Efficacy of Antimicrobial Photodynamic Therapy Mediated by Photosensitizers Conjugated with Inorganic Nanoparticles: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Data Extraction and Research Question
2.3. Eligibility Criteria
2.4. Search Strategy
- (((antimicrobial photodynamic therapy) AND (Drug delivery system)) AND (Metallic nanoparticles)) AND (metal nanoparticles)
- ((antimicrobial photodynamic therapy) AND (Drug delivery system)) AND (Metal oxides).
- ((antimicrobial photodynamic therapy) AND (Drug delivery system)) AND (Carbon quantum dots)
- (((antimicrobial photodynamic therapy) AND (Drug delivery system)) AND (Mesoporous silica) AND (silica nanoparticles)
2.5. Qualitative Analysis
2.6. Meta-Analysis and Quantitative Approaches
3. Results
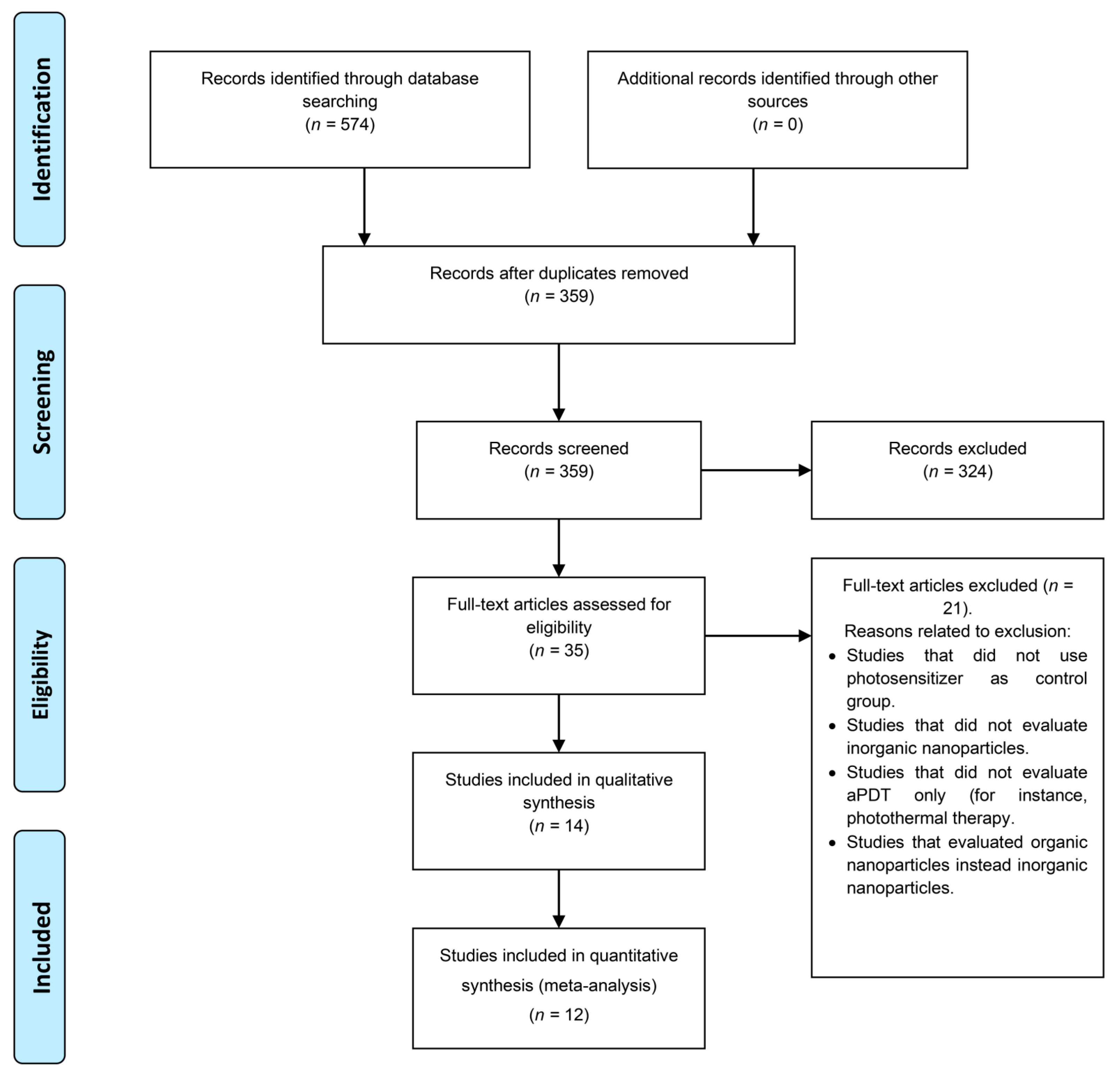
3.1. Search Results
3.2. Synthesis Results
3.3. Risk of Bias Assessment
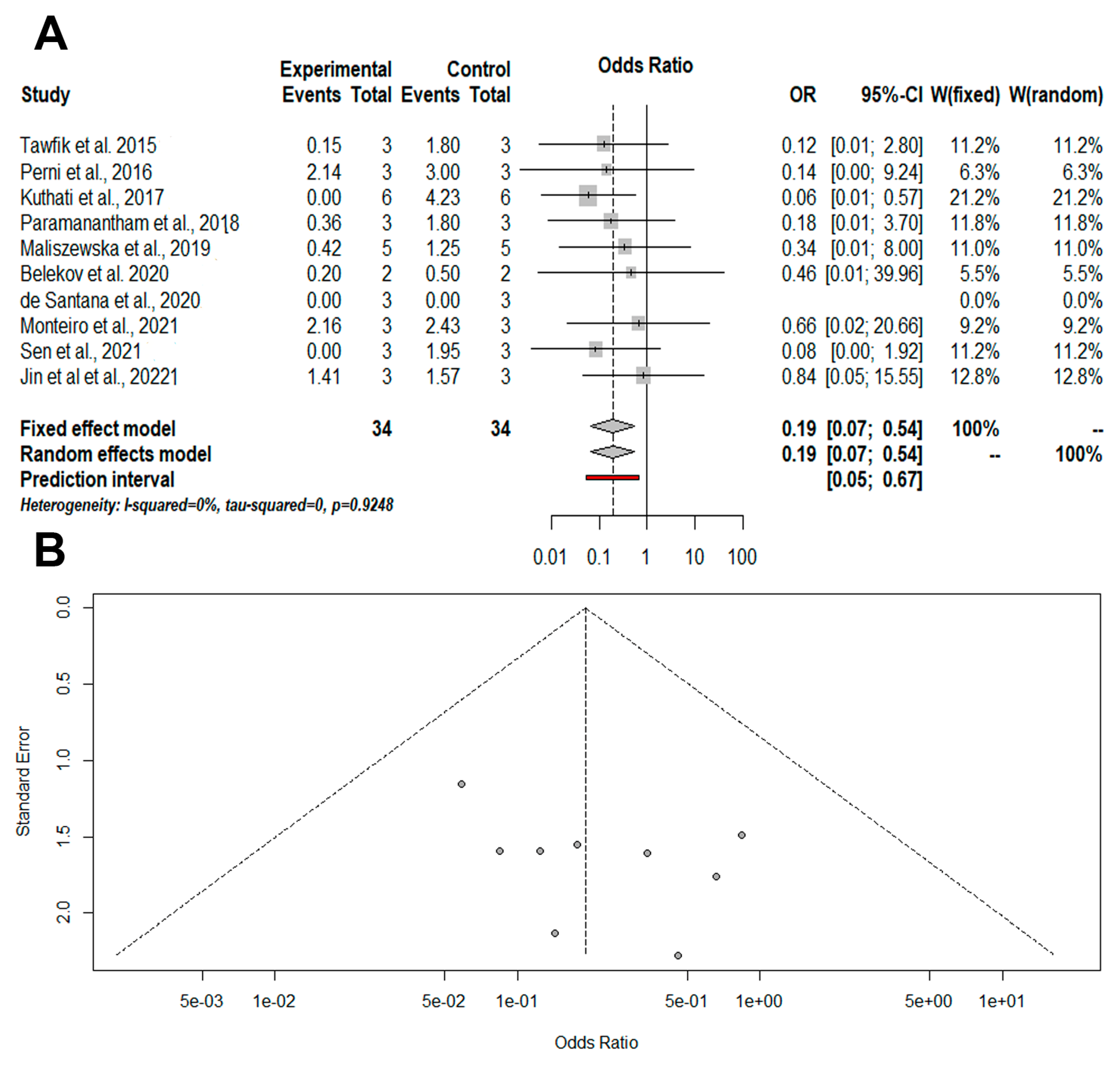
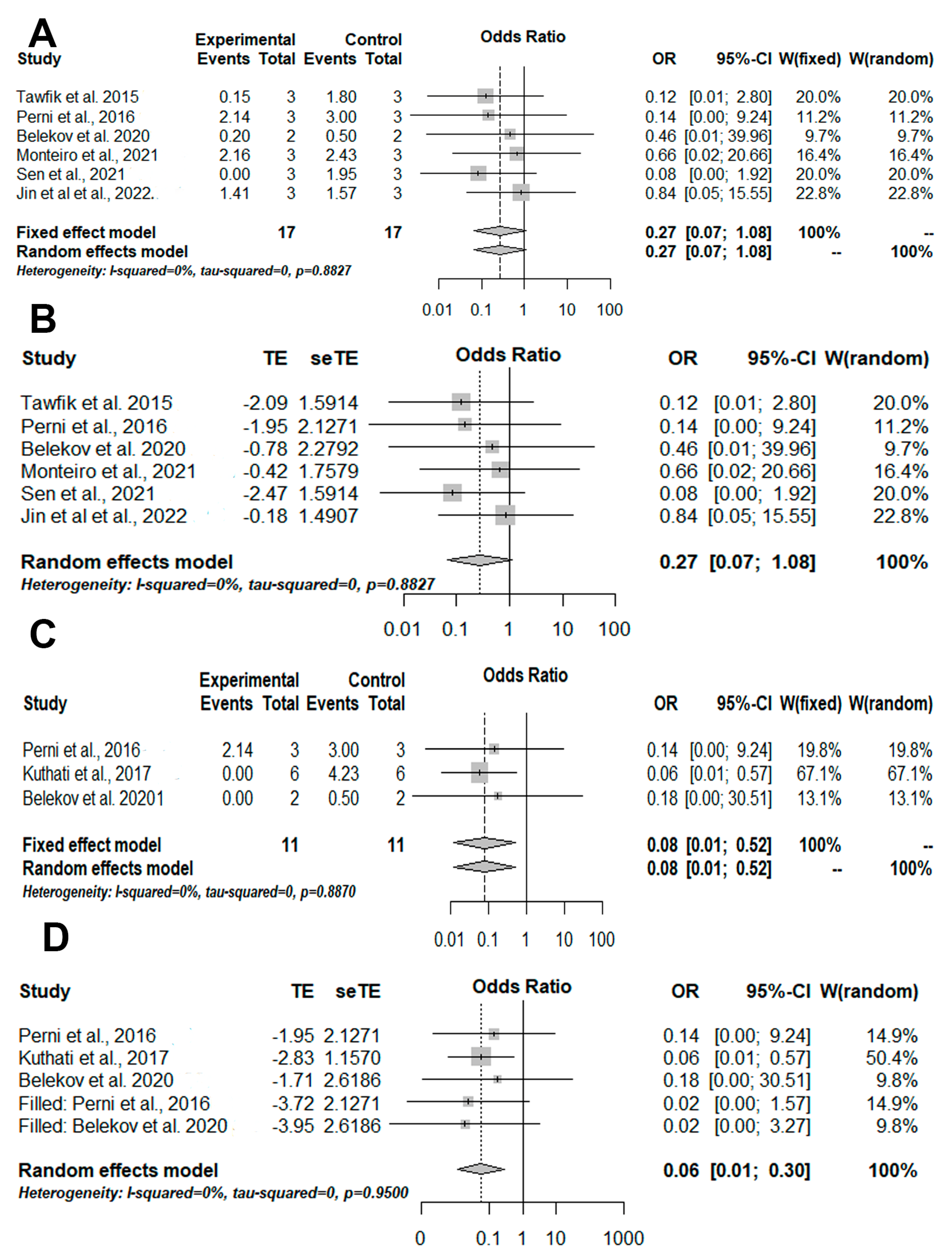
3.4. Meta-Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Oldenkamp, R.; Schultsz, C.; Mancini, E.; Cappuccio, A. Filling the gaps in the global prevalence map of clinical antimicrobial resistance. Proc. Natl. Acad. Sci. USA 2021, 118, e2013515118. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. London: Review on Antimicrobial Resistance. 2016. Available online: https://apo.org.au/sites/default/files/resource-files/2016-05/apo-nid63983.pdf. (accessed on 20 July 2020).
- Tiseo, G.; Brigante, G.; Giacobbe, D.R.; Maraolo, A.E.; Gona, F.; Falcone, M.; Giannella, M.; Grossi, P.; Pea, F.; Rossolini, G.M.; et al. Diagnosis and management of infections caused by multidrug-resistant bacteria: Guideline endorsed by the Italian Society of Infection and Tropical Diseases (SIMIT), the Italian Society of Anti-Infective Therapy (SITA), the Italian Group for Antimicrobial Stewardship (GISA), the Italian Association of Clinical Microbiologists (AMCLI) and the Italian Society of Microbiology (SIM). Int. J. Antimicrob. Agents 2022, 60, 106611. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Berman, J.; Krysan, D.J. Drug resistance and tolerance in fungi. Nat. Rev. Genet. 2020, 18, 319–331. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef]
- Surova, O.; Zhivotovsky, B. Various modes of cell death induced by DNA damage. Oncogene 2013, 32, 3789–3797. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Abrahamse, H. Can light-based approaches overcome antimicrobial resistance? Drug Dev. Res. 2019, 80, 48–67. [Google Scholar] [CrossRef]
- Nunes, L.; Nunes, G.P.; Ferrisse, T.M.; Strazzi-Sahyon, H.B.; Cintra, L.T.Â.; Dos Santos, P.H.; Sivieri-Araujo, G. Antimicrobial photodynamic therapy in endodontic reintervention: A systematic review and meta-analysis. Photodiagnos. Photodyn. Ther. 2022, 39, 103014. [Google Scholar] [CrossRef] [PubMed]
- Sales, L.S.; Miranda, M.L.; de Oliveira, A.B.; Ferrisse, T.M.; Fontana, C.R.; Milward, M.; Brighenti, F.L. Effect of the technique of photodynamic therapy against the main microorganisms responsible for periodontitis: A systematic review of in-vitro studies. Arch. Oral Biol. 2022, 138, 105425. [Google Scholar] [CrossRef] [PubMed]
- Ferrisse, T.M.; Dias, L.M.; de Oliveira, A.B.; Jordão, C.C.; Mima, E.G.O.; Pavarina, A.C. Efficacy of curcumin-mediated antibacterial photodynamic therapy for oral antisepsis: A systematic review and network meta-analysis of randomized clinical trials. Photodiagnos. Photodyn. Ther. 2022, 39, 102876. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.B.; Ferrisse, T.M.; Marques, R.S.; de Annunzio, S.R.; Brighenti, F.L.; Fontana, C.R. Effect of Photodynamic Therapy on Microorganisms Responsible for Dental Caries: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 3585. [Google Scholar] [CrossRef]
- Teixeira, C.G.D.S.; Sanitá, P.V.; Ribeiro, A.P.D.; Dias, L.M.; Jorge, J.H.; Pavarina, A.C. Antimicrobial photodynamic therapy effectiveness against susceptible and methicillin-resistant Staphylococcus aureus biofilms. Photodiagnos. Photodyn. Ther. 2020, 30, 101760. [Google Scholar] [CrossRef]
- Trigo-Gutierrez, J.; Vega-Chacón, Y.; Soares, A.; Mima, E. Antimicrobial Activity of Curcumin in Nanoformulations: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 7130. [Google Scholar] [CrossRef]
- Dias, L.M.; Ferrisse, T.M.; Medeiros, K.S.; Cilli, E.M.; Pavarina, A.C. Use of Photodynamic Therapy Associated with Antimicrobial Peptides for Bacterial Control: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 3226. [Google Scholar] [CrossRef]
- Silvestre, A.L.P.; Di Filippo, L.D.; Besegato, J.F.; de Annunzio, S.R.; de Camargo, B.A.F.; de Melo, P.B.G.; Rastelli, A.N.D.S.; Fontana, C.R.; Chorilli, M. Current applications of drug delivery nanosystems associated with antimicrobial photodynamic therapy for oral infections. Int. J. Pharm. 2020, 592, 120078. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Dias, H.B.; Bernardi, M.I.B.; Ramos, M.A.D.S.; Trevisan, T.C.; Bauab, T.M.; Hernandes, A.C.; Rastelli, A.N.D.S. Zinc oxide 3D microstructures as an antimicrobial filler content for composite resins. Microsc. Res. Tech. 2017, 99, 14434–14643. [Google Scholar] [CrossRef]
- Sábio, R.M.; Meneguin, A.B.; Ribeiro, T.D.C.; Silva, R.R.; Chorilli, M. New insights towards mesoporous silica nanoparticles as a technological platform for chemotherapeutic drugs delivery. Int. J. Pharm. 2019, 564, 379–409. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; The PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g764. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- NTP-OHAT. OHAT Risk of Bias Rating Tool for Human and Animal Studies; Office of Health Assessment and Translation: Morrisville, NC, USA, 2015.
- NTP-OHAT. Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration; National Toxicology Program-Office of Health Assessment and Translation: Morrisville, NC, USA, 2019.
- Ferrisse, T.M.; de Oliveira, A.B.; Surur, A.K.; Buzo, H.S.; Brighenti, F.L.; Fontana, C.R. Photodynamic therapy associated with nanomedicine strategies for treatment of human squamous cell carcinoma: A systematic review and meta-analysis. Nanomed. Nanotechnol. Biol. Med. 2021, 40, 102505. [Google Scholar] [CrossRef] [PubMed]
- Planas, O.; Bresolí-Obach, R.; Nos, J.; Gallavardin, T.; Ruiz-González, R.; Agut, M.; Nonell, S. Synthesis, Photophysical Characterization, and Photoinduced Antibacterial Activity of Methylene Blue-loaded Amino- and Mannose-Targeted Mesoporous Silica Nanoparticles. Molecules 2015, 20, 6284–6298. [Google Scholar] [CrossRef]
- Tawfik, A.A.; Alsharnoubi, J.; Morsy, M. Photodynamic antibacterial enhanced effect of methylene blue-gold nanoparticles conjugate on Staphylococcal aureus isolated from impetigo lesions in vitro study. Photodiagnos. Photodyn. Ther. 2015, 12, 215–220. [Google Scholar] [CrossRef]
- Perni, S.; Drexler, S.; Ruppel, S.; Prokopovich, P. Lethal photosensitisation of bacteria using silica-TBO nanoconjugates. Colloids Surf. A Physicochem. Eng. Asp. 2016, 510, 293–299. [Google Scholar] [CrossRef]
- Kuthati, Y.; Kankala, R.K.; Busa, P.; Lin, S.-X.; Deng, J.-P.; Mou, C.-Y.; Lee, C.-H. Phototherapeutic spectrum expansion through synergistic effect of mesoporous silica trio-nanohybrids against antibiotic-resistant gram-negative bacterium. J. Photochem. Photobiol. B Biol. 2017, 169, 124–133. [Google Scholar] [CrossRef]
- Paramanantham, P.; Antony, A.P.; Lal, S.S.; Sharan, A.; Syed, A.; Ahmed, M.; Alarfaj, A.A.; Busi, S.; Maaza, M.; Kaviyarasu, K. Antimicrobial photodynamic inactivation of fungal biofilm using amino functionalized mesoporus silica-rose bengal nanoconjugate against Candida albicans. Sci. Afr. 2018, 1, e00007. [Google Scholar] [CrossRef]
- Anju, V.T.; Paramanantham, P.; Sruthil Lal, S.B.; Sharan, A.; Syed, A.; Bahkali, N.A.; Alsaedi, M.H.; Kaviyarasu, K.; Busi, S. Antimicrobial photodynamic activity of toluidine blue-carbon nanotube conjugate against Pseudomonas aeruginosa and Staphylococcus aureus-Understanding the mechanism of action. Photodiagnos. Photodyn. Ther. 2019, 27, 305–316. [Google Scholar] [CrossRef]
- Maliszewska, I.; Wróbel, J.; Wanarska, E.; Podhorodecki, A.; Matczyszyn, K. Synergistic effect of methylene blue and biogenic gold nanoparticles against Enterococcus faecalis. Photodiagnos. Photodyn. Ther. 2019, 27, 218–226. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Parker, S.; Chiniforush, N.; Bahador, A. Photoexcitation triggering via semiconductor Graphene Quantum Dots by photochemical doping with Curcumin versus perio-pathogens mixed biofilms. Photodiagnos. Photodyn. Ther. 2019, 28, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Belekov, E.; Kholikov, K.; Cooper, L.; Banga, S.; Er, A.O. Improved antimicrobial properties of methylene blue attached to silver nanoparticles. Photodiagnos. Photodyn. Ther. 2020, 32, 102012. [Google Scholar] [CrossRef] [PubMed]
- de Santana, W.M.O.S.; Caetano, B.L.; de Annunzio, S.R.; Pulcinelli, S.H.; Ménager, C.; Fontana, C.R.; Santilli, C.V. Conjugation of superparamagnetic iron oxide nanoparticles and curcumin photosensitizer to assist in photodynamic therapy. Colloids Surf. B Biointerf. 2020, 196, 111297. [Google Scholar] [CrossRef]
- Monteiro, J.S.; Rangel, E.E.; de Oliveira, S.C.; Crugeira, P.J.; Nunes, I.P.; Fagnani, S.R.D.A.; Sampaio, F.J.; de Almeida, P.F.; Pinheiro, A.L. Enhancement of photodynamic inactivation of planktonic cultures of Staphylococcus aureus by DMMB-AuNPs. Photodiagnos. Photodyn. Ther. 2020, 31, 101930. [Google Scholar] [CrossRef]
- Sen, P.; Nyokong, T. Enhanced Photodynamic inactivation of Staphylococcus Aureus with Schiff base substituted Zinc phthalocyanines through conjugation to silver nanoparticles. J. Mol. Struct. 2021, 1232, 130012. [Google Scholar] [CrossRef]
- Sen, P.; Nyokong, T. Promising photodynamic antimicrobial activity of polyimine substituted zinc phthalocyanine and its polycationic derivative when conjugated to nitrogen, sulfur, co-doped graphene quantum dots against Staphylococcus aureus. Photodiagnos. Photodyn. Ther. 2021, 34, 102300. [Google Scholar] [CrossRef]
- Jin, Y.; Zhao, B.; Guo, W.; Li, Y.; Min, J.; Miao, W. Penetration and photodynamic ablation of drug-resistant biofilm by cationic Iron oxide nanoparticles. J. Control. Release 2022, 348, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Spiegelhalter, D.J. A re-evaluation of random-effects meta-analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 2009, 172, 137–159. [Google Scholar] [CrossRef]
- Ciofu, O.; Moser, C.; Jensen, P.Ø.; Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar] [CrossRef]
- Eisenreich, W.; Rudel, T.; Heesemann, J.; Goebel, W. Link Between Antibiotic Persistence and Antibiotic Resistance in Bacterial Pathogens. Front. Cell. Infect. Microbiol. 2022, 12, 900848. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, R.; Singh, A.K.; Singh, S.; Chakravortty, D.; Das, D. Enzymatic Dispersion of Biofilms: An Emerging Biocatalytic Avenue to Combat Biofilm-Mediated Microbial Infections. J. Biol. Chem. 2022, 102352. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, M.Q.; Dias, C.J.; Neves, M.G.P.M.S.; Almeida, A.; Faustino, M.A.F. Revisiting Current Photoactive Materials for Antimicrobial Photodynamic Therapy. Molecules 2018, 23, 2424. [Google Scholar] [CrossRef] [PubMed]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia Coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Ezzraimi, A.E.; Hannachi, N.; Mariotti, A.; Rolain, J.-M.; Camoin-Jau, L. Platelets and Escherichia coli: A Complex Interaction. Biomedicines 2022, 10, 1636. [Google Scholar] [CrossRef]
- Ballén, V.; Cepas, V.; Ratia, C.; Gabasa, Y.; Soto, S.M. Clinical Escherichia coli: From Biofilm Formation to New Antibiofilm Strategies. Microorganisms 2022, 10, 1103. [Google Scholar] [CrossRef]
- Schooling, S.R.; Beveridge, T.J. Membrane Vesicles: An Overlooked Component of the Matrices of Biofilms. J. Bacteriol. 2006, 188, 5945–5957. [Google Scholar] [CrossRef]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Gajdács, M. The Continuing Threat of Methicillin-Resistant Staphylococcus aureus. Antibiotics 2019, 8, 52. [Google Scholar] [CrossRef]
- Zhen, X.; Lundborg, C.S.; Zhang, M.; Sun, X.; Li, Y.; Hu, X.; Gu, S.; Gu, Y.; Wei, J.; Dong, H. Clinical and economic impact of methicillin-resistant Staphylococcus aureus: A multicentre study in China. Sci. Rep. 2020, 10, 3900. [Google Scholar] [CrossRef] [PubMed]
- Sedarat, Z.; Taylor-Robinson, A.W. Biofilm Formation by Pathogenic Bacteria: Applying a Staphylococcus aureus Model to Appraise Potential Targets for Therapeutic Intervention. Pathogens 2022, 11, 388. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.O.; Hengge, R. Stress responses go three dimensional–the spatial order of physiological differentiation in bacterial macrocolony biofilms. Environ. Microbiol. 2014, 16, 1455–1471. [Google Scholar] [CrossRef] [PubMed]
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef] [PubMed]
- Feezor, R.J.; Oberholzer, C.; Baker, H.V.; Novick, D.; Rubinstein, M.; Moldawer, L.L.; Pribble, J.; Souza, S.; Dinarello, C.A.; Ertel, W.; et al. Molecular Characterization of the Acute Inflammatory Response to Infections with Gram-Negative versus Gram-Positive Bacteria. Infect. Immun. 2003, 71, 5803–5813. [Google Scholar] [CrossRef] [PubMed]
- Bacellar, I.O.; Pavani, C.; Sales, E.M.; Itri, R.; Wainwright, M.; Baptista, M.S. Membrane damage efficiency of phenothiazinium photosensitizers. Photochem. Photobiol. 2014, 90, 801–813. [Google Scholar] [CrossRef]
- Boltes Cecatto, R.; Siqueira de Magalhães, L.; Fernanda Setúbal Destro Rodrigues, M.; Pavani, C.; Lino-Dos-Santos-Franco, A.; Teixeira Gomes, M.; Fátima Teixeira Silva, D. Methylene blue mediated antimicrobial photodynamic therapy in clinical human studies: The state of the art. Photodiagnos. Photodyn. Ther. 2020, 31, 101828. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one-photosensitizers, photochemistry and cellular localization. Photodiagnos. Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Krajczewski, J.; Rucińska, K.; Townley, H.E.; Kudelski, A. Role of various nanoparticles in photodynamic therapy and detection methods of singlet oxygen. Photodiagnos. Photodyn. Ther. 2019, 26, 162–178. [Google Scholar] [CrossRef]
- George, B.P.; Chota, A.; Sarbadhikary, P.; Abrahamse, H. Fundamentals and applications of metal nanoparticle- enhanced singlet oxygen generation for improved cancer photodynamic therapy. Front. Chem. 2022, 10, 964674. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Capeletti, L.B.; de Oliveira, L.F.; Gonçalves Kde, A.; de Oliveira, J.F.; Saito, Â.; Kobarg, J.; dos Santos, J.H.; Cardoso, M.B. Tailored silica-antibiotic nanoparticles: Overcoming bacterial resistance with low cytotoxicity. Langmuir 2014, 30, 7456–7464. [Google Scholar] [CrossRef]
- Saltaji, H.; Armijo-Olivo, S.; Cummings, G.G.; Amin, M.; Da Costa, B.R.; Flores-Mir, C. Influence of blinding on treatment effectsize estimate in randomized controlled trials of oral health interventions. BMC Med Res. Methodol. 2018, 18, 42. [Google Scholar] [CrossRef]
- Vetter, T.R. Fundamentals of Research Data and Variables: The Devil Is in the Details. Anesth Analg. 2017, 125, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Abreu-Pereira, C.A.; Klein, M.I.; Lobo, C.I.V.; Gorayb Pereira, A.L.; Jordão, C.C.; Pavarina, A.C. DNase enhances photodynamic therapy against fluconazole-resistant Candida albicans biofilms. Oral Dis. 2022; Epub ahead of print. [Google Scholar] [CrossRef]


| Study (year) | Study Design | Inorganic Nanoparticle (np) | Light Dose | Irradiation Time | WaveLength | PhotoSensitizer | Pre-Irradiation Time | Microorganism | Culture Type | Sample Size | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Planas et al., 2015 [28] | In vitro | Mesoporous Silica Nanoparticle (MSNP) modified with mannose sugars or amino groups | 16 J/cm2 | ND | 652 nm | Methylene Blue (MB) | 30 min | Escherichia coli Pseudomonas aeruginosa | Suspension | ND | Colony forming units (CFU) E. coli = reduction of 7 log10 using MB (10 µM) alone or associated with MSNP. P. aeruginosa = reduction of 8 log10 using MB (10 µM) alone or associated with MSNP targeting motifs with mannose sugars. Reduction of 5 log10 was observed using MB associated with MSNP targeting with amino groups. |
| Tawfik et al., 2015 [29] | In vitro | Gold nanoparticles (AuNPs) | 24 J/cm² | 2 min | 660 nm | Methylene blue (MB) | ND | Staphylococcus aureus | Suspension | 3 | Cell viability after treatment S. aureus Inhibition of 95% for MB+np and 40% after MB. |
| Perni et al., 2016 [30] | In vitro | Silica nanoparticle | ND | 0.5 min 1 min 2 min 3 min | 630 nm | Toluidine blue (TB) | ND | Staphylococcus aureus (MRSA), Staphylococcus epidermidis, and Escherichia coli | Suspension | 3 | Colony forming units (CFU) E. coli = Reduction of 2 log10 after 3 min of irradiation S. epidermidis = Reduction of 2 log10 after 2 min of irradiation, and after 3 min, the CFU fell below the limit detection. S. aureus = reduction of 2 log10 after 2 min of irradiation, and after 3 min, the CFU fell below the limit detection. |
| Kuthati et al., 2017 [31] | In vitro | Mesoporous silica (MSN) and silver nanoparticles (SNP) | 72 J/cm2 | 300 seg | 470 nm | Curcumin (Cur) | ND | Escherichia coli | Suspensions | 6 | Cell viability (log CFU/mL) -Cur: reduction of 6 log10 -Cur+np: total microbial reduction |
| Paramanantham et al., 2018 [32] | In vitro | Mesoporous silica (MSN) | 50 mW | 5 min | 540 nm = Rose Bengal (RB) free 532 nm = MSN-RB | Rose Bengal (RB) | 3 h | Candida albicans | Suspensions and biofilm | Reductions in microbial suspension -RB = 40.96 ± 2.71% -RB+np = 88.62 ± 3.4% Reductions in microbial biofilm -RB = 42.2 ± 2.6% -RB+np = 79.64 ± 3.05% | |
| Anju et al., 2019 [33] | In vitro | Carbon nanotubes | 58.49 J/cm² | 3 min | 630 nm | Toluidine blue (TB) | 3 h | Staphylococcus aureus Pseudomonas aeruginosa | Biofilm | 3 | Biofilm inhibition (crystal violet) S. aureus: Reduction of 75% for TB+np and 47% after TB P. aeroginosa: Reduction of 70% for TB+np and 32% after TB Cell viability inhibition (CFU/mL) S. aureus: inhibition of 65% for TB+np and 34% after TB P. aeroginosa: inhibition of 58% for TB+np and 30% after TB Inhibition of exopolysaccharide production S. aureus: inhibition of 53% for TB+np and 30% after TB. P. aeroginosa: inhibition of 50% for TB+np and 27% after TB. |
| Maliszewska et al., 2019 [34] | In vitro | Gold nanoparticles (AuNPs) | 55, 108, and 179 mW/cm2 | 5, 10, 15, 30, and 45 min | 660 nm | MB | 120 min | Enterococcus faecalis | Suspensions and biofilm | 5 | Cell viability in suspension (log10CFU/mL) -MB ~ 4.5 log10 of reduction -MB+np ~ 5.5 log10 of reduction Cell viability in biofilm (log10CFU/mL) -MB ~ 3 log10 of reduction -MB+np ~ 4 log10 of reduction |
| Pourhajibagher et al., 2019 [35] | In vitro | Graphene quantum dots (GQD) | 60–80 J/cm² | 1 min | 435 ± 20 nm | Curcumin (CUR) | 5 min | Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia | Biofilm | 3 | Cell viability inhibition Reduction of 93% for GQD-CUR and 82% after CUR Biofilm formation Reduction of 76% for GQD-CUR and 61.3% after CUR |
| Belekov et al., 2020 [36] | In vitro | Silver nanoparticle | ND | 5 min | 660 nm | Methylene blue (MB) | ND | Staphylococcus aureus Escherichia coli | Suspension | 2 | Colony forming units (CFU) S. aureus: Reduction of 90 % for MB+np and 75% for MB E. coli: Reduction of 100 % for MB+np and 75% for after MB |
| de Santana et al., 2020 [37] | In vitro | Superparamagnetic iron oxide nanoparticles (SPIONPs) | 3.12 J/cm² | 29 seg | 450 nm | Curcumin (CUR) | 5 min | Staphylococcus aureus | Suspension | 3 | Colony forming units (CFU) SPIONPs + aPDT promoted the complete elimination of S. aureus. aPDT mediated by CUR promoted complete elimination using the same parameters. |
| Monteiro et al., 2021 [38] | In vitro | Gold nanoparticles (AuNPs) | 125 mW; 12 J/cm2 | 192 seg | 630 nm ± 20 nm | 1,9-Dimethyl-Methylene Blue zinc chloride double salt (DMMB) | 5min | Staphylococcus aureus (MRSA) | Suspensions | 3 | Colony forming units(log CFU/mL) -DMBMB: reduction of 9 log10. -DMMB-AuNPs: reduction of 8 log10 |
| Sen et al., 2021 [39] (a) | In vitro | Silver nanoparticles | ND | 80 min | 680 nm | Phthalocyanines (complexes 2 and 3). | ND | Staphylococcus aureus | Suspension | ND | Colony forming units (CFU) 100% elimination of S. aureus employing light and the conjugate. 87.85% and 58.33% of reduction employing the Phthalocyanines complex numbers 2 and 3, respectively. |
| Sen et al., 2021 [40] (b) | In vitro | Nitrogen, sulfur co-doped GQDs (3@N,S-GQDs, 4@N,S-GQDs) | ND | 80 min | 687 and 685 nm | Phthalocyanines | ND | Staphylococcus aureus | Suspension | 3 | Colony forming units (CFU) ZnPC 3 + LED = 99.91% of reduction. ZnPC 4 + LED = 100% of reduction. Conjugated 3@N,S-GQDs + LED = 100% of reduction. Conjugated 4@N,S-GQDs + LED = 100% of reduction. |
| Jin et al., 2022 [41] | In vitro/ in vivo | Ce6@WCS-IONP | 100 mW/cm2 | 15 min | 660 nm | Chlorin e6 | ND | Staphylococcus aureus (MRSA) | Suspension and Biofilm | 3 | Colony forming units (log10 CFU/mL) (suspension) Reduction of 4.25 log10 for Ce6@WCS-IONP and 3.8 log10 after Chlorin e6 Cells in biofilm Reduction of 37.5% for Ce6@WCS-IONP and no reductions after Chlorin e6 Bacterial viability in an animal model Reduction of 85% for Ce6@WCS-IONP and 50% after Chlorin e6 |
| Question | Was Administered Dose or Exposure Level Adequately Randomized? | Was Allocation to Study Groups Adequately Concealed? | Were Experimental Conditions Identical across Study Groups? | Were Research Personnel Blinded to the Study Group during the Study? | Were Outcome Data Complete without Attrition or Exclusion from the Analysis? | Can We Be Confident in the Exposure Characterization? | Can We Be Confident in the Outcome Assessment (Including Blinding of Assessors?) | Were There No Other Potential Threats to Internal Validity? | |
|---|---|---|---|---|---|---|---|---|---|
| Study | |||||||||
| Planas et al., 2015 [28] | ++ | ++ | ++ | -- | ++ | ++ | - | -- | |
| Tawfik et al., 2015 [29] | ++ | ++ | ++ | -- | ++ | ++ | - | -- | |
| Perni et al., 2016 [30] | ++ | ++ | ++ | -- | ++ | ++ | - | -- | |
| Kuthati et al., 2017 [31] | ++ | ++ | ++ | -- | ++ | ++ | - | -- | |
| Paramanantham et al., 2018 [32] | ++ | ++ | ++ | -- | ++ | ++ | - | -- | |
| Anju et al., 2019 [33] | ++ | ++ | ++ | -- | ++ | ++ | - | -- | |
| Maliszewska et al., 2019 [34] | ++ | ++ | ++ | -- | ++ | ++ | - | -- | |
| Pourhajibagher et al., 2019 [35] | ++ | ++ | ++ | -- | ++ | ++ | - | -- | |
| Belekov et al., 2020 [36] | ++ | ++ | ++ | -- | ++ | ++ | - | -- | |
| de Santana et al., 2020 [37] | ++ | ++ | ++ | -- | ++ | ++ | - | -- | |
| Monteiro et al., 2021 [38] | ++ | ++ | ++ | -- | ++ | ++ | - | -- | |
| Sen et al., 2021 [39] (a) | ++ | ++ | ++ | -- | ++ | ++ | - | -- | |
| Sen et al., 2021 [40] (b) | ++ | ++ | ++ | -- | ++ | ++ | - | -- | |
| Jin et al., 2022 [41] | ++ | ++ | ++ | -- | ++ | ++ | - | -- | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrisse, T.M.; Dias, L.M.; de Oliveira, A.B.; Jordão, C.C.; Mima, E.G.d.O.; Pavarina, A.C. Efficacy of Antimicrobial Photodynamic Therapy Mediated by Photosensitizers Conjugated with Inorganic Nanoparticles: Systematic Review and Meta-Analysis. Pharmaceutics 2022, 14, 2050. https://doi.org/10.3390/pharmaceutics14102050
Ferrisse TM, Dias LM, de Oliveira AB, Jordão CC, Mima EGdO, Pavarina AC. Efficacy of Antimicrobial Photodynamic Therapy Mediated by Photosensitizers Conjugated with Inorganic Nanoparticles: Systematic Review and Meta-Analysis. Pharmaceutics. 2022; 14(10):2050. https://doi.org/10.3390/pharmaceutics14102050
Chicago/Turabian StyleFerrisse, Túlio Morandin, Luana Mendonça Dias, Analú Barros de Oliveira, Cláudia Carolina Jordão, Ewerton Garcia de Oliveira Mima, and Ana Claudia Pavarina. 2022. "Efficacy of Antimicrobial Photodynamic Therapy Mediated by Photosensitizers Conjugated with Inorganic Nanoparticles: Systematic Review and Meta-Analysis" Pharmaceutics 14, no. 10: 2050. https://doi.org/10.3390/pharmaceutics14102050
APA StyleFerrisse, T. M., Dias, L. M., de Oliveira, A. B., Jordão, C. C., Mima, E. G. d. O., & Pavarina, A. C. (2022). Efficacy of Antimicrobial Photodynamic Therapy Mediated by Photosensitizers Conjugated with Inorganic Nanoparticles: Systematic Review and Meta-Analysis. Pharmaceutics, 14(10), 2050. https://doi.org/10.3390/pharmaceutics14102050







