Bactericidal Activity of Carvacrol against Streptococcus pyogenes Involves Alteration of Membrane Fluidity and Integrity through Interaction with Membrane Phospholipids
Abstract
1. Introduction
2. Materials and Methods
2.1. Media and Chemicals
2.2. Bacterial Strains and Growth Conditions
2.3. Membrane Permeability
2.4. Fluorescence Microscopy of Bacterial Viability
2.5. Membrane Potential
2.6. Protoplast Experiments
2.6.1. Preparation of Protoplasts
2.6.2. Confirmation of Protoplast Formation
Microscopy of Protoplast Samples
Gel Electrophoresis of Protoplast Samples
2.6.3. Confirmation of the Effect of Carvacrol on Bacterial Membrane
Effect of Carvacrol on Protoplast Samples
Transmission Electron Microscopy (TEM) of Carvacrol-Treated Protoplast Samples
2.7. Fluorescence Anisotropy
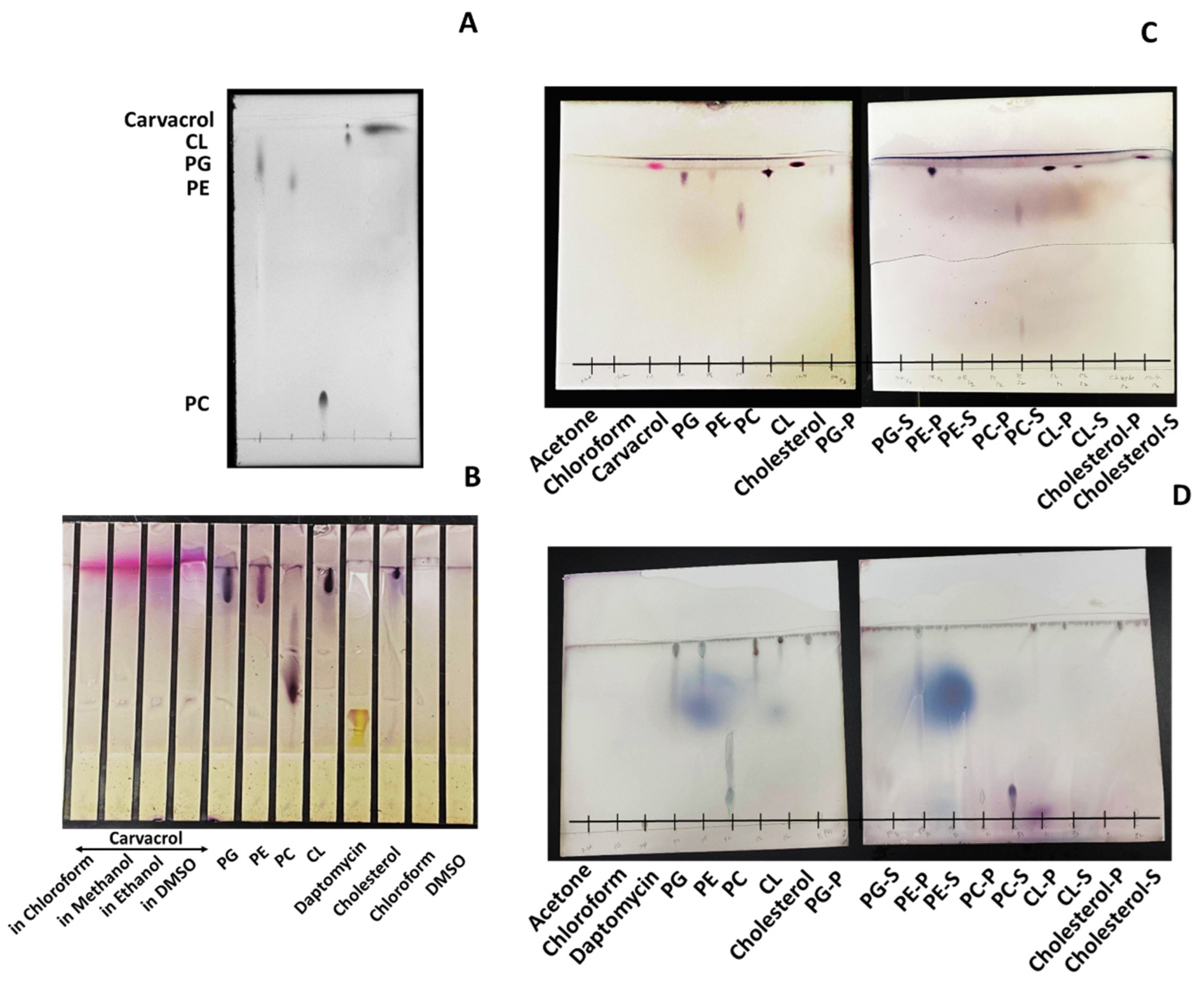
2.8. Thin-Layer Chromatography (TLC) Analysis
2.9. Statistical Analysis
3. Results
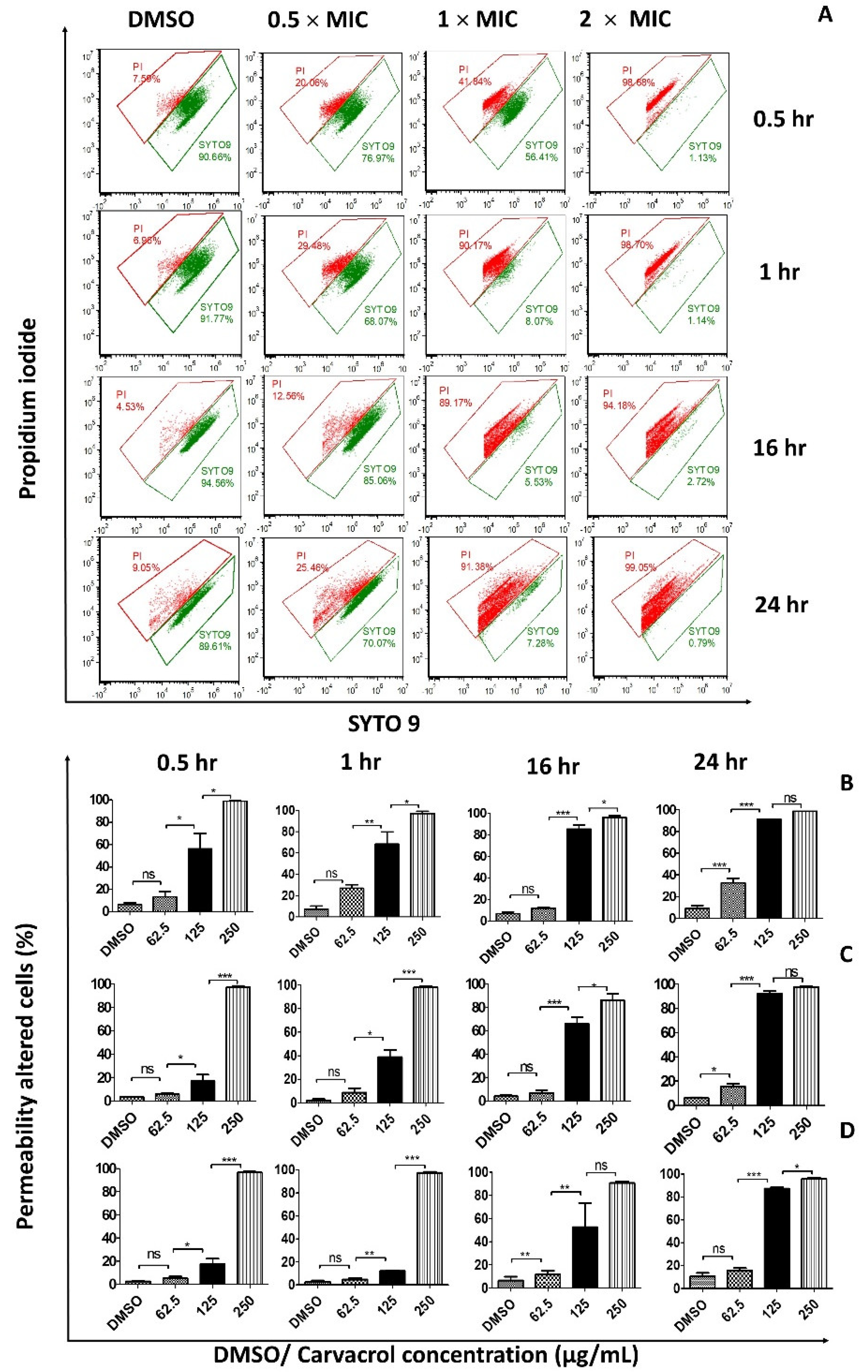
3.1. Carvacrol Increases the Permeability of the Bacterial Cell Membrane
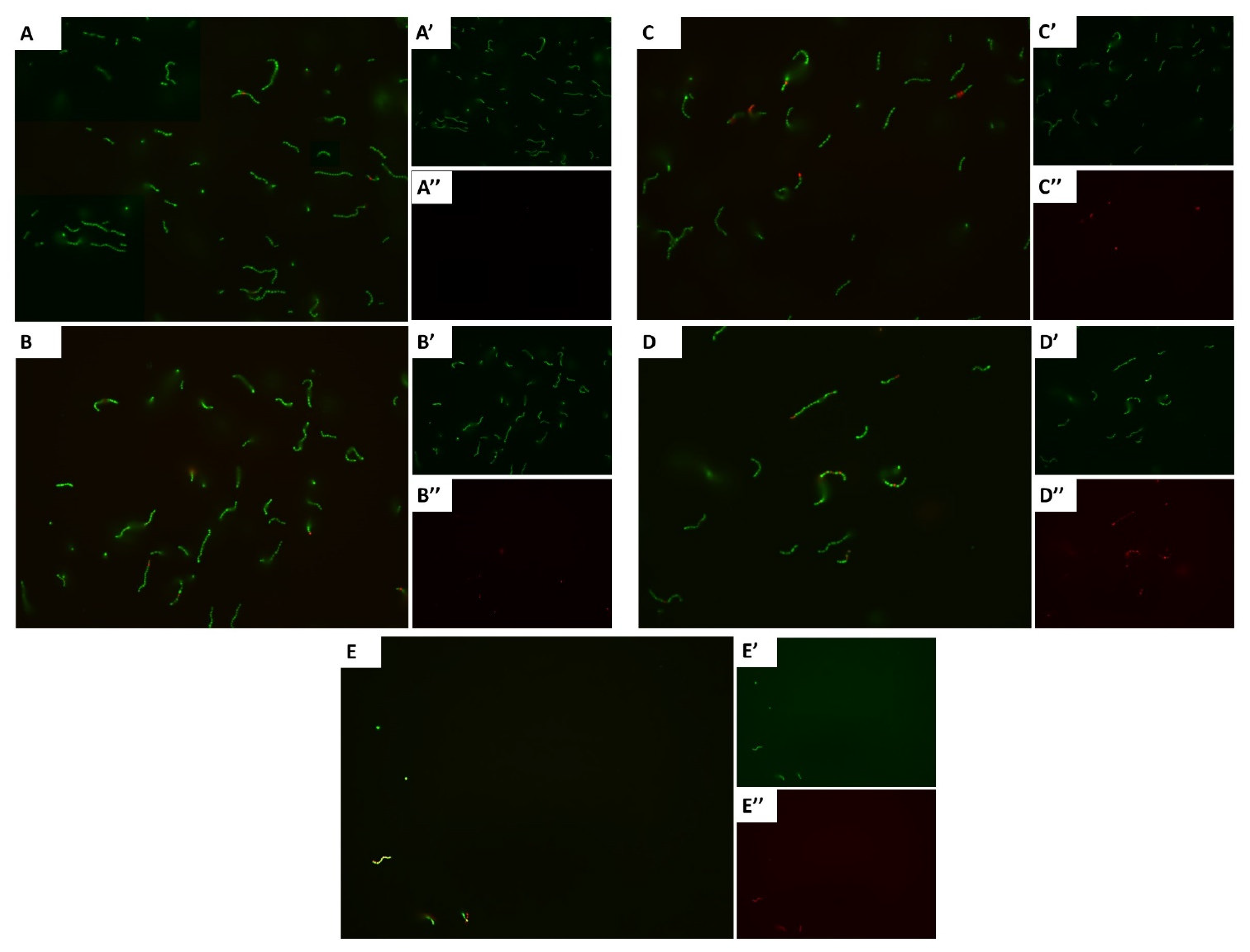
3.2. Carvacrol Shows a Concentration-Dependent Increment in Dead Cells
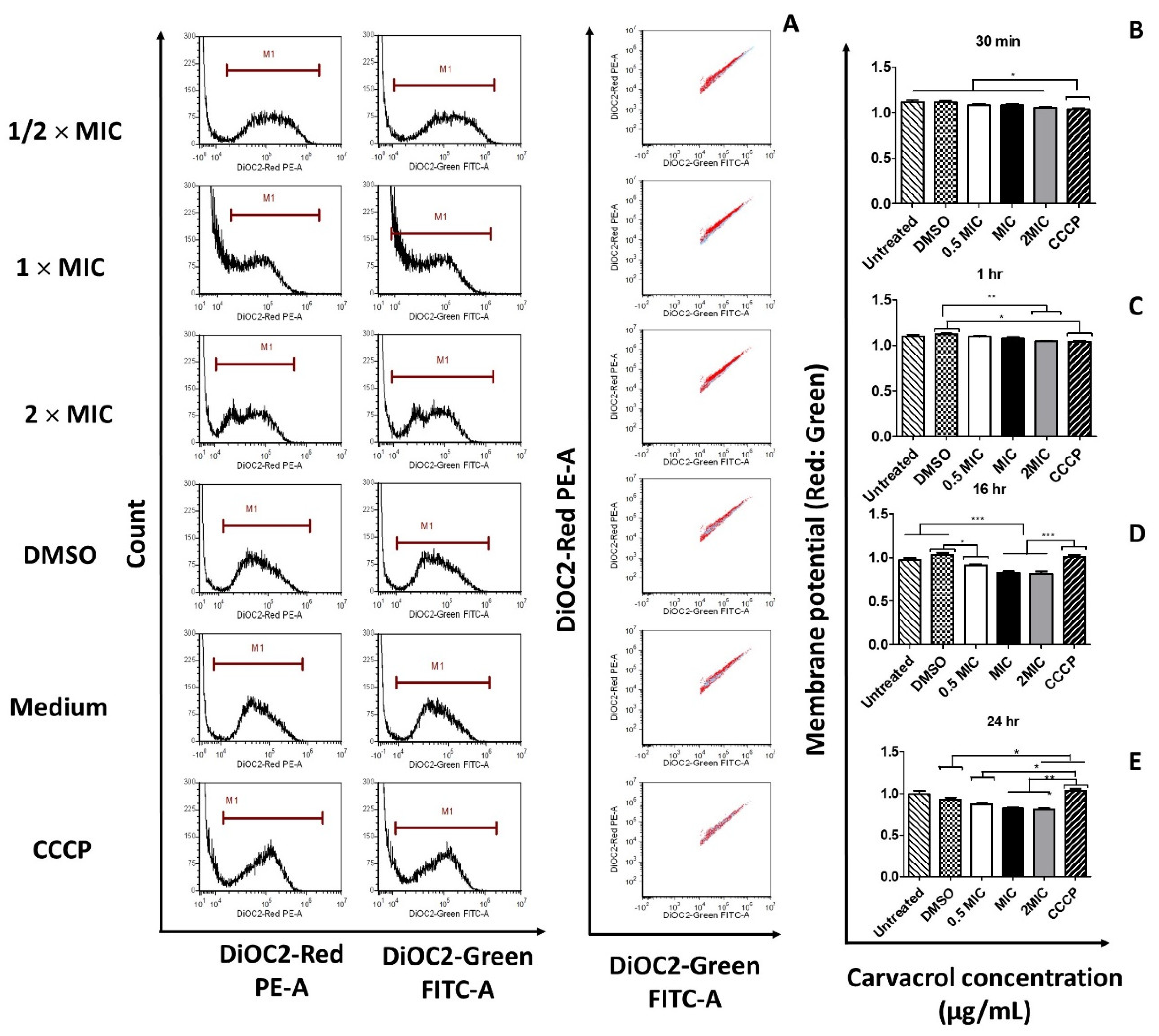
3.3. Carvacrol Causes Depolarization of the Cytoplasmic Membrane of S. pyogenes
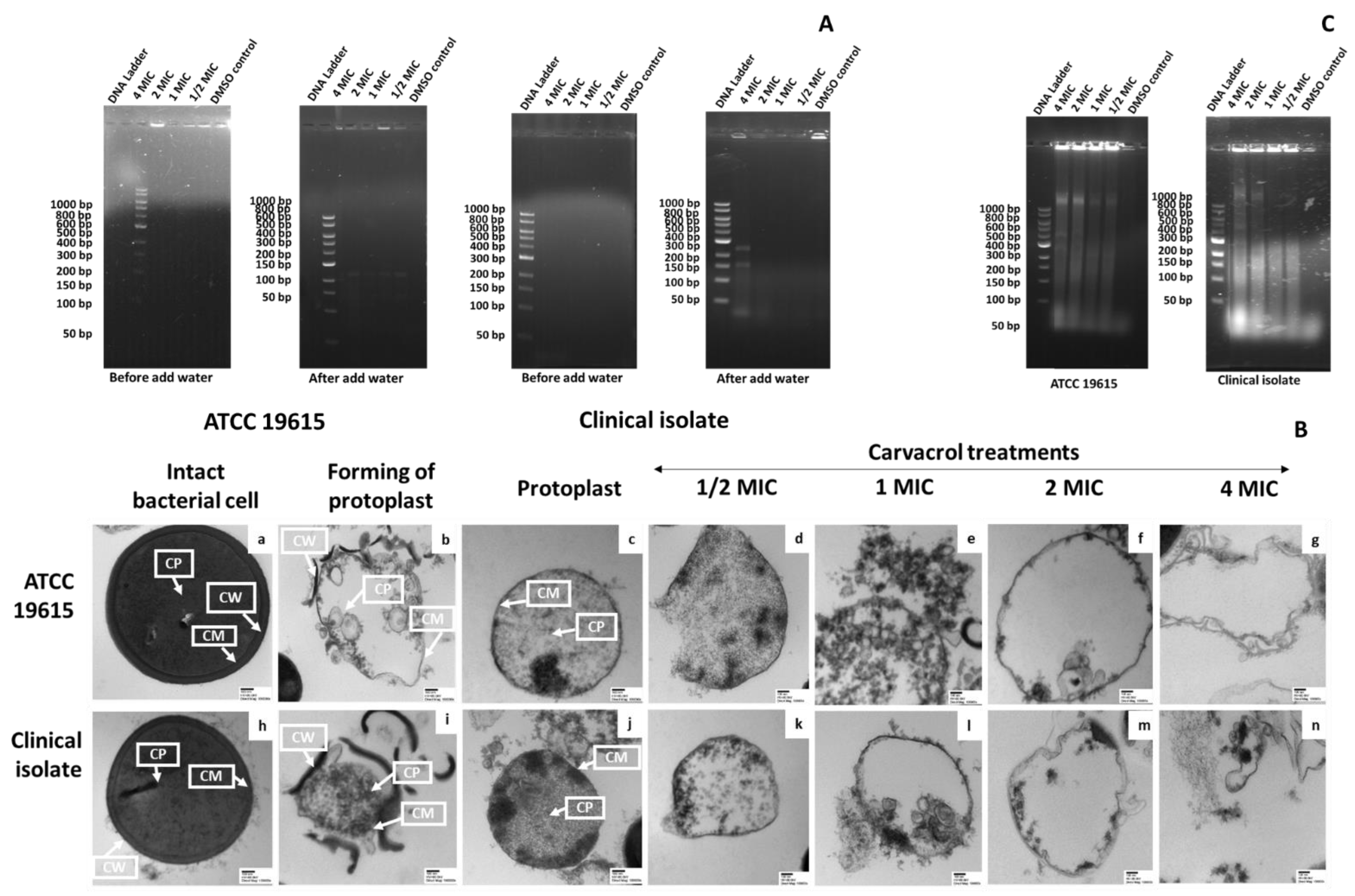
3.4. Carvacrol-Induced Membrane Damage in Protoplasts
3.4.1. Confirmation of Protoplast Formation
3.4.2. The Effect of Carvacrol on Protoplast Membrane
3.5. Carvacrol Causes Concentration-Dependent Membrane Fluidity Changes
3.6. Carvacrol Preferentially Binds to P.G., P.E., and Partially to CL in the Bacterial Membrane
4. Discussion
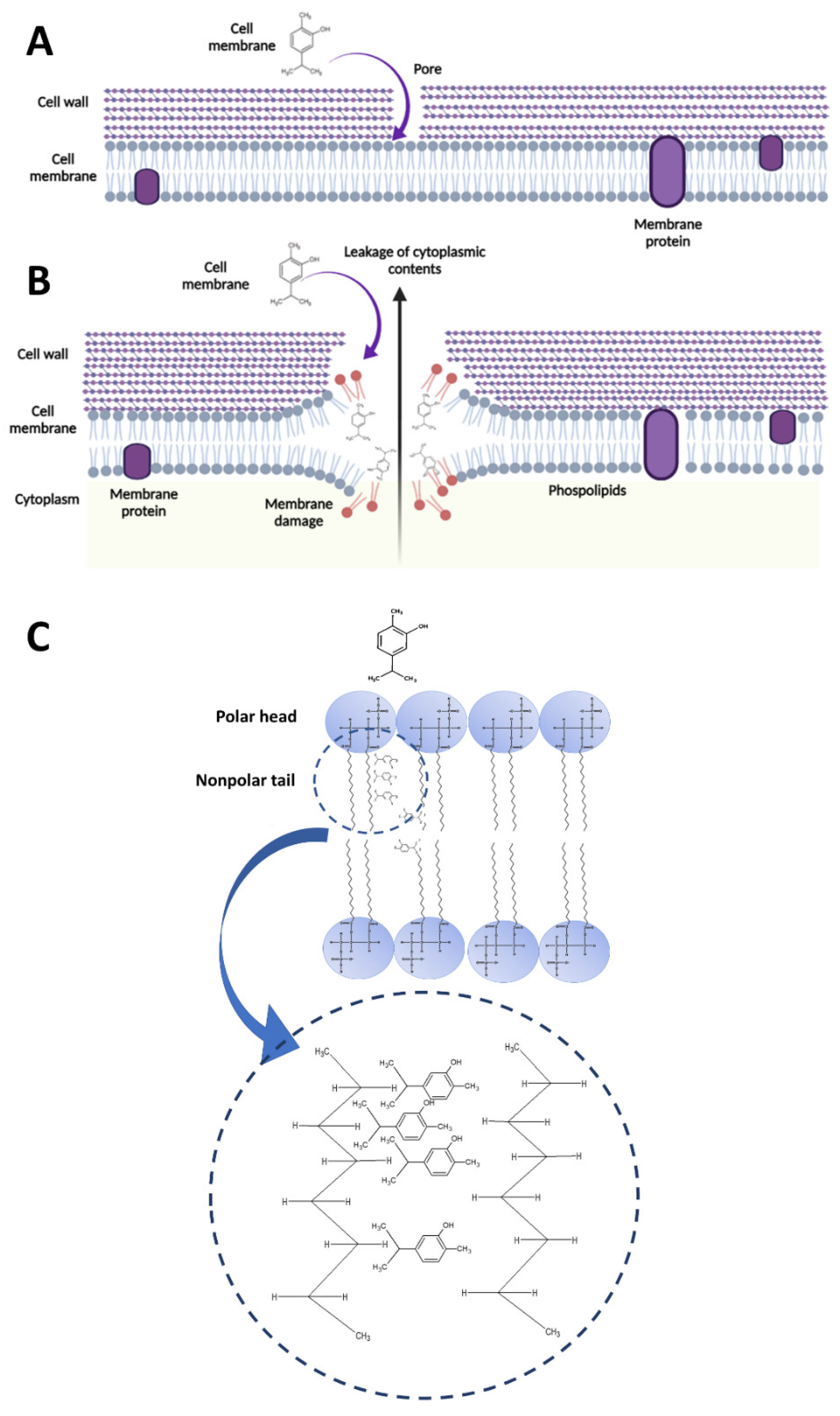
4.1. Carvacrol-Induced Cell Membrane Integrity Losses and Changes in Permeability, Potential, and Fluidity
4.2. Interaction between Carvacrol and Membrane Phospholipids
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wijesundara, N.M.; Rupasinghe, H.P.V. Essential oils from Origanum vulgare and Salvia officinalis exhibit antibacterial and anti-biofilm activities against Streptococcus pyogenes. Microb. Pathog. 2018, 117, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.G.; Lee, J. Carvacrol-rich oregano oil and thymol-rich thyme red oil inhibit biofilm formation and the virulence of uropathogenic Escherichia coli. J. Appl. Microbiol. 2017, 123, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, V.; Rojas, F.; Tedesco, V.; Giusiano, G.; Angiolella, L. Chemical characterization and antifungal activity of Origanum vulgare, Thymus vulgaris essential oils and carvacrol against Malassezia furfur. Nat. Prod. Res. 2019, 33, 3273–3277. [Google Scholar] [CrossRef] [PubMed]
- Wijesundara, N.M.; Rupasinghe, H.P.V. Bactericidal and anti-biofilm activity of ethanol extracts derived from selected medicinal plants against Streptococcus pyogenes. Molecules 2019, 24, 1165. [Google Scholar] [CrossRef]
- Li, H.; Yang, T.; Li, F.-Y.; Yao, Y.; Sun, Z.-M. Antibacterial activity and mechanism of action of Monarda punctata essential oil and its main components against common bacterial pathogens in respiratory tract. Int. J. Clin. Exp. Pathol. 2014, 7, 7389–7398. [Google Scholar]
- Al-Mnaser, A.A.; Woodward, M.J. Sub-lethal Concentrations of Phytochemicals (Carvacrol and Oregano) Select for Reduced Susceptibility Mutants of Escherichia coli O23:H52. Pol. J. Microbiol. 2020, 69, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wijesundara, N.M.; Lee, S.F.; Cheng, Z.; Davidson, R.; Rupasinghe, H.P.V. Carvacrol exhibits rapid bactericidal activity against Streptococcus pyogenes through cell membrane damage. Sci. Rep. 2021, 11, 1487. [Google Scholar] [CrossRef] [PubMed]
- Pfoh, E.; Wessels, M.R.; Goldmann, D.; Lee, G.M. Burden and economic cost of group A streptococcal pharyngitis. Pediatrics 2008, 121, 229–234. [Google Scholar] [CrossRef]
- Nelson, G.E.; Pondo, T.; Toews, K.-A.; Farley, M.M.; Lindegren, M.L.; Lynfield, R.; Aragon, D.; Zansky, S.M.; Watt, J.P.; Cieslak, P.R.; et al. Epidemiology of Invasive Group A Streptococcal Infections in the United States, 2005–2012. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 63, 478–486. [Google Scholar] [CrossRef] [PubMed]
- van Driel, M.L.; De Sutter, A.I.; Habraken, H.; Thorning, S.; Christiaens, T. Different antibiotic treatments for group A streptococcal pharyngitis. Cochrane Database Syst. Rev. 2016, 9, CD004406. [Google Scholar] [CrossRef]
- Shulman, S.T.; Bisno, A.L.; Clegg, H.W.; Gerber, M.A.; Kaplan, E.L.; Lee, G.; Martin, J.M.; Van Beneden, C. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2012, 55, 1279–1282. [Google Scholar] [CrossRef]
- Magi, G.; Marini, E.; Facinelli, B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci. Front. Microbiol. 2015, 6, 165. [Google Scholar] [CrossRef] [PubMed]
- Silva-Costa, C.; Friaes, A.; Ramirez, M.; Melo-Cristino, J. Macrolide-resistant Streptococcus pyogenes: Prevalence and treatment strategies. Expert Rev. Anti. Infect. 2015, 13, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Bahuguna, A.; Kumar, P.; Bajpai, V.K.; Kang, S.C. Antimicrobial potential of carvacrol against uropathogenic Escherichia coli via membrane disruption, depolarization, and reactive oxygen species generation. Front. Microbiol. 2017, 8, 2421. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Khan, M.; Ahmad, J.; Wahab, R.; Abd-Elkader, O.H.; Musarrat, J.; Alkhathlan, H.Z.; Al-Kedhairy, A.A. Thymol and carvacrol induce autolysis, stress, growth inhibition and reduce the biofilm formation by Streptococcus mutans. AMB Express 2017, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Di Pasqua, R.; Hoskins, N.; Betts, G.; Mauriello, G. Changes in membrane fatty acids composition of microbial cells induced by addiction of thymol, carvacrol, limonene, cinnamaldehyde, and eugenol in the growing media. J. Agric. Food Chem. 2006, 54, 2745–2749. [Google Scholar] [CrossRef]
- Linder, L.; Andersson, C.; Sund, M.L.; Shockman, G.D. Protoplast formation and localization of enzymes in Streptococcus mitis. Infect. Immun. 1983, 40, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Parks, L.C.; Shockman, G.D.; Higgins, M.L. Growth of Streptococcus mutans protoplasts is not inhibited by penicillin. J. Bacteriol. 1980, 143, 1491–1497. [Google Scholar] [CrossRef]
- Allaoua, M.; Etienne, P.; Noirot, V.; Carayon, J.L.; Tene, N.; Bonnafe, E.; Treilhou, M. Pharmacokinetic and antimicrobial activity of a new carvacrol-based product against a human pathogen, Campylobacter jejuni. J. Appl. Microbiol. 2018, 125, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Chavan, P.S.; Tupe, S.G. Antifungal activity and mechanism of action of carvacrol and thymol against vineyard and wine spoilage yeasts. Food Control 2014, 46, 115–120. [Google Scholar] [CrossRef]
- Stratford, J.P.; Edwards, C.L.A.; Ghanshyam, M.J.; Malyshev, D.; Delise, M.A.; Hayashi, Y.; Asally, M. Electrically induced bacterial membrane-potential dynamics correspond to cellular proliferation capacity. Proc. Natl. Acad. Sci. USA 2019, 116, 9552–9557. [Google Scholar] [CrossRef] [PubMed]
- Strahl, H.; Hamoen, L.W. Membrane potential is important for bacterial cell division. Proc. Natl. Acad. Sci. USA 2010, 107, 12281–12286. [Google Scholar] [CrossRef] [PubMed]
- Hurdle, J.G.; O’Neill, A.J.; Chopra, I.; Lee, R.E. Targeting bacterial membrane function: An underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol. 2011, 9, 62–75. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Dimroth, P.; Kaim, G.; Matthey, U. Crucial role of the membrane potential for ATP synthesis by F(1) F(o) ATP synthases. J. Exp. Biol. 2000, 203, 51–59. [Google Scholar] [CrossRef]
- Chawla, M.; Singh, A. Detection of membrane potential in Mycobacterium tuberculosis. Bio-Protocol 2013, 3, e785. [Google Scholar] [CrossRef]
- Khater, M.; Khater, S.S.; Gholap, H.; Patil, R.; Kulkarni, G. Comparative studies on measurement of membrane potential of bacterial cells treated with ZnO nanoparticles by spectrofluorometry, fluorescence microscopy and flowcytometry. J. Microbiol. Methods 2020, 173, 105920. [Google Scholar] [CrossRef]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial membrane potential probes and the proton gradient: A practical usage guide. Biotechniques 2011, 50, 98–115. [Google Scholar] [CrossRef]
- Clementi, E.A.; Marks, L.R.; Roche-Håkansson, H.; Håkansson, A.P. Monitoring changes in membrane polarity, membrane integrity, and intracellular ion concentrations in Streptococcus pneumoniae using fluorescent dyes. J. Vis. Exp. 2014, 84, e51008. [Google Scholar] [CrossRef]
- Nowotarska, S.W.; Nowotarski, K.; Grant, I.R.; Elliott, C.T.; Friedman, M.; Situ, C. Mechanisms of antimicrobial action of cinnamon and oregano oils, cinnamaldehyde, carvacrol, 2,5-Dihydroxybenzaldehyde, and 2-Hydroxy-5-Methoxybenzaldehyde against Mycobacterium avium subsp. paratuberculosis (Map). Foods 2017, 6, 72. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef]
- Casares, D.; Escribá, P.V.; Rosselló, C.A. Membrane Lipid Composition: Effect on Membrane and Organelle Structure, Function and Compartmentalization and Therapeutic Avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef] [PubMed]
- Denich, T.J.; Beaudette, L.A.; Lee, H.; Trevors, J.T. Effect of selected environmental and physico-chemical factors on bacterial cytoplasmic membranes. J. Microbiol. Methods 2003, 52, 149–182. [Google Scholar] [CrossRef]
- Gohrbandt, M.; Lipski, A.; Baig, Z.; Walter, S.; Kurre, R.; Strahl, H.; Deckers-Hebestreit, G. Low membrane fluidity triggers lipid phase separation and protein segregation in vivo. bioRxiv 2019, 852160. [Google Scholar] [CrossRef]
- Aricha, B.; Fishov, I.; Cohen, Z.; Sikron, N.; Pesakhov, S.; Khozin-Goldberg, I.; Dagan, R.; Porat, N. Differences in membrane fluidity and fatty acid composition between phenotypic variants of Streptococcus pneumoniae. J. Bacteriol. 2004, 186, 4638. [Google Scholar] [CrossRef]
- Svobodova, J.; Svoboda, P. Cytoplasmic membrane fluidity measurements on intact living cells of Bacillus subtilis by fluorescence anisotropy of 1,6-diphenyl-1,3,5-hexatriene. Folia Microbiol Praha 1988, 33, 1–9. [Google Scholar] [CrossRef]
- Nowotarska, S.W.; Nowotarski, K.J.; Friedman, M.; Situ, C. Effect of structure on the interactions between five natural antimicrobial compounds and phospholipids of bacterial cell membrane on model monolayers. Molecules 2014, 19, 7497–7515. [Google Scholar] [CrossRef]
- Sohlenkamp, C.; Geiger, O. Bacterial membrane lipids: Diversity in structures and pathways. FEMS Microbiol. Rev. 2015, 40, 133–159. [Google Scholar] [CrossRef]
- Gharib, R.; Auezova, L.; Charcosset, C.; Greige-Gerges, H. Effect of a series of essential oil molecules on DPPC membrane fluidity: A biophysical study. J. Iran. Chem. Soc. 2018, 15, 75–84. [Google Scholar] [CrossRef]
- Fan, X.; Wagner, K.; Sokorai, K.J.B.; Ngo, H. Inactivation of Gram-Positive Bacteria by Novel Phenolic Branched-Chain Fatty Acids. J. Food Prot. 2017, 80, 6–14. [Google Scholar] [CrossRef]
- Ledger, E.V.K.; Pader, V.; Edwards, A.M. Enterococcus faecalis and pathogenic streptococci inactivate daptomycin by releasing phospholipids. Microbiol. Read. 2017, 163, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Heidary, M.; Khosravi, A.D.; Khoshnood, S.; Nasiri, M.J.; Soleimani, S.; Goudarzi, M. Daptomycin. J. Antimicrob. Chemother. 2017, 73, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.D.; Palmer, M. The action mechanism of daptomycin. Bioorg. Med. Chem. 2016, 24, 6253–6268. [Google Scholar] [CrossRef] [PubMed]
| Samples | DPH Anisotropy Value * | |
|---|---|---|
| 1 h | 24 h | |
| PBS | 0.012 ± 0.01 | 0.011 ± 0.01 |
| DMSO | 0.012 ± 0.01 | 0.023 ± 0.01 |
| Carvacrol (1 × MIC) | 0.012 ± 0.01 | 0.018 ± 0.01 |
| Carvacrol (2 × MIC) | 0.005 ± 0.00 | 0.047 ± 0.00 |
| Triton X-100 | 0.009 ± 0.01 | 0.042 ± 0.00 |
| Tween-20 | 0.030 ± 0.00 | 0.034 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijesundara, N.M.; Lee, S.F.; Cheng, Z.; Davidson, R.; Langelaan, D.N.; Rupasinghe, H.P.V. Bactericidal Activity of Carvacrol against Streptococcus pyogenes Involves Alteration of Membrane Fluidity and Integrity through Interaction with Membrane Phospholipids. Pharmaceutics 2022, 14, 1992. https://doi.org/10.3390/pharmaceutics14101992
Wijesundara NM, Lee SF, Cheng Z, Davidson R, Langelaan DN, Rupasinghe HPV. Bactericidal Activity of Carvacrol against Streptococcus pyogenes Involves Alteration of Membrane Fluidity and Integrity through Interaction with Membrane Phospholipids. Pharmaceutics. 2022; 14(10):1992. https://doi.org/10.3390/pharmaceutics14101992
Chicago/Turabian StyleWijesundara, Niluni M., Song F. Lee, Zhenyu Cheng, Ross Davidson, David N. Langelaan, and H. P. Vasantha Rupasinghe. 2022. "Bactericidal Activity of Carvacrol against Streptococcus pyogenes Involves Alteration of Membrane Fluidity and Integrity through Interaction with Membrane Phospholipids" Pharmaceutics 14, no. 10: 1992. https://doi.org/10.3390/pharmaceutics14101992
APA StyleWijesundara, N. M., Lee, S. F., Cheng, Z., Davidson, R., Langelaan, D. N., & Rupasinghe, H. P. V. (2022). Bactericidal Activity of Carvacrol against Streptococcus pyogenes Involves Alteration of Membrane Fluidity and Integrity through Interaction with Membrane Phospholipids. Pharmaceutics, 14(10), 1992. https://doi.org/10.3390/pharmaceutics14101992









