A Review on Tumor Control Probability (TCP) and Preclinical Dosimetry in Targeted Radionuclide Therapy (TRT)
Abstract
:1. Introduction
2. TCP Modelling
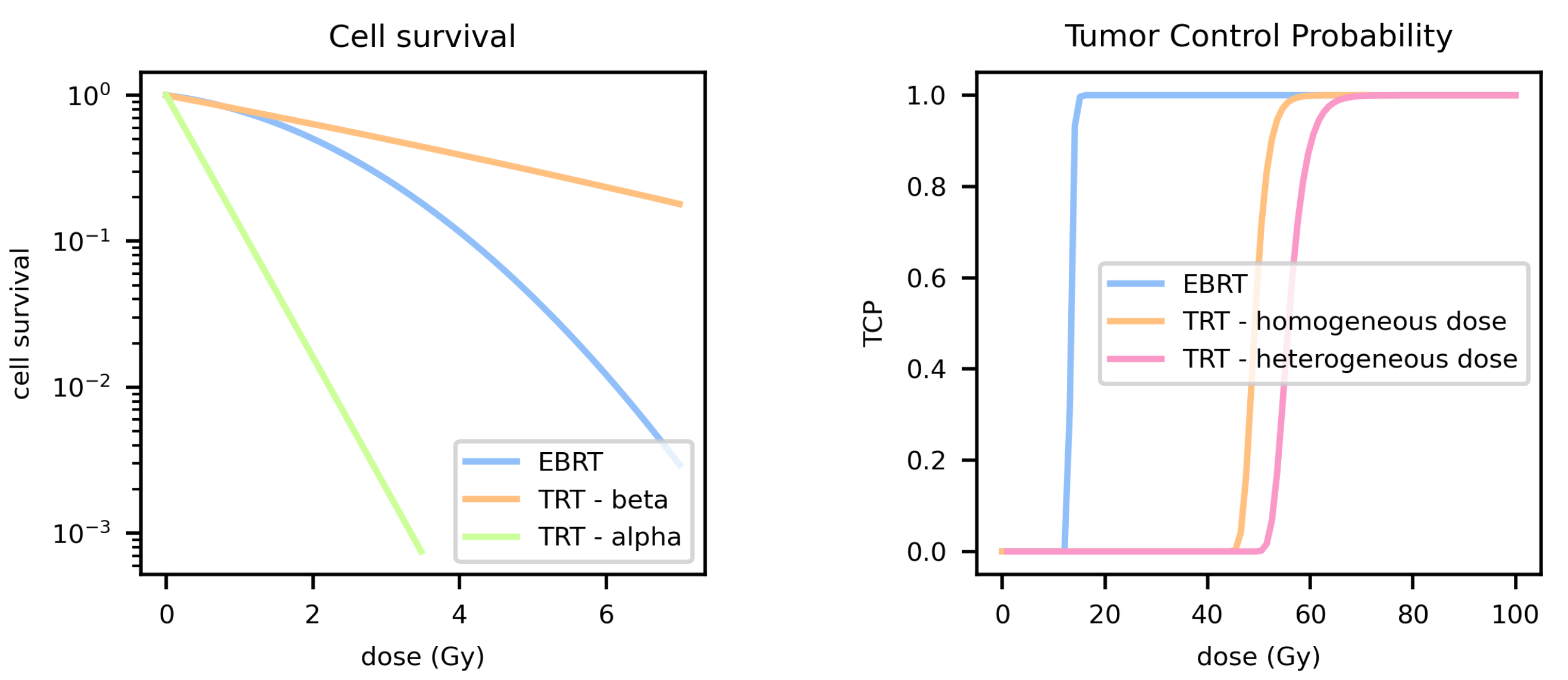
3. TCP for Targeted Radionuclide Therapy
3.1. Dose Rate
3.2. Heterogeneous Dose Distribution
3.2.1. Tumor/Tissue Dose Heterogeneity
3.2.2. Subcellular Dose Heterogeneity
3.3. Linear Energy Transfer
4. Extended Modelling
4.1. Repopulation
4.2. Heterogeneous Dose Response
4.3. Bystander Effect
5. Preclinical Dosimetry
5.1. MIRD Scheme
5.2. In Vitro Dosimetry
5.2.1. In Vitro Source Regions
5.2.2. Time-Integrated Activity
5.2.3. S-Value
5.3. In Vivo Dosimetry
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tommasino, F.; Nahum, A.; Cella, L. Increasing the power of tumour control and normal tissue complication probability modelling in radiotherapy: Recent trends and current issues. Transl. Cancer Res. 2017, 6, S807–S821. [Google Scholar] [CrossRef]
- Konijnenberg, M.W.; de Jong, M. Preclinical animal research on therapy dosimetry with dual isotopes. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 19–27. [Google Scholar] [CrossRef]
- Mayles, P.; Nahum, A.; Rosenwald, J. Handbook of Radiotherapy Physics; Taylor & Francis Group: Abingdon, UK, 2007. [Google Scholar]
- O’Rourke, S.F.C.; McAneney, H.; Hillen, T. Linear quadratic and tumour control probability modelling in external beam radiotherapy. J. Math. Biol. 2008, 58, 799–817. [Google Scholar] [CrossRef]
- Monte, U.D. Cell Cycle Does the cell number 109 still really fit one gram of tumor tissue? Cell Cycle 2009, 8, 505–506. [Google Scholar] [CrossRef]
- McMahon, S.J. The linear quadratic model: Usage, interpretation and challenges. Phys. Med. Biol. 2018, 64, 01TR01. [Google Scholar] [CrossRef]
- Dale, R. Use of the Linear-Quadratic Radiobiological Model for Quantifying Kidney Response in Targeted Radiotherapy. Cancer Biother. Radiopharm. 2004, 19, 363–370. [Google Scholar] [CrossRef]
- Dale, R.; Carabe-Fernandez, A. The Radiobiology of Conventional Radiotherapy and Its Application to Radionuclide Therapy. Cancer Biother. Radiopharm. 2005, 20, 47–51. [Google Scholar] [CrossRef]
- Bernhardt, P.; Svensson, J.; Hemmingsson, J.; van der Meulen, N.P.; Zeevaart, J.R.; Konijnenberg, M.W.; Müller, C.; Kindblom, J. Dosimetric Analysis of the Short-Ranged Particle Emitter 161Tb for Radionuclide Therapy of Metastatic Prostate Cancer. Cancers 2021, 13, 2011. [Google Scholar] [CrossRef]
- Elgqvist, J.; Timmermand, O.V.; Larsson, E.; Strand, S.E. Radiosensitivity of prostate cancer cell lines for irradiation from beta particle-emitting radionuclide 177Lu compared to alpha particles and gamma rays. Anticancer Res. 2016, 36, 103–110. [Google Scholar]
- Forand, A.; Dutrillaux, B.; Bernardino-Sgherri, J. Gamma-H2AX expression pattern in non-irradiated neonatal mouse germ cells and after low-dose gamma-radiation: Relationships between chromatid breaks and DNA double-strand breaks. Biol. Reprod. 2004, 71, 643–649. [Google Scholar] [CrossRef]
- Poty, S.; Francesconi, L.C.; McDevitt, M.R.; Morris, M.J.; Lewis, J.S. alpha-emitters for Radiotherapy: From Basic Radiochemistry to Clinical Studies—Part 1. J. Nucl. Med. 2018, 59, 878–884. [Google Scholar] [CrossRef] [Green Version]
- Solanki, J.H.; Tritt, T.; Pasternack, J.B.; Kim, J.J.; Leung, C.N.; Domogauer, J.D.; Colangelo, N.W.; Narra, V.R.; Howell, R.W. Cellular response to exponentially increasing and decreasing dose rates: Implications for rreatment planning in targeted radionuclide therapy. Radiat. Res. 2017, 188, 221–234. [Google Scholar] [CrossRef]
- Gholami, Y.; Willowson, K.; Forwood, N.J.; Harvie, R.; Hardcastle, N.; Bromley, R.; Ryu, H.; Yuen, S.; Howell, V.M.; Kuncic, Z.; et al. Comparison of radiobiological parameters for 90Y radionuclide therapy (RNT) and external beam radiotherapy (EBRT) in vitro. EJNMMI Phys. 2018, 5, 18. [Google Scholar] [CrossRef]
- Nonnekens, J.; van Kranenburg, M. Potentiation of peptide receptor radionuclide therapy by the PARP inhibitor olaparib. Theranostics 2016, 6, 1821–1832. [Google Scholar] [CrossRef]
- Dale, R.G. Dose-rate effects in targeted radiotherapy. Phys. Med. Biol. 1996, 41, 1871. [Google Scholar] [CrossRef]
- Timmermand, O.V.; Elgqvist, J.; Beattie, K.A.; Örbom, A.; Larsson, E.; Eriksson, S.E.; Thorek, D.L.J.; Beattie, B.J.; Tran, T.A.; Ulmert, D.; et al. Preclinical efficacy of hK2 targeted [177Lu]hu11B6 for prostate cancer theranostics. Theranostics 2019, 9, 2129–2142. [Google Scholar] [CrossRef] [PubMed]
- Feijtel, D.; Doeswijk, G.N.; Verkaik, N.S.; Haeck, J.C.; Chicco, D.; Angotti, C.; Konijnenberg, M.W.; de Jong, M.; Nonnekens, J. Inter- and intra-tumor somatostatin receptor 2 heterogeneity influences peptide receptor radionuclide therapy response. Theranostics 2021, 11, 491–505. [Google Scholar] [CrossRef]
- O’Neill, E.; Kersemans, V.; Allen, P.D.; Terry, S.Y.A.; Torres, J.B.; Mosley, M.; Smart, S.; Lee, B.Q.; Falzone, N.; Vallis, K.A.; et al. Imaging DNA Damage Repair In Vivo After 177Lu-DOTATATE Therapy. J. Nucl. Med. 2020, 61, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Nahum, A.E. Microdosimetry and radiocurability: Modelling targeted therapy with beta-emitters. Phys. Med. Biol 1996, 41, 1957–1972. [Google Scholar] [PubMed]
- Uusijarvi, H.; Bernhardt, P.; Forssell-Aronsson, E. Tumour control probability (TCP) for non-uniform activity distribution in radionuclide therapy. Phys. Med. Biol. 2008, 53, 4369–4381. [Google Scholar] [CrossRef]
- Howell, R.W.; Neti, P.V.; Pinto, M.; Gerashchenko, B.I.; Narra, V.R.; Azzam, E.I. Challanges and progress in predicting biological responses to incorporated radioactivity. Radiat. Prot. Dosim. 2006, 122, 521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamborino, G.; Nonnekens, J.; De Saint-Hubert, M.; Struelens, L.; Feijtel, D.; de Jong, M.; Konijnenberg, M.W. Dosimetric evaluation of receptor-heterogeneity on the therapeutic efficacy of peptide receptor radionuclide therapy: Correlation with DNA damage induction and in vivo survival. J. Nucl. Med. 2021, 63, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Wheldon, T.E.; O’donoghue, A.; Barrett, A.; Michalowski, A.S. The curability of tumours of differing size by targeted radiotherapy using 131I or 90Y. Radiother. Oncol. 1991, 21, 91–99. [Google Scholar] [CrossRef]
- Graf, F.; Fahrer, J.R.; Maus, S.; Morgenstern, A.; Bruchertseifer, F.; Venkatachalam, S.; Fottner, C.; Weber, M.M.; Huelsenbeck, J.; Schreckenberger, M.; et al. DNA Double Strand Breaks as Predictor of Efficacy of the Alpha-Particle Emitter Ac-225 and the Electron Emitter Lu-177 for Somatostatin Receptor Targeted Radiotherapy. PLoS ONE 2014, 9, e88239. [Google Scholar] [CrossRef]
- Miederer, M.; Henriksen, G.; Alke, A.; Mossbrugger, I.; Quintanilla-Martinez, L.; Senekowitsch-Schmidtke, R.; Essler, M. Preclinical Evaluation of the A-Particle Generator Nuclide 225 Ac for Somatostatin Receptor Radiotherapy of Neuroendocrine Tumors. Cancer Ther. Preclin. 2008, 14, 3555–3561. [Google Scholar] [CrossRef]
- Lechner, A.; Blaicknes, M.; Gianolini, S.; Poljanc, K.; Aiginger, H.; Georg, D. Targeted radionuclide therapy: Theoretical study of the relationship between tumour control probability and tumour radius for a 32P/33P radionuclide cocktail. Phys. Med. Biol. 2008, 53, 1961–1974. [Google Scholar] [CrossRef]
- Walrand, S.; Hanin, F.X.; Pauwels, S.; Jamar, F. Tumour control probability derived from dose distribution in homogeneous and heterogeneous models: Assuming similar pharmacokinetics, 125Sn–177Lu is superior to 90Y–177Lu in peptide receptor radiotherapy. Phys. Med. Biol. 2012, 57, 4263. [Google Scholar] [CrossRef]
- Aghevlian, S.; Boyle, A.J.; Reilly, R.M. Radioimmunotherapy of cancer with high linear energy transfer (LET) radiation delivered by radionuclides emitting alpha-particles or Auger electrons. Adv. Drug Deliv. Rev. 2017, 109, 102–118. [Google Scholar] [CrossRef]
- Bavelaar, B.M.; Lee, B.Q.; Gill, M.R.; Falzone, N.; Vallis, K.A. Subcellular targeting of theranostic radionuclides. Front. Pharmacol. 2018, 9, 996. [Google Scholar] [CrossRef]
- Tamborino, G.; Saint-Hubert, M.D.; Struelens, L.; Seoane, D.C.; Ruigrok, E.A.M.; Aerts, A.; van Cappellen, W.A.; de Jong, M.; Konijnenberg, M.W.; Nonnekens, J. Cellular dosimetry of [177Lu]Lu-DOTA-[Tyr3]octreotate radionuclide therapy: The impact of modeling assumptions on the correlation with in vitro cytotoxicity. EJNMMI Phys. 2020, 7, 8. [Google Scholar] [CrossRef]
- Freudenberg, R.; Runge, R.; Maucksch, U.; Berger, V.; Kotzerke, J. On the dose calculation at the cellular level and its implications for the RBE of 99mTc and 123I. Med. Phys. 2014, 41, 062503. [Google Scholar] [CrossRef] [PubMed]
- Borgna, F.; Haller, S.; Rodriguez, J.M.M.; Ginj, M.; Grundler, P.V.; Zeevaart, J.R.; Köster, U.; Schibli, R.; van der Meulen, N.P.; Müller, C. Combination of terbium-161 with somatostatin receptor antagonists—A potential paradigm shift for the treatment of neuroendocrine neoplasms. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Dale, R.G.; Jones, B. The assessment of RBE effects using the concept of biologically effective dose. Int. J. Radiat. Oncol. Biol. Phys. 1999, 43, 639–645. [Google Scholar] [CrossRef]
- Claesson, K.; Magnander, K.; Kahu, H.; Lindegren, S.; Hultborn, R.; Elmroth, K. RBE of alpha-particles from 211At for complex DNA damage and cell survival in relation to cell cycle position. Int. J. Radiat. Biol. 2011, 87, 372–384. [Google Scholar] [CrossRef]
- Verwijnen, S.; Capello, A.; Bernard, B.; van den Aardweg, G.; Konijnenberg, M.; Breeman, W.; Krenning, E.; de Jong, M. Low-Dose-Rate Irradiation by 131I Versus High-Dose-Rate External-Beam Irradiation in the Rat Pancreatic Tumor Cell Line CA20948. Cancer Biother. Radiopharm. 2004, 19, 285–292. [Google Scholar] [CrossRef]
- Chan, H.S.; de Blois, E.; Morgenstern, A.; Bruchertseifer, F.; de Jong, M.; Breeman, W.; Konijnenberg, M. In Vitro comparison of 213Bi-and 177Lu-radiation for peptide receptor radionuclide therapy. PLoS ONE 2017, 12, e0181473. [Google Scholar] [CrossRef]
- Nayak, T.K.; Norenberg, J.P.; Anderson, T.L.; Prossnitz, E.R.; Stabin, M.G.; Atcher, R.W. Somatostatin-receptor-targeted alpha-emitting 213Bi is therapeutically more effective than beta-emitting 177Lu in human pancreatic adenocarcinoma cells. Nucl. Med. Biol. 2007, 34, 185–193. [Google Scholar] [CrossRef]
- Dahmen, V.; Pomplun, E.; Kriehuber, R. Ioidine-125-labeled DNA-Triplex-forming oligonucleotides reveal increased cyto-and genotoxic effectiveness compared to Phosphorus-32. Int. J. Radiat. Biol. 2016, 92, 679–685. [Google Scholar] [CrossRef]
- Cai, Z.; Kwon, Y.L.; Reilly, R.M. Monte Carlo N-Particle (MCNP) Modeling of the Cellular Dosimetry of 64Cu: Comparison with MIRDcell S Values and Implications for Studies of its Cytotoxic Effects. J. Nucl. Med. 2017, 58, 339–345. [Google Scholar] [CrossRef]
- Franken, N.A.; Hovingh, S.; Ten Cate, R.; Krawczyk, P.; Stap, J.; Hoebe, R.; Aten, J.; Barendsen, G. Relative biological effectiveness of high linear energy transfer alpha-particles for the induction of DNA-double-strand breaks, chromosome aberrations and reproductive cell death in SW-1573 lung tumour cells. Oncol. Rep. 2012, 27, 769–774. [Google Scholar] [CrossRef]
- Zaider, M.; Minerbo, G.N. Tumour control probability: A formulation applicable to any temporal protocol of dose delivery. Phys. Med. Biol. 2000, 45, 279. [Google Scholar] [CrossRef]
- Maucksch, U.; Runge, R.; Oehme, L.; Kotzerke, J.; Freudenberg, R. Radiotoxicity of alpha particles versus high and low energy electrons in hypoxic cancer cells. NuklearMedizin 2018, 57, 56–63. [Google Scholar] [CrossRef]
- Dawson, A.; Hillen, T. Derivation of the tumour control probability (TCP) from a cell cycle model. Comput. Math. Methods Med. 2006, 7, 121–141. [Google Scholar] [CrossRef]
- Ebert, M.A.; Hoban, P.W. Some characteristics of tumour control probability for heterogeneous tumours. Phys. Med. Biol. 1996, 41, 2125. [Google Scholar] [CrossRef]
- Brenner, D.J.; Hlatky, L.R.; Hahnfeldt, P.J.; Hall, E.J.; Sachs, R.K. A convenient extension of the linear-quadratic model to include redistribution and reoxygenation. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 379–390. [Google Scholar] [CrossRef]
- Jiménez-Franco, L.D.; Glatting, G.; Prasad, V.; Weber, W.A.; Beer, A.J.; Kletting, P. Effect of Tumor Perfusion and Receptor Density on Tumor Control Probability in 177Lu-DOTATATE Therapy: An In Silico Analysis for Standard and Optimized Treatment. J. Nucl. Med. 2021, 62, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Widel, M.M. Radionuclides in radiation-induced bystander effect; may it share in radionuclide therapy? Neoplasma 2017, 64, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Bolch, W.E.; Eckerman, K.F. MIRD Pamphlet No. 21: A Generalized Schema for Radiopharmaceutical Dosimetry—Standardization of Nomenclature. J. Nucl. Med. 2009, 50, 477–484. [Google Scholar] [CrossRef]
- Jonkhoff, A.R.; Huijgens, P.C.; Versteegh, R.T.; van Lingen, A.; Ossenkoppele, G.J.; Dräger, A.M.; Teule, G.J.J. Radiotoxicity of 67-Gallium on myeloid leukemic blasts. Leuk. Res. 1995, 19, 169–174. [Google Scholar] [CrossRef]
- Costantini, D.L.; Chan, C.; Cai, Z.; Vallis, K.A.; Reilly, R.M. 111In-Labeled Trastuzumab (Herceptin) Modified with Nuclear Localization Sequences (NLS): An Auger Electron-Emitting Radiotherapeutic Agent for HER2/neu-Amplified Breast Cancer. J. Nucl. Med. 2007, 48, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Idrissou, M.B.; Pichard, A.; Tee, B.; Kibedi, T.; Poty, S.; Pouget, J.P. Targeted Radionuclide Therapy Using Auger Electron Emitters: The Quest for the Right Vector and the Right Radionuclide. Pharmaceutics 2021, 13, 980. [Google Scholar] [CrossRef]
- Woo, D.V.; Li, D.; Mattis, J.A.; Steplewski, Z. Selective Chromosomal Damage and Cytotoxicity of 125I-labeled Monoclonal Antibody 17-la in Human Cancer Cells1. Cancer Res. 1989, 49, 2952–2958. [Google Scholar]
- Capello, A.; Krenning, E.P.; Breeman, W.A.P.; Bernard, B.F.; Konijnenberg, M.W.; de Jong, M. Tyr 3-Octreotide and Tyr 3-Octreotate Radiolabeled with 177Lu or 90Y: Peptide Receptor Radionuclide Therapy Results In Vitro. Cancer Biother. Radiopharm. 2003, 18, 761–768. [Google Scholar] [CrossRef]
- Capello, A.; Krenning, E.P.; Breeman, W.A.P.; Bernard, B.F.; de Jong, M. Peptide Receptor Radionuclide Therapy In Vitro Using [111In-DTPA0] Octreotide. J. Nucl. Med. 2003, 44, 98–104. [Google Scholar]
- Fullerton, N.E.; Mairs, R.J.; Kirk, D.; Keith, W.N.; Carruthers, R.; McCluskey, A.G.; Brown, M.; Wilson, L.; Boyd, M. Application of targeted radiotherapy/gene therapy to bladder cancer cell lines. Eur. Urol. 2005, 47, 250–256. [Google Scholar] [CrossRef]
- Boyd, M.; Ross, S.C.; Dorrens, J.; Fullerton, N.E.; Tan, K.W.; Zalutsky, M.R.; Mairs, R.J. Radiation-Induced Biologic Bystander Effect Elicited In Vitro by Targeted Radiopharmaceuticals Labeled with alpha-, beta-, and Auger Electron-Emitting Radionuclides. J. Nucl. Med. 2006, 47, 1007–1015. [Google Scholar]
- Lee, Y.J.; Chung, J.K.; Kang, J.H.; Jeong, J.M.; Lee, D.S.; Lee, M.C. Wild-type p53 enhances the cytotoxic effect of radionuclide gene therapy using sodium iodide symporter in a murine anaplastic thyroid cancer model. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 235–241. [Google Scholar] [CrossRef]
- Klutz, K.; Willhauck, M.J.; Wunderlich, N.; Zach, C.; Anton, M.; Senekowitsch-Schmidtke, R.; Göke, B.; Spitzweg, C. Sodium Iodide Symporter (NIS)-Mediated Radionuclide (131I, 188Re) Therapy of Liver Cancer After Transcriptionally Targeted Intratumoral in Vivo NIS Gene Delivery. Hum. Gene Ther. 2011, 22, 1403–1412. [Google Scholar] [CrossRef]
- Mcmillan, D.D.; Maeda, J.; Bell, J.J.; Genet, M.D.; Phoonswadi, G.; Mann, K.A.; Kraft, S.L.; Kitamura, H.; Fujimori, A.; Yoshii, U.; et al. Validation of 64 Cu-ATSM damaging DNA via high-LET Auger electron emission. J. Radiat. Res. 2015, 56, 784–791. [Google Scholar] [CrossRef]
- Kiess, A.P.; Minn, I.; Vaidyanathan, G.; Hobbs, R.F.; Josefsson, A.; Shen, C.; Brummet, M.; Chen, Y.; Choi, J.; Koumarianou, E.; et al. (2S)-2-(3-(1-Carboxy-5-(4-211At-Astatobenzamido)Pentyl)Ureido)-Pentanedioic Acid for PSMA-Targeted alpha-Particle Radiopharmaceutical Therapy. J. Nucl. Med. 2016, 57, 1569–1575. [Google Scholar] [CrossRef]
- Waghorn, P.A.; Jackson, M.R.; Gouverneur, V.; Vallis, K.A. Targeting telomerase with radiolabeled inhibitors. Eur. J. Med. Chem. 2017, 125, 117–129. [Google Scholar] [CrossRef]
- Gill, M.R.; Menon, J.U.; Jarman, P.J.; Owen, J.; Skaripa-Koukelli, I.; Able, S.; Thomas, J.A.; Carlisle, R.; Vallis, K.A. 111In-labelled polymeric nanoparticles incorporating a ruthenium-based radiosensitizer for EGFR-targeted combination therapy in oesophageal cancer cells. Nanoscale 2018, 10, 10596. [Google Scholar] [CrossRef]
- bin Othman, M.F.; Verger, E.; Costa, I.; Tanapirakgul, M.; Cooper, M.S.; Imberti, C.; Lewington, V.J.; Blower, P.J.; Terry, S.Y.A. In vitro cytotoxicity of Auger electron-emitting [67Ga]Ga-trastuzumab. Nucl. Med. Biol. 2020, 80–81, 57–64. [Google Scholar] [CrossRef]
- Jackson, M.R.; Bavelaar, B.M.; Waghorn, P.A.; Gill, M.R.; El-Sagheer, A.H.; Brown, T.; Tarsounas, M.; Vallis, K.A. Radiolabeled Oligonucleotides Targeting the RNA Subunit of Telomerase Inhibit Telomerase and Induce DNA Damage in Telomerase-Positive Cancer Cells. Cancer Res. 2019, 79, 4627–4637. [Google Scholar] [CrossRef] [Green Version]
- Freudenberg, R.; Wendisch, M.; Kotzerke, J. Geant4-Simulations for cellular dosimetry in nuclear medicine. Z. Med. Phys. 2011, 21, 281–289. [Google Scholar] [CrossRef]
- Osytek, K.M.; Blower, P.J.; Costa, I.M.; Smith, G.E.; Abbate, V.; Terry, S.Y.A. In vitro proof of concept studies of radiotoxicity from Auger electron-emitter thallium-201. Eur. J. Nucl. Med. Mol. Imaging 2021, 11, 63. [Google Scholar] [CrossRef]
- Weeks, A.J.; Paul, R.L.; Marsden, P.K.; Blower, P.J.; Lloyd, D.R. Radiobiological effects of hypoxia-dependent uptake of 64Cu-ATSM: Enhanced DNA damage and cytotoxicity in hypoxic cells. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 330–338. [Google Scholar] [CrossRef]
- Weeks, A.J.; Blower, P.J.; Lloyd, D.R. P53-dependent radiobiological responses to internalised indium-111 in human cells. Nucl. Med. Biol. 2013, 40, 73–79. [Google Scholar] [CrossRef]
- Othman, M.F.; Mitry, N.R.; Lewington, V.J.; Blower, P.J.; Terry, S.Y.A. Re-assessing gallium-67 as a therapeutic radionuclide. Nucl. Med. Biol. 2017, 46, 12–18. [Google Scholar] [CrossRef]
- Thisgaard, H.; Olsen, B.B.; Dam, J.H.; Bollen, P.; Mollenhauer, J.; Høilund-Carlsen, P.F. Evaluation of Cobalt-Labeled Octreotide Analogs for Molecular Imaging and Auger Electron-Based Radionuclide Therapy. J. Nucl. Med. 2014, 55, 1311–1316. [Google Scholar] [CrossRef]
- Falzone, N.; Lee, B.Q.; Able, S.; Malcolm, J.; Terry, S.; Alayed, Y.; Vallis, K.A. Targeting micrometastases: The effect of heterogeneous radionuclide distribution on tumor control probability. J. Nucl. Med. 2019, 60, 250–258. [Google Scholar] [CrossRef]
- Costa, I.M.; Cheng, J.; Osytek, K.M.; Imberti, C.; Terry, S.Y.A. Methods and techniques for in vitro subcellular localization of radiopharmaceuticals and radionuclides. Nucl. Med. Biol. 2021, 98–99, 18–29. [Google Scholar] [CrossRef]
- Akudugu, J.M.; Neti, P.V.S.V.; Howell, R.W. Changes in Lognormal Shape Parameter Guide Design of Patient-Specific Radiochemotherapy Cocktails. J. Nucl. Med. 2011, 52, 642–649. [Google Scholar] [CrossRef]
- Neti, P.V.; Howell, R.W. Log Normal Distribution of Cellular Uptake of Radioactivity: Statistical Analysis of Alpha Particle Track Autoradiography. J. Nucl. Med. 2006, 47, 1049. [Google Scholar] [PubMed]
- Rajon, D.; Bolch, W.E.; Howell, R.W. Survival of tumor and normal cells upon targeting with electron-emitting radionuclides. Med. Phys. 2013, 40, 014101. [Google Scholar] [CrossRef]
- Guerriero, F.; Ferrari, M.E.; Botta, F.; Fioroni, F.; Grassi, E.; Versari, A.; Sarnelli, A.; Pacilio, M.; Amato, E.; Strigari, L.; et al. Kidney Dosimetry in 177Lu and 90Y Peptide Receptor Radionuclide Therapy: Influence of Image Timing, Time-Activity Integration Method, and Risk Factors. Biomed Res. Int. 2013, 2013, 935351. [Google Scholar] [CrossRef]
- Šefl, M.; Kyriakou, I.; Emfietzoglou, D. Technical Note: Impact of cell repopulation and radionuclide uptake phase on cell survival. Med. Phys. 2016, 43, 2715–2720. [Google Scholar] [CrossRef]
- Freudenberg, R.; Wendisch, M.; Runge, R.; Wunderlich, G.; Kotzerke, J. Reduction in clonogenic survival of sodium-iodide symporter (NIS)-positive cells following intracellular uptake of 99mTc versus 188Re. Int. J. Radiat. Biol. 2012, 88, 991–997. [Google Scholar] [CrossRef]
- Silva, F.; D’Onofrio, A.; Mendes, C.; Pinto, C.; Marques, A.; Campello, M.P.C.; Oliveira, M.C.; Raposinho, P.; Belchior, A.; Di Maria, S.; et al. Radiolabeled Gold Nanoseeds Decorated with Substance P Peptides: Synthesis, Characterization and In Vitro Evaluation in Glioblastoma Cellular Models. Int. J. Mol. Sci. 2022, 23, 617. [Google Scholar] [CrossRef]
- Yard, B.D.; Gopal, P.; Bannik, K.; Siemeister, G.; Hagemann, U.B.; Abazeed, M.E. Translational Science Cellular and Genetic Determinants of the Sensitivity of Cancer to alpha-Particle Irradiation. Cancer Res. 2019, 79, 5640–5651. [Google Scholar] [CrossRef]
- Nayak, T.; Norenberg, J.; Anderson, T.; Atcher, R. A Comparison of High- Versus Low-Linear Energy Transfer Somatostatin Receptor Targeted Radionuclide Therapy in vitro. Cancer Biother. Radiopharm. 2005, 20, 52–57. [Google Scholar] [CrossRef]
- Bailey, K.E.; Costantini, D.L.; Cai, Z.; Scollard, D.A.; Chen, Z.; Reilly, R.M.; Vallis, K.A. Epidermal Growth Factor Receptor Inhibition Modulates the Nuclear Localization and Cytotoxicity of the Auger Electron-Emitting Radiopharmaceutical 111In-DTPA-Human Epidermal Growth Factor. J. Nucl. Med. 2007, 48, 1562–1570. [Google Scholar] [CrossRef]
- Koosha, F.; Eynali, S.; Eyvazzadeh, N. Kamalabadi, M.A. The effect of iodine-131 beta-particles in combination with A-966492 and Topotecan on radio-sensitization of glioblastoma: An in vitro study. Appl. Radiat. Isot. 2021, 177, 109904. [Google Scholar] [CrossRef]
- Eke, I.; Ingargiola, M.; Föster, C.; Kunz-Schughart, L.A.; Baumann, M.; Runge, R.; Freudenberg, R.; Kotzerke, J.; Heldt, M.; Pietzsch, H.J.; et al. Cytotoxic properties of radionuclide-conjugated Cetuximab without and in combination with external irradiation in head and neck cancer cells in vitro. Int. J. Radiat. Biol. 2014, 90, 678–686. [Google Scholar] [CrossRef]
- Goddu, S.M.; Howell, R.W.; Rao, D.V. Cellular Dosimetry: Absorbed Fractions for Monoenergetic Electron and Alpha Particle Sources and S-Values for Radionuclides Uniformly Distributed in Different Cell Compartments. J. Nucl. Med. 1994, 35, 303–316. [Google Scholar]
- Katugampola, S.; Wang, J.; Rosen, A.; Howell, R.W. MIRD Pamphlet No. 27: MIRDcell V3, a revised software tool for multicellular dosimetry and bioeffect modeling. J. Nucl. Med. 2022, 63, jnumed.121.263253. [Google Scholar] [CrossRef]
- Šefl, M.; Incerti, S.; Papamichael, G.; Emfietzoglou, D. Calculation of cellular S-values using Geant4-DNA: The effect of cell geometry. Appl. Radiat. Isot. 2015, 104, 113–123. [Google Scholar] [CrossRef]
- Emfietzoglou, D.; Kostarelos, K.; Hadjidoukas, P.; Bousis, C.; Fotopoulos, A.; Pathak, A.; Nikjoo, H. Subcellular S-factors for low-energy electrons: A comparison of Monte Carlo simulations and continuous-slowing-down calculations. Int. J. Radiat. Biol. 2009, 84, 1034–1044. [Google Scholar] [CrossRef]
- Maucksch, U.; Runge, R.; Wunderlich, G.; Freudenberg, R.; Naumann, A.; Kotzerke, J. Comparison of the radiotoxicity of the Tc-MIBI Comparison of the radiotoxicity of the 99mTc-labeled compounds 99mTc-pertechnetate, 99mTc-HMPAO and 99mTc-MIBI. Int. J. Radiat. Biol. 2016, 92, 698–706. [Google Scholar] [CrossRef]
- Ruigrok, E.A.M.; Tamborino, G.; de Blois, E.; Roobol, S.J.; Verkaik, N.; De Saint-Hubert, M.; Konijnenberg, M.W.; van Weerden, W.M.; de Jong, M.; Nonnekens, J. In vitro dose effect relationships of actinium-225- and lutetium-177-labeled PSMA-Iamp. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3627–3638. [Google Scholar] [CrossRef]
- Falzone, N.; Fernández-Varea, J.M.; Flux, G.; Vallis, K.A. Monte Carlo Evaluation of Auger Electron-Emitting Theranostic Radionuclides. J. Nucl. Med. 2015, 56, 1441–1446. [Google Scholar] [CrossRef]
- Steffen, A.C.; Göstring, L.; Tolmachev, V.; Palm, S.; Stenerlöw, B.; Carlsson, J. Differences in radiosensitivity between three HER2 overexpressing cell lines. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1179–1191. [Google Scholar] [CrossRef]
- Palmer, T.L.; Tkacz-Stachowska, K.; Skartlien, R.; Omar, N.; Hassfjell, S.; Mjøs, A.; Bergvoll, J.; Brevik, E.M.; Hjelstuen, O. Microdosimetry modeling with Auger emitters in generalized cell geometry. Phys. Med. Biol. 2021, 66, 115023. [Google Scholar] [CrossRef]
- Marcatili, S.; Pichard, A.; Courteau, A.; Ladjohounlou, R.; Navarro-Teulon, I.; Repetto-Llamazares, A.; Heyerdahl, H.; Dahle, J.; Pouget, J.P.; Bardiès, M. Realistic multi-cellular dosimetry for 177Lu-labelled antibodies: Model and application. Phys. Med. Biol. 2016, 61, 6935. [Google Scholar] [CrossRef]
- Miller, B.W. Radiation Imagers for Quantitative, Single-particle Digital Autoradiography of Alpha- and Beta-particle Emitters. Semin. Nucl. Med. 2018, 48, 367–376. [Google Scholar] [CrossRef]
- Örbom, A.; Eriksson, S.E.; Elgström, E.; Ohlsson, T.; Nilsson, R.; Tennvall, J.; Strand, S.E. The Intratumoral Distribution of Radiolabeled 177 Lu-BR96 Monoclonal Antibodies Changes in Relation to Tumor Histology over Time in a Syngeneic Rat Colon Carcinoma Model. J. Nucl. Med. 2013, 54, 1404–1410. [Google Scholar] [CrossRef]
- Dewaraja, Y.K.; Frey, E.C.; Sgouros, G.; Brill, A.B.; Roberson, P.; Zanzonico, P.B.; Ljungberg, M. MIRD Pamphlet No. 23: Quantitative SPECT for Patient-Specific 3-Dimensional Dosimetry in Internal Radionuclide Therapy. J. Nucl. Med. 2012, 53, 1310–1325. [Google Scholar] [CrossRef]
- Umeda, I.O.; Kotaro, T.; Tsuda, K.; Kobayashi, M.; Ogata, M.; Kimura, S.; Yoshimoto, M.; Kojima, S.; Moribe, K.; Yamamoto, K.; et al. High resolution SPECT imaging for visualization of intratumoral heterogeneity using a SPECT/CT scanner dedicated for small animal imaging. Ann. Nucl. Med. 2012, 26, 67–76. [Google Scholar] [CrossRef]
- de Kemp, R.A.; Epstein, F.H.; Catana, C.; Tsui, B.M.W.; Ritman, E.L. Small-Animal Molecular Imaging Methods. J. Nucl. Med. 2010, 51, 18–32. [Google Scholar] [CrossRef]
- Bolch, W.E.; Bouchet, L.G.; Robertson, J.S.; Wessels, B.W.; Siegel, J.A.; Howell, R.W.; Erdi, A.K.; Aydogan, B.; Costes, S.; Watson, E.E. MIRD Pamphlet No. 17: The Dosimetry of Nonuniform Activity Distributions—Radionuclide S Values at the Voxel Level. J. Nucl. Med. 1999, 40, 118–368. [Google Scholar]
- Gupta, A.; Lee, M.S.; Kim, J.H.; Lee, D.S.; Lee, J.S. Preclinical Voxel-Based Dosimetry in Theranostics: A Review. Nucl. Med. Mol. Imaging 2020, 54, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Keenan, M.A.; Stabin, M.G.; Segars, W.P.; Fernald, M.J. RADAR realistic animal model series for dose assessment. J. Nucl. Med. 2010, 51, 471–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Radionuclide | Radiation Type | Energy | Range | LET |
|---|---|---|---|---|
| 90Y | beta | 50–2300 keV | 0.05–12 mm | 0.2 keV/mm |
| 111In | Auger | eV–keV | 2–500 nm | 4–6 keV/mm |
| 225Ac | alpha | 5–9 MeV | 40–100 μm | 80 keV/mm |
| Radionuclide | Cell Line | TAC | Cell Geometry | Target | RBEm | Ref. |
|---|---|---|---|---|---|---|
| I | CA20948 | sphere | entire cell | 1.2 | [36] | |
| Lu | U2OS + SSTR2 | realistic | nucleus | 1.7 | [31] | |
| Lu | U2OS + SSTR2 | sphere | nucleus | 0.5 | [19] | |
| Lu | CA20948 | sphere | nucleus | 3.5 | [19] | |
| Lu | CA20948 | sphere | entire cell | 0.4 | [37] | |
| Lu | Capan-2 | sphere | nucleus | 1 | [38] | |
| Lu | LNCaP | sphere | entire cell | 0.4 | [10] | |
| Lu | DU145 | sphere | entire cell | 1.5 | [10] | |
| Lu | PC3 | sphere | entire cell | 1.5 | [10] | |
| P | SCL-II | sphere | nucleus | 0.5 | [39] | |
| Y | HCT116 | sphere | nucleus | 0.2 | [14] | |
| Y | SW48 | sphere | nucleus | 0.2 | [14] | |
| Y | HT29 | sphere | nucleus | 0.2 | [14] | |
| Average RBE | 1.0 ± 0.9 | |||||
| I | PC Cl3 | sphere | nucleus | 3.4 | [32] | |
| I | PC Cl3 | sphere | cytoplasm | 1.7 | [32] | |
| I | PC Cl3 | sphere | entire cell | 1.9 | [32] | |
| Tc | PC Cl3 | sphere | nucleus | 2.2 | [32] | |
| Tc | PC Cl3 | sphere | cytoplasm | 0.7 | [32] | |
| Tc | PC Cl3 | sphere | entire cell | 0.8 | [32] | |
| I | SCL-II | sphere | nucleus | 4.5 | [39] | |
| Cu | MCF7/HER2-18 | sphere | nucleus | 0.6 | [40] | |
| Average RBE | 2.0 ± 1.3 | |||||
| Bi | CA20948 | sphere | entire cell | 2.7 | [37] | |
| Bi | CA20948 | sphere | entire cell | 3.5 | [37] | |
| Bi | Capan-2 | sphere | nucleus | 3.4 | [38] | |
| Am | LNCaP | sphere | entire cell | 8.1 | [10] | |
| Am | DU145 | sphere | entire cell | 15.2 | [10] | |
| Am | PC3 | sphere | entire cell | 14.0 | [10] | |
| Am | SW-1573 | sphere | nucleus | 14.7 | [41] | |
| Average RBE | 8.8 ± 5.3 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spoormans, K.; Crabbé, M.; Struelens, L.; De Saint-Hubert, M.; Koole, M. A Review on Tumor Control Probability (TCP) and Preclinical Dosimetry in Targeted Radionuclide Therapy (TRT). Pharmaceutics 2022, 14, 2007. https://doi.org/10.3390/pharmaceutics14102007
Spoormans K, Crabbé M, Struelens L, De Saint-Hubert M, Koole M. A Review on Tumor Control Probability (TCP) and Preclinical Dosimetry in Targeted Radionuclide Therapy (TRT). Pharmaceutics. 2022; 14(10):2007. https://doi.org/10.3390/pharmaceutics14102007
Chicago/Turabian StyleSpoormans, Kaat, Melissa Crabbé, Lara Struelens, Marijke De Saint-Hubert, and Michel Koole. 2022. "A Review on Tumor Control Probability (TCP) and Preclinical Dosimetry in Targeted Radionuclide Therapy (TRT)" Pharmaceutics 14, no. 10: 2007. https://doi.org/10.3390/pharmaceutics14102007
APA StyleSpoormans, K., Crabbé, M., Struelens, L., De Saint-Hubert, M., & Koole, M. (2022). A Review on Tumor Control Probability (TCP) and Preclinical Dosimetry in Targeted Radionuclide Therapy (TRT). Pharmaceutics, 14(10), 2007. https://doi.org/10.3390/pharmaceutics14102007






