polymers. Polymers 2020, 12, 2173. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, A.; Eder, P.; Rannier, L.; Cardoso, J.C.; Severino, P.; Silva, A.M.; Souto, E.B. Hydrogels for Modified-release Drug Delivery Systems. Curr. Pharm. Des. 2022, 28, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, A.; Bigucci, F.; Cerchiara, T.; Cruciani, F.; Vitali, B.; Luppi, B. Mucoadhesive chitosan/gelatin films for buccal delivery of propranolol hydrochloride. Carbohydr. Polym. 2012, 87, 581–588. [Google Scholar] [CrossRef]
- Hinrichs, W.L.J.; Lommen, E.J.C.M.P.; Wildevuur, C.R.H.; Feijen, J. Fabrication and characterization of an asymmetric polyurethane membrane for use as a wound dressing. J. Appl. Biomater. 1992, 3, 287–303. [Google Scholar] [CrossRef]
- Zorzi Bueno, C.; Maria Moraes, Â. Development of porous lamellar chitosan-alginate membranes: Effect of different surfactants on biomaterial properties. J. Appl. Polym. Sci. 2011, 122, 624–631. [Google Scholar] [CrossRef]
- Rattaya, S.; Benjakul, S.; Prodpran, T. Properties of fish skin gelatin film incorporated with seaweed extract. J. Food Eng. 2009, 95, 151–157. [Google Scholar] [CrossRef]
- Bodini, R.B.; Sobral, P.J.A.; Favaro-Trindade, C.S.; Carvalho, R.A. Properties of gelatin-based films with added ethanol–propolis extract. LWT-Food Sci. Technol. 2013, 51, 104–110. [Google Scholar] [CrossRef]
- Lakra, R.; Kiran, M.S.; Usha, R.; Mohan, R.; Sundaresan, R.; Korrapati, P.S. Enhanced stabilization of collagen by furfural. Int. J. Biol. Macromol. 2014, 65, 252–257. [Google Scholar] [CrossRef]
- de Menezes, A.S.; Remédios, C.M.R.; Sasaki, J.M.; da Silva, L.R.D.; Góes, J.C.; Jardim, P.M.; Miranda, M.A.R. Sintering of nanoparticles of α-Fe2O3 using gelatin. J. Non-Cryst. Solids 2007, 353, 1091–1094. [Google Scholar] [CrossRef]
- Jalaja, K.; Naskar, D.; Kundu, S.C.; James, N.R. Fabrication of cationized gelatin nanofibers by electrospinning for tissue regeneration. RSC Adv. 2015, 5, 89521–89530. [Google Scholar] [CrossRef]
- Yakimets, I.; Wellner, N.; Smith, A.C.; Wilson, R.H.; Farhat, I.; Mitchell, J. Mechanical properties with respect to water content of gelatin films in glassy state. Polymer 2005, 46, 12577–12585. [Google Scholar] [CrossRef]
- Dashnyam, K.; Perez, R.; Lee, E.-J.; Yun, Y.-R.; Jang, J.-H.; Wall, I.B.; Kim, H.-W. Hybrid scaffolds of gelatin–siloxane releasing stromal derived factor-1 effective for cell recruitment. J. Biomed. Mater. Res. A 2014, 102, 1859–1867. [Google Scholar] [CrossRef]
- de Macedo, L.M.; Santos, É.M.d.; Militão, L.; Tundisi, L.L.; Ataide, J.A.; Souto, E.B.; Mazzola, P.G. Rosemary (Rosmarinus officinalis L., syn Salvia rosmarinus Spenn.) and Its Topical Applications: A Review. Plants 2020, 9, 651. [Google Scholar] [CrossRef]
- Ergene Öz, B.; Saltan İşcan, G.; Küpeli Akkol, E.; Süntar, İ.; Keleş, H.; Bahadır Acıkara, Ö. Wound healing and anti-inflammatory activity of some Ononis taxons. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 91, 1096–1105. [Google Scholar] [CrossRef]
- Henriques, B.O.; Corrêa, O.; Azevedo, E.P.; Pádua, R.M.; de Oliveira, V.L.; Oliveira, T.H.; Boff, D.; Dias, A.C.; de Souza, D.G.; Amaral, F.A.; et al. In Vitro TNF-α Inhibitory Activity of Brazilian Plants and Anti-Inflammatory Effect of Stryphnodendron adstringens in an Acute Arthritis Model. Evid. -Based Complement. Altern. Med. Ecam 2016, 2016, 9872598. [Google Scholar] [CrossRef]
- Sánchez-Fidalgo, S.; da Silva, M.S.; Cárdeno, A.; Aparicio-Soto, M.; Salvador, M.J.; Frankland Sawaya, A.C.; Souza-Brito, A.R.; de la Lastra, C.A. Abarema cochliacarpos reduces LPS-induced inflammatory response in murine peritoneal macrophages regulating ROS-MAPK signal pathway. J. Ethnopharmacol. 2013, 149, 140–147. [Google Scholar] [CrossRef]
- Su, W.H.; Cheng, M.H.; Lee, W.L.; Tsou, T.S.; Chang, W.H.; Chen, C.S.; Wang, P.H. Nonsteroidal anti-inflammatory drugs for wounds: Pain relief or excessive scar formation? Mediat. Inflamm. 2010, 2010, 413238. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.H.; Huang, B.S.; Horng, H.C.; Yeh, C.C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. JCMA 2018, 81, 94–101. [Google Scholar] [CrossRef]
- Shurtz-Swirski, R.; Sela, S.; Herskovits, A.T.; Shasha, S.M.; Shapiro, G.; Nasser, L.; Kristal, B. Involvement of peripheral polymorphonuclear leukocytes in oxidative stress and inflammation in type 2 diabetic patients. Diabetes Care 2001, 24, 104–110. [Google Scholar] [CrossRef]
- Sela, S.; Shurtz-Swirski, R.; Awad, J.; Shapiro, G.; Nasser, L.; Shasha, S.M.; Kristal, B. The involvement of peripheral polymorphonuclear leukocytes in the oxidative stress and inflammation among cigarette smokers. Isr. Med. Assoc. J. IMAJ 2002, 4, 1015–1019. [Google Scholar] [PubMed]
- Kapoor, M.; Howard, R.; Hall, I.; Appleton, I. Effects of epicatechin gallate on wound healing and scar formation in a full thickness incisional wound healing model in rats. Am. J. Pathol. 2004, 165, 299–307. [Google Scholar] [CrossRef]
- Kim, H.; Kawazoe, T.; Han, D.W.; Matsumara, K.; Suzuki, S.; Tsutsumi, S.; Hyon, S.H. Enhanced wound healing by an epigallocatechin gallate-incorporated collagen sponge in diabetic mice. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2008, 16, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Klass, B.R.; Branford, O.A.; Grobbelaar, A.O.; Rolfe, K.J. The effect of epigallocatechin-3-gallate, a constituent of green tea, on transforming growth factor-beta1-stimulated wound contraction. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2010, 18, 80–88. [Google Scholar] [CrossRef]
- Hajiaghaalipour, F.; Kanthimathi, M.S.; Abdulla, M.A.; Sanusi, J. The Effect of Camellia sinensis on Wound Healing Potential in an Animal Model. Evid. -Based Complement. Altern. Med. Ecam 2013, 2013, 386734. [Google Scholar] [CrossRef]
- Schmidt, C.A.; Murillo, R.; Bruhn, T.; Bringmann, G.; Goettert, M.; Heinzmann, B.; Brecht, V.; Laufer, S.A.; Merfort, I. Catechin derivatives from Parapiptadenia rigida with in vitro wound-healing properties. J. Nat. Prod. 2010, 73, 2035–2041. [Google Scholar] [CrossRef]
- Gantwerker, E.A.; Hom, D.B. Skin: Histology and physiology of wound healing. Clin. Plast. Surg. 2012, 39, 85–97. [Google Scholar] [CrossRef]
- Tanigawa, T.; Kanazawa, S.; Ichibori, R.; Fujiwara, T.; Magome, T.; Shingaki, K.; Miyata, S.; Hata, Y.; Tomita, K.; Matsuda, K.; et al. (+)-Catechin protects dermal fibroblasts against oxidative stress-induced apoptosis. BMC Complement. Altern. Med. 2014, 14, 133. [Google Scholar] [CrossRef] [PubMed]
- Silva Santos, L.F.; Stolfo, A.; Calloni, C.; Salvador, M. Catechin and epicatechin reduce mitochondrial dysfunction and oxidative stress induced by amiodarone in human lung fibroblasts. J. Arrhythmia 2017, 33, 220–225. [Google Scholar] [CrossRef]
- Morin, M.P.; Grenier, D. Regulation of matrix metalloproteinase secretion by green tea catechins in a three-dimensional co-culture model of macrophages and gingival fibroblasts. Arch. Oral Biol. 2017, 75, 89–99. [Google Scholar] [CrossRef]



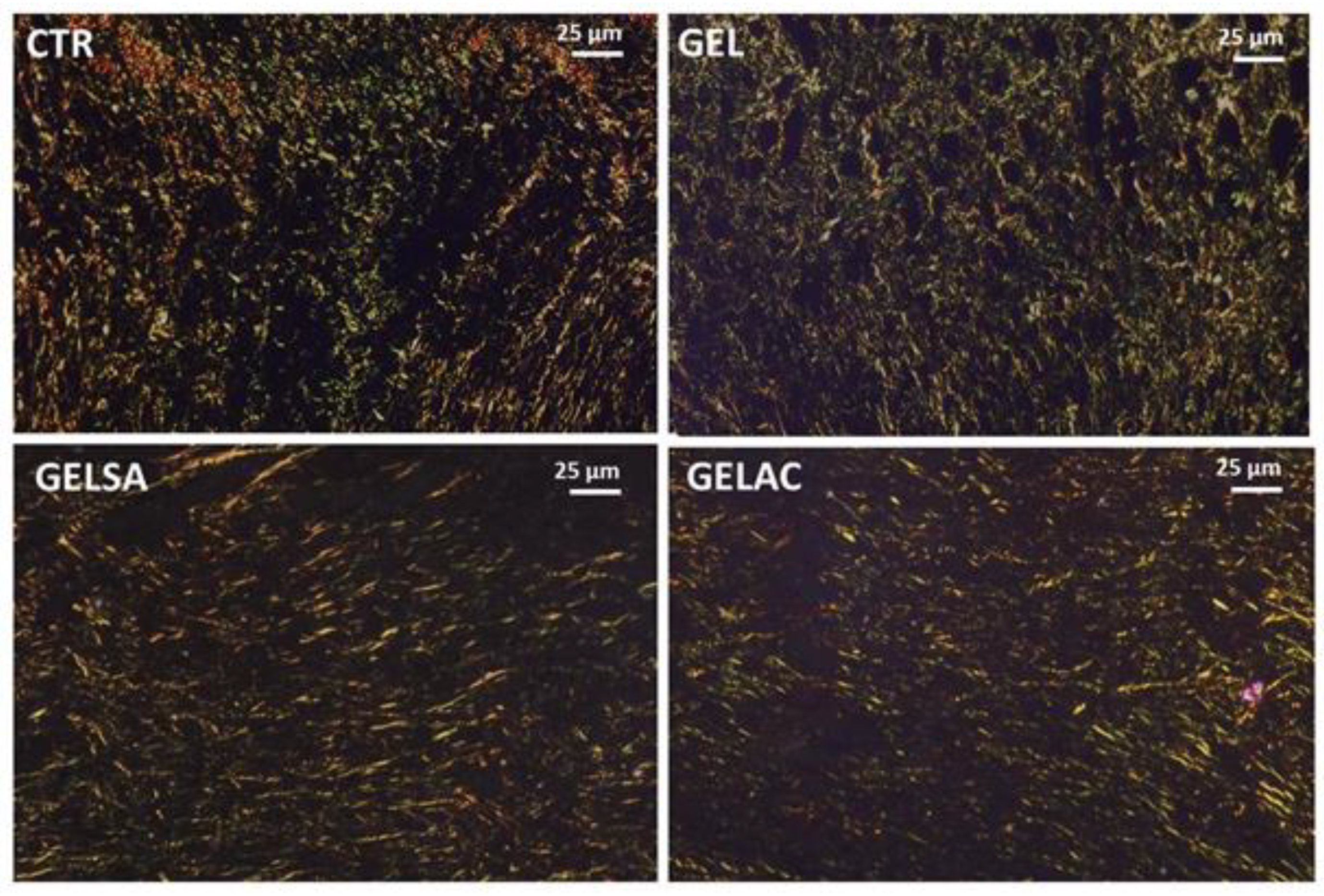
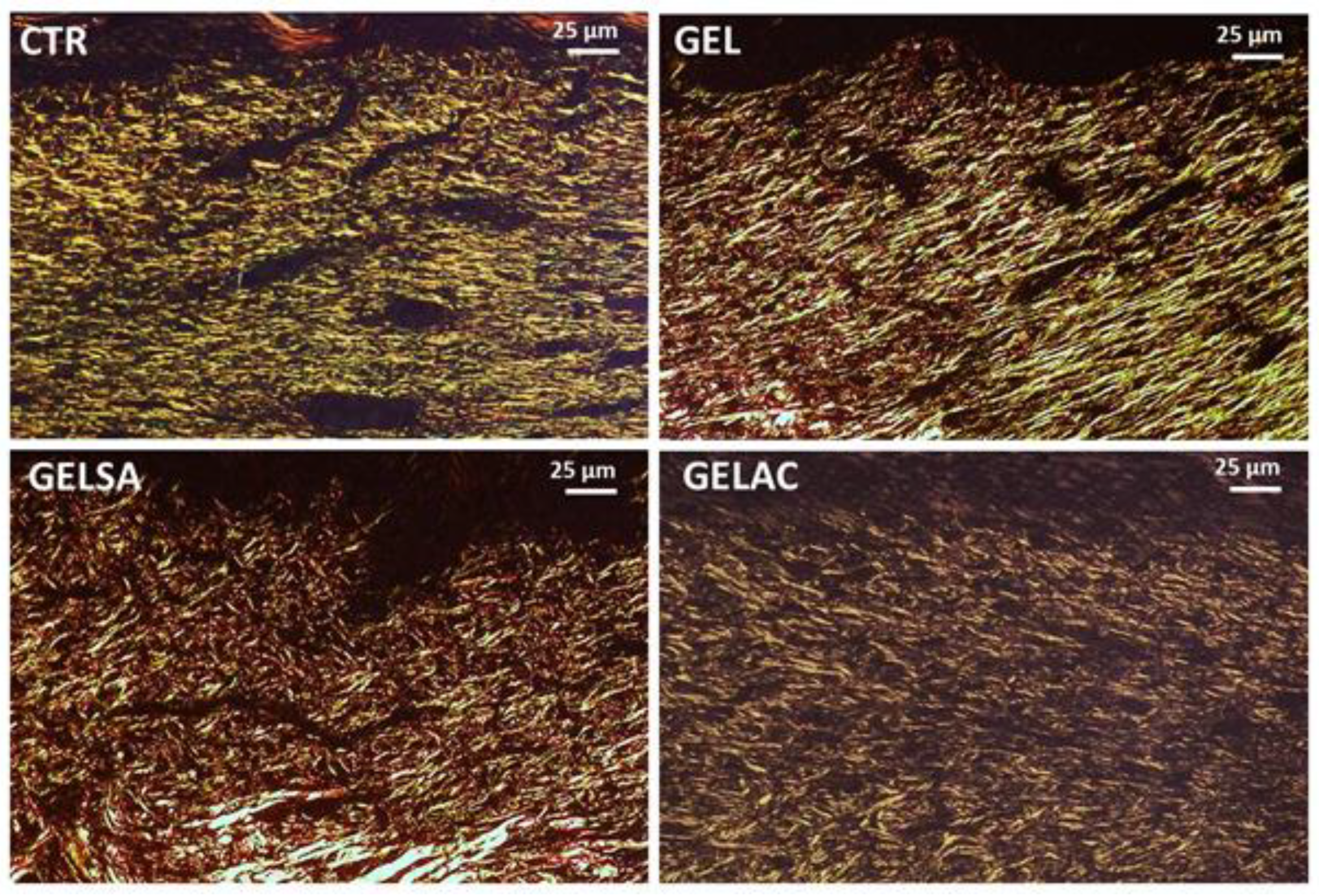
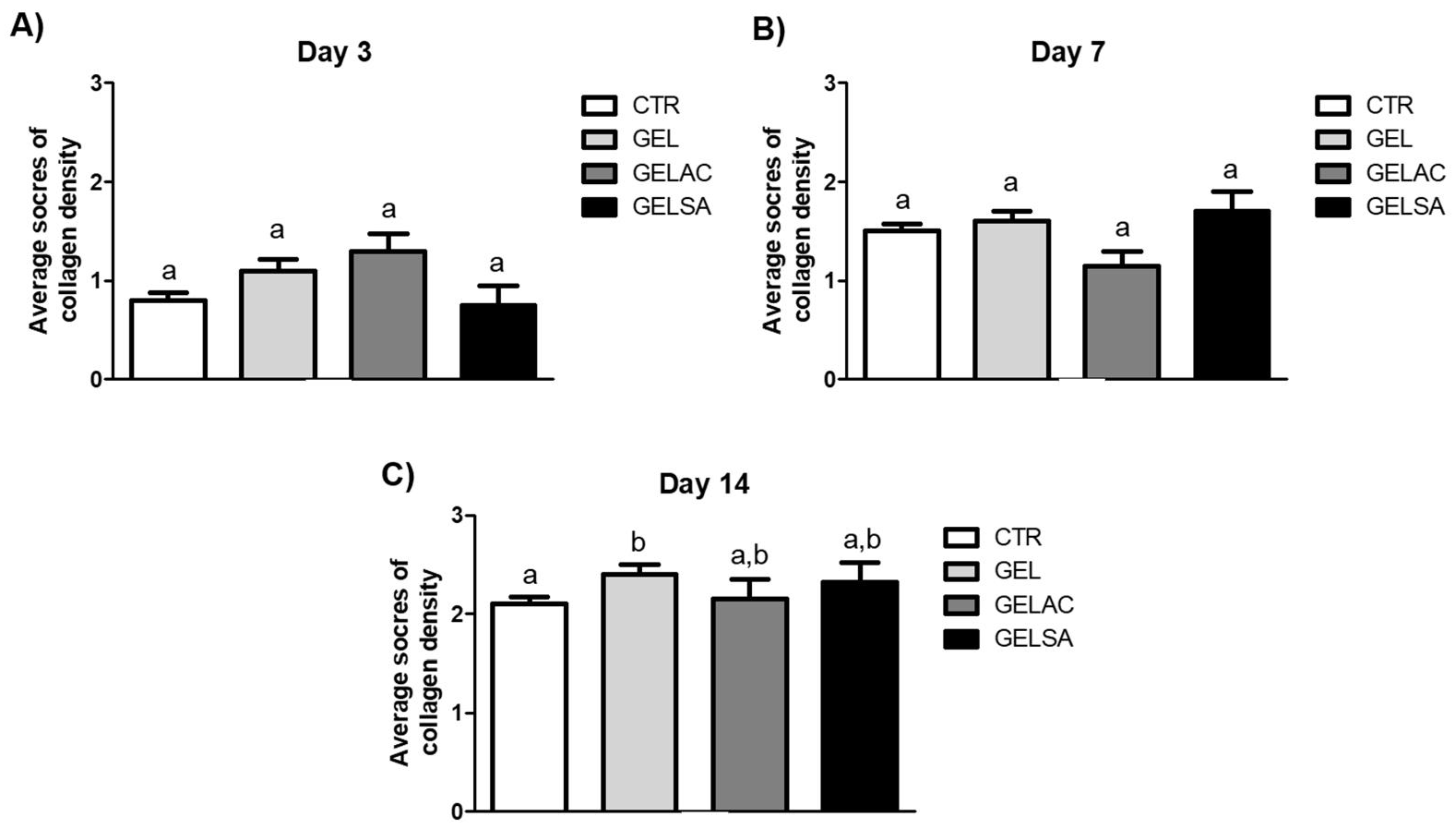
| Histological Criteria | Score System | Histological Method |
|---|---|---|
| Inflammatory infiltrate | Plenty—1, moderate—2, a few—3 | Light microscopy (HE) |
| Amount of granulation tissue | Profound—1, moderate—2, scanty—3, absent—4 | Light microscopy (HE) |
| Orientation of collagen fibers | Vertical—1, mixed—2, horizontal—3 | Polarized light (sirius red) |
| Pattern of collagenization | Reticular—1, mixed—2, fascicle—3 | Polarized light (sirius red) |
| Amount of early collagen (type III) | Profound—1, moderate—2, minimum—3, absent—4 | Polarized light (sirius red) |
| Amount of mature collagen (type I) | Profound—1, moderate—2, minimum—3 | Polarized light (sirius red) |
| Biomembranes | Continuity | Homogeneicity | Handiness | Flexibility |
|---|---|---|---|---|
| GEL | +++ | +++ | ++ | ++ |
| GELSA | +++ | +++ | ++ | ++ |
| GELAC | +++ | +++ | +++ | +++ |
| Membrane | ∆L | ∆a | ∆b | ∆E |
|---|---|---|---|---|
| GEL | 0 | 0 | 0 | 0 |
| GELSA | 13.85 ± 0.94 (a) | −7.48 ± 0.61 (a) | −14.73 ± 0.95 (a) | 21.56 ± 1.41 (a) |
| GELAC | 8.42 ± 1.46 (b) | −4.53 ± 0.99 (b) | −11.59 ± 1.57 (b) | 15.04 ± 2.24 (b) |
| Parameter | GEL | GELSA | GELAC |
|---|---|---|---|
| Thickness (µm) | 27.41 ± 0.4 (a) | 17.43 ± 0.18 (b) | 29.94 ± 0.38 (c) |
| Young’s Modulus (MPa) | 1185.06 ± 268.7 (a) | 2387.74 ± 321.09 (b) | 1084.95 ± 248.35 (a) |
| Maximum Voltage (MPa) | 43.33 ± 9.0 (a) | 65.32 ± 20.76 (b) | 32.28 ± 15.11 (a) |
| Deformation (%) | 7.12 ± 2.3 (a) | 6.2 ± 1.99 (a) | 5.4 ± 1.84 (a) |
| Permeability (g·mm/day·m2·KPa) | 9.68 ± 3.2 (a) | 10.39 ± 2.77 (a) | 11.82 ± 1.83 (b) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, M.C.M.A.; Nascimento, M.F.; de Almeida, B.M.; Alves, M.M.A.; Lima-Verde, I.B.; Costa, D.S.; Araújo, D.C.M.; de Paula, M.N.; Mello, J.C.P.d.; Cano, A.; et al. Hydrophilic Scaffolds Containing Extracts of Stryphnodendron adstringens and Abarema cochliacarpa for Wound Healing: In Vivo Proofs of Concept. Pharmaceutics 2022, 14, 2150. https://doi.org/10.3390/pharmaceutics14102150
Alves MCMA, Nascimento MF, de Almeida BM, Alves MMA, Lima-Verde IB, Costa DS, Araújo DCM, de Paula MN, Mello JCPd, Cano A, et al. Hydrophilic Scaffolds Containing Extracts of Stryphnodendron adstringens and Abarema cochliacarpa for Wound Healing: In Vivo Proofs of Concept. Pharmaceutics. 2022; 14(10):2150. https://doi.org/10.3390/pharmaceutics14102150
Chicago/Turabian StyleAlves, Maria C. M. A., Marismar F. Nascimento, Bernadeth M. de Almeida, Matheus M. A. Alves, Isabel B. Lima-Verde, Daniela S. Costa, Daniela C. Medeiros Araújo, Mariana N. de Paula, João C. P. de Mello, Amanda Cano, and et al. 2022. "Hydrophilic Scaffolds Containing Extracts of Stryphnodendron adstringens and Abarema cochliacarpa for Wound Healing: In Vivo Proofs of Concept" Pharmaceutics 14, no. 10: 2150. https://doi.org/10.3390/pharmaceutics14102150
APA StyleAlves, M. C. M. A., Nascimento, M. F., de Almeida, B. M., Alves, M. M. A., Lima-Verde, I. B., Costa, D. S., Araújo, D. C. M., de Paula, M. N., Mello, J. C. P. d., Cano, A., Severino, P., Albuquerque-Júnior, R. L. C. d., Souto, E. B., & Cardoso, J. C. (2022). Hydrophilic Scaffolds Containing Extracts of Stryphnodendron adstringens and Abarema cochliacarpa for Wound Healing: In Vivo Proofs of Concept. Pharmaceutics, 14(10), 2150. https://doi.org/10.3390/pharmaceutics14102150









