Biologic Disease-Modifying Antirheumatic Drugs for Preventing Radiographic Progression in Psoriatic Arthritis: A Systematic Review and Network Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection
2.3. Data Extraction and Risk of Bias Assessment
2.4. Statistical Analysis
3. Results
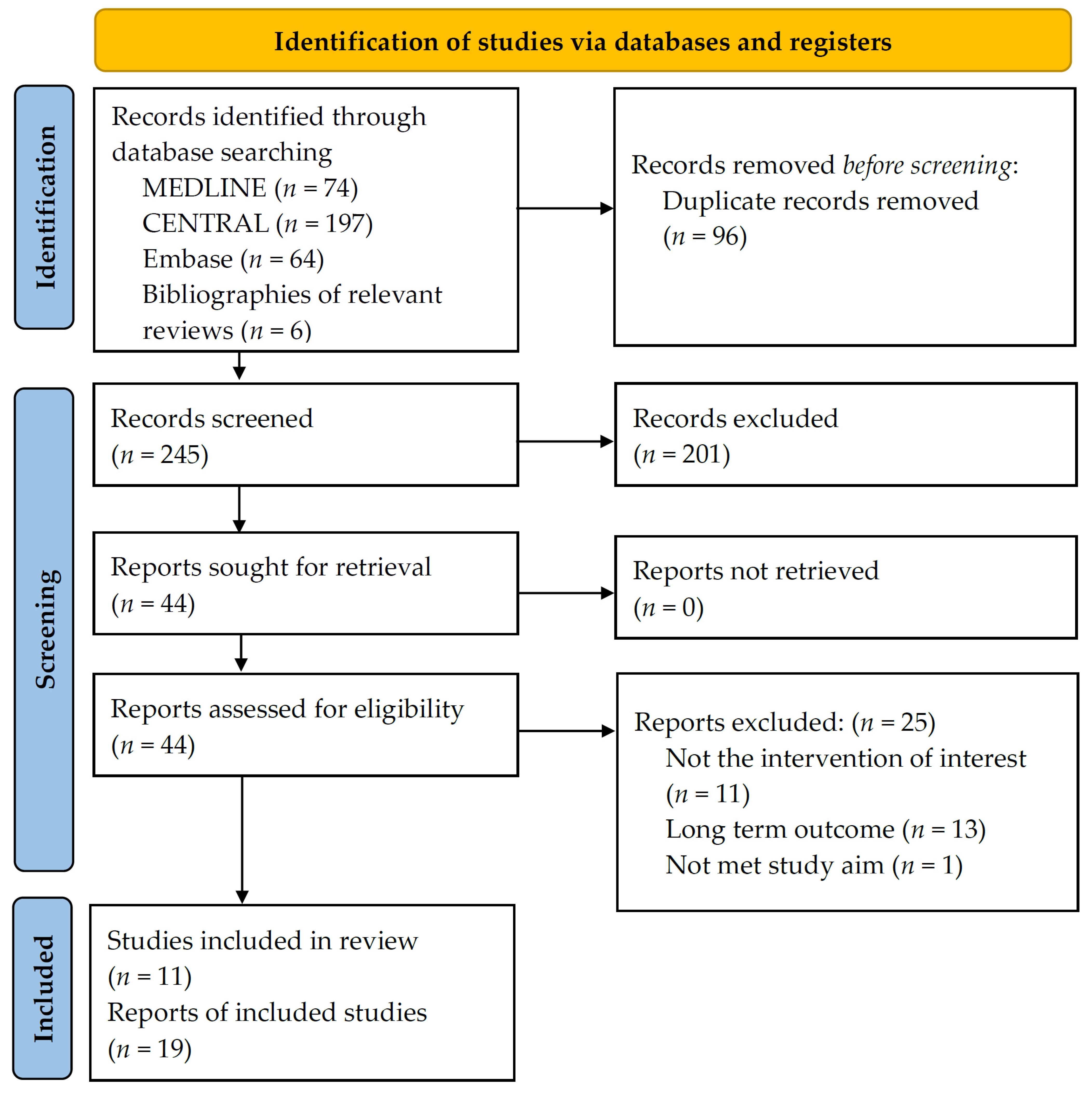
3.1. Search Results and Study Characteristics
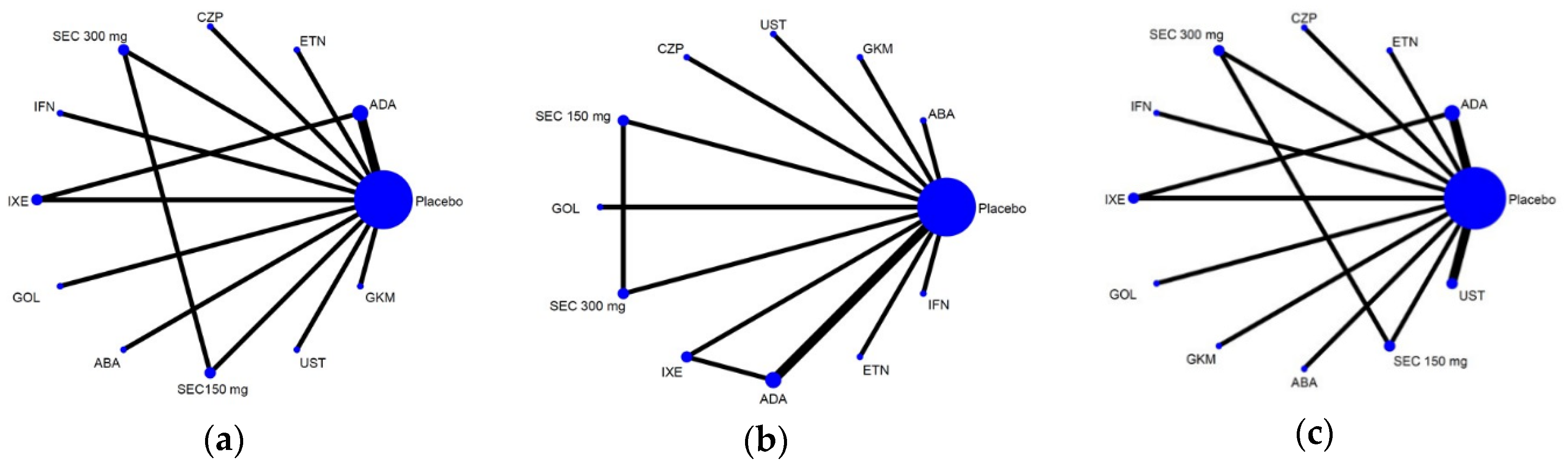
3.2. Overall Geometric Structure of the Whole Network
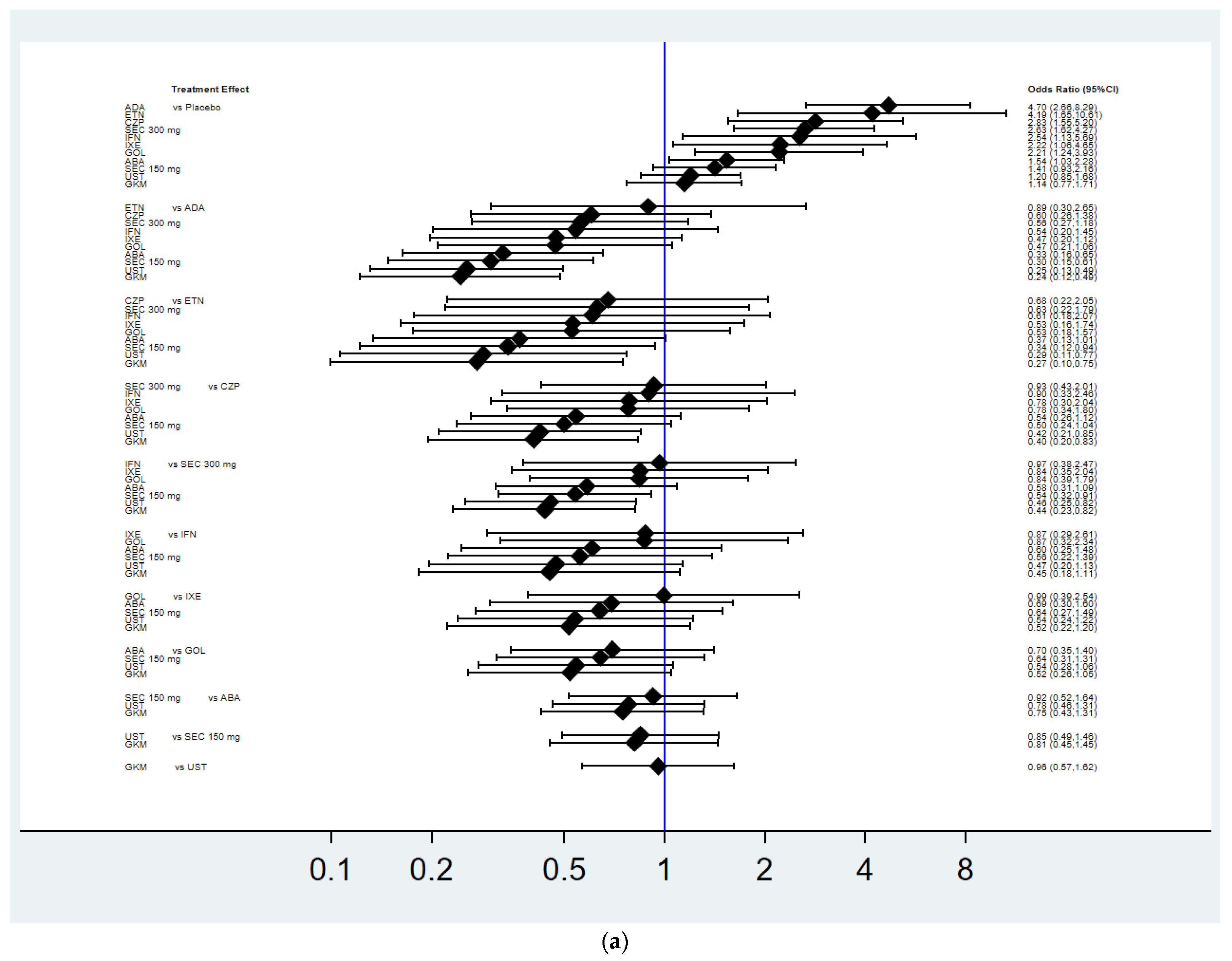
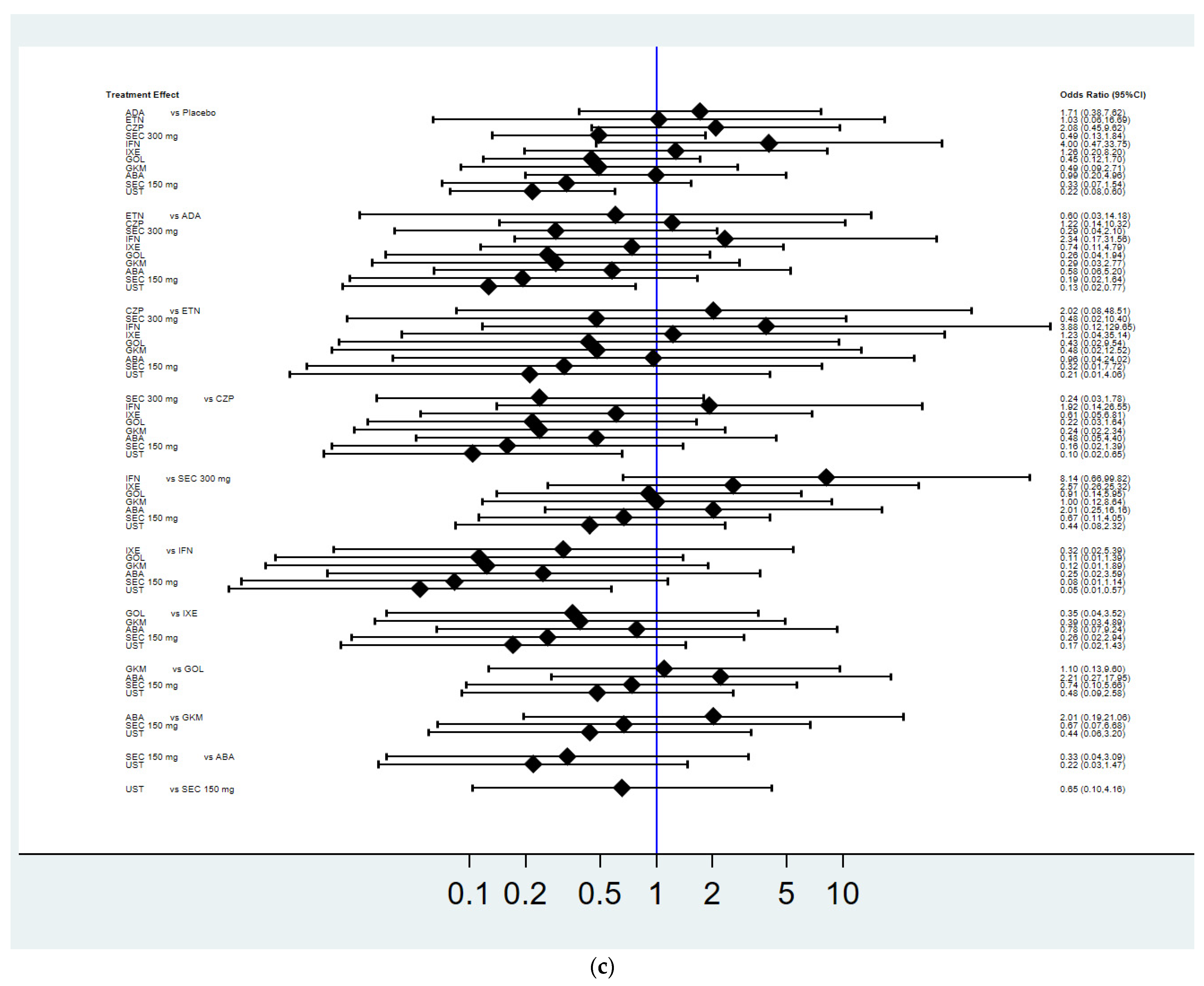
3.3. Achievement of Radiographic Non-Progression
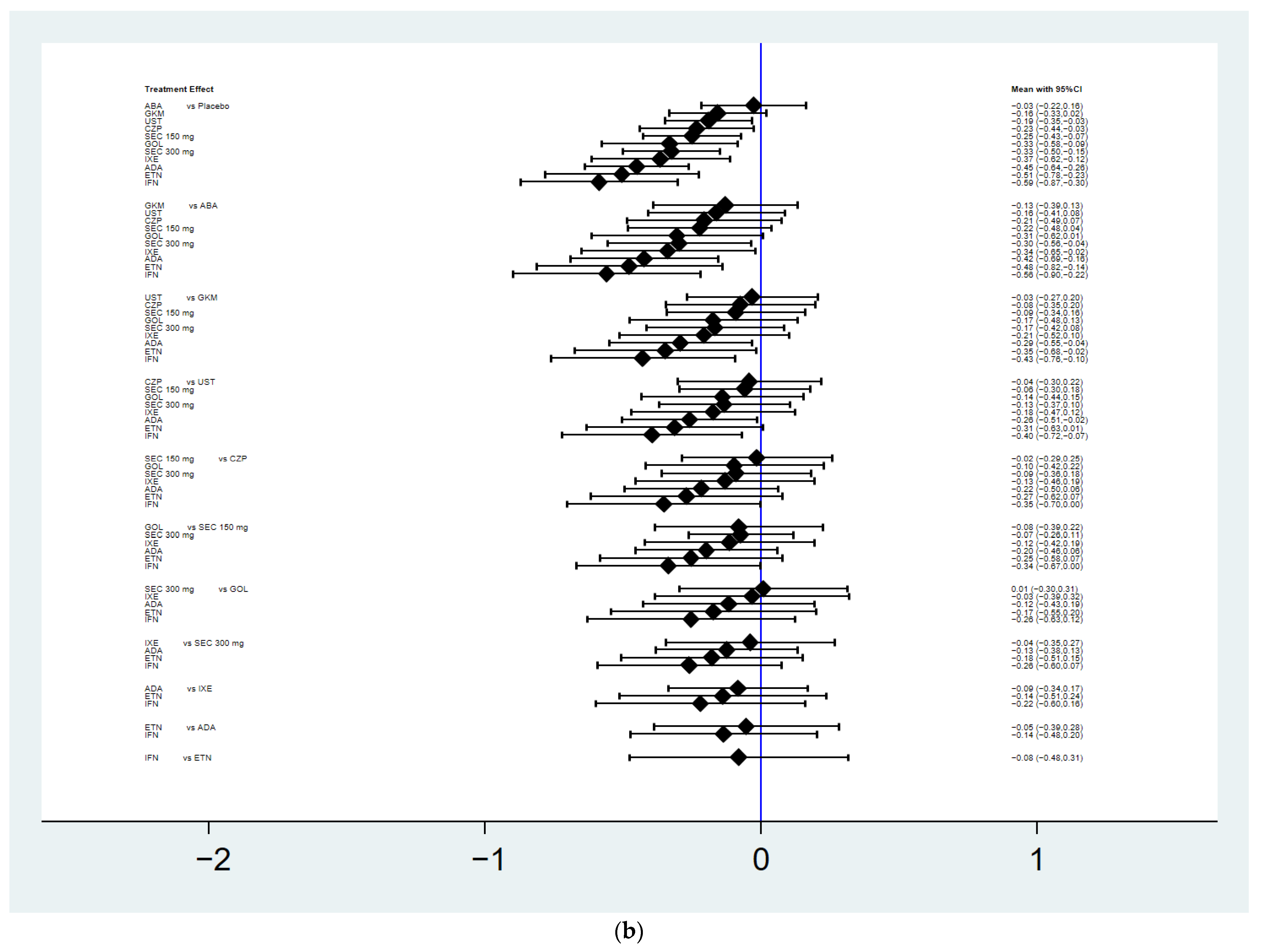
3.4. Mean Change in the Total Radiographic Score
3.5. Safety
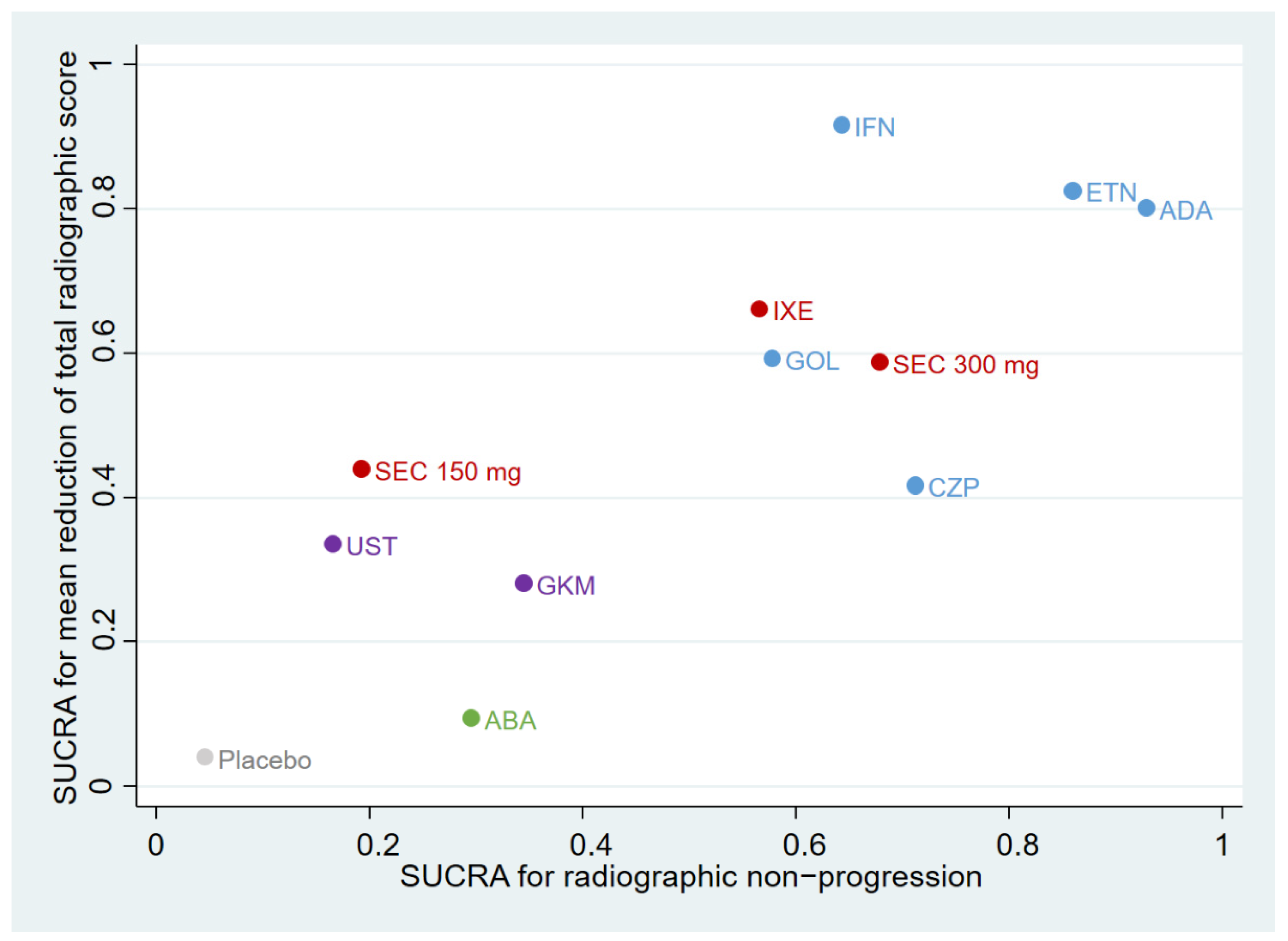
3.6. Ranking Plot of Different Treatments
3.7. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alinaghi, F.; Calov, M.; Kristensen, L.E.; Gladman, D.D.; Coates, L.C.; Jullien, D.; Gottlieb, A.B.; Gisondi, P.; Wu, J.J.; Thyssen, J.P.; et al. Prevalence of psoriatic arthritis in patients with psoriasis: A systematic review and meta-analysis of observational and clinical studies. J. Am. Acad. Dermatol. 2019, 80, 251–265.e219. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, J.M.; Gladman, D.D.; Mease, P.J.; Smith, N.; Margolis, D.J.; Nijsten, T.; Stern, R.S.; Feldman, S.R.; Rolstad, T. Epidemiology of psoriatic arthritis in the population of the United States. J. Am. Acad. Dermatol. 2005, 53, 573. [Google Scholar] [CrossRef]
- Lo, Y.; Wang, T.S.; Li, K.J.; Tsai, T.F. Correlation of clinical diagnosis of dactylitis by the dermatologist and ultrasonographic diagnosis by the rheumatologist in patients with psoriasis arthritis: Experience of a single clinic. Dermatol. Sin. 2021, 39, 27–32. [Google Scholar] [CrossRef]
- Yang, S.F.; Chen, T.H.; Tsai, S.H.; Chen, P.E.; Chi, C.C.; Tung, T.H. Risk of chronic kidney disease and end-stage renal disease in patients with psoriasis: A systematic review and meta-analysis of cohort studies. Dermatol. Sin. 2021, 39, 19–26. [Google Scholar] [CrossRef]
- Coates, L.C.; Helliwell, P.S. Psoriatic arthritis: State of the art review. Clin. Med. 2017, 17, 65–70. [Google Scholar] [CrossRef]
- Day, M.S.; Nam, D.; Goodman, S.; Su, E.P.; Figgie, M. Psoriatic arthritis. J. Am. Acad. Orthop. Surg. 2012, 20, 28–37. [Google Scholar] [CrossRef]
- Van der Heijde, D.; Sharp, J.; Wassenberg, S.; Gladman, D.D. Psoriatic arthritis imaging: A review of scoring methods. Ann. Rheum. Dis. 2005, 64 (Suppl. S2), ii61–ii64. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.F.; Hsieh, T.Y.; Chi, C.C.; Chou, C.T.; Hsieh, L.F.; Chen, H.H.; Hui, R.C.; Lee, C.H.; Liu, C.H.; Liu, H.C.; et al. Recommendations for psoriatic arthritis management: A joint position paper of the Taiwan Rheumatology Association and the Taiwanese Association for Psoriasis and Skin Immunology. J. Formos. Med Assoc. 2021, 120, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Coates, L.C.; Navarro-Coy, N.; Brown, S.R.; Brown, S.; McParland, L.; Collier, H.; Skinner, E.; Law, J.; Moverley, A.; Pavitt, S.; et al. The TICOPA protocol (TIght COntrol of Psoriatic Arthritis): A randomised controlled trial to compare intensive management versus standard care in early psoriatic arthritis. BMC Musculoskelet. Disord. 2013, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Kane, D.; Stafford, L.; Bresnihan, B.; FitzGerald, O. A prospective, clinical and radiological study of early psoriatic arthritis: An early synovitis clinic experience. Rheumatology 2003, 42, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Gladman, D.D.; Farewell, V.T.; Wong, K.; Husted, J. Mortality studies in psoriatic arthritis: Results from a single outpatient center. II. Prognostic indicators for death. Arthritis Rheum. 1998, 41, 1103–1110. [Google Scholar] [CrossRef]
- Gottlieb, A.; Korman, N.J.; Gordon, K.B.; Feldman, S.R.; Lebwohl, M.; Koo, J.Y.; van Voorhees, A.S.; Elmets, C.A.; Leonardi, C.L.; Beutner, K.R.; et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 2. Psoriatic arthritis: Overview and guidelines of care for treatment with an emphasis on the biologics. J. Am. Acad. Dermatol. 2008, 58, 851–864. [Google Scholar] [CrossRef]
- Gossec, L.; Baraliakos, X.; Kerschbaumer, A.; de Wit, M.; McInnes, I.; Dougados, M.; Primdahl, J.; McGonagle, D.G.; Aletaha, D.; Balanescu, A.; et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann. Rheum. Dis. 2020, 79, 700–712. [Google Scholar] [CrossRef]
- Yu, C.L.; Lin, Y.T.; Chi, C.C. Recommendations on use of systemic treatments for immune-mediated dermatologic disorders in patients with confirmed COVID-19 infection: A rapid review. Dermatol. Sin. 2022, 40, 67–70. [Google Scholar] [CrossRef]
- Mease, P.J.; Gladman, D.D.; Ritchlin, C.T.; Ruderman, E.M.; Steinfeld, S.D.; Choy, E.H.; Sharp, J.T.; Ory, P.A.; Perdok, R.J.; Weinberg, M.A.; et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: Results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005, 52, 3279–3289. [Google Scholar] [CrossRef] [PubMed]
- Gladman, D.D.; Mease, P.J.; Ritchlin, C.T.; Choy, E.H.; Sharp, J.T.; Ory, P.A.; Perdok, R.J.; Sasso, E.H. Adalimumab for long-term treatment of psoriatic arthritis: Forty-eight week data from the adalimumab effectiveness in psoriatic arthritis trial. Arthritis Rheum. 2007, 56, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; Kivitz, A.J.; Burch, F.X.; Siegel, E.L.; Cohen, S.B.; Ory, P.; Salonen, D.; Rubenstein, J.; Sharp, J.T.; Tsuji, W. Etanercept treatment of psoriatic arthritis: Safety, efficacy, and effect on disease progression. Arthritis Rheum. 2004, 50, 2264–2272. [Google Scholar] [CrossRef]
- Van der Heijde, D.; Kavanaugh, A.; Gladman, D.D.; Antoni, C.; Krueger, G.G.; Guzzo, C.; Zhou, B.; Dooley, L.T.; de Vlam, K.; Geusens, P.; et al. Infliximab inhibits progression of radiographic damage in patients with active psoriatic arthritis through one year of treatment: Results from the induction and maintenance psoriatic arthritis clinical trial 2. Arthritis Rheum. 2007, 56, 2698–2707. [Google Scholar] [CrossRef]
- Kavanaugh, A.; Antoni, C.E.; Gladman, D.; Wassenberg, S.; Zhou, B.; Beutler, A.; Keenan, G.; Burmester, G.; Furst, D.E.; Weisman, M.H.; et al. The infliximab multinational psoriatic arthritis controlled trial (IMPACT): Results of radiographic analyses after 1 year. Ann. Rheum. Dis. 2006, 65, 1038–1043. [Google Scholar] [CrossRef]
- Van der Heijde, D.; Fleischmann, R.; Wollenhaupt, J.; Deodhar, A.; Kielar, D.; Woltering, F.; Stach, C.; Hoepken, B.; Arledge, T.; Mease, P.J. Effect of different imputation approaches on the evaluation of radiographic progression in patients with psoriatic arthritis: Results of the RAPID-PsA 24-week phase III double-blind randomised placebo-controlled study of certolizumab pegol. Ann. Rheum. Dis. 2014, 73, 233–237. [Google Scholar] [CrossRef]
- Kavanaugh, A.; van der Heijde, D.; McInnes, I.B.; Mease, P.; Krueger, G.G.; Gladman, D.D.; Gómez-Reino, J.; Papp, K.; Baratelle, A.; Xu, W.; et al. Golimumab in psoriatic arthritis: One-year clinical efficacy, radiographic, and safety results from a phase III, randomized, placebo-controlled trial. Arthritis Rheum. 2012, 64, 2504–2517. [Google Scholar] [CrossRef]
- Van der Heijde, D.; Mease, P.J.; Landewe, R.B.M.; Rahman, P.; Tahir, H.; Singhal, A.; Boettcher, E.; Navarra, S.; Zhu, X.; Ligozio, G.; et al. Secukinumab provides sustained low rates of radiographic progression in psoriatic arthritis: 52-week results from a phase 3 study, FUTURE 5. Rheumatology 2020, 59, 1325–1334. [Google Scholar] [CrossRef]
- Kavanaugh, A.; Ritchlin, C.; Rahman, P.; Puig, L.; Gottlieb, A.B.; Li, S.; Wang, Y.; Noonan, L.; Brodmerkel, C.; Song, M.; et al. Ustekinumab, an anti-IL-12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: Results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double-blind, placebo-controlled PSUMMIT-1 and PSUMMIT-2 trials. Ann. Rheum. Dis. 2014, 73, 1000–1006. [Google Scholar] [PubMed]
- Mease, P.J.; Gottlieb, A.B.; van der Heijde, D.; FitzGerald, O.; Johnsen, A.; Nys, M.; Banerjee, S.; Gladman, D.D. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann. Rheum. Dis. 2017, 76, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; Rahman, P.; Gottlieb, A.B.; Kollmeier, A.P.; Hsia, E.C.; Xu, X.L.; Sheng, S.; Agarwal, P.; Zhou, B.; Zhuang, Y.; et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): A double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2020, 395, 1126–1136. [Google Scholar] [CrossRef]
- Mease, P.J.; van der Heijde, D.; Ritchlin, C.T.; Okada, M.; Cuchacovich, R.S.; Shuler, C.L.; Lin, C.Y.; Braun, D.K.; Lee, C.H.; Gladman, D.D. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: Results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann. Rheum. Dis. 2017, 76, 79–87. [Google Scholar] [CrossRef]
- Ritchlin, C.; Rahman, P.; Kavanaugh, A.; McInnes, I.B.; Puig, L.; Li, S.; Wang, Y.; Shen, Y.-K.; Doyle, M.K.; Mendelsohn, A.M.; et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann. Rheum. Dis. 2014, 73, 990–999. [Google Scholar] [CrossRef]
- Goulabchand, R.; Mouterde, G.; Barnetche, T.; Lukas, C.; Morel, J.; Combe, B. Effect of tumour necrosis factor blockers on radiographic progression of psoriatic arthritis: A systematic review and meta-analysis of randomised controlled trials. Ann. Rheum. Dis. 2014, 73, 414–419. [Google Scholar] [CrossRef]
- Wu, D.; Li, C.; Zhang, S.; Wong, P.; Cao, Y.; Griffith, J.F.; Zhang, X.; Gu, J.; Tam, L.S. Effect of biologics on radiographic progression of peripheral joint in patients with psoriatic arthritis: Meta-analysis. Rheumatology 2020, 59, 3172–3180. [Google Scholar] [CrossRef]
- Mease, P.J.; McInnes, I.B.; Tam, L.S.; Eaton, K.; Peterson, S.; Schubert, A.; Chakravarty, S.D.; Parackal, A.; Karyekar, C.S.; Nair, S.; et al. Comparative effectiveness of guselkumab in psoriatic arthritis: Results from systematic literature review and network meta-analysis. Rheumatology 2021, 60, 2109–2121. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Salaffi, F.; Carotti, M.; Beci, G.; di Carlo, M.; Giovagnoni, A. Radiographic scoring methods in rheumatoid arthritis and psoriatic arthritis. Radiol. Med. 2019, 124, 1071–1086. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- White, I.R. Network meta-analysis. Stata J. 2015, 15, 951–985. [Google Scholar] [CrossRef]
- Chen, H.L.; Tu, Y.K.; Chang, H.M.; Lee, T.H.; Wu, K.L.; Tsai, Y.C.; Lee, M.H.; Yang, C.J.; Hung, J.Y.; Chong, I.W. Systematic review and network meta-analysis of immune checkpoint inhibitors in combination with chemotherapy as a first-line therapy for extensive-stage small cell carcinoma. Cancers 2020, 12, 3629. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2021. [Google Scholar]
- Salanti, G.; Ades, A.E.; Ioannidis, J.P. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 2011, 64, 163–171. [Google Scholar] [CrossRef]
- Mbuagbaw, L.; Rochwerg, B.; Jaeschke, R.; Heels-Andsell, D.; Alhazzani, W.; Thabane, L.; Guyatt, G.H. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst. Rev. 2017, 6, 79. [Google Scholar] [CrossRef]
- Riley, R.D.; Jackson, D.; Salanti, G.; Burke, D.L.; Price, M.; Kirkham, J.; White, I.R. Multivariate and network meta-analysis of multiple outcomes and multiple treatments: Rationale, concepts, and examples. BMJ 2017, 358, j3932. [Google Scholar] [CrossRef]
- Bax, L.; Ikeda, N.; Fukui, N.; Yaju, Y.; Tsuruta, H.; Moons, K.G.M. More than numbers: The power of graphs in meta-analysis. Am. J. Epidemiol. 2009, 169, 249–255. [Google Scholar] [CrossRef]
- Mease, P.J.; Kivitz, A.J.; Burch, F.X.; Siegel, E.L.; Cohen, S.B.; Ory, P.; Salonen, D.; Rubenstein, J.; Sharp, J.T.; Dunn, M.; et al. Continued inhibition of radiographic progression in patients with psoriatic arthritis following 2 years of treatment with etanercept. J. Rheumatol. 2006, 33, 712–721. [Google Scholar]
- Mease, P.; van der Heijde, D.; Landewe, R.; Mpofu, S.; Rahman, P.; Tahir, H.; Singhal, A.; Boettcher, E.; Navarra, S.; Meiser, K.; et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: Primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann. Rheum. Dis. 2018, 77, 890–897. [Google Scholar] [CrossRef]
- Araujo, E.G.; Englbrecht, M.; Hoepken, S.; Finzel, S.; Kampylafka, E.; Kleyer, A.; Bayat, S.; Schoenau, V.; Hueber, A.; Rech, J.; et al. Effects of ustekinumab versus tumor necrosis factor inhibition on enthesitis: Results from the enthesial clearance in psoriatic arthritis (ECLIPSA) study. Semin. Arthritis Rheum. 2019, 48, 632–637. [Google Scholar] [CrossRef]
- Boutet, M.-A.; Nerviani, A.; Gallo Afflitto, G.; Pitzalis, C. Role of the IL-23/IL-17 axis in psoriasis and psoriatic arthritis: The clinical importance of its divergence in skin and joints. Int. J. Mol. Sci. 2018, 19, 530. [Google Scholar] [CrossRef]
- Silvagni, E.; Missiroli, S.; Perrone, M.; Patergnani, S.; Boncompagni, C.; Bortoluzzi, A.; Govoni, M.; Giorgi, C.; Alivernini, S.; Pinton, P.; et al. From bed to bench and back: TNF-alpha, IL-23/IL-17A, and JAK-dependent inflammation in the pathogenesis of ssoriatic synovitis. Front. Pharmacol. 2021, 12, 672515. [Google Scholar] [CrossRef]
- Chimenti, M.S.; Caso, F.; Alivernini, S.; de Martino, E.; Costa, L.; Tolusso, B.; Triggianese, P.; Conigliaro, P.; Gremese, E.; Scarpa, R.; et al. Amplifying the concept of psoriatic arthritis: The role of autoimmunity in systemic psoriatic disease. Autoimmun. Rev. 2019, 18, 565–575. [Google Scholar] [CrossRef]
- Fewtrell, M.S.; Kennedy, K.; Singhal, A.; Martin, R.M.; Ness, A.; Hadders-Algra, M.; Koletzko, B.; Lucas, A. How much loss to follow-up is acceptable in long-term randomised trials and prospective studies? Arch. Dis. Child. 2008, 93, 458–461. [Google Scholar] [CrossRef]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations; The GRADE Working Group: Stockholm, Sweden, 2013. [Google Scholar]
- Schett, G.; Lories, R.J.; D’Agostino, M.A.; Elewaut, D.; Kirkham, B.; Soriano, E.R.; McGonagle, D. Enthesitis: From pathophysiology to treatment. Nat. Rev. Rheumatol. 2017, 13, 731–741. [Google Scholar] [CrossRef]
- Ibrahim, A.; Gladman, D.D.; Thavaneswaran, A.; Eder, L.; Helliwell, P.; Cook, R.J.; Chandran, V. Radiographic scoring instruments have moderate sensitivity but high specificity for detecting change in axial psoriatic arthritis. Arthritis Care Res. 2017, 69, 1700–1705. [Google Scholar] [CrossRef]
| Study | Treatment | n | Prior bDMARD use (%) | Age (years) | Baseline Radiographic Score | Duration of Psoriatic Arthritis (Years) | Tender Joint Count | Swollen Joint Count | C-Reactive Protein (mg/dL) |
|---|---|---|---|---|---|---|---|---|---|
| Anti-tumor necrosis factor agents | |||||||||
| Gladman (2007) | Adalimumab 40 mg Q2W | 151 | 0% | 48.6 ± 12.5 | 22.7 ± 46 | 9.8 ± 8.3 | 23.9 ± 17.3 | 14.3 ± 12.2 | 1.4 ± 2.1 |
| ADEPT | Placebo | 162 | 0% | 49.2 ± 11.1 | 19.1 ± 35.5 | 9.2 ± 8.7 | 25.8 ± 18 | 14.3 ± 11.1 | 1.4 ± 1.7 |
| van der Heijde (2014) | Certolizumab pegol 200 mg Q2W | 138 | 22.50% | 48.2 ± 12.3 | 18.0 ± 30.6 | 9.6 ± 8.5 | 21.5 ± 15.3 | 11.0 ± 8.8 | 0.87 (0.01–8.70) |
| RAPID-PSA | Certolizumab pegol 400 mg Q4W | 135 | 17% | 47.1 ± 10.8 | 22.8 ± 46.5 | 8.1 ± 8.3 | 19.6 ± 14.8 | 10.5 ± 7.5 | 0.70 (0.02–23.80) |
| Placebo | 136 | 19.10% | 47.3 ± 11.1 | 24.4 ± 49.7 | 7.9 ± 7.7 | 19.9 ± 14.7 | 10.4 ± 7.6 | 0.90 (0.02–13.10) | |
| Mease (2006) | Etanercept 25 mg BIW | 101 | 0% | 47.6 | 25.89 | 9.0 | Not reported | Not reported | Not reported |
| Placebo | 104 | 0% | 47.3 | 18.30 | 9.2 | Not reported | Not reported | Not reported | |
| Kavanaugh (2012) | Golimumab 50 mg Q4W | 146 | 0% | 45.7 ± 10.7 | 23.85 ± 35.41 | 7.2 ± 6.8 | 24.0 ± 17.1 | 14.1 ± 11.4 | 1.3 ± 1.6 |
| GO-REVEAL | Placebo | 113 | 0% | 47.0 ± 10.6 | 18.15 ± 27.76 | 7.6 ± 7.9 | 21.9 ± 14.7 | 13.4 ± 9.8 | 1.3 ± 1.6 |
| van der Heijde (2007) | Infliximab 5 mg/kg Q8W | 100 | 0% | 47.1 ± 12.8 | 30.3 ± 61.4 | 8.4 ± 7.2 | 24.6 ± 14.1 | 13.9 ± 7.9 | 1.9 ± 2.1 |
| IMPACT 2 | Placebo | 100 | 0% | 46.5 ± 11.3 | 39.1 ± 82.8 | 7.5 ± 7.8 | 25.1 ± 13.3 | 14.4 ± 8.9 | 2.3 ± 3.4 |
| IL-17A inhibitors | |||||||||
| Mease (2017) | Ixekizumab 80 mg Q4W | 107 | 0% | 49.1 ± 10.1 | 19.2 ± 32.7 | 6.2 ± 6.4 | 20.5 ± 13.7 | 11.4 ± 8.2 | 1.28 ± 1.64 |
| SPIRIT-P1 | Adalimumab 40 mg Q2W | 101 | 0% | 48.6 ± 12.4 | 15.9 ± 27.4 | 6.9 ± 7.5 | 19.3 ± 13.0 | 9.9 ± 6.5 | 1.32 ± 1.91 |
| Placebo | 106 | 0% | 50.6 ± 12.3 | 17.6 ± 28.6 | 6.3 ± 6.9 | 19.2 ± 13.0 | 10.6 ± 7.3 | 1.51 ± 2.36 | |
| Mease (2018) | Secukinumab 300 mg (LD) Q4W | 222 | 30.70% | 48.9 ± 12.8 | 12.9 ± 23.7 | 6.7 ± 8.3 | 19.8 ± 15.1 | 10.0 ± 8.0 | Not reported |
| FUTURE 5 | Secukinumab 150 mg (LD) Q4W | 220 | 29.50% | 48.4 ± 12.9 | 13.6 ± 25.9 | 6.7 ± 7.1 | 21.2 ± 15.9 | 12.1 ± 10.5 | Not reported |
| Placebo | 332 | 29.50% | 49.0 ± 12.1 | 15 ± 38.2 | 6.6 ± 7.6 | 21.2 ± 16.2 | 11.7 ± 10.8 | Not reported | |
| p19 subunit of IL-23 inhibitor | |||||||||
| Mease (2020) | Guselkumab 100 mg Q8W | 248 | 0% | 44.9 ± 11.9 | 23.0 ± 37.8 | 5.1 ± 5.5 | 19.8 ± 11.9 | 11.7 ± 6.8 | 1.3 (0.7–2.5) |
| DISCOVER-2 | Placebo | 246 | 0% | 46.3 ± 11.7 | 23.8 ± 37.8 | 5.8 ± 5.6 | 21.6 ± 13.1 | 12.3 ± 6.9 | 1.2 (0.5–2.6) |
| IL-12/23 inhibitor | |||||||||
| Kavanaugh (2014) | PSUMMIT-1: Ustekinumab 45 mg Q12W | 205 | 0% | 48.0 (39.0–55.0) | 30.1 ± 51.7 | 3.4 (1.2–9.2) | 18.0 (12.0–28.0) | 10.0 (7.0–15.0) | 1.00 (0.59–2.11) |
| PSUMMIT-1: Placebo | 206 | 0% | 48.0 (39.0–57.0) | 29.9 ± 59.3 | 3.6 (1.0–9.7) | 22.0 (13.0–33.0) | 12.0 (8.0–19.0) | 0.96 (0.60–1.86) | |
| PSUMMIT-2: Ustekinumab 45 mg Q12W | 103 | 58.25% | 49.0 (40.0–56.0) | 31.1 ± 48.9 | 5.3 (2.3–12.2) | 22.0 (15.0–33.0) | 12.0 (8.0–19.0) | 1.30 (0.45–3.63) | |
| PSUMMIT-2: Placebo | 104 | 59.61% | 48.0 (38.5–56.0) | 24.3 ± 48.0 | 5.5 (2.3–12.2) | 21.0 (11.0–30.0) | 11.0 (7.0–18.0) | 0.85 (0.46–2.20) | |
| Selective T-cell costimulation modulator | |||||||||
| Mease (2017) | Abatacept 125 mg QW | 213 | 60.60% | 51.0 ± 10.7 | 20.0 ± 46.8 | 8.3 ± 8.1 | 21.0 ± 13.4 | 12.1 ± 7.8 | 1.40 ± 2.09 |
| ASTRAEA | Placebo | 211 | 61.60% | 49.8 ± 11.3 | 17.7 ± 39.6 | 8.8 ± 8.3 | 19.3 ± 13.1 | 11.1 ± 7.2 | 1.43 ± 3.03 |
| Intervention | Efficacy | Safety DAE (%) | |
|---|---|---|---|
| Non-Progression (%) | Total Sharp Score (%) | ||
| ADA | 92.8 | 80.2 | 27.8 |
| ETN | 85.9 | 82.6 | 44.9 |
| CZP | 71.1 | 41.7 | 23.7 |
| SEC 300 mg | 67.8 | 58.8 | 66.4 |
| IFN | 64.2 | 91.7 | 13.9 |
| IXE | 56.5 | 66.2 | 37.7 |
| GOL | 57.7 | 59.3 | 68.9 |
| GKM | 34.4 | 28.1 | 67.2 |
| ABA | 29.5 | 9.5 | 43.9 |
| SEC 150 mg | 19.2 | 44 | 75.3 |
| UST | 16.5 | 33.6 | 88.6 |
| PBO | 4.5 | 4.1 | 41.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-H.; Yu, C.-L.; Wang, T.-Y.; Yang, C.-H.; Chi, C.-C. Biologic Disease-Modifying Antirheumatic Drugs for Preventing Radiographic Progression in Psoriatic Arthritis: A Systematic Review and Network Meta-Analysis. Pharmaceutics 2022, 14, 2140. https://doi.org/10.3390/pharmaceutics14102140
Wang S-H, Yu C-L, Wang T-Y, Yang C-H, Chi C-C. Biologic Disease-Modifying Antirheumatic Drugs for Preventing Radiographic Progression in Psoriatic Arthritis: A Systematic Review and Network Meta-Analysis. Pharmaceutics. 2022; 14(10):2140. https://doi.org/10.3390/pharmaceutics14102140
Chicago/Turabian StyleWang, Szu-Hsuan, Chia-Ling Yu, Tzu-Yu Wang, Chung-Han Yang, and Ching-Chi Chi. 2022. "Biologic Disease-Modifying Antirheumatic Drugs for Preventing Radiographic Progression in Psoriatic Arthritis: A Systematic Review and Network Meta-Analysis" Pharmaceutics 14, no. 10: 2140. https://doi.org/10.3390/pharmaceutics14102140
APA StyleWang, S.-H., Yu, C.-L., Wang, T.-Y., Yang, C.-H., & Chi, C.-C. (2022). Biologic Disease-Modifying Antirheumatic Drugs for Preventing Radiographic Progression in Psoriatic Arthritis: A Systematic Review and Network Meta-Analysis. Pharmaceutics, 14(10), 2140. https://doi.org/10.3390/pharmaceutics14102140





